Abstract
Objective:
Arsenic is an endocrine-disrupting chemical associated with diabetes risk. Increased adiposity is a significant risk factor for diabetes and its comorbidities. Here, the impact of chronic arsenic exposure on adiposity and metabolic health was assessed in mice.
Methods:
Male C57BL/6J mice were provided ad libitum access to a normal- or high-fat diet and water +/− 50 mg/L sodium arsenite (iAs). Changes in body weight, body composition, insulin sensitivity, energy expenditure, and locomotor activity were measured. Measures of adiposity were compared to accumulated arsenic in the liver.
Results:
Despite uniform arsenic exposure, internal arsenic levels varied significantly among arsenic-exposed mice. Hepatic arsenic levels in exposed mice negatively correlated with overall weight gain, individual adipose depots’ masses, and hepatic triglyceride accumulation. No effects were observed in mice on a normal diet. For mice on a high-fat diet, arsenic exposure reduced fasting insulin levels, HOMA-IR, HOMA-β, and systemic insulin resistance. Arsenic exposure did not alter energy expenditure or activity.
Conclusions:
Collectively, these data indicate that arsenic is anti-obesogenic, and that concentration at the source poorly predicts arsenic accumulation and phenotypic outcomes. In future studies, investigators should consider internal accumulation of arsenic, rather than source concentration, when assessing the outcomes of arsenic exposure.
Keywords: Obesity, Triglyceride, Insulin Sensitivity, Adipose Tissue, High-Fat Diet
Introduction
The global burden of diabetes mellitus is projected to grow to a staggering 629 million individuals by 2045 (1). The consequences of this pandemic are devastating on an individual and collective basis, with diabetes costing $327 billion annually in the United States alone (2). As such, identifying and addressing diabetes risk factors is paramount for addressing this public health crisis. Both genetics and lifestyle factors are widely recognized for their roles in promoting diabetes risk. Less appreciated, however, is the contribution of diabetogenic endocrine-disrupting chemicals (EDCs), such as arsenic (3, 4). Arsenic is a complex metalloid contaminating the groundwater under an estimated 140 million people worldwide (5). Trivalent, inorganic arsenite (iAs) is thought to be the most toxic and prevalent form in drinking water. Several epidemiological studies have reported positive associations between arsenic exposure and metabolic abnormalities, including insulin resistance, glucose intolerance, and diabetes (6, 7, 8, 9, 10, 11, 12). These epidemiological studies are supported by animal models demonstrating arsenic-induced disruptions in glucose homeostasis (13, 14, 15, 16, 17).
Given the links between increased adiposity and diabetes as well as widespread persistent global exposure to arsenic, the potential relationship between arsenic exposure, adiposity, and metabolic health is of interest. Whether arsenic exposure via drinking water specifically promotes obesity is a controversial topic, with mixed results in both clinical studies (8, 18, 19, 20) and animal models [reviewed in (21, 22)]. Studies have previously demonstrated that arsenic accumulates in the liver, pancreas, muscle, and other metabolically-relevant tissues (23, 24, 25, 26); however, to our knowledge there have been no reports evaluating a potential dose-response relationship between tissue arsenic levels and adiposity or other metabolic phenotypes. To clarify arsenic’s impact on adipose biology, the present study examines how trivalent, inorganic arsenite impacts weight gain, adipose accretion, liver lipids, and metabolic function under normo- and hyper-caloric conditions, with a specific focus on the hepatic accumulation of arsenic as a continuous independent variable of arsenic exposure.
Methods
Animal care and arsenic exposure
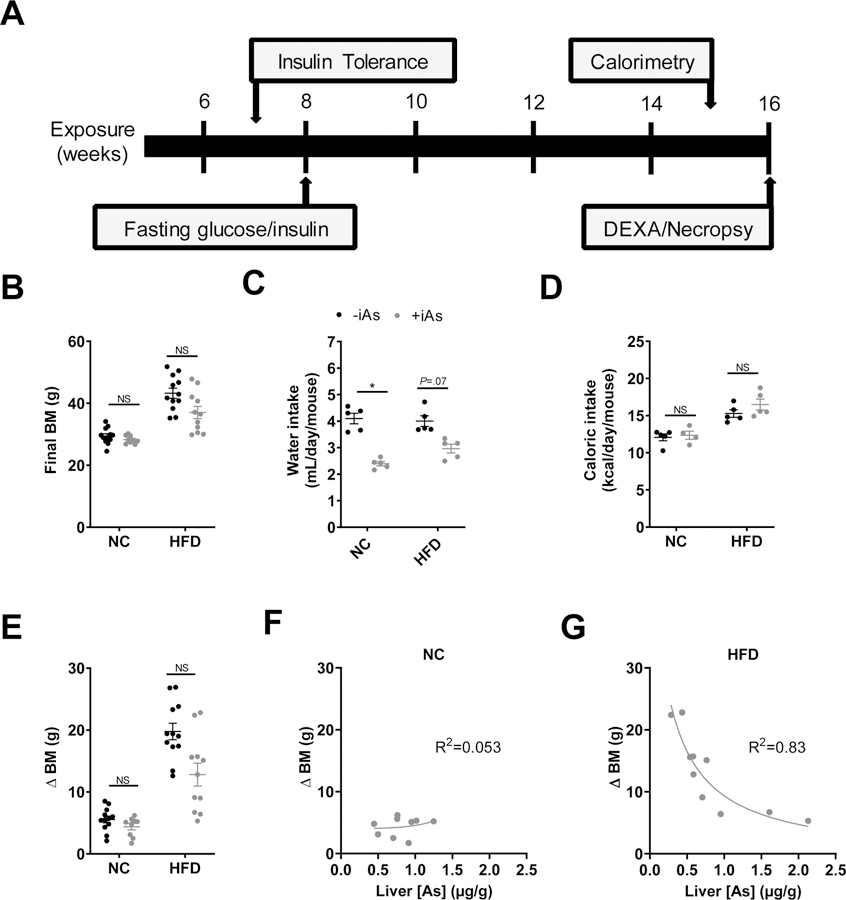
Seven-week-old male C57BL6/J mice from the Jackson Laboratory (Bar Harbor, ME) were group-housed under 12-hour light/dark cycles at 22.2 ± 1.1°C. At 8 weeks of age mice were provided bottles of reverse osmosis-purified drinking water +/− 50 mg/L sodium arsenite (NaAsO2, iAs, Sigma Aldrich, St. Louis, MO). Though to date a variety of genetic backgrounds have been utilized for arsenic studies in mice, the most common has been the C57BL/6 background (17, 21, 27), which was selected on that basis for the present study. This exposure protocol has been used previously, resulting in liver arsenic accumulation comparable with previous mouse studies and below levels observed in some chronically-exposed humans (16, 23). Mice had ad libitum access to a purified normal (NC) or high-fat diet (HFD). Nutrient details for each diet can be found in Table 1. Body mass, water intake, and food intake were measured weekly until sacrifice. As previous studies have demonstrated that sex and sex steroid hormones affect susceptibility to the effects of arsenic (24, 28), this study focused on male mice. All animal protocols were approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Chicago and University of Illinois at Chicago. Mice were euthanized after 16 weeks of iAs exposure and their tissues were collected as described below. Figure 1A describes the experimental timeline.
