Abstract
Objective
In contrast to intentionally restricting energy intake, restricting the eating window may be an option for treating obesity. By comparing time-restricted eating (TRE) to an unrestricted (non-TRE) control, we hypothesized that TRE facilitates weight loss, alters body composition, and improves metabolic measures.
Methods
Participants [17F/3M, mean(SD) 45.5 years(12.1), BMI 34.1 kg/m2(7.5)] with a prolonged eating window [15.4 hours(0.9)] were randomized to TRE (n=11: 8 hour window, unrestricted eating within window) vs non-TRE (n=9: unrestricted eating), for 12 weeks. Weight, body composition (Dual X-ray Absorptiometry), lipids, blood pressure, 2-hour oral glucose tolerance test, 2-week continuous glucose monitoring, and 2-week physical activity (actigraphy-assessed) were measured pre- and end-intervention.
Results
The TRE group significantly reduced their eating window [end-intervention window: 9.9 hours(2.0)] compared to the non-TRE group [end-intervention window: 15.1 hours(1.1)](p<0.01). Compared to non-TRE, TRE decreased the number of eating occasions (EO), weight, lean mass and visceral fat (all p≤0.05). Compared to pre-intervention, the TRE group reduced the number of EO [−21.9%(30.1)], weight [−3.7%(1.8)], fat mass [−4%(2.9)], lean mass [−3.0%(2.7)] and visceral fat [11.1%(13.4)] (all p≤0.05). Physical activity and metabolic measures remained unchanged.
Conclusions
In the setting of a randomized trial, TRE presents a simplified view of food intake which reduces weight.
Keywords: Obesity, Time restricted eating, Weight loss, Body composition, Metabolic measures
Introduction
Obesity affects 36% of the United States’ adult population and presents a significant health care burden(1). Intentional energy restriction plays an important role in obesity treatment. Yet, intentional energy restriction is hampered by lack of patient adherence(2). Therefore, obesity treatment options beyond intentional energy restriction are needed.
In contrast to intentionally restricting energy intake, Time Restricted Eating (TRE) presents a simplified approach towards eating by restricting the eating window. As adult humans (n=156) documented a median daily eating window of 14.75 hours(3), there is interest in the implications of restricting the eating window to 10 hours or less. Human TRE studies providing all food to maintain isoenergetic intake report weight stability with variable effects on glycemic measures(4, 5, 6). Human TRE studies with ad libitum intake report modest weight loss (~ 3%, 3 kg)(3, 7, 8, 9) while glycemic measures remain unchanged(7, 8, 9).
The findings from the TRE literature are promising. Yet, several features may limit generalizability. One limitation is the provision of all food(5, 6), reducing applicability to the real-world setting. Administration TRE with ad libitum intake as the primary intervention have primarily compared outcomes with pre-intervention values(3, 8, 9), historical control(8) or assigned group(7). Although some studies have compared TRE with ad libitum intake vs non-TRE in a randomized setting(10, 11), these studies were within the setting of resistance training, potentially masking TRE’s effects(10, 11).
For an intervention, the “gold standard” reference is a randomized control group(12). Therefore, we conducted a 12 week feasibility study randomizing TRE (ad libitum intake during 8 hour window) or non-TRE (ad libitum) in humans who are overweight or obese with a documented eating window ≥14 hours. We hypothesized that TRE with ad libitum intake would facilitate weight loss, reduce body fat, and improve metabolic measures compared with non-TRE.
Methods
Study Design
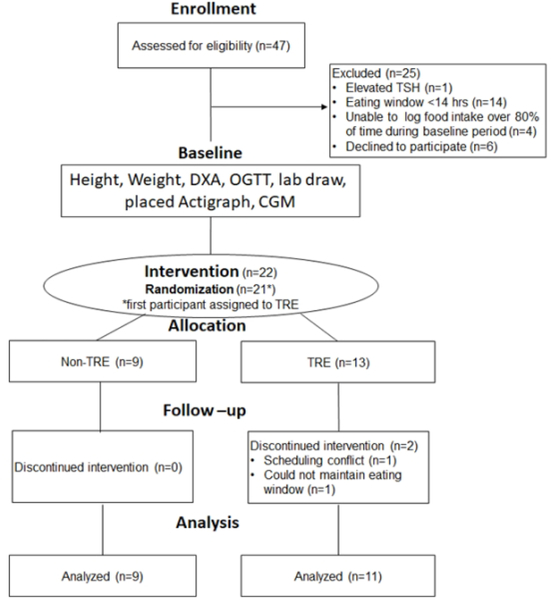
For this feasibility study, we recruited participants who were overweight or obese (18–65 years, BMI≥25kg/m2). Figure 1 describes the study design. The University of Minnesota’s Institutional Review Board approved the protocol. All participants provided written informed consent before participation.
Figure 1:
Participant Flow
At the screening visit (Figure 1), we measured the participant’s weight/height and provided the myCircadianClock (mCC) application(www.mycircadianclock.org)(3). We asked each participant to document all oral intake using the mCC application for at least 1 week. Inclusion criteria included: stable work and sleep schedule (awake/sleep within a 2 hour time frame 6 out of 7 days with ≤4 hour variance on the 7th day), owns a smartphone compatible with the mCC application, and capable of giving informed consent. Exclusion criteria included: eating window <14 hours, poor logging adherence (unable to document ≥2 eating events ≥5 hours apart per day >1 day/week), current/anticipated pregnancy or clinically significant medical issues [self-reported uncontrolled cardiovascular or pulmonary disease, pre-intervention thyroid stimulating hormone (TSH) or creatinine outside the reference range, pre-intervention glycemic measures (fasting glucose, 2 hour OGTT glucose, HbA1c) suggestive of diabetes(13)]. Enrollment began in October 2017 and ended in September 2018. We stopped recruitment after meeting pre-specified recruitment goals. We completed all follow-up by December 2018.
