Abstract
Background:
Elevated cerebral fractional tissue oxygen extraction (cFTOE) is an adaptation to anemia of prematurity (AOP). cFTOE ≥0.4 is associated with brain injury in infants ≤30 weeks. This longitudinal study sought to investigate the utility of cFTOE in the evaluation of AOP.
Methods:
Infants ≤30 weeks estimated gestational age (EGA) underwent weekly hemoglobin, cerebral saturation, and pulse oximetry recordings from the second through 36 weeks post-menstrual age (PMA). Recordings were excluded if they were under 1 hour or if hemoglobin was not measured within 7 days of recording. Mean cFTOE was calculated for each recording. Statistical analysis used linear mixed-effects modeling and receiver operating characteristic analysis.
Results:
144 recordings from 39 infants (mean EGA 27.6 ± 2.2 weeks, BW 1139 ± 286g) were included of whom 39% (15/39) were transfused. The mean recording length was 2.8 ± 1.3 hours. There was a significant negative correlation between hemoglobin and cFTOE (R = −0.423, p=<0.001). In a multivariate model, adjusting for EGA, PMA, and patent ductus arteriosus treatment the AUC was 0.821. A critical increase in cFTOE occurred at a hemoglobin level of 9.6 g/dL.
Conclusions:
AOP is associated with a critical increase in cFTOE that occurs at a significantly higher hemoglobin level than standard clinical thresholds for transfusion.
Keywords: Anemia of prematurity, prematurity, cerebral NIRS, oxygen extraction
Introduction
Anemia of prematurity (AOP) continues to present a common and significant management challenge in the NICU as demonstrated by the substantial variation in clinical transfusion practices [1-4]. Though transfusions in some populations have decreased, the burden remains high with more than half of preterm infants < 30 weeks estimated gestational age (EGA) and up to 85% of those < 26 weeks EGA receiving packed red blood cell (pRBC) transfusions during their NICU hospitalization [5,6]. Conflicting data on appropriate transfusion thresholds for preterm infants and emerging data on potential sequelae of both severe anemia and transfusion make it difficult for clinicians to decide when to transfuse an individual patient [7-12]. Published randomized trials demonstrate heterogenous results; some studies support a reduction in short-term brain injury and apnea outcomes with liberal transfusion practices [13], others demonstrate no statistically significant difference in neurologic injury or neurodevelopmental outcomes [10,14], and yet others suggest a difference in longer term neurodevelopmental outcomes [9]. Two ongoing studies (Transfusions of Prematures (TOP) NCT01702805, and Effects of Transfusion Thresholds on Neurocognitive Outcome of Extremely Low Birth Weight Infants (ETTNO) NCT01393496) aim to definitively answer this question with adequate statistical power.
Nevertheless, even the existing data are compelling enough to prompt an evaluation into the mechanism which might underlie the link between anemia and brain injury risk. While the brain has a remarkable ability to compensate for fluctuations in the delivery of oxygen by increasing the fraction of oxygen extracted from the blood, this autoregulatory system can be exceeded [15,16]. The central focus of this project was to investigate the impact of anemia of prematurity (AOP) on cerebral tissue hypoxia and compensatory oxygen extraction.
While available data on cerebral compensation for AOP are limited, cross-sectional studies have revealed lower cerebral saturations and higher cerebral oxygen extraction in the setting of anemia and that this pathologic state normalizes with pRBC transfusion [17-20]. Existing studies comparing transfusion thresholds have relied on standard clinical monitoring of vital signs (heart rate, pulse-oximetry) and have not examined compensatory responses to progressive anemia or its impact on end-organ tissue oxygenation. While pulse oximetry provides important information about arterial oxygen saturation, it does not assess oxygen delivery or consumption at the tissue-specific level. In contrast, near-infrared spectroscopy (NIRS) provides a noninvasive measure of regional (cerebral) tissue oxygenation (C-rSO2) and allows for calculation of cerebral tissue oxygen extraction [21-24].
Prior work by our group demonstrated a strong relationship between hemoglobin and cerebral saturation in transfusion-naïve preterm infants where worsening anemia was associated with a progressive decrease in C-rSO2. In that study, we demonstrated that a hemoglobin concentration of 9.5g/dL or less results in cerebral desaturation >2 SD below the normative mean [25,26]. This study was limited by a lack of simultaneous pulse-oximetry, which is needed to calculate the cerebral fractional tissue oxygen extraction (cFTOE). Without this information, it is difficult to assess the magnitude of the compensatory response.
We sought to address this shortcoming in the present study by focusing on the longitudinal impact of anemia of prematurity on cFTOE in preterm infants. We hypothesized that preterm infants are able to compensate for mild anemia, but that there is a critical threshold, beyond which pathologic oxygen extraction occurs, increasing the risk for brain injury and that NIRS would provide insight into this loss of compensation. Using simultaneous NIRS and pulse oximetry, we prospectively evaluated the longitudinal impact of increasing anemia on cFTOE in a cohort of preterm infants ≤30 completed weeks EGA from the second week of life through 36 weeks post-menstrual age (PMA).
Methods
Patient selection
In this prospective observational study, preterm infants born at or before 30 weeks completed gestation were recruited in the first 14 days of life from the St. Louis Children’s Hospital NICU, a level IV unit serving an urban, suburban, and rural population. Infants were excluded if they had known congenital or chromosomal anomalies or were clinically unstable and not expected to survive the first week of life. Infants with severe IVH (grade III/IV on the Papile scale [27]) were also excluded from the analysis. Prior reports have demonstrated concern that the large pool of deoxygenated blood in the ventricles /parenchyma following severe IVH regionally alters the total hemoglobin and may depress the measured cerebral saturations on the ipsilateral hemisphere as the hemorrhage [28-30]. Additionally, the hemorrhage independently increases fractional oxygen extraction for an extended time period [31], further confounding measurement in these infants.