Table 1.
Diet details
| Nutrient | NC | HFD |
|---|---|---|
| Company | Envigo | Envigo |
| Product # | TD.97184 | TD.16009 |
| Carbohydrates (% of kilocalories) | 64 | 21 |
| Fat (% of kilocalories) | 17 | 60 |
| Protein (% of kilocalories) | 19 | 18 |
| Caloric Density (total kcal/g) | 3.8 | 5.1 |
| Copper (mg/kg diet) | 6.2 | 8.5 |
| Iron (mg/kg diet) | 37.1 | 50.8 |
| Iodine (mg/kg diet) | .21 | 0.28 |
| Manganese (mg/kg diet) | 10.5 | 14.5 |
Figure 1. Arsenic reduces body mass following high fat feeding.
(A) Experimental timeline. (B) Final body mass, (C) daily water intake, and (D) caloric intake during 8 weeks of normal or high fat feeding +/− 50 mg/L arsenic exposure. (E) Total change in body mass of each mouse during the 8-week feeding protocol. Plot of the change in body mass versus liver arsenic concentration in arsenic-exposed mice with (F) normal diet or (G) high fat feeding. (F, G) Each plot was fitted with a 4-parameter logistic inhibitor dose-response curve using GraphPad Prism. Statistics: (B-E) Kruskal-Wallis test comparing groups within each dietary treatment, adjusted for multiple testing. *P<0.05 for the comparison indicated. Error bars are ±SEM.
Tissue collection
Mice were fasted for 5–6 hours and then euthanized with isoflurane anesthesia followed by exsanguination via cardiac puncture. Serum was collected from whole blood as described previously (13). Adipose tissue depots (perigonadal, perirenal, mesenteric, subcutaneous, and intrascapular brown adipose) were dissected, weighed, and flash-frozen in liquid nitrogen. Liver was collected, dissected and flash-frozen immediately. A slice of the left lobe of each liver was separately collected for liver arsenic accumulation measurements. Tissue samples were stored at −80°C.
Fasting Glucose and Insulin
Mice were fasted for 6 hours and then blood glucose was measured by tail vein sampling using a Freestyle Lite glucometer (Abbott Laboratories, Abbott Park, IL, range 20–500 mg/dL). Prior to all tail vein blood sampling, 2% viscous lidocaine was applied to minimize animal stress (2% viscous lidocaine, Water-Jel, Carlstadt, NJ). Plasma was obtained from whole blood as described previously (13). Plasma insulin concentrations were measured using the Mouse Ultrasensitive Insulin ELISA kit according to the manufacturer’s instructions ([80-INSMSU-E01], ALPCO, Salem, NH, range 0.025–6.9 ng/mL). Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using fasting blood glucose and fasting plasma insulin levels as previously described (29).
Insulin tolerance tests (ITTs)
Mice were fasted for 3 hours and then blood glucose levels were measured via tail vein sampling. Mice were then injected intraperitoneally (i.p.) with Humalog insulin (0.5 U/kg body mass; Eli Lilly, Indianapolis, IN). Post-injection blood glucose readings were taken at 15, 30, 45, 60, 90, and 120 minutes.
Indirect Calorimetry
After 14–15 weeks of exposure, indirect calorimetric measurements were carried out on a separate cohort of individually housed mice using the LabMaster System (TSE Systems, Chesterfield, MO) maintaining a 12-hour light-dark cycle (6 AM to 6 PM), 25 ± 0.5°C environment as described previously (30). Mice were provided ad libitum access to their respective experimental diets and water. After a 2-day acclimation period, O2 consumption, CO2 production, energy expenditure, locomotor activity (X/Y-axis movement activity and Z-axis rearing activity), as well as food and water consumption were monitored at 20-minute intervals for 3 consecutive days. The respiratory exchange ratio (RER) was calculated as the ratio of O2 consumption to CO2 production at each interval. These values were then averaged for each mouse during each 12-hour cycle (light or dark).
Body composition
Body composition was assessed after 9 weeks of iAs exposure with the assistance of the Metabolic Testing Facility of the Diabetes Research and Training Center (DRTC) at the University of Chicago. After administration of anesthesia (80 mg/kg ketamine and 5 mg/kg xylazine) and determination of mouse mass and length, body composition was measured by dual-energy x-ray absorptiometry scanning (DEXA, Lunar PIXImus densitometer system; GE Healthcare) using the PIXImus 2 software package following system calibration according to the manufacturer’s instructions.
Serum analyses
Serum triglycerides were measured by colorimetric assay (Cayman Chemical, Ann Arbor, MI, range 1.1–2000 mg/dL) according to the manufacturers’ instructions.
Liver Triglycerides
Snap-frozen pieces of liver (20–40 mg) were homogenized in 750 uL of 10% extraction buffer (chloroform:isopropanol:NP40; 7:11:0.1) using a bead mill apparatus. Extracts were centrifuged 10 min at 15,000 x g. Lipids were dried then reconstituted in sample buffer (Cell Biolabs INC, cat#STA-384) and then subjected to triglyceride measurement using a commercially available kit (L-Type Triglyceride M, WAKO Chemical). Measurable range of the kit was 0.11 to 200 mg/L.