Pre-intervention measures (Figure 1) included: height, weight, body composition [dual X-ray absorptiometry (DXA)], lipid profile, TSH, creatinine, oral glucose tolerance test (OGTT) and hemoglobin A1c (HbA1c). Participants fasted for at least 8 hours before plasma blood measurements. Sensors to monitor physical activity (ActiGraph Link: ActiGraph, Pensacola FL) and ambulatory glucose profile [continuous glucose monitoring system(CGM): Freestyle LibrePro, Abbott, Chicago IL] were placed. The sensors were worn up to 14 days prior to randomization. The participants continued dietary documentation by mCC while wearing the sensors.
At the randomization visit, the Actigraph and CGM were removed. Participants were randomized to either TRE or non-TRE stratified by sex (male/female) and age (<45 vs ≥45 years old). The intervention duration was 12 weeks. As this was a feasibility study, the first participant was assigned to the TRE to determine TRE feasibility. Subsequent participants were randomized by randomization assignments generated in advance using SAS’s pseudo-random number generator procedure.
For the TRE group, each participant self-selected an 8-hour eating window for ad libitum intake. There was no restriction on the eating window selection other than maintaining this daily window during the intervention. For the non-TRE group, participants were instructed to eat ad libitum per their usual habits. Other than establishing the daily eating window (TRE) or instructing ad libitum intake (non-TRE), we did not provide any additional instruction on diet intake, diet quality or energy intake.
Outcome measures (height, body weight, lipid profile, DXA, OGTT, HbA1c, Actigraphy, CGM) were repeated at study conclusion. The CGM and Actigraph were initiated at Week 10 and removed at Week 12.
Documentation of Food Intake:mCC application
During the study (pre-intervention to Week 12), each participant was instructed to document all oral intake using the mCC application. The utility of the mCC application has been established(3). In brief, the participant used their phone camera to document all oral intake, including food, beverages, water and medications, along with an identifying text entry. Participants were randomly reminded (1–2 times/day) to input recent food intake. The picture, text entry and time-stamp were encrypted, deidentified, and continuously uploaded to a cloud-based server, allowing the study team to remotely monitor for data fidelity and intervention compliance.
Every week, participants were notified by either phone call, email, or text, about their eating window compliance (TRE group) and logging adherence (TRE and non-TRE group). An eating event was defined as any oral intake (including coffee, tea, diet drinks, noncaloric drinks), excluding medications or water. Adherence was derived from the mCC data. Participants were considered adherent to logging if they documented ≥2 meals ≥5 hours apart >1 day/ week. Participants were considered adherent to the intervention if they met logging criteria during their designated eating window. We reported adherence within ±15 minutes, ±30 minutes, and ±60 minutes of the eating window in Results.
Physical Activity:ActiGraph Link
We quantified physical activity pre and end-intervention using actigraphy. Each participant wore the Actigraph Link (sampling time 60 seconds) on the non-dominant wrist. We used the ActiLife software package (v6.11.9) to validate wear time and calculate physical activity (sedentary, light, moderate, vigorous)(14) as a percent of validated wear time.
Outcomes:Eating Occasions (EO)
We estimated eating frequency by documenting eating occasions from the mCC data(15). An EO was considered distinct when food or drink was documented ≥15 minutes apart from another EO(15). The average number of EO per day was determined over a 14 day period just prior to randomization and just prior (Weeks 10–12) to end-intervention.
Outcomes:Body Weight/Composition
Body weight was measured at pre-intervention and end-intervention (Week 12). Body composition was measured by DXA (GE Healthcare Lunar iDXA, Madison, WI) and analyzed by the enCore™ software (Version 16.2). Participants fasted for at least 8 hours before scanning. Visceral fat was estimated by established methods(16).
Outcomes:Metabolic measures
Metabolic outcomes were measured pre and end-intervention. Blood pressure was measured after 5 minutes of rest in a sitting position with feet flat on the floor[Welch Allyn Connex Spot Monitor (Skaneateles Falls, NY) or Philips SureSign VS2+ (Amsterdam, Netherlands)]. All blood samples were analyzed at the University of Minnesota Acute Care Laboratory. Fasting lipid profile was determined using the Siemens Dimension Vista 1500 (Erlangen, Germany) with LDL cholesterol calculated using the Friedewald equation(17). HbA1c was measured by a turbidimetric inhibition immunoassay on the Siemens Dimension Vista 1500 (Erlangen, Germany). Participants underwent a 75 gram 2-hour OGTT with glucose and insulin sampled every 30 minutes. Plasma glucose was measured using a modified hexokinase method on the Siemens Dimension Vista 1500 (Erlangen, Germany) and serum insulin was measured using a two-site chemiluminescent sandwich immunoassay on the Siemens Advia Centaur (Erlangen, Germany). We calculated the homeostatic model assessment of insulin resistance (HOMA-IR)(18) and Matsuda Insulin Sensitivity Index(19, 20) from the OGTT results. The CGM provided the ambulatory glucose profile: 1) average glucose, 2) coefficient of variation, 3) standard deviation, 4) percent time glucose below target (<70 mg/dL), 5) percent time glucose within target (70–180 mg/dL), 6) percent time glucose above target (≥180 mg/dL)(21).