Informed written consent was obtained from parents for all participants. The study protocol and procedures were reviewed and approved by the Human Subjects Research Protection Office at Washington University.
Sample characteristics
Comprehensive sample characteristics were collected for all infants in the cohort. Antenatal characteristics included mode of delivery, antenatal corticosteroid exposure, delayed cord clamping, and the five-minute Apgar score. Patient characteristics included EGA in completed weeks, birth weight, small for gestational age (SGA) status (defined as birth weight <10th centile), gender, and race/ethnicity. Clinical factors included Clinical Risk Index for Babies II (CRIB-II) score (using the algorithm developed by Parry et al.[32]), IVH, respiratory support type and duration, medication administration, patent ductus arteriosus (PDA) requiring treatment (defined as moderate or large diameter ductus with concurrent left atrial and ventricular enlargement on echocardiogram), hemoglobin measurements, transfusions, and mortality. All hemoglobin values were obtained at clinical provider discretion.
Institutional practices and guidelines
Cerebral NIRS is not part of routine clinical monitoring of preterm infants at our institution. Cranial ultrasound evaluations are performed at least twice in the first ten days, generally on the third and tenth day of life. All studies are performed through the anterior fontanelle and the mastoid window to identify IVH and cerebellar hemorrhage.
Institutional transfusion guidelines for premature infants recommend pRBC transfusion for hemoglobin ≤10 g/dL in critically-ill infants (defined as invasive mechanical ventilation and/or inotropic support) and for hemoglobin ≤8 g/dL in stable, non-intubated infants. In order to reduce exposure to multiple donors, single units of blood are split into five equal aliquots and are stored until used or expired (6 weeks from split). Transfusions at our institution are generally given via peripheral IV and administered over 2 hours. All transfusions in the study cohort occurred at clinician discretion.
Data collection
Cerebral oximetry data were collected using NIRS via the INVOS 5100C oximeter with the OxyAlert Infant/Neonatal Sensor (Covidien, Mansfield, MA). The device utilizes a two-wavelength (730 and 810 nm) LED-based emitter and two optical detectors located 30 and 40 mm from the emitter, sampled at a rate of 0.2 Hz. The sensor was placed on the left frontoparietal scalp as standard. Weekly cerebral NIRS recordings 6-8 hours in length were conducted from the second week of life through 36 weeks PMA.
Pulse oximetry data (systemic oxygen saturation, SpO2) were obtained using an adhesive probe place on the infant’s hand or foot (Neonatal-Adult SpO2 sensor, Covidien, Mansfield, MA) and the Nellcor Oximax algorithm integrated into the patient’s bedside monitor (Phillips IntelliVue monitor MP70 or MX800, Andover, MA). Time-linked cerebral oximetry and pulse-oximetry data were captured from the infant’s monitor using ixTrend software (ixellence, Wildau, Germany).
Cerebral fractional tissue oxygen extraction (cFTOE) is the ratio of cerebral oxygen consumption to oxygen delivery. It is a composite measure influenced by factors which impair oxygen delivery (hypotension, hypoxia, acidosis, anemia) and oxygen utilization by the brain (from increased cerebral activity [33]). The autoregulatory system of the brain provides a compensatory mechanism which increases oxygen extraction in impaired states (inadequate delivery and/or increased metabolism). In order to define a pathologic or “critical” cFTOE, we utilized normative and outcome data published in the literature. We acknowledge that biologic systems are not truly binary and that the final threshold represents a balance between a practical definition and the evidence in the literature.
Numerous references support the relationship between impaired oxygen delivery from a variety of mechanisms (including anemia, hypotension, and respiratory distress syndrome) and increasing cFTOE [34-37] which is consistent even when using other methodologies for measurement of oxygen extraction such as MRI [38]. Although there are minor variations by gestational age, reference curves of nearly 1000 infants published by Alderliesten et al. suggest a mean cFTOE of 0.32 and that a threshold of 0.40 represents values at least two standard deviations beyond the normative distribution [25]. Increasing FTOE, particularly at levels ≥ 0.4, is also associated with an increased risk of ultrasound-identified brain injury in preterm infants ≤ 30 weeks [19,20,39,40] and adverse neurodevelopment at 2-3 years of age [41].
Recording analysis
Prior to analysis, all recordings were visually evaluated for quality and were eliminated if they were corrupted, of insufficient duration (<1 hour), if a hemoglobin measurement was not obtained within 1 week of the recording, or if a transfusion occurred between hemoglobin measurement and monitoring. The one-hour length threshold for sufficiency of raw data was empirically determined by examining the duration of C-rSO2 data required to obtain a stabilized mean within 10% of the value for a recording 6-8 hours in length, a methodology utilized by this group in a prior study [26].
After visual quality assessment, recordings underwent three-step error correction for data sufficiency, systemic desaturation, and motion artifact. Error correction was performed by dividing each recording into 60-second epochs. Each epoch was examined for data sufficiency and epochs were discarded if greater than 50% of either the C-rSO2 or SpO2 data was missing (e.g. probes not in good contact with skin or removed). Remaining epochs were next examined for systemic desaturation and any 60-second epoch with a mean SpO2 value <85% was eliminated as desaturation-related changes in arterial and venous contributions to C-rSO2 make NIRS values unreliable in this setting [42]. Finally, the remaining epochs were examined for motion artifact contamination, which was identified by sudden, non-physiologic changes in the measured SpO2. Any epoch where the measured SpO2 had second-to-second variation ≥5% was discarded for motion contamination, as established in a prior study [43]. After error-correction, a mean cFTOE was calculated for each recording along with corrected recording length and error correction rates. Calculation of cFTOE was performed using the standard equation: FTOE = (SpO2 – C-rSO2)/SpO2 [25].