Liver Arsenic Quantification
Mouse liver samples were weighed and predigested overnight with 1.5 mL of high purity concentrated nitric acid (HNO3). The next morning, another 1.5 mL of HNO3 was added to each sample. Samples were completely digested using a microwave-assisted heating method without pressurization (MARS V, CEM, Matthews, NC), then allowed to cool to room temperature and diluted to 10 mL with double-deionized (>18 MΩ.cm) water. Digested liver samples, calibration standards, and several digested reference materials were diluted with a reagent containing a gallium internal standard, 0.005% Triton-X-100 (TX-100), and 2% (v/v) HNO3 and analyzed for total arsenic on a PE ELAN DRC II Inductively Coupled Plasma – Mass Spectrometer (ICP-MS) (PerkinElmer, Shelton, CT). The ICP-MS instrument was operated in dynamic reaction cell (DRC) mode with a mixture of 10% (v/v) H2 gas in argon, to eliminate a polyatomic interference (40Ar35Cl) at the same mass-to-charge ratio as 75As. The ICP-MS method limit of detection (LOD) for arsenic in liver was 0.12 μg/g, while method repeatability (%RSD) was 4.1%. Levels below the LOD were assigned a value equal to the LOD divided by the square root of two (0.08 μg/g). Validation for arsenic in liver was established using NRC Certified Reference Material (CRM) TORT 3 - Lobster Hepatopancreas (National Research Council, Ontario, Canada), and New York State Caprine Liver Reference Materials, G99–3 and G99–14 (NYS DOH, Wadsworth Center, Albany, NY). Staff were blinded to the arsenic exposure and diet status of samples until after all measurement and analyses were completed.
Statistics
For insulin tolerance tests, area-under-the-curve (AUC) of blood glucose over time was calculated using the trapezoidal rule using GraphPad Prism, version 7.0. Statistical significance was tested using the Kruskal-Wallis test for comparisons of exposure groups within each diet (NC or HFD). In collaboration with the Statistical Laboratory at the University of Illinois at Chicago, Analysis of Response Profile, which does not make a parametric assumption on the form of the mean trajectory, was used to analyze effects on insulin sensitivity using R (R Foundation for Statistical Computing, Vienna, Austria). The main goal in the Analysis of Response Profiles is to characterize the patterns of change in the mean response over time in the two groups and to determine whether the shapes of the mean response profiles do or do not differ for the two groups. The model employed for these analyses used time and treatment as main effects, and treatment-by-time interaction effects. For indirect calorimetry data, linear regression was performed on energy expenditure versus lean body mass as a possible covariate and the slopes of each line within each treatment group were compared within each dietary group. (31). Slopes and intercepts of linear regressions were evaluated for significance. Mean energy expenditure data were then ln-normalized and subjected to Student’s t-tests to compare arsenic exposure groups within each dietary group. Statistical analyses were performed using GraphPad Prism, version 7.0, unless otherwise noted. The specific statistical tests performed are described in each figure legend. Data are presented as means ± SEM.. P<0.05 was considered statistically significant for all experiments.
Results
Inverse relationship between liver arsenic and change in body mass after HFD feeding
Within each diet group, 16 weeks of iAs exposure did not have a significant effect on final body mass (Figure 1B). Average water intake in the NC group was significantly lower with iAs exposure, and trended lower in the HFD group (P=0.07) (Figure 1C). Arsenic did not affect mean caloric intake in either dietary group (Figure 1D). Notably, mass gain in iAs-exposed mice fed a HFD varied markedly from 5.0 – 22.8 g and was significantly lower than the weight gain in HFD control mice (Figure 1E). No association was observed between the change in body mass and hepatic arsenic concentration in the NC group (Figure 1F). In the HFD group, however, there was a marked inverse relationship between these two parameters, suggesting an inhibitory effect of arsenic on body mass accretion (Figure 1G).
Inverse relationship between liver arsenic and adiposity following HFD feeding
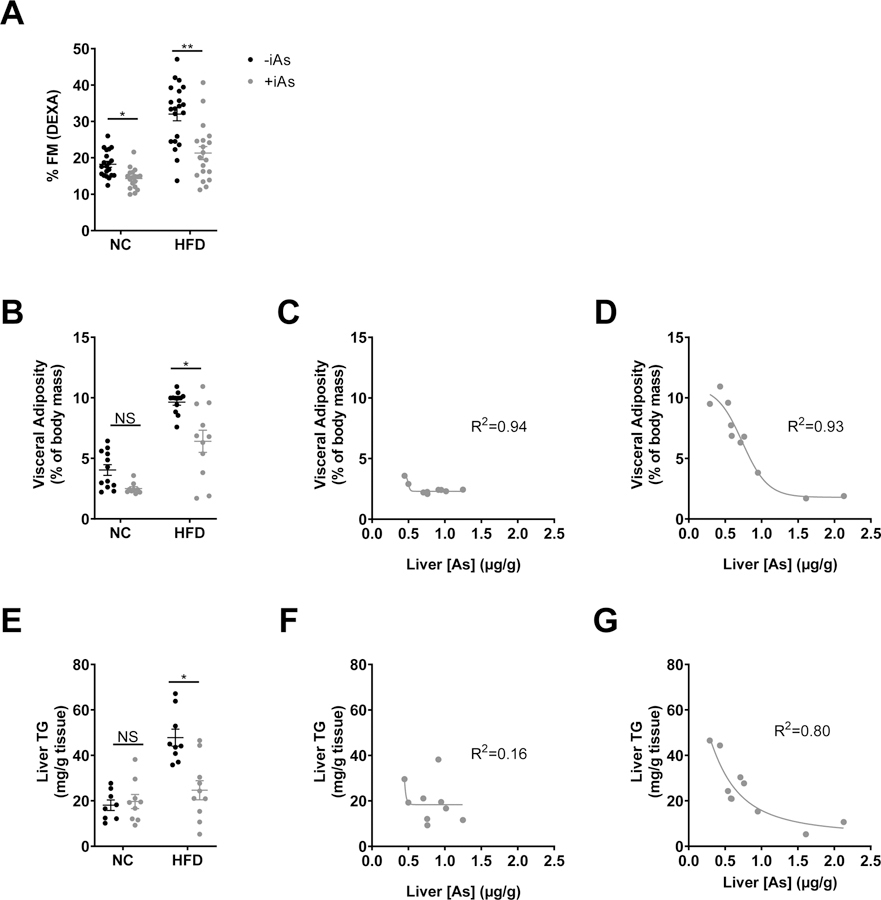
Within each dietary group, arsenic exposure significantly decreased adiposity as measured by DEXA (Figure 2A) without significant effects on bone mineral density or animal length (data not shown). Exposure to iAs in the HFD group significantly decreased the combined masses of visceral adipose tissues (Figure 2B). In both dietary groups we observed a similar inverse relationship between changes in visceral adiposity and liver arsenic concentrations (Figures 2C and 2D). Arsenic exposure also decreased liver triglyceride content relative to HFD controls (Figure 2E). In the HFD group (R2=0.80), but not the NC group (R2=0.16), hepatic triglyceride levels correlated with hepatic arsenic levels (Figure 2F and 2G).