Statistical analysis
The primary hypothesis was the TRE group would have greater body weight loss than the non-TRE group. As this is a feasibility study, we determined a-priori that at least 20 completed study participants was needed. A pre-study power computation showed that 20 participants (11 TRE and 9 non-TRE) gave 80% power to detect a minimum difference of 1.33 SD in body weight with two-sided alpha (false positive rate) 0.05 using a two-sample t test.
Treatment groups were compared on pre-intervention demographic and clinical characteristics using two sample t-tests for continuous characteristics and chi-square or Fisher’s exact tests for categorical characteristics. Changes in body weight, body composition, and glycemic measures from pre to end-intervention were compared between treatment groups using two-sample t tests. We examined whether pre-intervention differences in diastolic blood pressure, TSH, 2 hour OGTT glucose or fasting triglycerides may affect the observed findings using multivariate linear regression models. If the results were altered by the pre-intervention value, we presented the adjusted p-value (Table 2). For assessment of change in EO over time, we used linear mixed models to account for correlations among repeated measures. Pearson’s correlation within the TRE group measured the association between eating window restriction and body composition changes. Statistical analyses were performed in SAS (v. 9.3, SAS Institute Inc, Cary NC). A p-value less than 0.05 was considered statistically significant.
Table 2:
Comparison of Pre and End Intervention Measures
| Mean (SD) | TRE (n=11) | P* value relative to pre - intervention | Non-TRE (n=9) | P* value relative to pre-intervention | P value comparing pre-post change between TRE and non-TRE | ||
|---|---|---|---|---|---|---|---|
| Pre-Intervention | End Intervention | Pre-Intervention | End Intervention | ||||
| Weight | |||||||
| Weight | 95.2 (22.6) | 91.6 (21.5) | <0.01 | 100.9 (28.1) | 99.4 (28.1) | 0.09 | 0.04 |
| Body Composition | |||||||
| Lean Mass:Total (kg) | 50.0 (9.8) | 48.6 (9.9) | <0.01 | 51.1 (8.7) | 51.0 (7.8) | 0.81 | 0.03 |
| Lean Mass:Trunk (kg) | 23.5 (4.6) | 22.9 (4.7) | 0.08 | 23.6 (4.3) | 23.3 (3.6) | 0.24 | 0.50 |
| Lean Mass:Arms (kg) | 5.6 (1.5) | 5.4 (1.4) | 0.07 | 5.8 (1.0) | 5.7 (1.1) | 0.38 | 0.36 |
| Lean Mass:Legs (kg) | 17.8 (3.6) | 17.2 (3.7) | <0.01 | 18.6 (3.9) | 18.8 (3.6) | 0.22 | <0.01 |
| Fat mass: Total (kg) | 41.1 (16.8) | 39.4 (16.4) | <0.01 | 45.6 (20.7) | 44.7 (20.8) | 0.10 | 0.31 |
| Fat mass: Visceral (kg) | 1.7 (1.3) | 1.4 (1.1) | 0.04 | 1.1 (0.6) | 1.1 (0.6) | 0.63 | 0.04 |
| %body fat | 44.0 (9.5) | 43.7 (9.6) | 0.51 | 45 (8.5) | 44.8 (9.4) | 0.14 | 0.48 |
| Eating Habits | |||||||
| Eating Window (hours) | 15.2 (0.7) | 9.9 (2.0) | <0.01 | 15.5 (1.1) | 15.1 (1.1) | 0.24 | <0.01 |
| Eating Occasions (# occasions/day) | 5.4 (1.6) | 3.8 (0.8) | <0.01 | 5.6 (2.0) | 4.9 (1.6) | 0.01 | 0.02 |
| Blood Pressure | |||||||
| Systolic blood pressure (mmHg) | 132 (13.0) | 121 (16) | 0.12 | 123 (13) | 115 (12) | 0.07 | 0.78 |
| Diastolic blood pressure (mmHg) | 85# (4) | 79 (15) | 0.22 | 79 (8) | 72 (7) | 0.02 | 0.77 |
| Glycemic measures | |||||||
| Hemoglobin A1c (%) | 5.4 (0.4) | 5.4 (0.4) | 0.74 | 5.6 (0.4) | 5.6 (0.4) | 0.59 | 0.57 |
| HOMA-IR | 2.5 (1.6) | 2.4 (1.9) | 0.75 | 2.3 (1.2) | 2.2 (1.9) | 0.90 | 0.95 |
| Matsuda Index | 4.4 (2.6) | 5.2 (3.4) | 0.12 | 5.2 (3.4) | 5.9 (4.3) | 0.30 | 0.70 |
| Fasting glucose (mg/dl) | 95 (10) | 87 (9) | <0.01 | 95 (13) | 88 (3) | 0.17 | 0.98 |
| Fasting insulin (mU/L) | 11 (6) | 11 (7) | 0.84 | 10 (5) | 10 (8) | 0.97 | 0.84 |
| 2 hour OGTT glucose value (mg/dL) | 142# (45) | 126 (21) | 0.14 | 96 (24) | 107 (26) | 0.14 | 0.98† |
| Physical activity | |||||||
| % time in Sedentary Activity | 46.0 (5.5) | 45.8 (3.7) | 0.87 | 47.6 (5.6) | 49.1 (4.6) | 0.42 | 0.47 |
| % time in Light Activity | 41.1 (1.9) | 42.0 (3.1) | 0.37 | 39.4 (4.6) | 38.9 (3.3) | 0.75 | 0.42 |
| % time in Moderate+Vigorous Activity | 12.9 (4.5) | 12.2 (3.5) | 0.52 | 13.1 (3.5) | 12.0 (4.1) | 0.57 | 0.83 |