Statistical Approach
The goal of this project was to identify the impact of longitudinally decreasing hemoglobin (with progressive AOP) on the risk of developing critical cerebral oxygen extraction, defined here as a cFTOE ≥0.4. Given the repeated-measures design of the study, a mixed-effects logistic regression model was used. In this model we evaluate the hemoglobin around the time of the NIRS measurement and the resulting cFTOE. This model was adjusted for important confounding fixed effects. These variables, selected from the comprehensive clinical data collected for the project, were included if they were statistically significant in univariate analysis, improved model discrimination, and/or were known determinants of cFTOE such as gestational age at birth and a hemodynamically significant PDA requiring treatment [44,45]. The repeated and randomly sampled nature of the observations are accounted for using the additional fixed effect of PMA at time of recording and a random effect by participant to control for within-subjects correlation.
To determine the hemoglobin threshold where the cFTOE increased beyond 0.4, we computed receiver-operating characteristics (ROC) of hemoglobin values by plotting sensitivity vs. 1 – specificity. To identify the hemoglobin value that best correlated with cFTOE ≥0.4, we selected the point with the highest sum of sensitivity and specificity (Youden’s J statistic) [46]. Differences between ROC curves were assessed using the DeLong method [47].
All statistical tests were two-tailed and considered significant at p<0.05. All statistical analysis was performed using R version 3.5.3 (R Foundation for Statistical Computing, Vienna, Austria). Mixed modeling was done using the lme4 package for R. As a similar study has not previously been performed, power was not calculated a priori but was rather a convenience sample over a fixed time interval.
Results
Patient characteristics
This study included 39 infants with mean ± SD EGA of 27.6 ± 2.1 weeks and mean ± SD birth weight of 1139 ± 286 grams. Of the included infants, 12 (31%) had grade I-II IVH. During monitoring, 15% (6/39) had a measured hemoglobin ≤8 g/dL and 69% (27/39) had a measured hemoglobin ≤10 g/dL with a median hemoglobin of 9.9 g/dL (range 7.1-17.0 g/dL). During hospitalization, 15/39 (39%) infants received a pRBC transfusion. Full cohort characteristics are listed in Table 1.
Table 1.
Cohort Clinical Characteristics
| n=39 | |
|---|---|
| EGA, mean ± SD | 27.6 ± 2.1 weeks |
| BW, mean ± SD | 1139 ± 286 g |
| Male sex, n (%) | 12 (31%) |
| Race, n (%) | |
| African-American | 15 (39%) |
| Caucasian | 24 (61%) |
| Antenatal steroids, n (%) | |
| Any | 25 (64%) |
| Complete | 20 (51%) |
| Cesarean delivery, n (%) | 28 (72%) |
| Delayed cord clamping, n (%) | 11 (29%) |
| 5 minute Apgar, median (range) | 6 (1-9) |
| CRIB-II score, median (range) | 9 (3-15) |
| Small for gestational age1, n (%) | 2 (5%) |
| Anemia during monitoring, n (%) | |
| Hemoglobin ≤8 g/dL | 6 (15%) |
| Hemoglobin ≤10 g/dL | 27 (69%) |
| Transfused, n (%) | 15 (39%) |
| Intraventricular hemorrhage, n (%) | 12 (31%) |
| Inotropic medications, n (%) | 2 (5%) |
| PDA treatment, n (%) | 5 (13%) |
| Necrotizing enterocolitis, n (%) | 1 (3%) |
| Bronchopulmonary dysplasia, n (%) | 17 (46%) |
| Mortality, n (%) | 1 (3%) |
Defined as BW <10th percentile.
Data quality
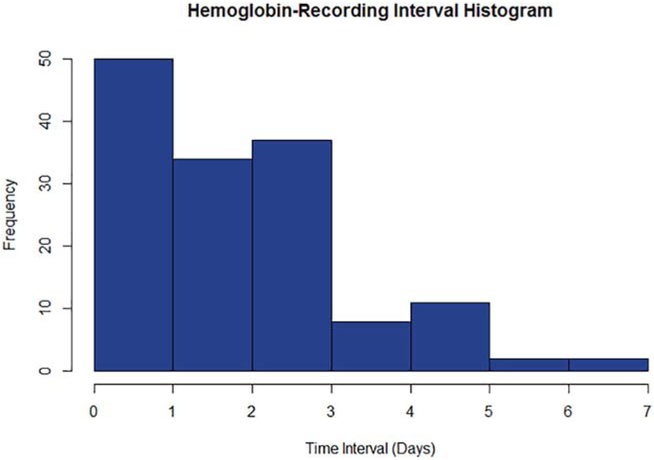
A total of 214 C-rSO2 recordings were obtained from the 39 infants included in our study. 144 recordings (67%) were included in the final analysis. The remaining 70 recordings were excluded due to missing/corrupted data (n=24), insufficient raw recording length (n=8), or absence of a hemoglobin measurement within 7 days (n=38). The mean raw recording length was 4.6 ± 1.0 hours with a mean data rejection rate of 40%. Of the identified errors, 34% were due to systemic desaturation, 34% were due to the NIRS probe not being in contact with the skin, and 32% were due to motion artifact detected in the pulse oximetry data. After error correction, the mean recording length was 2.8 ± 1.3 hours. The mean interval between recording and hemoglobin measurement was 2.3 ± 1.5 days (Figure 1).
Figure 1.
Histogram illustrating the latency between hemoglobin measurement and cerebral NIRS recording in days.