Figure 2. Arsenic exposure reduced adiposity.
(A) % fat mass measured by DEXA, (B) and visceral adiposity as a % of body mass calculated by summating the individual masses of perigonadal, perirenal, and mesenteric fat pads and dividing by total body mass at the time of necropsy. Plot of visceral adiposity versus liver arsenic concentration in arsenic-exposed mice with (C) normal diet or (D) high fat feeding. (E) Liver triglycerides after 8 weeks +/− iAs exposure. Plot of liver triglycerides versus liver arsenic concentration in arsenic-exposed mice with (F) normal diet or (G) high fat feeding. Each plots C, D, F, and G were fitted with a 4-parameter logistic inhibitor dose-response curve using GraphPad Prism. Statistics: (A, B, E) Kruskal-Wallis test comparing groups within each dietary treatment, adjusted for multiple testing. *P<0.05, **P<0.01 for the comparison indicated. Error bars are ±SEM.
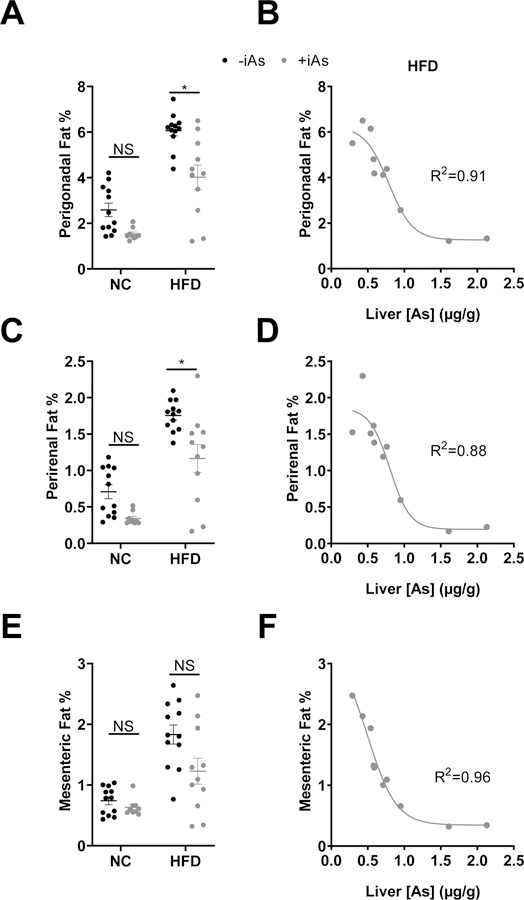
In HFD mice, iAs exposure reduced the masses of perigonadal and perirenal depots; however, these differences were not observed in the NC diet group (Figures 3A and 3C). Exposure to iAs did not change the mass of the mesenteric adipose depot (Figure 3E). Strikingly, in the HFD group, hepatic arsenic levels inversely correlated strongly with adipose mass; in perigonadal, perirenal, and mesenteric depots, the R2 value for these curves were 0.91, 0.88, and 0.96, respectively (Figures 3B, 3D, and 3F).
Figure 3. Arsenic exposure decreased visceral adipose depot masses.
(A) Perigonadal fat, (C) perirenal fat, (E) and mesenteric fat pad sizes represented as a % of total body mass. (B, D, F) Plots of corresponding fat pads versus liver arsenic concentration in arsenic exposed mice on a high-fat diet were fitted with a 4-parameter logistic inhibitor dose-response curve using GraphPad Prism. Statistics: (A, C, E) Kruskal-Wallis test comparing groups within each dietary treatment, adjusted for multiple testing. *P<0.05 for the comparison indicated. Error bars are ±SEM.
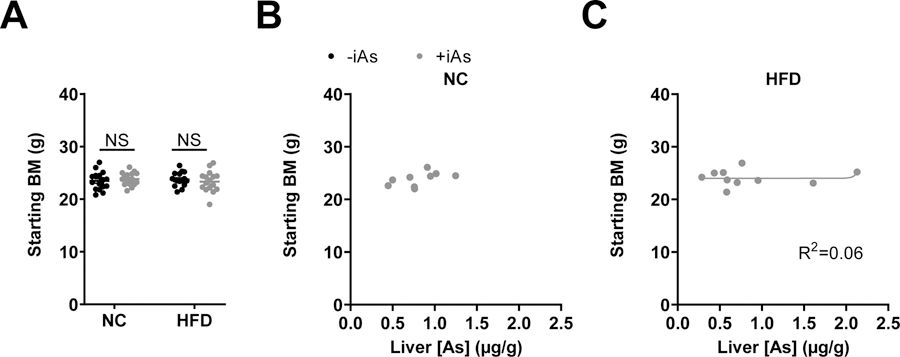
In consideration of whether differences in starting mass may be related to the accumulation of arsenic and its subsequent effect on adiposity, the starting masses of mice, which were not significantly different (Figure 4A), were compared to the final liver arsenic concentrations. In both the NC or HFD groups, hepatic arsenic content did not correlate with starting body mass (Figures 4B and 4C, respectively).