| CGMS measures | |||||||
| Average glucose (mg/dL) | 93 (10) | 96 (9) | 0.39 | 91 (13) | 92 (9) | 0.66 | 0.67 |
| Coefficient of Variation (%) | 17.7 (3.8) | 16.3 (2.3) | 0.32 | 27.0 (27.4) | 17.0 (3.6) | 0.33 | 0.40 |
| Standard Deviation (mg/dL) | 16.5 (3.5) | 15.7 (3.2) | 0.58 | 15.7 (4.0) | 15.5 (2.9) | 0.85 | 0.76 |
| % time where blood sugars below target (<70 mg/dL) | 6.4 (5.1) | 3.0 (2.1) | 0.07 | 7.7 (8.8) | 8.3 (9.2) | 0.76 | 0.14 |
| % time where blood sugars within target (70–180 mg/dL) | 92.9 (5.5) | 97 (2.1) | 0.03 | 92.0 (9.3) | 92 (9.2) | 0.86 | 0.09 |
| % time where blood sugars above target (>180 mg/dL) | 0.7 (1.4) | 0 (0) | 0.12 | 0.3 (1.0) | 0 (0) | 0.35 | |
| Lipid Measures | |||||||
| HDL (mg/dL) | 50 (14) | 51 (14) | 0.97 | 60 (18) | 67 (19) | 0.57 | 0.66 |
| Triglycerides (mg/dL) | 144# (54) | 106 (39) | 0.02 | 87 (21) | 87 (27) | 0.90 | 0.62†† |
| LDL (mg/dL) | 95 (24) | 104 (31) | 0.55 | 105 (19) | 106 (19) | 0.68 | 0.84 |
p value is for significance of absolute change relative to pre-intervention
indicates significantly different between TRE and non-TRE groups pre-intervention p<0.05
After adjusting for pre-intervention 2 hour OGTT glucose value, unadjusted p value is 0.05
After adjusting for pre-intervention triglyceride value, unadjusted p value is 0.07
We used multiple imputation to address missing data for subjects assigned to the TRE group (n=2) who did not complete the study [Supplemental Methods 1]. Given that multiply-imputing missing data did not substantially affect the results, the participants who completed the study are described in the Results section.
Results
Forty-seven participants were assessed for eligibility (Figure 1). Participants tracked their food intake using the mCC application during a pre-intervention period [28.5 days (8.8)] to establish logging adherence and their eating window. Twenty-five were excluded due to failure to meet study criteria (n=19) or declined to participate (n=6). Exclusion criteria included eating window <14 hours (n=14, 29.7% of screened participants), inconsistent logging of food intake (n=4, 8.5% of screened participants), and elevated TSH (n=1). Twenty-two participants received the intervention. After randomization, one participant in the TRE group stopped because of scheduling conflicts. One other participant in the TRE group stopped because of inability to maintain the prescribed 8-hour eating window. Twenty participants [17F/3M, mean(SD): age: 45.5 years(12.1), BMI: 34.1 kg/m2(7.5)] completed the study.
Participant Characteristics
Table 1 reports basic participant characteristics of the TRE group (n=11, 9F/2M) and non-TRE group (n=9, 8F/1M) pre-intervention.
Table 1:
Characteristics of Randomized Participants*
| Results: mean (SD) | TRE (n=11) | non TRE (n=9) | p-value |
|---|---|---|---|
| Age (Years) | 46.5 (12.4) | 44.2 (12.3) | 0.69 |
| Sex | 9F/2M | 8F/1M | 0.66 |
| BMI (kg/m2) | 33.8 (7.6) | 34.4 (7.8) | 0.86 |
| Pre-intervention duration (Days) | 28.5 (9.0) | 28.6 (9.1) | 0.98 |
| Thyroid Stimulating Hormone (mU/L) | 2.2 (0.8) | 1.4 (0.6) | 0.01 |
| Creatinine (mg/dL) | 0.7 (0.1) | 0.8 (0.1) | 0.61 |
Remainder of Baseline Characteristics are noted in Table 2 to permit pre-post intervention comparison
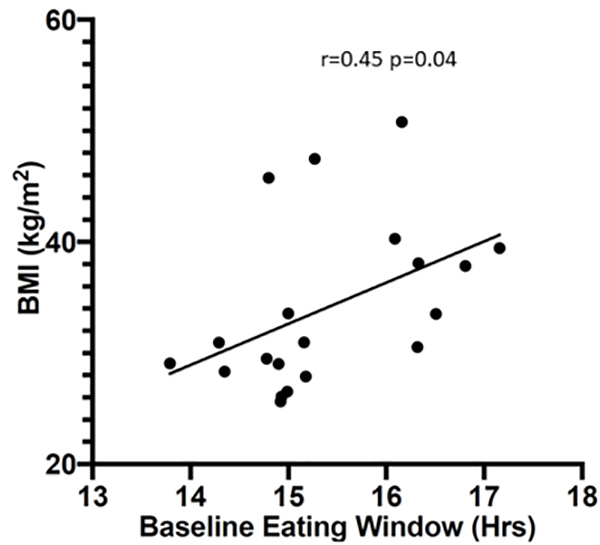
Table 2 reports detailed participant characteristics pre and end-intervention. Pre-intervention, the TRE group had higher fasting triglycerides, TSH, diastolic blood pressure, and 2 hour OGTT values than the non-TRE group (p<0.05), whereas the remaining characteristics were not significantly different. In the combined population, average glucose as measured by CGMS [92 mg/dL(8.7)], fasting glucose [95 mg/dL(11.2)], HbA1c [5.5%(0.4)] and insulin sensitivity, as measured by HOMA-IR [2.4(1.4)](18) and by the Matsuda index [4.8(3.0)] were within the normal range(22). In the TRE group, the two hour OGTT results were suggestive of prediabetes [142 mg/dL(45)]. The pre-intervention eating window was similar [15.4 hours(0.9) in combined population] between groups and was positively associated with BMI (r=0.45, p = 0.04) (Figure 2).