Statistical Analysis
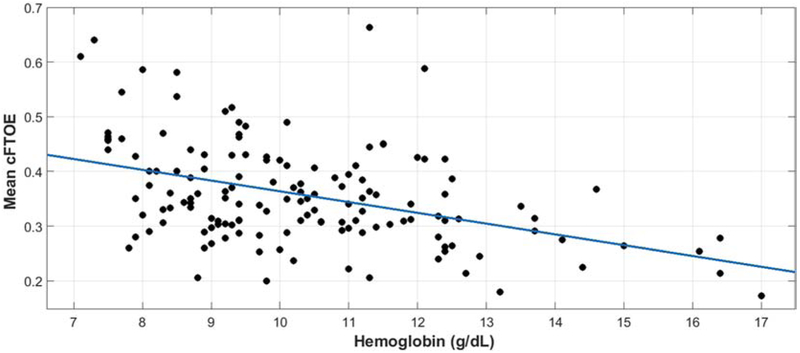
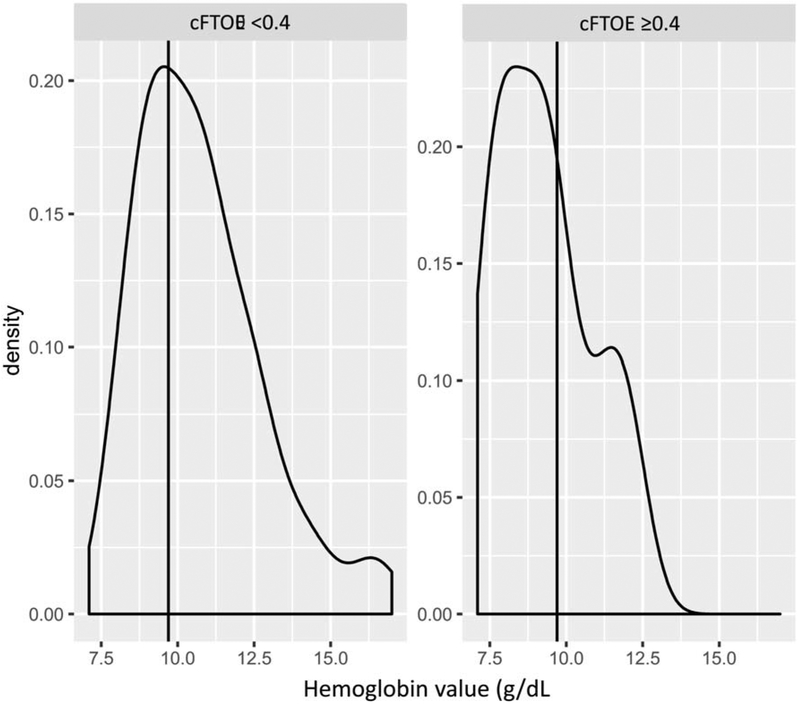
Unadjusted linear regression revealed a moderate statistically significant negative correlation between hemoglobin and cFTOE (r = −0.423, p<0.001). A scatter plot of hemoglobin and cFTOE in the cohort is illustrated in Figure 2. Unadjusted ROC analysis demonstrated moderate discrimination (AUC 0.708, p<0.001) with a threshold hemoglobin of 9.6 g/dL for critically increased cerebral oxygen extraction (cFTOE ≥0.4) with a sensitivity of 66% and a specificity of 67%. Density plots demonstrating the distribution of measured hemoglobin around the time of recording, divided into groups for those with a mean cFTOE <0.4 and ≥0.4 are illustrated in Figure 3.
Figure 2.
Scatter plot illustrating the population-level correlation between hemoglobin and cFTOE.
Figure 3.
Plots illustrating the normalized densities of hemoglobin values amongst recordings with cFTOE <0.4 (left) and cFTOE ≥0.4 (right).
The final mixed-effect model included fixed effects of EGA, hemoglobin around the time of recording, presence of a hemodynamically significant PDA requiring treatment, and PMA at time of recording. The model also included a random effect by participant. After adjustment for all fixed and random effects, hemoglobin remained a significant predictor of a critically increased cFTOE (p=0.02). Full characteristics of the mixed model are listed in Table 2.
Table 2.
Statistical characteristics of the mixed-model
| Variable | β-coefficient | z-value | p-value |
|---|---|---|---|
| Hemoglobin | −0.77 | −1.49 | 0.13 |
| EGA | −1.02 | −2.25 | 0.02* |
| PMA | −0.21 | −1.36 | 0.17 |
| PDA treatment | 3.12 | 1.36 | 0.17 |
Model = Critical_FTOE ~ EGA + Hgb + PMA + PDA_tx + (1 ∣ StudyID), AIC = 110.6,
denotes significance at p<0.05.
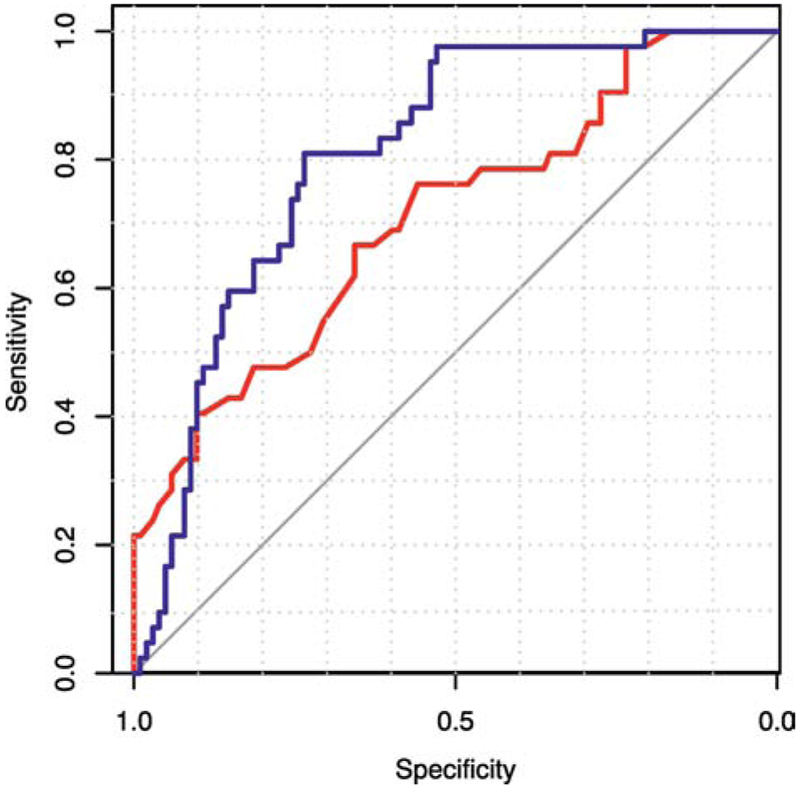
ROC analysis adjusting for EGA, PMA, and PDA treatment status demonstrated good discrimination (AUC 0.821, p<0.001). Both the unadjusted and adjusted ROC curves are illustrated in Figure 4. The adjusted ROC curve performed significantly better than the unadjusted (p<0.001). The model is not affected by history of transfusion, with similar performance when the two groups of infants are compared (never transfused AUC = 0.805, transfused AUC = 0.809).