Figure 4. No apparent relationship between starting body mass and final arsenic accumulation.
(A) Starting body masses of mice in each treatment group. Plots of corresponding fat pads versus liver arsenic concentration in arsenic exposed mice on a normal (B) or high-fat (C) diet were fitted with a 4-parameter logistic inhibitor dose-response curve using GraphPad Prism. In panel B, this curve could not be plotted. Statistics: (A) Kruskal-Wallis test comparing groups within each dietary treatment, adjusted for multiple testing. *P<0.05 for the comparison indicated. Error bars are ±SEM.
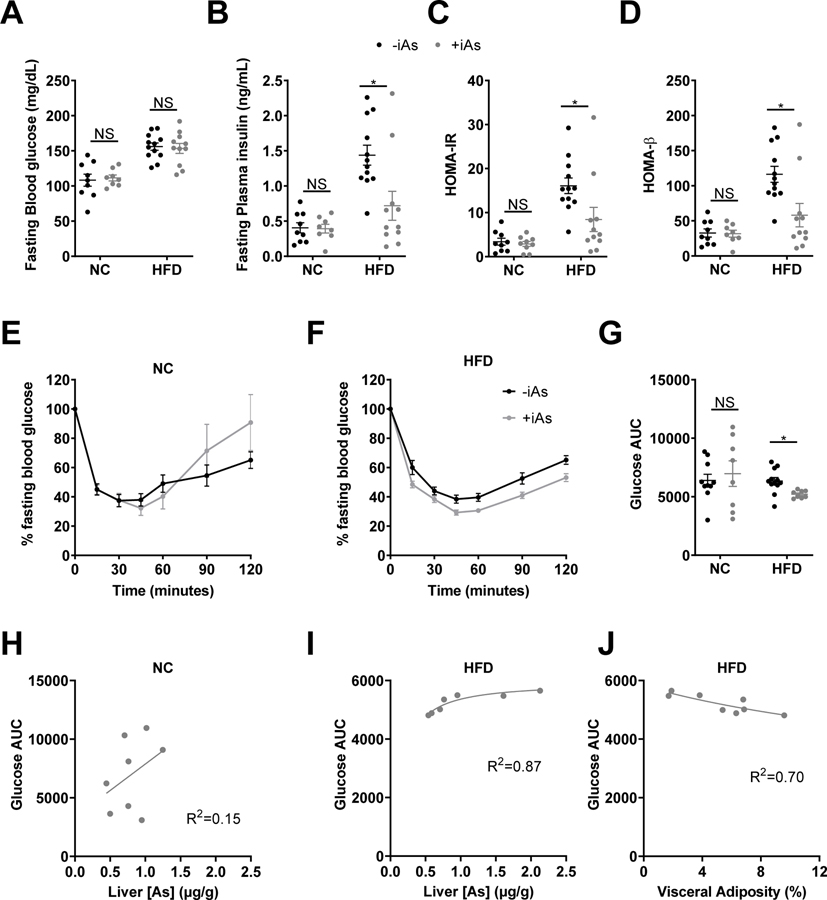
Arsenic affected measures of β-cell performance and insulin tolerance
Fasting blood glucose was not significantly different between iAs groups in either dietary group (Figure 5A); however, arsenic exposure decreased fasting plasma insulin in HFD mice (Figure 5B). Arsenic decreased both HOMA-IR and HOMA-β in HFD mice (Fig 5C and 5D). An insulin tolerance test revealed no differences in insulin sensitivity between iAs exposure groups in the NC diet group (Figures 5E and 5G), however iAs-exposed mice on a HFD were significantly more insulin sensitive than HFD controls (Figures 5F and 5G). There was no correlation between glucose AUC during the insulin tolerance test and liver arsenic in the NC group (Figure 5H, R2=0.15). In contrast, despite overall improvements in insulin sensitivity relative to controls in the HFD group during the insulin tolerance test, glucose AUC was positively correlated with liver arsenic, suggesting that arsenic dose-dependently impairs insulin sensitivity (Figure 5I, R2=0.87). However, the slope of this relationship was shallow suggesting a minimal effect across these arsenic concentrations. Among HFD-fed mice, visceral adiposity was inversely correlated with glucose AUC (Figure 5J, R2=0.70), revealing that mice with lower visceral adiposity were more insulin resistant. As with the relationship between glucose AUC and hepatic arsenic content, the slope of this relationship was shallow, suggestive of a minor relationship to insulin sensivity.
Figure 5. Arsenic exposure improved insulin sensitivity and decreased HOMA-β.
(A) Whole blood glucose and (B) plasma insulin were measured following 6 hr of fasting. (C) HOMA-IR and (D) HOMA-β were calculated from glucose and insulin values by the HOMA2 Calculator. Blood glucose levels during an intraperitoneal insulin tolerance test following 8 weeks on (E) normal or (F) high-fat diet. (G) Area under the curve (AUC) for insulin tolerance test blood glucose values. Plots of glucose AUC vs liver arsenic concentration in arsenic exposed mice on a (H) normal or (I) high-fat diet. (J) Plot of glucose AUC vs visceral adiposity in HFD mice exposed to arsenic. (H-J) A 4-parameter logistic inhibitor dose-response curve was fitted using Graphpad Prism for Glucose AUC measured during ITT versus liver arsenic on (H) normal diet or (I) high-fat diet. (J) The same curve was also fitted to glucose AUC from the ITT versus visceral adiposity measured during necropsy for mice on high-fat diet. Statistics: (A-D, G) Kruskal-Wallis test comparing groups within each dietary treatment, adjusted for multiple testing. (E, F) Response analysis. For ITT data, two major outliers with AUC values greater than 2 standard deviations above the median AUC value were excluded from time course and AUC charts. *P<0.05 for the comparison indicated. Error bars are ±SEM.
