Abstract
Objective: Intravenous (IV) acetaminophen (paracetamol; APAP) is well-documented to cause hypotension. Since the patients receiving intravenous APAP are usually critically ill, any severe haemodynamic changes, as with those associated with APAP, can be life threatening. The mechanism underlying this dangerous iatrogenic effect of APAP was unknown. Approach and Results: Here, we show that intravenous APAP caused transient hypotension in rats, which was attenuated by the Kv7 channel blocker, linopirdine. APAP metabolite N-acetyl-p-benzoquinone imine (NAPQI) caused a vasodilatation of rat mesenteric arteries ex vivo. This vasodilatation was sensitive to linopirdine, and also the calcitonin gene-related peptide (CGRP) antagonist, BIBN 4096. Further investigation revealed NAPQI stimulates CGRP release from perivascular nerves, causing a cAMP-dependent activation of Kv7 channels. We also show that NAPQI enhances Kv7.4 and Kv7.5 channels overexpressed in oocytes, suggesting that it can activate Kv7.4 and Kv7.5 channels directly, in order to elicit a vasodilatation. Conclusions: Direct and indirect activation of Kv7 channels by the APAP metabolite NAPQI decreases arterial tone, which can lead to a drop in blood pressure. Our findings provide a molecular mechanism and potential preventive intervention for the clinical phenomenon of intravenous APAP-dependent transient hypotension.
Keywords: Kv7 channels, KCNQ, NAPQI, smooth muscle, vascular, acetaminophen, paracetamol, Vascular Biology, Pharmacology, Blood Pressure
Graphical Abstract

Introduction
Acetaminophen (paracetamol; APAP) is the most frequently used analgesic worldwide and the most common drug ingredient used in the United States, with a presence in over 600 medications. Orally administered APAP can increase blood pressure when used often 1–3. In contrast, APAP is well-documented to cause severe and sometimes fatal hypotension in patients when administered intravenously, such as is common in surgical intensive care units 4–8. Although >33% of patients that had APAP-induced hypotension required therapeutic intervention 6,9, the mechanisms underlying the severe haemodynamic changes during IV APAP administration are still unknown 4.
APAP’s analgesic mechanism of action is regarded to be through cyclooxygenase (COX) inhibition 10; however this hypothesis has come under scrutiny since APAP lacks the adverse effects of nonsteroidal anti-inflammatory drugs (NSAIDs) that are associated with COX inhibition. Novel targets of certain APAP metabolites have been identified to underlie its analgesic effects. N-acetyl-p-benzoquinone imine (NAPQI) and N-arachidonoylphenolamine (AM404) are two such metabolites of APAP identified to activate TRPA1 and TRPV1, which were associated with its antinociceptive effects 11–13. Furthermore, NAPQI was identified recently to enhance the activity of the neuronal voltage-gated Kv7 potassium channels, Kv7.2 and Kv7.3 in dorsal root ganglion and spinal dorsal horn neurones 14. By enhancing Kv7 channel activity, NAPQI hyperpolarised the membrane potential and reduced action potential firing, which might underlie the analgesic action of APAP as well as contributing to APAPs recently discovered anticonvulsant properties 14,15.
Since Kv7 channels are important regulators of arterial tone 16–21, we hypothesised that APAP-induced hypotension was due to APAP metabolites activating Kv7 channels, thereby causing a vasodilation and a drop in blood pressure. Herein, we provide in vivo, ex vivo and in vitro evidence that APAP metabolites can cause hypotension and relax arteries by activating Kv7 channels directly and indirectly.
Materials and Methods
Disclosure Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
To avoid the potential confounding effects of sex-specific Kv7 channel function in the vasculature45 that are still not understood, male Wistar rats (Janvier Labs, France) aged 12–14 weeks were used in accordance with Directive 2010/63EU on the protection of animals used for scientific purposes and approved by the national ethics committee, Denmark. Rats were group-housed with regular 12-hour light/dark cycles, in clear plastic containers with ad libitum access to food and water, and underwent at least one week of habituation.
Surgical preparation for rat in vivo experiments
In vivo experiments were performed on 12-week-old male Wistar rats (300–400g). The rats were anaesthetized with 5% isoflurane delivered in 35% oxygen and 65% nitrogen, intubated and connected to a respirator (~65 breaths/min; tidal volume 8 ml/kg). The left carotid artery was cannulated with a catheter connected to a pressure transducer (Statham P23-dB) for continuous monitoring of the arterial blood pressure. The left jugular vein was cannulated to allow for continuous infusion of a muscle relaxant, cisatracurium (0.85 mg/ml), and saline infusion or i.v. administration of the drugs. The rat was placed on a heating plate to maintain body temperature at 37°C and the trachea was. After surgery, the anaesthesia was maintained at 2 % isoflurane and the rat was allowed to rest for 30 minutes before initiating the experimental protocol. After the experimental protocol the rat was euthanized.
Experimental protocol for in vivo rat experiments
After the 30 min rest period and obtaining a stable blood pressure baseline, APAP was infused at 0.33mg/min for 15 minutes to give a total dose of 4.95 mg of APAP, equivalent to 14.14 mg/kg in a 350 g rat, which corresponds to a 70 kg human receiving 1 g of APAP. Hereafter, another 30-min recovery period under infusion of saline was initiated to recover the blood pressure to baseline. Subsequently, the Kv7 channel blocker linopirdine (0.9 mg/min) was pre-infused for 10 minutes followed by co-infusion of linopridine (0.9 mg/min) and APAP (0.33 mg/min) for another 15 minutes. The infusion rate of the saline and drugs, including cisatracurium, was constant throughout the experiment at 40 μl/min.
Channel subunit cRNA preparation and Xenopus laevis oocyte injection
cRNA transcripts encoding human Kv7.4 and Kv7.5 were generated by in vitro transcription using the T7 polymerase mMessage mMachine kit (Thermo Fisher Scientific), after vector linearization, from cDNA and sub-cloned into plasmids incorporating Xenopus laevis β-globin 5’ and 3’ UTRs flanking the coding region to enhance translation and cRNA stability. We quantified cRNA by spectrophotometry. Defolliculated stage V and VI Xenopus laevis oocytes (Xenoocyte, Dexter, MI, US) were injected with Kv7.4 and/or Kv7.5 cRNA (20 ng total per oocyte). Oocytes were incubated at 16 °C in Barth’s saline solution (Ecocyte Bioscience, Austin, TX) containing penicillin and streptomycin, and washed daily for 3–5 days prior to two-electrode voltage-clamp (TEVC) recording.
Isometric Tension Recordings
Rats were euthanized by cervical dislocation and third-order mesenteric arteries were isolated and mounted in a wire myograph (Danish Myo Technology, Aarhus Denmark) for isometric tension recording as described previously 20. Briefly, arterial segments were removed from the animals and cleaned of adherent tissue in physiological salt solution (PSS) containing (in mM): 121 NaCl, 2.8 KCl, 1.6 CaCl2, 25 NaHCO3, 1.2 KH2HPO4, 1.2 MgSO4, 0.03 EDTA, and 5.5 glucose. Following dissection, vessels were cut into 2 mm segments and mounted on 40 μm stainless steel wires in a myograph for isometric tension recordings. The chambers of the myograph contained PSS maintained at 37°C and aerated with 95% O2/5% CO2. Changes in tension were recorded continuously by PowerLab and Chart software (ADInstruments, Oxford, United Kingdom). The arteries were equilibrated for 30 minutes and normalized to passive force 46.