Figure 4.
ROC curves for cFTOE by total hemoglobin (red) and by total hemoglobin adjusting for EGA, PMA, and PDA treatment status (blue) for critically increased oxygen extraction (cFTOE ≥0.4, p<0.001 for both).
Pre- and post-transfusion NIRS recordings were available for nine of the fifteen transfused infants. The mean cFTOE before transfusion was 0.44 which improved to 0.35 after transfusion, a ΔcFTOE of 0.09. This finding is comparable to other published studies [48,49].
Discussion
This study demonstrates an association between worsening anemia and increased cerebral oxygen extraction in preterm infants ≤30 weeks. The identified threshold for critical cerebral oxygen extraction in preterm infants is approximately 9.6 g/dL, which is consistent with the threshold we previously identified for critical cerebral desaturation is non-transfused preterm infants [26]. We defined cFTOE ≥ 0.4 as critical since this threshold has been associated with an increased risk of brain injury in premature infants [19-21]. This result is consistent with existing cross-sectional studies which have demonstrated lower C-rSO2 and higher cFTOE in anemic preterm infants [18,21,26,50]. It is additionally supported by a 2010 cross-sectional study by van Hoften et al. of 33 preterm infants which found a pre-transfusion hemoglobin ≤9.7 g/dL was associated with an increased incidence of cFTOE ≥0.4 [49]. Using cFTOE rather than C-rSO2, we were able to identify a threshold that was significant in preterm infants regardless of their transfusion status making it a more generalizable metric than C-rSO2 for identifying compromised cerebral oxygenation.
The identified hemoglobin threshold for critically increased cFTOE is significantly greater that that used in existing randomized control trials, such as the PINT trial where thresholds levels for the majority of infants ≥15 days were 8.5g/dL or less [1]. One possible reason for the PINT study’s equivocal short-term outcomes may be that the selected thresholds were below the level where critical cerebral oxygen extraction occurs. As cerebral oxygenation was not considered in that study, infants in both groups may have experienced anemia that overwhelmed their cerebral compensatory mechanisms and potentially caused injury without meeting study criteria for transfusion thus preventing a distinction in outcomes between groups.
Although we have identified a hemoglobin threshold below which there is a significantly increased risk of critical cerebral oxygen extraction, the intent is not to provide a new threshold for transfusion. Instead, this study argues that cerebral oxygenation should be an important component of the overall decision to transfuse and should be used in conjunction with clinical and laboratory findings. Cerebral oximetry, and cFTOE in particular, offers a means to assess the often silent loss of compensation to anemia in an infant who may otherwise lack standard signs of physiologic decompensation such as tachycardia, apnea, and respiratory distress. NIRS monitoring could be crucial in helping clinicians determine if an individual patient is experiencing cerebral tissue hypoxia and therefore an elevated risk of brain injury from their anemia that would prompt transfusion [22-24]. Use of cFTOE could individualize transfusion thresholds to patient physiology and provide guidance in a current area of clinical ambiguity. That the group threshold identified in this study is above current clinical practice merely suggests that this compromise will likely be found at less severe degrees of anemia than might be anticipated in the current clinical paradigm.
This study has a few limitations. First, this was an observational study that relied on clinically obtained hemoglobin data with elimination of recordings that lacked a hemoglobin measurement within 7 days. During even the most acute phases of anemia of prematurity (the first six weeks), the hemoglobin is estimated to drop by no more than approximately 1g/dL/week [51,52]. The overwhelming majority of recordings were made within 72 hours of the most recent hemoglobin measurement, and most of those were within 48 hours; differences in hemoglobin between such short intervals is within the margin of error of most commercial analyzers (approximately 0.25-0.5 g/dL) [53]. Aggressive error correction was used to eliminate segments of data where C-rSO2 or pulse oximetry were unreliable such as probe displacement, systemic desaturation (SpO2 <85%), or motion artifact to ensure recorded results were not contaminated by errors. Although we were not able to control for other determinants of cFTOE (hypercapnia, sedation, and hypotension), the recordings were made after the first week of life and the majority (94%) occurred in extubated infants minimizing the risk of these confounding factors. Finally, infants with severe IVH were eliminated to ensure that cFTOE alterations seen in the population truly represented globally compromised physiology rather than local pathology. Further study is needed as to the reliability and norms of C-rSO2 and cFTOE in infants with severe IVH.
Future directions for this work will include longitudinal measures of hemoglobin and cerebral tissue hypoxia burden using NIRS in preterm infants and correlations with short term outcomes using MRI-based assessment of brain injury at term-equivalent age and long-term follow-up of neurodevelopmental outcomes. This will allow for a more rigorous evaluation of the link between anemia, cerebral hypoxia, and brain injury in this population and provide further validation for the use of NIRS in the assessment of the anemic preterm infant.