Figure 2:
Pre-intervention Eating Window Is Associated with BMI
Study Compliance
During the pre-intervention period, participants were adherent to logging on 92.3%(8.5) of days. During the 12 week intervention, the TRE group was adherent to logging 83.1%(13.4) of days while the non-TRE group was adherent to logging 90.9%(8.3) of days; this difference in logging adherence was not statistically significant (p=0.15).The TRE group was adherent ±15 minutes of their 8-hour eating window for 55.5%(22.4) of days, ±30 minutes for 60%(23) of days, and ±60 minutes for 66.3%(20.7) of days. The end time of the daily eating window did not correlate with either logging adherence [r=0.37, p=0.26] or eating window adherence [r=0.09, p=0.8].
TRE effects: Eating window
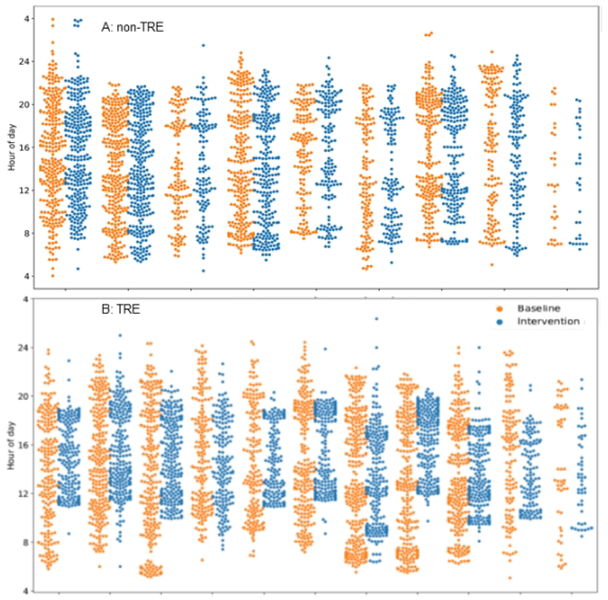
Within the TRE group, the self-defined eating window generally started at 10:40AM and ended at 18:40PM. The earliest self-defined window was from 9:00AM to 17:00PM and latest window from 12:00PM to 20:00PM (Figure 3). Based on the mCC data, the end-intervention eating window in the TRE group significantly declined from pre-intervention [−5.4 hours(2.2):p<0.01] whereas the end-intervention eating window in the non-TRE group remained unchanged (Table 2).
Figure 3:
Time of eating events in the non-TRE (A) and TRE group (B) Pre and End-Intervention. This figure depicts the clock hour for eating events at pre-intervention (orange; 2 weeks prior to randomization) and end-intervention (blue; 2 weeks prior to study conclusion) for each participant (Panel A: non-TRE, Panel B: TRE). Y axis: Clock hour for eating event (4=4AM, 24=midnight). X-axis: Each orange/blue combination represents an individual participant.
TRE effects: Eating Occasions
During pre-intervention, the number of EO per day were similar between the TRE and non-TRE groups (Table 2). At end-intervention, EO significantly reduced in both the TRE [21.9% (30.1) p<0.01] and non-TRE groups [7.6%(22.0) less EO relative to pre-intervention, p=0.01] (Table 2). The reduction in EO in the TRE group was significantly lower than the non-TRE group (p=0.02).
TRE effects: Physical activity
Pre-intervention, the percent time spent in sedentary, light, and moderate+vigorous activity was not different between the TRE group and non-TRE group. This physical activity distribution did not change at end-intervention (Table 2).
TRE effects: Body weight, body composition, and metabolic measures
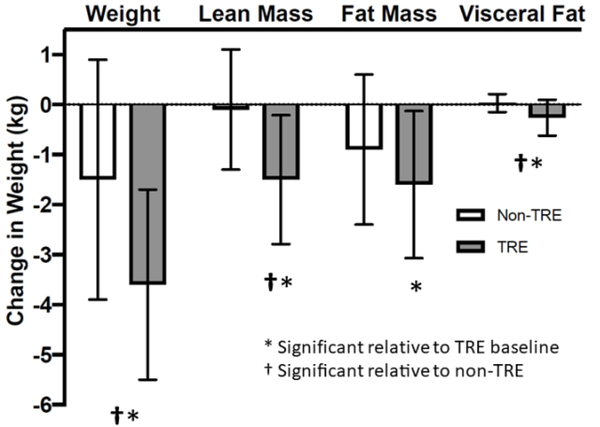
Compared to the non-TRE group, the TRE group lost body weight, visceral fat, and lean mass (all p< 0.05: Figure 4). Compared to pre-intervention (Table 2), the TRE group reduced body weight [−3.7%(1.8)], fat mass [−4.0%(2.9)], lean mass [−3.0%(2.7)] and visceral fat [11.1%(13.4)]. The lean mass loss in the TRE group was from the legs [−3.7%(3.6)] which was significant compared with pre-intervention (p<0.01) and non-TRE (p<0.01). TRE did not result in any significant lean mass loss from the trunk or arms. The effect of TRE on changes in fat mass, lean mass and visceral fat were not significant after adjusting for body weight loss, suggesting that TRE’s effect on these measures is mediated by body weight loss.