Arteries were contracted with 20 μM α1-adrenergic receptor agonist, methoxamine, before increasing concentrations of APAP (1.0 – 100 μM), NAPQI (0.1 – 10 μM) or AM404 (0.1 – 30 μM) were added to vessels in the absence or presence of the Kv7 channel blocker, linopirdine (10 μM). The effects of NAPQI and AM404 were also tested on preconstricted segments of mesenteric arteries in presence of the CGRP receptor antagonist, BIBN 4096 (1 μM) or TRPV1 channel blocker AMG9810 (0.1 μM). Furthermore, NAPQI relaxations were tested after repeated capsaicin (10 μM) stimulations to deplete CGRP levels. To test the effects of NAPQI and AM404 on contractions elicited by high external potassium, the PSS was replaced with a high postassium solution (KPSS) containing (in mM): 123.7 KCl, 1.6 CaCl2, 25 NaHCO3, 1.2 KH2HPO4, 1.2 MgSO4, 0.03 EDTA, and 5.5 glucose. Once a stable contraction was achieved, either NAPQI or AM404 was applied.
Two-electrode voltage clamp (TEVC)
TEVC was performed at room temperature using an OC-725C amplifier (Warner Instruments, Hamden, CT) and pClamp11 software (Molecular Devices, Sunnyvale, CA) 3–5 days after cRNA injection as described in the section above. For recording, oocytes were placed in a small-volume oocyte bath (Warner) and viewed with a dissection microscope. Bath solution was (in mM): 96 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2, 10 HEPES (pH 7.6). NAPQI and AM404 were solubilised in DMSO and diluted to working concentrations each experimental day. NAPQI and AM404 were introduced into the oocyte recording bath by gravity perfusion at a constant flow of 1 ml per minute for 3 minutes prior to recording. Pipettes were of 1–2 MΩ resistance when filled with 3 M KCl. We recorded currents in response to voltage pulses between −120 or −80 mV and + 40 mV at 20 mV intervals from a holding potential of −80 mV, to yield current-voltage relationships, current magnitude, and for quantifying activation rate. Electrophysiology data analysis was performed with Clampfit (Molecular Devices) and Graphpad Prism software (GraphPad, San Diego, CA, USA); values are stated as mean ± SEM. Raw or normalized tail currents were plotted versus prepulse voltage and fitted with a single Boltzmann function:
| Eq. 1 |
where g is the normalized tail conductance, A1 is the initial value at −∞, A2 is the final value at +∞, V1/2 is the half-maximal voltage of activation and Vs the slope factor. We fitted activation and deactivation kinetics with single exponential functions. Based on our previous studies of Kv7 channel pharmacology using the oocyte expression 21 system, the homogeneity, stability and reproducibility of the system with respect to recordings of drug effects compared to an internal reference, baseline recording from the same oocyte means that the variability is minimal and n≥5 gives an accurate representation allowing conclusions to be made.
Chemicals and Reagents
Paracetamol (acetaminophen, APAP), N-arachidonoylaminophenol (AM404), N-acetyl-p-benzoquinone imine (NAPQI), linopirdine, methoxamine, indomethacin and capsaicin were purchased from Sigma-Aldrich (Søborg, DK). CGRP (rat), glibencamide, AMG9810 and BIBN 4096 were purchased from Tocris (UK). Stock solutions of APAP, AM404, NAPQI, linopirdine BIBN4096, glibencamide, indomethacin, AMG9810 and capsaicin were prepared in DMSO. Methoxamine and CGRP were prepared in Milli-Q water.
Statistics
All data passed the F test for equal variance and with no sphericity assumed. Normality was not assessed as the n values were too low for an accurate D’Agostino & Pearson test. In TEVC experiments, the changes in membrane potential (EM) were compared with a Student’s unpaired t test. Maximum relaxation (Rmax) and EC50 values from the isometric tension experiments were compared by either a Student’s unpaired t test or one-way ANOVA followed by a Tukey or Dunnett’s multiple comparison test. For the in vivo experiments, changes in mean, systolic and diastolic arterial pressures, and heart rate and pulse pressure were compared by a Wilcoxon matched-pairs signed rank test. All data are mean±S.E.M.
Results
Intravenous APAP causes hypotension in rats, which is attenuated by Kv7 channel inhibition
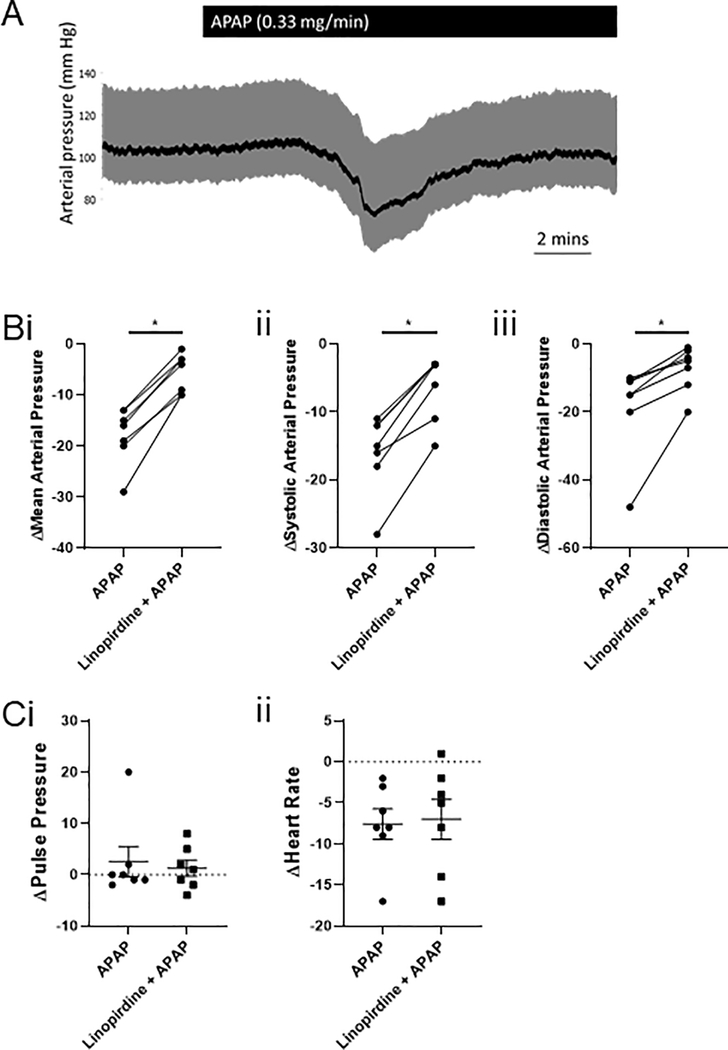
Although there are inherent differences in human and rat metabolism as well as differences in the ratios of total water to total plasma levels, we attempted to administer a dose of APAP to the anaesthetized rats that corresponds to that given to humans. In humans, typical intravenous doses for APAP are of the order of 1 g, which for a 70 kg person, would equate to 14.28 mg/kg. Thus, in the rats, which had an average weight of 350 g, we administered 0.33 mg/min for 15 min to give a dose of 14.12 mg/kg and a total of 4.95 mg of APAP. APAP induced a transient drop in mean arterial pressure from 97 ± 4 mmHg to 79 ± 4 mmHg (n=7; P=0.015 according to a Wilcoxon matched-pairs signed rank test; figure 1). APAP did not affect the heart rate (340 ± 13 bpm before vs. 333 ± 13 bpm during APAP-induced hypotension) or pulse pressure (28 ± 5 mmHg before vs. 30 ± 7 mmHg during APAP-induced hypotension).