In conclusion, cerebral fractional tissue oxygen extraction as measurement by NIRS presents a valuable means for identifying compromised cerebral oxygenation in this population of hemodynamically stable, preterm infants with anemia of prematurity. Decreasing hemoglobin was associated with a progressive increase in cFTOE in all infants regardless of their transfusion status with a hemoglobin threshold for critical cerebral oxygen extraction (cFTOE ≥0.4) of 9.6 g/dL.
Highlights.
Cerebral fractional tissue oxygen extraction (cFTOE), derived from regional cerebral saturations measured using near-infrared spectroscopy (NIRS) and pulse oximetry, is a marker of regional tissue oxygen delivery and metabolic activity.
Elevated cFTOE is a known adaptation to anemia, but values ≥0.4 are associated with brain injury in preterm infants.
Current clinical threshold hemoglobin levels for pRBC transfusions in anemia of prematurity are population based and not based on individual patient physiology.
This study identified the threshold for critical oxygen extraction (cFTOE ≥0.4) as 9.6 g/dL in stable preterm infants ≤ 30 weeks with anemia of prematurity, significantly higher than current standard clinical thresholds for transfusion (<8 g/dL).
Longitudinal evaluation of premature infants with anemia of prematurity using concurrent hemoglobin levels and cerebral NIRS may help define patient specific transfusion thresholds of hemoglobin in preterm infants.
Acknowledgements
The authors would like to thank our study coordinator, Anthony Barton, and our research assistant, Laura Atwood, for their tireless efforts in patient recruitment and data collection. We also thank all the patients and families who participated in this study.
Funding Sources:
This work was supported by the following grants:
Washington University Institute of Clinical and Translational Sciences KL2 Training Program (NIH/NCATS KL2 TR000450)- Vesoulis
The Gerber Foundation- Mathur
Cerebral Palsy Alliance Project Grant- Vesoulis
The Barnes-Jewish Hospital Foundation and the Washington University Institute of Clinical and Translational Sciences Clinical and Translational Funding Program (NIH/NCATS UL1 TR000448)- Vesoulis
NIH award R01HL124078- Maheshwari
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Aher S, Malwatkar K, Kadam S, Neonatal anemia, Semin Fetal Neonatal Med. 13 (2008) 239–247. doi: 10.1016/j.siny.2008.02.009. [DOI] [PubMed] [Google Scholar]
- [2].Christensen RD, Carroll PD, Josephson CD, Evidence-based advances in transfusion practice in neonatal intensive care units, Neonatology. 106 (2014) 245–253. doi: 10.1159/000365135. [DOI] [PubMed] [Google Scholar]
- [3].Colombatti R, Sainati L, Trevisanuto D, Anemia and transfusion in the neonate, Semin Fetal Neonatal Med. 21 (2016) 2–9. doi: 10.1016/j.siny.2015.12.001. [DOI] [PubMed] [Google Scholar]
- [4].Juul S, Erythropoiesis and the approach to anemia in premature infants, J. Matern. Fetal. Neonatal. Med 25 (2012) 97–99. doi: 10.3109/14767058.2012.715467. [DOI] [PubMed] [Google Scholar]
- [5].Fabres J, Wehrli G, Marques MB, Phillips V, Dimmitt RA, Westfall AO, Schelonka RL, Estimating blood needs for very-low-birth-weight infants, Transfusion. 46 (2006) 1915–1920. doi: 10.1111/j.1537-2995.2006.00997.x. [DOI] [PubMed] [Google Scholar]
- [6].Keir AK, Yang J, Harrison A, Pelausa E, Shah PS, Canadian Neonatal Network, Temporal changes in blood product usage in preterm neonates born at less than 30 weeks’ gestation in Canada, Transfusion. 55 (2015) 1340–1346. doi: 10.1111/trf.12998. [DOI] [PubMed] [Google Scholar]
- [7].Ibrahim M, Ho SKY, Yeo CL, Restrictive versus liberal red blood cell transfusion thresholds in very low birth weight infants: a systematic review and meta-analysis, J Paediatr Child Health. 50 (2014) 122–130. doi: 10.1111/jpc.12409. [DOI] [PubMed] [Google Scholar]
- [8].Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, Zimmerman MB, Georgieff MK, Lindgren SD, Richman LC, Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions, Arch Pediatr Adolesc Med. 165 (2011) 443–450. doi: 10.1001/archpediatrics.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang Y-C, Chan O-W, Chiang M-C, Yang P-H, Chu S-M, Hsu J-F, Fu R-H, Lien R, Red Blood Cell Transfusion and Clinical Outcomes in Extremely Low Birth Weight Preterm Infants, Pediatr Neonatol. 58 (2017) 216–222. doi: 10.1016/j.pedneo.2016.03.009. [DOI] [PubMed] [Google Scholar]
- [10].Whyte RK, Kirpalani H, Asztalos EV, Andersen C, Blajchman M, Heddle N, LaCorte M, Robertson CMT, Clarke MC, Vincer MJ, Doyle LW, Roberts RS, PINTOS Study Group, Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion, Pediatrics. 123 (2009) 207–213. doi: 10.1542/peds.2008-0338. [DOI] [PubMed] [Google Scholar]