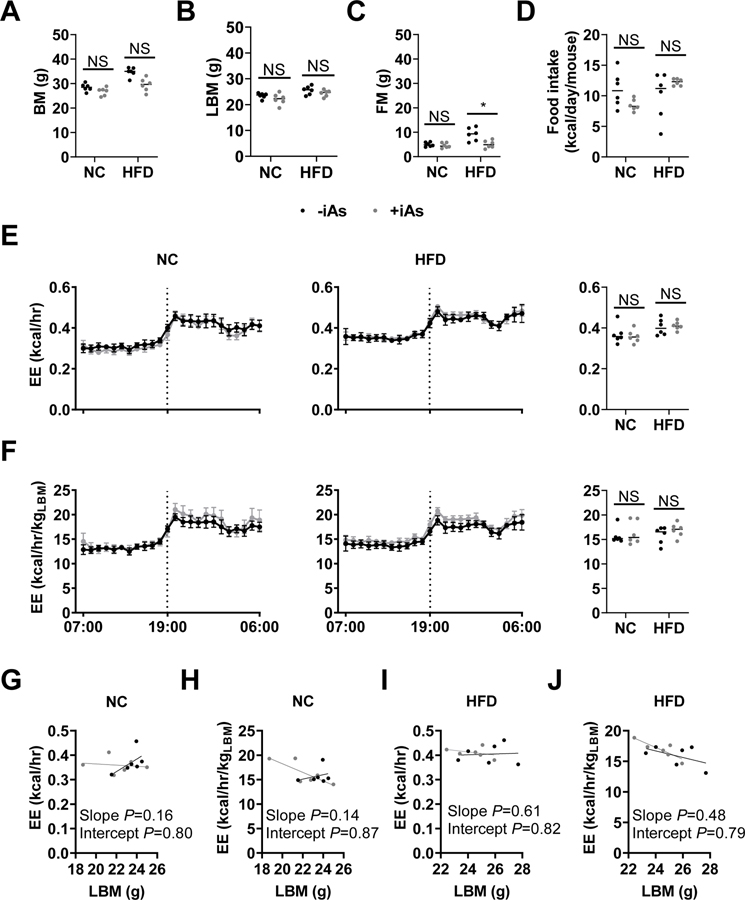
Exposure to iAs did not affect indirect calorimetry measurements
Mice exposed to iAs showed no significant differences in mean respiratory exchange ratio, locomotor activity, or rearing activity compared to their controls within each dietary group (data not shown). Total body mass (Figure 6A) and lean body mass (Figure 6B) in this cohort were not significantly different; however, fat mass was significantly lower in iAs-exposed mice fed a HFD versus controls (Figure 6C). There were no significant differences in food intake observed. Arsenic did not appear to affect energy expenditure per mouse (Figure 6E) or energy expenditure adjusted for lean body mass (Figure 6G) within dietary groups. There were no significant differences observed in linear regression analyses examining lean body mass as a potential covariate for energy expenditure in the NC diet (Figures 6G and 6H) or the HFD (Figures 6I and 6J), in agreement with hourly and mean values. Furthermore, the slope of each regression model was not significantly non-zero, suggesting that, in this model, lean body mass was not a significant covariate for energy expenditure.
Figure 6. Indirect calorimetry data.
Black: no arsenic; Gray: 14 weeks of 50 mg/L arsenic in drinking water. Final (A) Body mass, (B) lean body mass, (C) fat mass, and (D) mean 24-hour food intake. (E-F) Energy expenditure (E) per mouse and (F) per kg lean body mass (left panels) on normal or (center panels) high-fat diet. (Right panels) Mean 24-hr energy expenditure derived from left and center panels. (G-J) Linear regression analysis of lean body mass as a potential covariate. Plots of (G, I) energy expenditure versus lean body mass and (H, J) lean body mass-adjusted energy expenditure versus lean body mass in (G, H) normo-caloric or (I, J) HFD dietary groups. Statistics: (A-D) Kruskal-Wallis test comparing only iAs exposure groups within each dietary treatment, adjusted for multiple testing. (E, F) Statistical analyses were not performed on hourly charts. (E, F, right panels) Student’s t-test was performed between groups within each dietary treatment following natural log-normalization of 24-hr mean values. (G-J) P values are given in each chart for linear regression analysis comparing the two lines of best fit. *P<0.05 for the comparison indicated. Error bars are ±SEM.
Discussion
Arsenic exposure during HFD feeding significantly decreased weight gain, adiposity, and insulin resistance, while also decreasing β-cell performance. These findings largely agree with recent studies showing that arsenic-induced impairment of β-cell function is offset by improvements in insulin sensitivity at higher levels of arsenic exposure and in the context of hypercaloric feeding (32, 33). Given the uncertain relationship between arsenic exposure and obesity (21, 34, 35), we believe that this study provides new evidence that arsenic exposure in the context of diet-induced weight gain has an anti-obesogenic effect in mice. Accounting for the dramatic inter-animal variations in liver arsenic levels and treating other phenotypic endpoints as dependent variables enhanced our ability to interpret the effects of arsenic exposure on metabolic outcomes. These findings highlight the potential for inter-animal differences in arsenic accumulation to confound interpretation of data from arsenic-exposed mice. Furthermore, these analyses illuminate more complex exposure-phenotype relationships than reported previously. Given this improved power to relate tissue-level arsenic to other phenotypic data, our results support the conclusion that trivalent arsenic, which is present as the arsenite oxyanion in aqueous solution, is an anti-obesogenic compound that potentially exerts insulin-sensitizing effects in the context of HFD feeding.