Figure 1: Intravenous APAP decreases mean, systolic and diastolic arterial pressure, which linopirdine attenuated.
(A) (i) MAP (ii) SAP and (iii) DAP are decreased in anaesthetised rats infused with APAP intravenously (n=7). This effect is inhibited by the pre- and co-infusion of linopirdine for MAP, SAP and DAP (according to a Wilcoxon matched-pairs signed rank test, with * denoting P<0.05).
(B) (i) Pulse pressure and (ii) heart rate were not different between groups (n=7).
To determine if the APAP-induced hypotension could be prevented by Kv7 channel inhibition, we pre-infused linopirdine at 39.1mg/ml for 10 minutes, before co-infusion of linopridine (0.9 mg/min) and APAP (0.33 mg/min). Under these conditions, APAP still decreased mean arterial pressure from 90 ± 5 mmHg to 85 ± 4 mmHg (n=7), however this decrease in mean arterial pressure was much smaller compared to that in the absence of linopirdine (−5 ± 1 mmHg vs −18 ± 2 mmHg; P=0.015 according to a Wilcoxon matched-pairs signed rank test; figure 1). APAP-induced decreases in systolic and diastolic arterial pressures were also attenuated by pre- and co-infusion of linopirdine (figure 1). Linopirdine had no effect on the heart rate or pulse pressure during pre-infusion or co-infusion with APAP (figure 1).
APAP has no effect on tone in rat mesenteric arteries
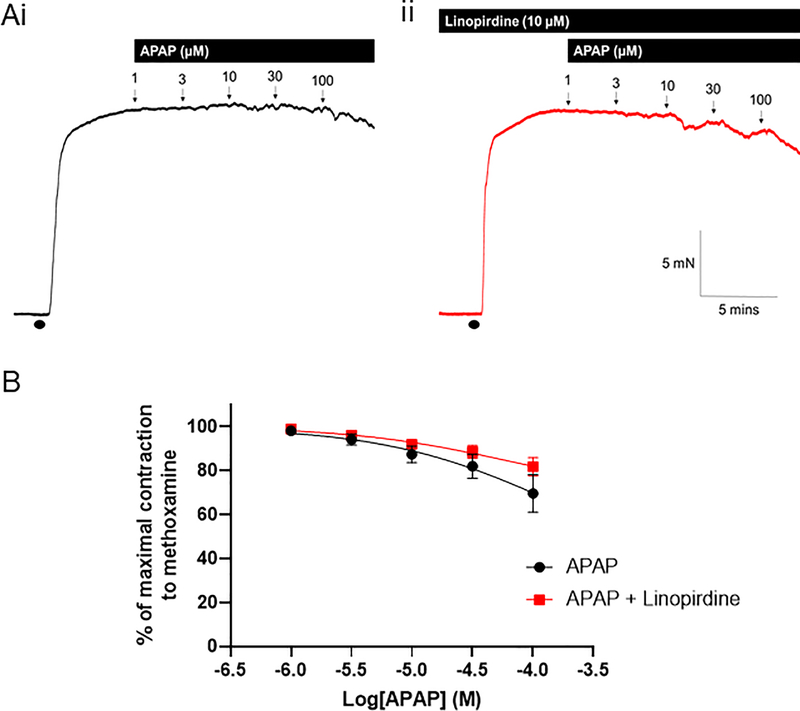
Application of increasing concentrations of APAP to rat mesenteric arteries pre-constricted with methoxamine caused a diminutive relaxation at 100 μM, which was not affected by the Kv7 channel blocker, 10 μM linopirdine (n=7; figure 2).
Figure 2: APAP has no effect on preconstricted mesenteric arteries.
(A) Representative isometric tension recordings showing the effect of increasing concentrations of APAP in rat mesenteric arteries preconstricted with 10 μM methoxamine (•) in the (i) absence and (ii) presence of the Kv7 channel inhibitor, linopirdine (10 μM).
(B) Mean data showing the effect of APAP (n=7) and APAP in the presence of linopirdine (n=7) on the tension of preconstricted mesenteric arteries.
NAPQI relaxes mesenteric artery segments
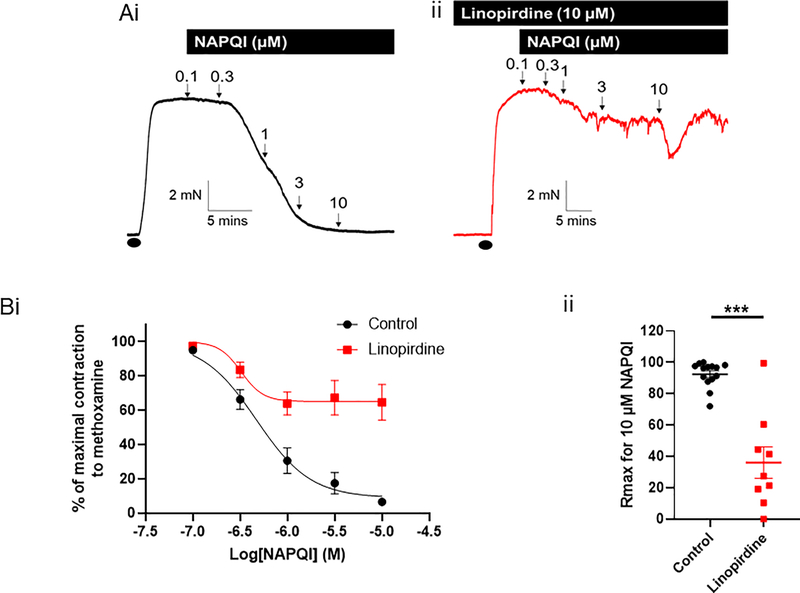
We applied increasing concentrations of NAPQI to mesenteric artery segments pre-constricted with methoxamine (figure 3). NAPQI caused vasorelaxation of the arterial segments (n=14), which was inhibited by pretreatment with 10 μM linopirdine (n=9; Rmax P=0.0004 according to an unpaired Student’s t test; figure 3). Application of 10 μM NAPQI in high K+-containing PSS had no effect (Supplementary figure I). To determine whether the NAPQI-relaxations were due to COX inhibition, we performed experiments in the presence of the COX inhibitor indomethacin. Application of 10 μM indomethacin had no effect on NAPQI relaxations (n=7; supplementary figure II).
Figure 3: NAPQI relaxes preconstricted mesenteric arteries, which is attenuated by Kv7 channel inhibition.
(A) Representative isometric tension recordings showing the effect of increasing concentrations of NAPQI in rat mesenteric arteries preconstricted with 10 μM methoxamine (•) in the (i) absence and (ii) presence of the Kv7 channel inhibitor, linopirdine (10 μM).