- [11].Lust C, Vesoulis Z, Jackups R, Liao S, Rao R, Mathur AM, Early red cell transfusion is associated with development of severe retinopathy of prematurity, J Perinatol. 39 (2019) 393–400. doi: 10.1038/s41372-018-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, Easley KA, Josephson CD, Association of Red Blood Cell Transfusion, Anemia, and Necrotizing Enterocolitis in Very Low-Birth-Weight Infants, JAMA. 315 (2016) 889–897. doi: 10.1001/jama.2016.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, Cress GA, Johnson KJ, Kromer IJ, Zimmerman MB, Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants, Pediatrics. 115 (2005) 1685–1691. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, Peliowski A, Rios A, LaCorte M, Connelly R, Barrington K, Roberts RS, The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants, J. Pediatr 149 (2006) 301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- [15].Aaslid R, Lindegaard KF, Sorteberg W, Nornes H, Cerebral autoregulation dynamics in humans, Stroke. 20 (1989) 45–52. [DOI] [PubMed] [Google Scholar]
- [16].Greisen G, Autoregulation of cerebral blood flow in newborn babies, Early Human Development. 81 (2005) 423–428. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [17].Fredrickson LK, Bell EF, Cress GA, Johnson KJ, Zimmerman MB, Mahoney LT, Widness JA, Strauss RG, Acute physiological effects of packed red blood cell transfusion in preterm infants with different degrees of anaemia, Arch. Dis. Child. Fetal Neonatal Ed 96 (2011) F249–253. doi: 10.1136/adc.2010.191023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bailey S, Hendricks-Muñoz K, Wells J, Mally P, Packed Red Blood Cell Transfusion Increases Regional Cerebral and Splanchnic Tissue Oxygen Saturation in Anemic Symptomatic Preterm Infants, American Journal of Perinatology. 27 (2010) 445–453. doi: 10.1055/s-0030-1247598. [DOI] [PubMed] [Google Scholar]
- [19].El-Dib M, Aly S, Govindan R, Mohamed M, du Plessis A, Aly H, Brain Maturity and Variation of Oxygen Extraction in Premature Infants, Am J Perinatol. 33 (2016) 814–820. doi: 10.1055/S-0036-1572542. [DOI] [PubMed] [Google Scholar]
- [20].Wardle SP, Yoxall CW, Weindling AM, Determinants of cerebral fractional oxygen extraction using near infrared spectroscopy in preterm neonates, J. Cereb. Blood Flow Metab 20 (2000) 272–279. doi: 10.1097/00004647-200002000-00008. [DOI] [PubMed] [Google Scholar]
- [21].Andersen CC, Hodyl NA, Kirpalani HM, Stark MJ, A Theoretical and Practical Approach to Defining “Adequate Oxygenation” in the Preterm Newborn, Pediatrics. 139 (2017). doi: 10.1542/peds.2016-1117. [DOI] [PubMed] [Google Scholar]
- [22].Dix LML, van Bel F, Lemmers PMA, Monitoring Cerebral Oxygenation in Neonates: An Update, Front Pediatr. 5 (2017) 46. doi: 10.3389/fped.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF, Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit, J Neonatal Perinatal Med. 7 (2014) 89–100. doi: 10.3233/NPM-1477213. [DOI] [PubMed] [Google Scholar]
- [24].van Bel F, Lemmers P, Naulaers G, Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls, Neonatology. 94 (2008) 237–244. doi: 10.1159/000151642. [DOI] [PubMed] [Google Scholar]
- [25].Alderliesten T, Dix L, Baerts W, Caicedo Dorado A, van Huffel S, Naulaers G, Groenendaal F, van Bel F, Lemmers P, Reference Values of Regional Cerebral Oxygen Saturation during the First 3 Days of Life in Preterm Neonates, Pediatric Research. (2015). doi: 10.1038/pr.2015.186. [DOI] [PubMed] [Google Scholar]
- [26].Whitehead HV, Vesoulis ZA, Maheshwari A, Rao R, Mathur AM, Anemia of prematurity and cerebral near-infrared spectroscopy: should transfusion thresholds in preterm infants be revised?, J Perinatol. 38 (2018) 1022–1029. doi: 10.1038/s41372-018-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Papile LA, Burstein J, Burstein R, Koffler H, Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm, J. Pediatr 92 (1978) 529–534. [DOI] [PubMed] [Google Scholar]
- [28].Elser HE, Holditch-Davis D, Brandon DH, Cerebral Oxygenation Monitoring: A Strategy to Detect Intraventricular Hemorrhage and Periventricular Leukomalacia, Newborn and Infant Nursing Reviews. 11 (2011) 153–159. doi: 10.1053/j.nainr.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sorensen LC, Maroun LL, Borch K, Lou HC, Greisen G, Neonatal cerebral oxygenation is not linked to foetal vasculitis and predicts intraventricular haemorrhage in preterm infants, Acta Paediatr. 97 (2008) 1529–1534. doi: 10.1111/j.1651-2227.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- [30].Austin T, Gibson AP, Branco G, Yusof RM, Arridge SR, Meek JH, Wyatt JS, Delpy DT, Hebden JC, Three dimensional optical imaging of blood volume and oxygenation in the neonatal brain, Neuroimage. 31 (2006) 1426–1433. doi: 10.1016/j.neuroimage.2006.02.038. [DOI] [PubMed] [Google Scholar]
- [31].Verhagen EA, ter Horst HJ, Keating P, Martijn A, Van Braeckel KNJA, Bos AF, Cerebral Oxygenation in Preterm Infants With Germinal Matrix–Intraventricular Hemorrhages, Stroke. 41 (2010) 2901–2907. doi: 10.1161/STROKEAHA.110.597229. [DOI] [PubMed] [Google Scholar]
- [32].Parry G, Tucker J, Tarnow-Mordi W, CRIB II: an update of the clinical risk index for babies score, Lancet. 361 (2003) 1789–1791. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- [33].ter Horst HJ, Verhagen EA, Keating P, Bos AF, The relationship between electrocerebral activity and cerebral fractional tissue oxygen extraction in preterm infants, Pediatr. Res 70 (2011) 384–388. doi: 10.1203/PDR.0b013e3182294735. [DOI] [PubMed] [Google Scholar]