Figure 4:
TRE Alters Body Composition. Average change in body weight, lean mass, fat mass, and visceral fat in the TRE vs. the non-TRE groups. Results reported as mean (SD). *indicates significance relative to TRE pre-intervention. † indicates significant relative to non-TRE group end-intervention.
Table 2 reports the effect of TRE on metabolic measures. Relative to pre-intervention TRE significantly lowered fasting glucose [−7.7% (6.9)] and fasting triglyceride concentration [−23.6% (21.7)] (both p<0.05). Relative to pre-intervention, TRE increased the amount of time[+4.1% of time (5.5), p=0.03] within CGMS target range (70–180 mg/dL)]. The TRE intervention did not alter HbA1c or insulin sensitivity relative to pre-intervention or the non-TRE group. The observed changes in metabolic measures were not significant when comparing between TRE and non-TRE group; these findings remained not significant after adjusting for pre-intervention differences.
The non-TRE group had no significant changes in body composition or glycemic or lipid measures relative to pre-intervention. In the non-TRE group, diastolic blood pressure decreased significantly relative to pre-intervention (p=0.02); however, this measure was not significantly different from the TRE group.
Association between restriction of eating window and body composition changes
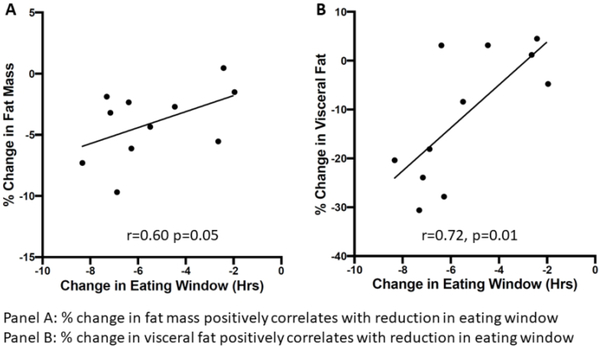
Within the TRE group, we examined the relationship between eating window restriction and body composition changes. Body weight loss (absolute change: r=0.41, p=0.21, percent change: r=0.18, p=0.60) did not significantly correlate with eating window restriction. Restriction of the eating window correlated with fat mass loss (r=0.60, p=0.05) and visceral fat loss (r=0.72, p=0.01) (Figure 5).
Figure 5:
Restriction of Eating Window Affects Body Composition
Discussion
We compared TRE with ad libitum intake relative to a randomized, non-TRE control in humans who were overweight or obese and had an eating window≥ 14 hours. We found that TRE resulted in fewer eating occasions, greater body weight loss, lean mass loss and visceral fat loss compared to non-TRE and pre-intervention values. TRE also reduced fasting glucose and triglyceride concentration relative to pre-intervention values, although this was not significant compared with the non-TRE group. Within the TRE group, greater eating window restriction was associated with greater loss of fat mass and visceral fat.
In humans, the effect of TRE depends on the prescribed intervention. TRE studies either impose an eating window while maintaining isoenergetic intake(5, 6) or impose a daily eating window with ad libitum intake(3, 7, 8). When all food is provided to maintain isoenergetic intake and weight stability, TRE improves body composition and select metabolic parameters(5, 6). Specifically, a randomized cross-over study in healthy, normal-weight, middle-aged adults showed that consuming an entire day’s energy intake at dinner (4 hour TRE for 8 weeks) preserved fat-free mass and decreased fat mass (~13%) compared with eating the same energy amount over breakfast, lunch and dinner(6). Another cross-over study involving men with prediabetes showed that isoenergetic TRE (6-hour eating window for 5 weeks with food intake completed by 15:00 PM) improved glucose tolerance, insulin sensitivity, blood pressure, and oxidative stress and increased fasting triglyceride concentration compared with the same energy intake over a 12 hour eating window(5). When studies prescribe TRE with ad libitum intake(3, 8, 9), weight loss (~3 kg, ~2–3% loss in body weight) similar to our study are observed. When assessing TRE’s effect on body composition, TRE with ad libitum intake reduced bioelectrical impedence measured percent body fat and visceral fat relative to pre-intervention(9) but did not alter DXA-measured fat mass, lean mass or visceral fat compared with a historical control group(8). Our study adds to the literature by detailing the impact of TRE with ad libitum intake, including DXA quantified body composition, actigraphy-measured physical activity and multifaceted glycemic measures (CGM/OGTT/HbA1c) relative to a randomized non-TRE group.