(B) (i) Mean data showing the effect of NAPQI in the absence (n=14) and presence of linopirdine (n=9) o the tension of preconstricted rat mesenteric arteries. (ii) Linopirdine significantly attenuates the Rmax for NAPQI (according to a Student’s unpaired t test, *** denotes P<0.001).
NAPQI stimulates CGRP release from perivascular nerves
NAPQI can activate TRPA1 and TRPV1, which are found on perivascular nerves and induce release of calcitonin gene-related peptide (CGRP), a potent vasodilator 22. We therefore investigated whether the relaxation to NAPQI was attributable to CGRP release from the perivascular nerves.
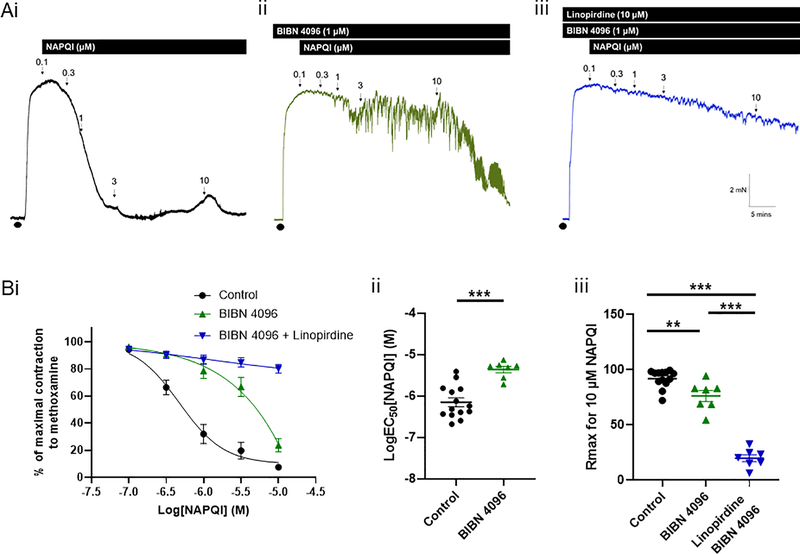
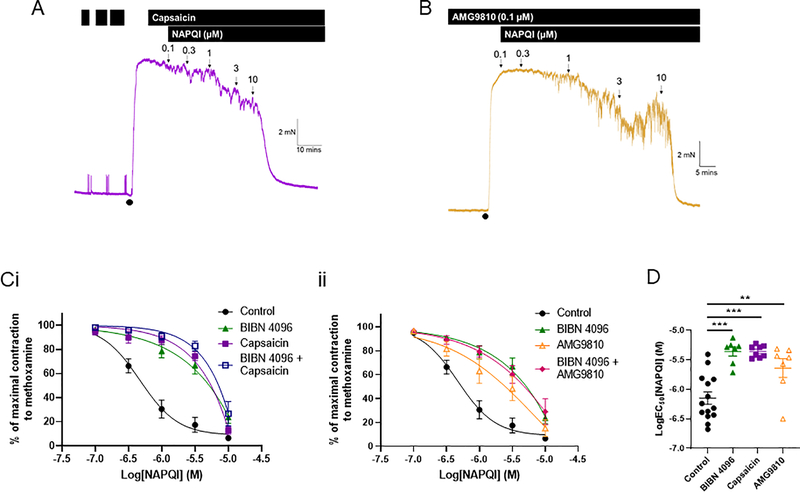
Direct inhibition of the CGRP receptor with 1 μM BIBN 4096 (n=7) attenuated the NAPQI-induced vasorelaxations (control n=14; EC50 P<0.0001 according to an unpaired Student’s t test; figure 4). At 10 μM, NAPQI was able to relax the arterial segments in the presence of BIBN 4096, although this relaxation was still attenuated compared to control (Rmax P=0.033 according to a one-way ANOVA followed by a Tukey multiple comparisons test; figure 4). When BIBN 4096 was applied with linopirdine (n=7), the relaxation to NAPQI was abolished completely (Rmax P<0.0001 according to a one-way ANOVA followed by a Tukey multiple comparisons test; figure 4).
Figure 4: NAPQI-mediated relaxations are dependent on CGRP receptor activation as well as Kv7 channel activation.
(A) Representative isometric tension recordings showing the effect of increasing concentrations of NAPQI in rat mesenteric arteries preconstricted with 10 μM methoxamine (•) (i) under control conditions, (ii) in the presence of the CGRP receptor antagonist, BIBN 4096, and (iii) in the presence of BIBN 4096 and the Kv7 channel inhibitor, linopirdine (10 μM).
(B) (i) Mean data showing the effect of NAPQI on changes in tension of preconstricted rat mesenteric arteries in control arteries (n=14), in the presence of BIBN 4096 (n=7) and in the presence of BIBN 4096 and linopirdine (n=7). (ii) BIBN 4096 significantly attenuates the LogEC50 for NAPQI (according to an unpaired t test, *** denotes P<0.001). (iii) BIBN 4096 and BIBN 4096 with linopirdine attenuated the Rmax for NAPQI (according to a one-way ANOVA followed by a Tukey multiple comparisons test, ** denotes P<0.01 and *** denotes P<0.001).
To confirm that the BIBN 4096-sensitive relaxations were due to CGRP release from the perivascular nerves, we depleted CGRP from the perivascular nerves using the TRPV1 activator, capsaicin. After repeated capsaicin stimulations, we precontracted the arterial segment, confirmed that capsaicin was unable to relax the artery and applied increasing concentrations of NAPQI (n=7; figure 5). The effect of NAPQI after capsaicin-stimulations was the same as in the presence of BIBN 4096 (NAPQI EC50 for BIBN 4096 vs capsaicin was −5.36±0.07 vs −5.36±0.03; P=0.99 according to a one-way ANOVA followed by a Tukey multiple comparisons test; figure 5). When we performed the capsaicin experiments in the presence of BIBN 4096, there was no effect on the NAPQI relaxations observed with capsaicin alone (figure 5). We also confirmed this pathway by inhibiting the TRPV1 channel with AMG9810 to prevent NAPQI from stimulating this channel thereby provoking CGRP release (figure 5). The relaxations to NAPQI in the presence of 0.1 μM AMG9810 (EC50 = −5.6±0.16; n=7; P=0.0095 compared to control according to a one-way ANOVA followed by a Tukey multiple comparisons test) were comparable to those in the presence of BIBN 4096 and capsaicin (P=0.39 and P=0.35, respectively, according to a one-way ANOVA followed by a Tukey multiple comparisons test; figure 5). In addition, BIBN 4096 did not affect the NAPQI-mediated relaxations in the presence of AMG9810 (n=7; figure 5). These results confirm comprehensively that this component of the relaxation was due to CGRP release from the perivascular nerves and not a direct effect of NAPQI on the CGRP receptor.
Figure 5: NAPQI relaxes preconstricted mesenteric arteries by stimulating CGRP release.
Representative isometric tension recordings showing the effect of increasing concentrations of NAPQI in preconstricted (•) rat mesenteric arteries (A) pre-treated with, and in the presence of capsaicin, and (B) in the presence of AMG9810.