In the context of using hepatic arsenic as a continuous independent variable of arsenic exposure, we noted a few surprising features in our dataset. Arsenic reduced adiposity and improved insulin sensitivity in mice on a HFD; however, within this group, hepatic arsenic levels were positively correlated with insulin resistance. This suggests a non-monotonic dose response relationship in which arsenic exerts separate, opposing effects across the exposure range. Arsenic dose-dependently decreases adiposity overall, promoting insulin sensitivity, while simultaneously exerting separate, deleterious effects on insulin sensitivity that manifest within this narrower band of improved sensitivity. Perhaps a β-cell defect in insulin secretion (36, 37) chronically reduces insulin, manifesting as decreased HOMA-β, which decreases glucose uptake and storage of dietary lipids (38). This could explain the overall reductions in adiposity and improvements in insulin sensitivity. Secondarily to that, however, there may be other effects of arsenic that inhibit insulin sensitivity. Prior publications have revealed that arsenic is capable of dysregulating hepatic glucose output (32, 39) or inducing muscle insulin resistance (15), which could explain the positive association between insulin resistance and liver arsenic accumulation in our mice within the narrower band of improved sensitivity. Though it is tempting to draw parallels between reduced adiposity and a potential increase in hepatic glucose output, our data also shows that hepatic triglycerides were significantly lower in iAs-exposed mice, which would not be consistent with a lipodystrophy-induced hepatic glucose output mechanism. These complex, concentration-dependent effects argue strongly for more robust studies examining arsenic’s impact on metabolic physiology across the full spectrum of exposure relevant for human populations with specific attention to divergent effects on the various metabolic tissues that regulate glucose and lipid homeostasis.
The causes of inter-animal variability with respect to hepatic arsenic accumulation remain unevaluated by our group; however, some possibilities include differences in water intake, arsenic metabolism, and/or arsenic excretion. We favor the hypothesis that inter-individual variation in arsenic metabolism and/or excretion are most likely to explain the observed variation, as water intake variability was not nearly as high as differences in arsenic accumulation. Our inter-animal variation in hepatic arsenic accumulation quantitatively agreed with prior publications (21, 40). Recently, Stýblo et al reported that mouse strain differences may cause up to a 7-fold difference in liver arsenic accumulation (17), showing similar variability in 4 of the 12 strains evaluated by their group (SE values of approximately 20%).
Given the marked differences in hepatic arsenic accumulation and adiposity following HFD feeding, there are some limitations and unanswered questions that merit attention. We have not examined these parameters in female mice, which need to be explored. Additionally, the high drinking water concentration of sodium arsenite is both a strength and limitation of this study. While this exposure leads to liver arsenic concentrations that approximate levels observed in highly exposed human populations, and provided generated a wide range of internal arsenic levels, future studies will need to examine lower levels of exposure to fully model effects relevant for human health. Whether lower drinking water concentrations recapitulate both the observed reductions in adipose mass and the effects on insulin sensitivity will require further study.
Arsenic exposure is of immense global public health concern due to the ubiquity of exposure and its adverse health effects, including associations with diabetes. In the present study, we demonstrate that arsenic exposure is associated with reductions in weight gain, adipose mass, and hepatic liver triglyceride content in the context of HFD feeding. Moreover, our data provide evidence that tissue-level arsenic accumulation may be a more powerful method for assessing the impact of arsenic exposure on metabolic outcomes, which could ultimately increase the predictive power of future studies. Furthermore, evidence that exposure improved insulin sensitivity, while arsenic levels in the liver were positively correlated with insulin resistance suggests multiple modes of action that intersect at the level of insulin action. Therefore, the clinically relevant effects of chronic, low-dose arsenic exposure in animal models may be obscured when utilizing higher doses of arsenic. More work is required to illuminate the mechanisms of arsenic-induced metabolic dysfunction. Uncovering these mechanisms will be important for informing our understanding of the consequences of arsenic exposure and shaping public policy.
STUDY IMPORTANCE QUESTIONS:
What is already known about the subject?
There are conflicting clinical and animal model data about whether environmental exposure to arsenic is obesogenic.
Current animal model studies most commonly utilize source drinking water exposure as the primary metric of exposure.
What are the new findings in the manuscript?
Arsenic decreases adiposity in the context of high-fat feeding.
Hepatic arsenic content inversely correlates with adiposity.
How might your results change the direction of research or the focus of clinical practice?
Investigators may improve the quality of their animal model studies by using tissue-level arsenic accumulation as a continuous independent exposure variable rather than arsenic concentration at the source.
Acknowledgements
The authors wish to thank Graeme Bell and the Diabetes Research and Training Center at the University of Chicago for their expert assistance with the performance of metabolism cage testing and body composition analyses. The authors also wish to thank Kirstie Danielson, PhD, for her guidance regarding statistical analyses.
FUNDING: This work was supported by the National Institutes of Health (R01ES028879; P30ES027792; and P30DK020595) and the American Diabetes Association (17-JDF-033).
Footnotes
DISCLOSURE: The authors declare no conflict of interest.
References
- 1.Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018. [DOI] [PubMed]
- 2.Association AD. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018;41: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonini MG, Sargis RM. Environmental Toxicant Exposures and Type 2 Diabetes Mellitus: Two Interrelated Public Health Problems on the Rise. Curr Opin Toxicol 2018;7: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruiz D, Regnier SM, Kirkley AG, Hara M, Haro F, Aldirawi H, et al. Developmental exposure to the endocrine disruptor tolylfluanid induces sex-specific later-life metabolic dysfunction. Reprod Toxicol 2019;89: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravenscroft P, Brammer H, Richards K. Arsenic Pollution: A Global Synthesis. Wiley-Blackwell, 2009. [Google Scholar]