We reported that both TRE and non-TRE groups reduced eating occasions, with greater reductions observed in the TRE group. These findings suggest that TRE may cause involuntary reductions of energy intake via reduced eating occasions. Previously, dietary records have been used by previous TRE studies with ad libitum intake to document reduced energy intake (~350–650 kcal/day)(8, 10). Alternatively, reduced energy intake have been estimated using reference nutritional values given the image or text entry without considering food volume(3). As we could not ascertain food volume or energy density from the mCC-based images, we could not assess TRE effects on energy intake. We acknowledge that our observed reduction in eating occasions may be influenced by reduced logging. Nevertheless, our significant findings relative to the randomized non-TRE group offsets this concern, assuming that both the TRE and non-TRE groups had similar reductions in logging. Moving forward, future TRE studies with ad libitum intake should consider energy restriction as a comparison group.
We used DXA to document TRE-alterations in body composition. We observed a significant loss of fat mass and lean mass which is greater than the technical error of DXA measurement (~1–3%)(23). Both dietary restriction(24, 25, 26) and bariatric surgery(27, 28) are associated with lean mass loss. This loss has been attributed to alterations in protein intake or protein turnover(24, 29), which can be mitigated either by high protein diets(25, 30, 31) or exercise intervention(32, 33). Indeed, studies incorporating TRE with resistance training and either unrestricted intake (10), TRE with slight caloric restriction (200–250 kcal/day) and randomized whey protein supplementation(11) or TRE with instructions to maintain isoenergetic intake (34) demonstrate preserved or increased lean mass.
Although TRE was associated with lower fasting glucose, greater time within CGMS target range and lower fasting triglycerides relative to pre-intervention; these findings were not significant compared with non-TRE. The literature reports TRE having varying effects on metabolic measures. In a healthy normal-weight population, isoenergetic TRE was associated with higher fasting glucose and greater impairment of glucose tolerance(4).In participants with obesity(8) or the metabolic syndrome(9), TRE with ad libitum intake did not alter fasting glucose, fasting triglycerides or HOMA-IR. In men with prediabetes, isoenergetic TRE with completion of all food intake by 3PM improved insulin sensitivity(5).
The study has several strengths. TRE with ad libitum intake altered weight and body composition relative to a randomized non-TRE group despite the small sample size (n=20), short duration (12 weeks) and achieved eating window restriction (~10 hours, despite instructed 8 hour window). As all participants documented intake using the mCC application, we were able to monitor intervention compliance and use the non-TRE referent group to account for mCC application use. The logging also documented the extent of eating window restriction, which we correlated with observed outcomes. Lastly, we performed detailed clinical phenotyping [body composition (DXA), physical activity (actigraphy), glycemic measures (CGM/OGTT/HbA1c)] to assess TRE’s effects.
Several study limitations exist. Our reliance on the mCC application assumed consistent use. Therefore, we only enrolled participants who displayed high logging adherence during the pre-intervention period. Although logging adherence was lower in the TRE group than the non-TRE group, this difference was not statistically significant. Another study limitation is the use of eating occasions as a surrogate for energy intake, as food volume could not be ascertained. Yet, another limitation is our inclusion of participants with an eating window≥14 hours. Therefore, these findings may be attenuated and less applicable to humans with a shorter eating window. As all participants self-selected an 8-hour eating window to include the evening meal, we were unable to address the contribution of TRE timing (early vs late eating)(5, 35) or effect on circadian clock genes(35). Lastly, we acknowledge the short time frame (12 weeks), preponderance of females, and small sample size may potentially limit translation. Nevertheless, our significant results in relevant measures (body weight, body composition, eating occasions) within the context of a randomized intervention provides important data informing the design of future TRE studies.
Conclusions
Adherence to TRE presents a simplified view of food intake which reduces eating occasions and is associated with weight loss.
Trial registration: Clinicaltrials.gov NCT03129581
Supplementary Material
Online Only Supplemental Materials
Supplemental Methods 1: Multiple Imputation Analysis
Supplemental Materials 2: Inclusion/Exclusion of first participant assigned to TRE
What is known about this subject?
Time restricted eating presents a simplified view of eating by focusing on restricting the eating window, rather than intentionally restricting calories.
What does your study add?
In humans who are overweight with a pre-intervention eating window ≥ 14 hours, we found that allowing unrestricted intake within an 8-hour eating window resulted in fewer eating occasions [mean(SD): −21.9%(30.1)], weight loss [−3.7%(1.8)], fat mass [−4%(2.9)], lean mass [−3.0%(2.7)], and visceral fat [11.1%(13.4)] compared to their counterparts randomized to unrestricted intake.
Greater restriction of the eating window was associated with greater loss of fat mass and visceral fat as measured by Dual X-ray Absorptiometry
Using mobile-phone based application to document eating events “in real time,” we successfully restricted the eating window within a community population to provide detailed information about intervention compliance and outcomes for informing future time-restricted eating studies.
How might your results change the direction of research or the focus of clinical practice?
By not focusing on intentional energy restriction, time restricted eating presents a simplified view of food intake which reduces weight.
Acknowledgements
Dr. Amy Karger for her description on assay methodology.
Deidentified data will be shared after request review by Dr.Chow.
Funding Sources: This work was support by the Healthy Foods Healthy Lives program (17SFR-2YR50LC to LC) and the National Institutes of Health (NIH National Center for Advancing Translational Sciences, UL1TR002494).