- [34].Massa-Buck B, Amendola V, McCloskey R, Rais-Bahrami K, Significant Correlation between Regional Tissue Oxygen Saturation and Vital Signs of Critically Ill Infants, Front Pediatr. 5 (2017) 276. doi: 10.3389/fped.2017.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kissack CM, Garr R, Wardle SP, Weindling AM, Cerebral Fractional Oxygen Extraction is Inversely Correlated with Oxygen Delivery in the Sick, Newborn, Preterm Infant, J Cereb Blood Flow Metab. 25 (2005) 545–553. doi: 10.1038/sj.jcbfm.9600046. [DOI] [PubMed] [Google Scholar]
- [36].Lemmers PMA, Toet M, van Schelven LJ, van Bel F, Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome, Exp Brain Res. 173 (2006) 458–467. doi: 10.1007/s00221-006-0388-8. [DOI] [PubMed] [Google Scholar]
- [37].Mintzer JP, Parvez B, La Gamma EF, Regional Tissue Oxygen Extraction and Severity of Anemia in Very Low Birth Weight Neonates: A Pilot NIRS Analysis, Am J Perinatol. 35 (2018) 1411–1418. doi: 10.1055/s-0038-1660458. [DOI] [PubMed] [Google Scholar]
- [38].Morris EA, Juttukonda MR, Lee CA, Patel NJ, Pruthi S, Donahue MJ, Jordan LC, Elevated brain oxygen extraction fraction in preterm newborns with anemia measured using noninvasive MRI, J Perinatol. 38 (2018) 1636–1643. doi: 10.1038/s41372-018-0229-1. [DOI] [PubMed] [Google Scholar]
- [39].Balegar KK, Stark MJ, Briggs N, Andersen CC, Early cerebral oxygen extraction and the risk of death or sonographic brain injury in very preterm infants, J. Pediatr 164 (2014) 475–480.e1. doi: 10.1016/j.jpeds.2013.10.041. [DOI] [PubMed] [Google Scholar]
- [40].Verhagen EA, Keating P, ter Horst HJ, Martijn A, Bos AF, Cerebral oxygen saturation and extraction in preterm infants with transient periventricular echodensities, Pediatrics. 124 (2009) 294–301. doi: 10.1542/peds.2008-2057. [DOI] [PubMed] [Google Scholar]
- [41].Verhagen EA, Van Braeckel KNJA, van der Veere CN, Groen H, Dijk PH, Hulzebos CV, Bos AF, Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years, Dev Med Child Neurol. 57 (2015) 449–455. doi: 10.1111/dmcn.12622. [DOI] [PubMed] [Google Scholar]
- [42].Wong FY, Alexiou T, Samarasinghe T, Brodecky V, Walker AM, Cerebral Arterial and Venous Contributions to Tissue Oxygenation Index Measured Using Spatially Resolved Spectroscopy in Newborn Lambs:, Anesthesiology. 113 (2010) 1385–1391. doi: 10.1097/ALN.0b013e3181fc5567. [DOI] [PubMed] [Google Scholar]
- [43].Vesoulis ZA, Lust CE, Liao SM, Trivedi SB, Mathur AM, Early hyperoxia burden detected by cerebral near-infrared spectroscopy is superior to pulse oximetry for prediction of severe retinopathy of prematurity, J Perinatol. 36 (2016) 966–971. doi: 10.1038/jp.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lemmers PM, Toet MC, van Bel F, Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants, Pediatrics. 121 (2008) 142–7. doi:121/1/142 [pii] 10.1542/peds.2007-0925. [DOI] [PubMed] [Google Scholar]
- [45].Vanderhaegen J, De Smet D, Meyns B, Van De Velde M, Van Huffel S, Naulaers G, Surgical closure of the patent ductus arteriosus and its effect on the cerebral tissue oxygenation, Acta Paediatr. 97 (2008) 1640–4. doi:APA1021 [pii] 10.1111/j.1651-2227.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- [46].Schisterman EF, Perkins NJ, Liu A, Bonded H, Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples, Epidemiology. 16 (2005) 73–81. [DOI] [PubMed] [Google Scholar]
- [47].DeLong ER, DeLong DM, Clarke-Pearson DL, Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach, Biometrics. 44 (1988) 837–845. [PubMed] [Google Scholar]
- [48].Seidel D, Bläser A, Gebauer C, Pulzer F, Thome U, Knüpfer M, Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants, Journal of Perinatology. 33 (2013) 282–287. doi: 10.1038/jp.2012.108. [DOI] [PubMed] [Google Scholar]
- [49].van Hoften JCR, Verhagen EA, Keating P, ter Horst HJ, Bos AF, Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion, Arch. Dis. Child. Fetal Neonatal Ed 95 (2010) F352–358. doi: 10.1136/adc.2009.163592. [DOI] [PubMed] [Google Scholar]
- [50].Roche-Labarbe N, Carp SA, Surova A, Patel M, Boas DA, Grant PE, Franceschini MA, Noninvasive optical measures of CBV, StO2, CBF index, and rCMRO2 in human premature neonates’ brains in the first six weeks of life, Human Brain Mapping. 31 (2010) 341–352. doi: 10.1002/hbm.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dallman PR, Anemia of prematurity, Annu. Rev. Med 32 (1981) 143–160. doi: 10.1146/annurev.me.32.020181.001043. [DOI] [PubMed] [Google Scholar]
- [52].Widness JA, Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity, Neoreviews. 9 (2008) e520. doi: 10.1542/neo.9-11-e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Patel KP, Hay GW, Cheteri MK, Holt DW, Hemoglobin test result variability and cost analysis of eight different analyzers during open heart surgery, J Extra Corpor Technol. 39 (2007) 10–17. [PMC free article] [PubMed] [Google Scholar]