(C) (i) Mean data showing the effect of NAPQI on changes in tension of preconstricted rat mesenteric arteries in control arteries (n=14), in the presence of BIBN 4096 (n=7), capsaicin (n=7) and BIBN 4096 with capsaicin (n=7). (ii) Mean data showing the effect of NAPQI on changes in tension of preconstricted rat mesenteric arteries in control arteries (n=14), in the presence of BIBN 4096 (n=7), AMG9810 (n=7) and BIBN 4096 with AMG9810 (n=7). (D) BIBN 4096, capsaicin and AMG9810 all attenuated the LogEC50 for NAPQI significantly (according to a one-way ANOVA followed by a Tukey multiple comparisons test, ** and *** denote P<0.01 and P<0.001, respectively).
We confirmed that CGRP receptor stimulation could lead to Kv7 channel activation by applying increasing concentrations of CGRP in the absence and presence of linopirdine. We also tested the effect of the KATP channel blocker glibencamide, since these channels have been implicated in CGRP-dependent relaxations 23. CGRP caused a concentration-dependent relaxation of mesenteric artery segments, which was inhibited by linopirdine (supplementary figure III). Glibencamide had no effect on CGRP-dependent relaxations (supplementary figure III). Furthermore, glibencamide had no effect on NAPQI-induced relaxations (supplementary figure IV).
AM404 stimulates CGRP release to elicit vasorelaxations that are linopirdine-sensitive
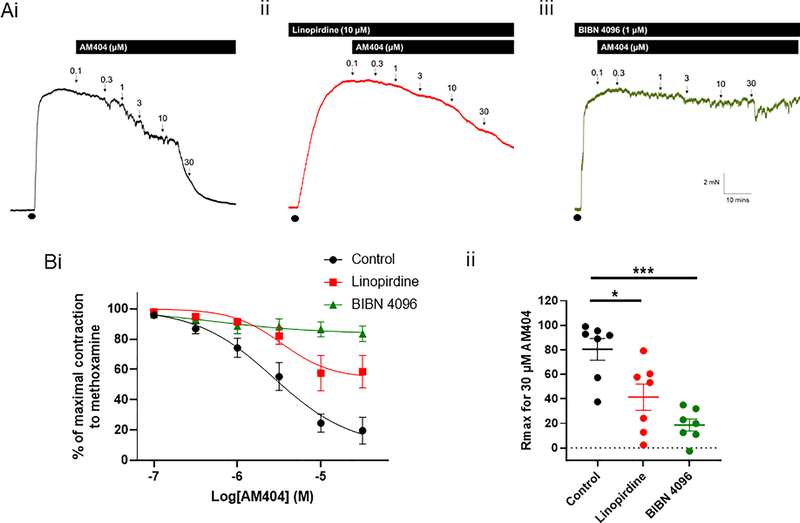
The second APAP metabolite, AM404, caused a concentration-dependent relaxation of pre-constricted mesenteric artery segments (n=7; figure 6). The Kv7 channel blocker, linopirdine, attenuated this relaxation (n=7; Rmax P=0.01 according to a one-way ANOVA followed by a Dunnett’s multiple comparisons test; figure 6); Application of the CGRP receptor antagonist, BIBN 4096, fully inhibited AM404-induced relaxations (n=7; Rmax P=0.0007 according to a one-way ANOVA followed by a Dunnett’s multiple comparisons test; figure 6). Application of 30 μM AM404 in high K+-containing PSS had no effect (supplementary figure I).Taken together, these data suggest that AM404 was eliciting its relaxation through CGRP release, which activates vascular Kv7 channels and other cAMP-dependent vasorelaxant mechanisms.
Figure 6: AM404-mediated relaxations are dependent on CGRP receptor activation.
(A) Representative isometric tension recordings showing the effect of increasing concentrations of AM404 in rat mesenteric arteries preconstricted with 10 μM methoxamine (•) (i) under control conditions, (ii) in the presence of the Kv7 channel inhibitor, linopirdine (10 μM) and (iii) in the presence of the CGRP receptor antagonist, BIBN 4096.
(B) (i) Mean data showing the effect of AM404 on changes in tension of preconstricted rat mesenteric arteries in control arteries (n=7), in the presence of linopirdine (n=7) and in the presence of BIBN 4096 (n=7). (ii) Linopirdine and BIBN 4096 attenuated the Rmax for AM404 (according to a one-way ANOVA followed by a Tukey multiple comparisons test, * denotes P<0.05 and *** denotes P<0.001).
NAPQI enhances Kv7.5 channel activity
Vascular smooth muscle cells express Kv7.4 and Kv7.5 channels, which are important regulators of vascular tone 24,25. We tested whether NAPQI and/or AM404 were able to enhance Kv7.4 or Kv7.5 channels overexpressed in oocytes. At 1 μM, NAPQI had no effect on Kv7.4 current density and no effect on Kv7.4 V0.5activation. Conversely, 100 μM NAPQI increased Kv7.4 currents 20-fold at −60 mV and shifted Kv7.4 V0.5activation −19.1 ± 2.7 mV (Figure 7A–C). Consistent with the effect on Kv7.4 V0.5activation, NAPQI hyperpolarised the EM of oocytes expressing Kv7.4 (Figure 7D). Kv7.4 currents were also augmented 4-fold at +40 mV by 100 μM NAPQI (Figure 7B).
Figure 7. NAPQI activates KCNQ4 and KCNQ5 channels.
(A) Mean traces showing effects of NAPQI (1 versus 100 μM, as indicated) on Kv7.4 (n=5–7).
(B) Raw and normalized (G/Gmax) Kv7.4 tail currents versus prepulse voltage relationships calculated from traces as in A in the presence (red) versus absence (black) of NAPQI (1 versus 100 μM, as indicated; n=5–7).
(C) Effects of NAPQI (1 versus 100 μM, as indicated) on Kv7.4 quantified as current fold-increase versus voltage (n=5–7).
(D) Effects of NAPQI (1 versus 100 μM, as indicated) on resting membrane potential (EM) of unclamped X. laevis oocytes expressing Kv7.4 (n=5–7).
(E) Mean traces showing effects of NAPQI (1 versus 100 μM, as indicated) on Kv7.5 (n=5–10).
(F) Raw and normalized (G/Gmax) Kv7.5 tail currents versus prepulse voltage relationships calculated from traces as in E in the presence (red) versus absence (black) of NAPQI (1 versus 100 μM, as indicated; n=5–10).
(G) Effects of NAPQI (1 versus 100 μM, as indicated) on Kv7.5 quantified as current fold-increase versus voltage (n=5–10).
(H) Effects of NAPQI (1 versus 100 μM, as indicated) on resting membrane potential (EM) of unclamped X. laevis oocytes expressing Kv7.5 (n=5–10).
(I) Current fold-increase versus [NAPQI] for KCNQ4, Kv7.5, or heteromeric Kv7.4/ Kv7.5 channels at −60 mV (n=3–6).
(J) Mean traces showing effects of N-4-Hydroxyphenyl arachidonylamide (AM404; 100 μM) on Kv7.4 and Kv7.5 (n=5).
(K) Raw and normalized (G/Gmax) Kv7.4 and Kv7.5 tail currents versus prepulse voltage relationships calculated from traces as in J in the presence (blue) versus absence (black) of N-4-Hydroxyphenyl arachidonylamide (AM404; 100 μM; n=5).