Abbreviations
- BMI
Body mass index
- CGM
Continuous glucose monitoring
- DXA
Dual X-ray absorptiometry
- EO
Eating Occasion
- Fig
Figure
- HbA1c
Hemoglobin A1c
- HOMA-IR
homeostatic model assessment of insulin resistance
- mCC
MyCircadianClock
- OGTT
Oral glucose tolerance testing
- TRE
Time restricted eating
- TSH
Thyroid stimulating hormone
Footnotes
Disclosure Summary: SP has authored a book “The Circadian Code” for which he receives author royalty and specifically recommends time restricted eating.
References
- 1.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315: 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heymsfield SB, Harp JB, Reitman ML, Beetsch JW, Schoeller DA, Erondu N, et al. Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. The American journal of clinical nutrition 2007;85: 346–354. [DOI] [PubMed] [Google Scholar]
- 3.Gill S, Panda S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metabolism 2015;22: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson O, Martin B, Stote KS, Golden E, Maudsley S, Najjar SS, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism: clinical and experimental 2007;56: 1729–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metabolism 2018;27: 1212−+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. The American journal of clinical nutrition 2007;85: 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. Journal of nutritional science 2018;7: e22. [Google Scholar]
- 8.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutrition and healthy aging 2018;4: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-Hour Time-Restricted Eating Reduces Weight, Blood Pressure, and Atherogenic Lipids in Patients with Metabolic Syndrome. Cell Metabolism 2020;31: 92–104.e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, et al. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. European journal of sport science 2017;17: 200–207. [DOI] [PubMed] [Google Scholar]
- 11.Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, et al. Time-restricted feeding plus resistance training in active females: a randomized trial. The American journal of clinical nutrition 2019;110: 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbaum PR. Observational Studies. Springer: New York, 1995. [Google Scholar]
- 13.ADA. Glycemic Targets: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41: S55. [DOI] [PubMed] [Google Scholar]
- 14.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40: 181–188. [DOI] [PubMed] [Google Scholar]
- 15.Leech RM, Worsley A, Timperio A, McNaughton SA. Characterizing eating patterns: a comparison of eating occasion definitions. The American journal of clinical nutrition 2015;102: 1229–1237. [DOI] [PubMed] [Google Scholar]
- 16.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, et al. Dual-Energy X-Ray Absorptiometry for Quantification of Visceral Fat. Obesity 2012;20: 1313–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18: 499–502. [PubMed] [Google Scholar]
- 18.Bonora E, Saggiani F, Targher G, Zenere MB, Alberiche M, Monauni T, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity - Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000;23: 57–63. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28: 412–419. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22: 1462–1470 PMCID not available. [DOI] [PubMed] [Google Scholar]
- 21.Bergenstal RM, Ahmann AJ, Bailey T, Beck RW, Bissen J, Buckingham B, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. Journal of diabetes science and technology 2013;7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. New England Journal of Medicine 2004;350: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nana A, Slater GJ, Hopkins WG, Burke LM. Effects of Exercise Sessions on DXA Measurements of Body Composition in Active People. Medicine and Science in Sports and Exercise 2013;45: 178–185. [DOI] [PubMed] [Google Scholar]
- 24.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. The American journal of clinical nutrition 2006;83: 260–274. [DOI] [PubMed] [Google Scholar]
- 25.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. The American journal of clinical nutrition 2012;96: 1281–1298. [DOI] [PubMed] [Google Scholar]
- 26.D’Alessio DA, Seeley RJ, Daniels SR, Brehm BJ. A Randomized Trial Comparing a Very Low Carbohydrate Diet and a Calorie-Restricted Low Fat Diet on Body Weight and Cardiovascular Risk Factors in Healthy Women. The Journal of Clinical Endocrinology & Metabolism 2003;88: 1617–1623. [DOI] [PubMed] [Google Scholar]
- 27.Zalesin KC, Franklin BA, Lillystone MA, Shamoun T, Krause KR, Chengelis DL, et al. Differential loss of fat and lean mass in the morbidly obese after bariatric surgery. Metabolic syndrome and related disorders 2010;8: 15–20. [DOI] [PubMed] [Google Scholar]
- 28.Carnero EA, Dubis GS, Hames KC, Jakicic JM, Houmard JA, Coen PM, et al. Randomized trial reveals that physical activity and energy expenditure are associated with weight and body composition after RYGB. Obesity (Silver Spring, Md) 2017;25: 1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bopp MJ, Houston DK, Lenchik L, Easter L, Kritchevsky SB, Nicklas BJ. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J Am Diet Assoc 2008;108: 1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soenen S, Martens EA, Hochstenbach-Waelen A, Lemmens SG, Westerterp-Plantenga MS. Normal protein intake is required for body weight loss and weight maintenance, and elevated protein intake for additional preservation of resting energy expenditure and fat free mass. The Journal of nutrition 2013;143: 591–596. [DOI] [PubMed] [Google Scholar]
- 31.Pasiakos SM, Cao JJ, Margolis LM, Sauter ER, Whigham LD, McClung JP, et al. Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2013;27: 3837–3847. [DOI] [PubMed] [Google Scholar]
- 32.Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of Lifestyle Activity vs Structured Aerobic Exercise in Obese WomenA Randomized Trial. JAMA 1999;281: 335–340. [DOI] [PubMed] [Google Scholar]
- 33.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. New England Journal of Medicine 2017;376: 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, et al. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. Journal of Translational Medicine 2016;14: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019;11: 1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Only Supplemental Materials
Supplemental Methods 1: Multiple Imputation Analysis
Supplemental Materials 2: Inclusion/Exclusion of first participant assigned to TRE