- 6.Wang SL, Chiou JM, Chen CJ, Tseng CH, Chou WL, Wang CC, et al. Prevalence of non-insulin-dependent diabetes mellitus and related vascular diseases in southwestern arseniasis-endemic and nonendemic areas in Taiwan. Environ Health Perspect 2003;111: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai M-S, Hsueh Y-M, Chen C-J, Shyu M-P, Chen S-Y, Kuo T-L, et al. Ingested Inorganic Arsenic and Prevalence of Diabetes Mellitus. American Journal of Epidemiology 1994;139: 484–492. [DOI] [PubMed] [Google Scholar]
- 8.Islam R, Khan I, Hassan SN, McEvoy M, D’Este C, Attia J, et al. Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health 2012;11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BK, Kim Y. Association of diabetes mellitus with a combination of vitamin d deficiency and arsenic exposure in the korean general population: analysis of 2008–2009 korean national health and nutrition examination survey data. Ann Occup Environ Med 2013;25: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Xie Z, Lin Y, Zhang D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: a meta-analysis. J Epidemiol Community Health 2014;68: 176–184. [DOI] [PubMed] [Google Scholar]
- 11.Pan WC, Seow WJ, Kile ML, Hoffman EB, Quamruzzaman Q, Rahman M, et al. Association of low to moderate levels of arsenic exposure with risk of type 2 diabetes in Bangladesh. Am J Epidemiol 2013;178: 1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng W, Cui X, Liu B, Liu C, Xiao Y, Lu W, et al. Association of urinary metal profiles with altered glucose levels and diabetes risk: A population-based study in China. PLoS ONE 2015;10. [DOI] [PMC free article] [PubMed]
- 13.Kirkley AG, Carmean CM, Ruiz D, Ye H, Regnier SM, Poudel A, et al. Arsenic Exposure Induces Glucose Intolerance and Alters Global Energy Metabolism. Am J Physiol Regul Integr Comp Physiol 2017: ajpregu005222016. [DOI] [PMC free article] [PubMed]
- 14.Huang MC, Douillet C, Dover EN, Stýblo M. Prenatal arsenic exposure and dietary folate and methylcobalamin supplementation alter the metabolic phenotype of C57BL/6J mice in a sex-specific manner. Arch Toxicol 2018. [DOI] [PMC free article] [PubMed]
- 15.Huang MC, Douillet C, Dover EN, Zhang C, Beck R, Tejan-Sie A, et al. Metabolic Phenotype of Wild-Type and As3mt-Knockout C57BL/6J Mice Exposed to Inorganic Arsenic: The Role of Dietary Fat and Folate Intake. Environ Health Perspect 2018;126: 127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul DS, Hernández-Zavala A, Walton FS, Adair BM, Dedina J, Matousek T, et al. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol 2007;222: 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stýblo M, Douillet C, Bangma J, Eaves LA, de Villena FP, Fry R. Differential metabolism of inorganic arsenic in mice from genetically diverse Collaborative Cross strains. Arch Toxicol 2019. [DOI] [PMC free article] [PubMed]
- 18.Bulka CM, Mabila SL, Lash JP, Turyk ME, Argos M. Arsenic and Obesity: A Comparison of Urine Dilution Adjustment Methods. Environ Health Perspect 2017;125: 087020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Audouze K, Brunak S, Grandjean P. A computational approach to chemical etiologies of diabetes. Sci Rep 2013;3: 2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Rubio P, Roberge J, Arendell L, Harris RB, O’Rourke MK, Chen Z, et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol 2011;252: 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carmean CM, Seino S. Braving the Element: Pancreatic β-Cell Dysfunction and Adaptation in Response to Arsenic Exposure. Front Endocrinol (Lausanne) 2019;10: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 2012;120: 1658–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santra A, Das Gupta J, De BK, Roy B, Guha Mazumder DN. Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol 1999;18: 152–155. [PubMed] [Google Scholar]
- 24.Douillet C, Huang MC, Saunders RJ, Dover EN, Zhang C, Stýblo M. Knockout of arsenic (+3 oxidation state) methyltransferase is associated with adverse metabolic phenotype in mice: the role of sex and arsenic exposure. Arch Toxicol 2017;91: 2617–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonaventura MM, Bourguignon NS, Bizzozzero M, Rodriguez D, Ventura C, Cocca C, et al. Arsenite in drinking water produces glucose intolerance in pregnant rats and their female offspring. Food Chem Toxicol 2017;100: 207–216. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Mu X, Xu W, Martin FL, Alamdar A, Liu L, et al. Exposure to arsenic via drinking water induces 5-hydroxymethylcytosine alteration in rat. Sci Total Environ 2014;497–498: 618–625. [DOI] [PubMed] [Google Scholar]
- 27.Paul DS, Walton FS, Saunders RJ, Stýblo M. Characterization of the impaired glucose homeostasis produced in C57BL/6 mice by chronic exposure to arsenic and high-fat diet. Environ Health Perspect 2011;119: 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang CF, Yang CY, Chan DC, Wang CC, Huang KH, Wu CC, et al. Arsenic Exposure and Glucose Intolerance/Insulin Resistance in Estrogen-Deficient Female Mice. Environ Health Perspect 2015;123: 1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28: 412–419. [DOI] [PubMed] [Google Scholar]
- 30.Savic D, Ye H, Aneas I, Park SY, Bell GI, Nobrega MA. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res 2011;21: 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, Chan L, et al. A guide to analysis of mouse energy metabolism. Nat Methods 2011;9: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu S, Guo X, Wu B, Yu H, Zhang X, Li M. Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci Rep 2014;4: 6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong Y, Liu J, Xue Y, Zhuang Z, Qian S, Zhou W, et al. Non-monotonic dose-response effects of arsenic on glucose metabolism. Toxicol Appl Pharmacol 2019;377: 114605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahangarpour A, Zeidooni L, Samimi A, Alboghobeish S, Khorsandi LS, Moradi M. Chronic exposure to arsenic and high fat diet additively induced cardiotoxicity in male mice. Res Pharm Sci 2018;13: 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahangarpour A, Alboghobeish S, Rezaei M, Khodayar MJ, Oroojan AA, Zainvand M. Evaluation of Diabetogenic Mechanism of High Fat Diet in Combination with Arsenic Exposure in Male Mice. Iran J Pharm Res 2018;17: 164–183. [PMC free article] [PubMed] [Google Scholar]
- 36.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007;56: 1783–1791. [DOI] [PubMed] [Google Scholar]
- 37.Carmean CM, Yokoi N, Takahashi H, Oduori OS, Kang C, Kanagawa A, et al. Arsenic modifies serotonin metabolism through glucuronidation in pancreatic β-cells. Am J Physiol Endocrinol Metab 2018. [DOI] [PMC free article] [PubMed]
- 38.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008;31 Suppl 2: S262–268. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Fennel EMJ, Douillet C, Stýblo M. Exposures to arsenite and methylarsonite produce insulin resistance and impair insulin-dependent glycogen metabolism in hepatocytes. Arch Toxicol 2017;91: 3811–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinkai Y, Sumi D, Toyama T, Kaji T, Kumagai Y. Role of aquaporin 9 in cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. Toxicol Appl Pharmacol 2009;237: 232–236. [DOI] [PubMed] [Google Scholar]