(L) Effects of N-4-Hydroxyphenyl arachidonylamide (AM404; 100 μM) on Kv7.4 and Kv7.5quantified as current fold-increase versus voltage (n=5). All error bars indicate SEM.
In comparison, Kv7.5 exhibited even greater sensitivity to NAPQI, with 1 μM increasing Kv7.5 currents 4-fold at −60 mV and shifting the V0.5activation −10.1 ± 1.4 mV (Figure 7E–G). At 1 μM, NAPQI hyperpolarised by −10 mV the EM of oocytes expressing Kv7.5 (Figure 7H). Strikingly, 100 μM NAPQI resulted in potent augmentation of Kv7.5 currents even between −120 to −80 mV, increasing Kv7.5 currents 100–130-fold (Figure 7E–G) and hyperpolarising by >−20 mV the EM of oocytes expressing Kv7.5 (Figure 7H). Like Kv7.4, maximal Kv7.5 currents at +40 mV were also augmented (11-fold by 100 μM NAPQI) (Figure 7F).
We were unable to establish an accurate NAPQI EC50 value for Kv7.4 or Kv7.5 due to the nature of the dose response curve, which suggests NAPQI is a highly efficacious Kv7.4 and Kv7.5 activator, but with a relatively high EC50 (Figure 7I). Heteromeric Kv7.4/Kv7.5 channels exhibited a NAPQI sensitivity that was intermediate between that of the respective homomers (Figure 7I).
Consistent with data in figure 6, which suggested that Kv7 channel activation by AM404 was entirely indirect via perivascular nerve stimulation, AM404 had negligible effects on oocyte-expressed Kv7.4 and Kv7.5 activity (Figure 7J) or voltage dependence (Figure 7K), as also evidenced by the lack of changes it induced in Kv7.4 or Kv7.5 current magnitude, across the voltage range (Figure 7L).
Discussion
APAP is the most commonly used analgesic in the world. Apart from well-known dose-dependent hepatotoxicity, it is considered a safe drug; yet, the precise modes of action for various APAP metabolites are still unknown and new targets are still being discovered 11–13. One such target revealed recently was the “neuronal” Kv7 channels, Kv7.2 and Kv7.3, which are enhanced by the APAP metabolite NAPQI 14. In this study, we have found that Kv7 channel activation contributes to APAP-induced hypotension and that NAPQI, but not AM404, is able to enhance Kv7.4 and Kv7.5 channels directly.
The Kv7 channel family of voltage-gated K+ channels exists of five isoforms encoded by the KCNQ1–5 genes (Kv7.1-Kv7.5), with each isoform having a characteristic tissue distribution and function. In the vasculature, Kv7 channels, particularly Kv7.4 and Kv7.5 channels, are important regulators of vascular tone 16–21. Kv7 channel activation in smooth muscle produces hyperpolarisation of the membrane potential, decreasing the open probability of the voltage-gated calcium channels, which ultimately results in decreased Ca2+ entry and decreased contractility. Moreover, Kv7 channels in vascular smooth muscle are functional endpoints for numerous endogenous vasodilators. Agonists of Gs-coupled receptors, such as calcitonin-gene-related peptide (CGRP), isoprenaline and adenosine, increase vascular Kv7 channel activity and promote vasodilation 26–31.
APAP is available in intravenous form for inpatient management of acute pain. In the critically ill patients to which it is administered, intravenous APAP can cause iatrogenic hypotension, which hemodynamically compromises the patient, increasing mortality 4,5,9,32. Up to 61% of patients treated with acetaminophen develop transient hypotension 5–8, which is also known to affect non-critically ill patients 33. The severity of the APAP-induced transient hypotension is highlighted by studies identifying >33% of patients that had APAP-induced hypotension required therapeutic intervention 6,9. The iatrogenic and molecular mechanisms of APAP-induced hypotension remain unknown, with decreases in both cardiac output and peripheral resistance being implicated 4. In the present study, we found that intravenous administration of APAP to anaesthetised rats caused a transient decrease in mean arterial pressure of −17 ± 2 mmHg, similar to that described in humans 4,9. We were able to attenuate this hypotension with pre- and co-infusion of the Kv7 channel inhibitor, linopirdine. These findings suggest the APAP-induced hypotension is due to activation of the Kv7 channels on vascular smooth muscle, which would decrease total peripheral resistance. We did not observe any APAP-induced changes in heart rate or pulse pressure, suggesting that cardiac output is maintained and is not responsible for the hypotension we observed in the anaesthetised rats.
Using ex vivo segments of mesenteric artery, we were able to investigate thoroughly the Kv7 channel activation that occurs with APAP in vivo. We report that two different APAP metabolites, AM404 and NAPQI, can induce CGRP release from CGRPergic perivascular nerves found in the adventitial layer of mesenteric arteries 22,34, thereby relaxing preconstricted mesenteric arteries 22,34–36. This relaxation was dependent predominantly, but not completely, on Kv7 channel activation. Several studies have shown that Kv7 channels are important downstream effectors of cAMP-mediated vasorelaxations 26–31. A study by Chadha et al. (2014), found that CGRP-mediated dilation of rat middle cerebral arteries requires functional Kv7 channels 27 and in this study we demonstrate that linopirdine attenuated CGRP-relaxations in isolated rat mesenteric arteries. We also investigated whether KATP channels were indirectly involved in the NAPQI- and AM404-dependent relaxations, downstream of CGRP release 23, but found no effect of the KATP inhibitor, glibencamide. This was in line with no effect of glibencamide on CGRP-relaxations, which is supported by other studies 35,37. The results of our study support a role for Kv7 channel recruitment following CGRP receptor activation; however we do not negate a role for other K+ channels in CGRP-mediated vasodilations 35. In this study, we also found that NAPQI can activate Kv7 channels on the arterial smooth muscle directly to produce a vasorelaxation, which is seen at 10 μM NAPQI in the presence of the CGRP antagonist, BIBN 4096, after CGRP depletion with capsaicin and in the presence of the TRPV1 blocker, AMG9810. The ability of NAPQI to activate Kv7.4 and Kv7.5 channels directly was confirmed by two-electrode voltage clamp experiments on oocytes overexpressing these channels, whereas AM404 had no effect on the overexpressed channels.
We suggest that NAPQI is the most likely metabolite to elicit the APAP-induced hypotension that was linopirdine-sensitive. The role of NAPQI, and not the alternative metabolite AM404, is supported by the current data and it is unlikely that these effects are mediated by the other known metabolites of APAP (the glucuronic acid and sulfate adducts), as these have well established non-toxic effects. NAPQI is a highly reactive electrophile and undergoes very ready adduction to proteins, particularly at Cys residues, which are the most reactive common nucleophiles on proteins and known to be very sensitive to modification. In this regard, it is worth noting that Kv7.4 and 7.5 channels have a triad of reactive Cys residues that are known to be sensitive to modification 38, which could act as the potential site of NAPQI interaction. Such Cys residues would not be expected to be modified by the glucuronic acid, sulfate or AM404 metabolites because of the poor reactivity of these species relative to NAPQI. Taken together, these striking findings show that NAPQI is the most likely APAP metabolite to cause vasodilatation by directly activating Kv7 channels on vascular smooth muscle, whereas both NAPQI and AM404 are able to activate Kv7 channels indirectly through stimulation of CGRP-release from perivascular nerves.
APAP is taken in its oral form predominantly. When taken orally, APAP is metabolized extensively by the liver via three main hepatic pathways: glucuronidation, sulfation, and CYP450 oxidation 39,40. At regular doses, between 80%–90% of APAP is conjugated with glucuronic acid or sulfate to form nontoxic metabolites that can be excreted through the kidneys 41. A minor component is metabolised by CYP450 enzymes to NAPQI, which is toxic. The most important of these CYP450 enzymes are CYP1A2, CYP2E1 and CYP3A4, although at high concentrations of APAP, metabolism via CYP2E1 predominates. Under normal therapeutic doses, NAPQI is detoxified rapidly in the liver by glutathione and excreted through the bile 40,41. Thus, NAPQI is unlikely to have systemic haemodynamic effects when APAP is taken orally.
When administered intravenously, APAP needs to be metabolised outside of the liver to cause a Kv7 channel-dependent decrease in total peripheral resistance and concomitant decrease in blood pressure. The endothelial cells of the vascular wall contain several CYP450 enzymes with prominent roles in vascular regulation, particularly those in the CYP 2 gene family (e.g., CYP 2B, 2C8, 2C9, 2C10, and 2J2) 42. We speculate that these CYP450 enzymes in the endothelium have the capacity to metabolise APAP to NAPQI, thereby resulting in a direct and indirect (through CGRP release) activation of Kv7 channels, as we showed in our myograph studies. One limitation of our study is that we have not investigated the role of endothelium CYP450 enzymes in APAP-induced hypotension. Future studies will clarify the role of these enzymes in generating NAPQI localised throughout the vasculature in order to activate Kv7 channels and elicit the observed effects. In addition to CYP450 enzymes, direct oxidation of APAP can lead to the formation of radical species and NAPQI as a product species. One such pathway of APAP oxidation involves the leukocyte-derived species myeloperoxidase 43, which is released in response to inflammatory stimuli and can lead to the generation of the powerful oxidants HOCl (hypochlorous acid, from chloride ions), HOBr (hypobromous acid from bromide ions) and HOSCN (hypothiocyanous acid from thiocyanate ions). This might prove to be a crucial pathway underlying NAPQI formation when APAP is administered intravenously to critically ill patients that have systemic inflammation, such as sepsis. It will be important for future studies to investigate whether systemic inflammation augments APAP-induced hypotension through increased NAPQI formation, which would provide a clear contraindication for the intravenous APAP in the critically ill.
Our data raise the possibility of a Kv7 channel blocker, such as linopirdine, being used to prevent the iatrogenic APAP-induced hypotension. A trial performed with linopirdine in Alzheimer’s disease patients reported elevations of liver function tests as the only adverse clinical event occurring significantly more often in linopirdine patients than in those receiving placebo 44. Although these data suggest linopirdine is well tolerated in patients, the effects of Kv7 channel blockers are not understood fully, particularly in the critically ill, which is a limitation of our study. Future work will investigate whether linopirdine is tolerated intravenously and if it is able to inhibit APAP-induced hypotension in humans. Additionally, with the effect and target of NAPQI now identified in this study, future work could develop a novel, specific Kv7.4/7.5 channel inhibitor that has a short half-life and is administered intravenously with paracetamol.
In summary, we provide novel molecular insight to explain how intravenous APAP administration causes transient hypotension. Our findings demonstrate that direct and indirect activation of Kv7 channels by the APAP metabolite NAPQI can result in decreased arterial tone, which can lead to a drop in blood pressure. Since patients receiving intravenous APAP are usually critically ill, any severe haemodynamic changes, such as those associated with APAP, can be life threatening. Importantly, we show that blockade of vascular Kv7 channels with linopirdine can protect against a severe hypotensive episode, without affecting other cardiovascular parameters. Thus, we suggest that inhibiting Kv7 channels with intravenous linopirdine could be a novel therapy to prevent the potentially life threatening APAP-induced hypotension.
Supplementary Material
Highlights.
Intravenous acetaminophen administration causes transient hypotension in the clinic but the mechanism responsible for this iatrogenic effect was unknown.
We show that the metabolite, NAPQI, is a potent vasodilator, which can activate Kv7.4 and Kv7.5 channels directly. We also discovered that NAPQI can induce release of calcitonin gene-related peptide from perivascular nerves, which causes relaxation of smooth muscle cells, in part, through the activation of Kv7 channels.
Thus, we provide novel molecular insight to explain how intravenous acetaminophen administration causes transient hypotension and suggest a possible therapeutic solution.
Acknowledgments
We would like to thank Karin Larsen and Associate Professor Charlotte Mehlin Sørensen (Department of Biomedical Sciences, University of Copenhagen) for their valuable input and assistance with the in vivo experiments. We are also grateful to Prof. Michael J. Davies (Department of Biomedical Sciences, University of Copenhagen) for sharing his expert insight into APAP metabolism and NAPQI reactivity.
Funding: TAJ was funded by the Lundbeck Foundation (R323-2018-3674). JvH was funded by TALENT Doctoral Fellowship Programme - European Union’s Horizon 2020. RWM and GWA were supported by U.S. National Institutes of Health, National Institute of General Medical Sciences and National Institute of Neurological Disorders and Stroke (GM130377 and NS107671 to GWA).
Nonstandard abbreviations
- APAP
acetaminophen/paracetamol
- NAPQI
N-acetyl-p-benzoquinone imine
- AM404
N-arachidonoylphenolamine
- TRPA1
transient receptor potential ankyrin 1 channel
- TRPV1
transient receptor potential vanilloid 1 channel
- CGRP
calcitonin gene-related peptide
- CYP
cytochrome P450
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Disclosures: None.
References
- 1.Chalmers JP, West MJ, Wing LM, Bune AJ, Graham JR. Effects of indomethacin, sulindac, naproxen, aspirin, and paracetamol in treated hypertensive patients. Clin Exp Hypertens A. 1984;6:1077–93. [DOI] [PubMed] [Google Scholar]
- 2.Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med. 2002;162:2204–8. [DOI] [PubMed] [Google Scholar]
- 3.McCrae JC, Morrison EE, MacIntyre IM, Dear JW, Webb DJ. Long-term adverse effects of paracetamol – a review. Br. J. Clin. Pharmacol. 2018;84:2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maxwell EN, Johnson B, Cammilleri J, Ferreira JA. Intravenous Acetaminophen-Induced Hypotension: A Review of the Current Literature. Ann Pharmacother. 2019;53:1033–1041. [DOI] [PubMed] [Google Scholar]
- 5.Krajčová A, Matoušek V, Duška F. Mechanism of paracetamol-induced hypotension in critically ill patients: A prospective observational cross-over study. Aust Crit Care. 2013;26:136–141. [DOI] [PubMed] [Google Scholar]
- 6.Boyle M, Nicholson L, O’Brien M et al. Paracetamol induced skin blood flow and blood pressure changes in febrile intensive care patients: An observational study. Aust Crit Care. 2010;23:208–214. [DOI] [PubMed] [Google Scholar]
- 7.De Maat MM, Tijssen TA, Brüggemann RJ, Ponssen HH. Paracetamol for intravenous use in medium- and intensive care patients: Pharmacokinetics and tolerance. Eur J Clin Pharmacol. 2010;66:713–719. [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie I, Forrest K, Thompson F, Marsh R. Effects of acetaminophen administration to patients in intensive care. Intensive Care Med. 2000;26:1408. [DOI] [PubMed] [Google Scholar]
- 9.Cantais A, Schnell D, Vincent F et al. Acetaminophen-Induced Changes in Systemic Blood Pressure in Critically Ill Patients: Results of a Multicenter Cohort Study. Crit Care Med. 2016;44:2192–2198. [DOI] [PubMed] [Google Scholar]
- 10.Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383–390. [DOI] [PubMed] [Google Scholar]
- 11.Andersson DA, Gentry C, Alenmyr L et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ 9 -tetrahydrocannabiorcol. Nat Commun. 2011;2:551. [DOI] [PubMed] [Google Scholar]
- 12.Eberhardt MJ, Schillers F, Eberhardt EM et al. Reactive metabolites of acetaminophen activate and sensitize the capsaicin receptor TRPV1. Sci Rep. 2017;7:12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stueber T, Meyer S, Jangra A, Hage A, Eberhardt M, Leffler A. Activation of the capsaicin-receptor TRPV1 by the acetaminophen metabolite N -arachidonoylaminophenol results in cytotoxicity. Life Sci. 2018;194:67–74. [DOI] [PubMed] [Google Scholar]
- 14.Ray S, Salzer I, Kronschläger MT, Boehm S. The paracetamol metabolite N-acetylp-benzoquinone imine reduces excitability in first- and second-order neurons of the pain pathway through actions on KV7 channels. Pain. 2019;160:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suemaru K, Yoshikawa M, Tanaka A, Araki H, Aso H, Watanabe M. Anticonvulsant effects of acetaminophen in mice: Comparison with the effects of nonsteroidal anti-inflammatory drugs. Epilepsy Res. 2018;140:22–28. [DOI] [PubMed] [Google Scholar]
- 16.Ng FL, Davis AJ, Jepps TA et al. Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol. 2011;162:42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung SYM, Pucovský V, Moffatt JD et al. Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol. 2007;151:758–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackie AR, Brueggemann LI, Henderson KK et al. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther. 2008;325:475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrese V, Stott JB, Greenwood IA. KCNQ-Encoded Potassium Channels as Therapeutic Targets. Annu Rev Pharmacol Toxicol. 2018;58:625–648. [DOI] [PubMed] [Google Scholar]
- 20.Jepps TA, Bentzen BH, Stott JB et al. Vasorelaxant effects of novel Kv 7.4 channel enhancers ML213 and NS15370. Br J Pharmacol. 2014;171:4413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manville RW, van der Horst J, Redford KE, Katz BB, Jepps TA, Abbott GW. KCNQ5 activation is a unifying molecular mechanism shared by genetically and culturally diverse botanical hypotensive folk medicines. Proc Natl Acad Sci. 2019;116:21236–21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki H, Takasaki K, Saito A, Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988;335:164–167. [DOI] [PubMed] [Google Scholar]
- 23.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. [DOI] [PubMed] [Google Scholar]
- 24.Brueggemann LI, Mackie AR, Cribbs LL et al. Differential Protein Kinase C-dependent Modulation of Kv7.4 and Kv7.5 Subunits of Vascular Kv7 Channels. J Biol Chem. 2014;289:2099–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jepps TA, Carr G, Lundegaard PR, Olesen S-P, Greenwood IA. Fundamental role for the KCNE4 ancillary subunit in Kv7.4 regulation of arterial tone. J Physiol. 2015;593:5325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanamiri S, Soltysinska E, Jepps TA et al. Contribution of KV7 channels to basal coronary flow and active response to ischemia. Hypertension. 2013;62:1090–7. [DOI] [PubMed] [Google Scholar]
- 27.Chadha PS, Jepps TA, Carr G et al. Contribution of Kv7.4/Kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol. 2014;34:887–893. [DOI] [PubMed] [Google Scholar]
- 28.Chadha PS, Zunke F, Zhu H-L et al. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired β-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertens (Dallas, Tex 1979). 2012;59:877–84. [DOI] [PubMed] [Google Scholar]
- 29.Stott J, Greenwood I. Complex role of Kv7 channels in cGMP and cAMP-mediated relaxations. Channels. 2015;9:117–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stott JB, Barrese V, Greenwood IA. Kv7 Channel Activation Underpins EPAC-Dependent Relaxations of Rat Arteries. Arterioscler Thromb Vasc Biol. 2016;36:2404–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mani BK, Robakowski C, Brueggemann LI et al. Kv7.5 Potassium Channel Subunits Are the Primary Targets for PKA-Dependent Enhancement of Vascular Smooth Muscle Kv7 Currents. Mol Pharmacol. 2016;89:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaman A, Demir B, Belen FB et al. Paracetamol infusion-related severe hypotension and cardiac arrest in a child. Turk J Pediatr. 2016;58:550–553. [DOI] [PubMed] [Google Scholar]
- 33.Chiam E, Weinberg L, Bailey M, McNicol L, Bellomo R. The haemodynamic effects of intravenous paracetamol (acetaminophen) in healthy volunteers : a double-blind, randomized, triple crossover trial. Br J Clin Pharmacol. 2016;81:605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawasaki H, Takatori S, Zamami Y et al. Paracrine control of mesenteric perivascular axo-axonal interaction. Acta Physiol (Oxf). 2011;203:3–11. [DOI] [PubMed] [Google Scholar]
- 35.Bol M, Leybaert L, Vanheel B. Influence of methanandamide and CGRP on potassium currents in smooth muscle cells of small mesenteric arteries. Pflugers Arch Eur J Physiol. 2012;463:669–677. [DOI] [PubMed] [Google Scholar]
- 36.Wang LH, Luo M, Wang Y, Galligan JJ, Wang DH. Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice. J Hypertens. 2006;24:2399–2408. [DOI] [PubMed] [Google Scholar]
- 37.Lei S, Mulvany MJ, Nyborg NC. Characterization of the CGRP receptor and mechanisms of action in rat mesenteric small arteries. Pharmacol Toxicol. 1994;74:130–5. [DOI] [PubMed] [Google Scholar]
- 38.Gamper N, Zaika O, Li Y et al. Oxidative modification of M-type K+ channels as a mechanism of cytoprotective neuronal silencing. EMBO J. 2006;25:4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Athersuch TJ, Antoine DJ, Boobis AR et al. Paracetamol metabolism, hepatotoxicity, biomarkers and therapeutic interventions: A perspective. Toxicol Res (Camb). 2018;7:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandran A, Jaeschke H. Acetaminophen toxicity: Novel insights into mechanisms and future perspectives. Gene Expr. 2018;18:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleming I Cytochrome P450 enzymes in vascular homeostasis. Circ. Res 2001;89:753–762. [DOI] [PubMed] [Google Scholar]
- 43.Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: Therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21:201–232. [DOI] [PubMed] [Google Scholar]
- 44.Rockwood K, Beattie BL, Eastwood MR et al. A randomized, controlled trial of linopirdine in the treatment of Alzheimer’s disease. Can J Neurol Sci. 1997;24:140–5. [DOI] [PubMed] [Google Scholar]
- 45.Abbott GW, Jepps TA. Kcne4 Deletion Sex-Dependently Alters Vascular Reactivity. J Vasc Res. 2016;53:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.