Abstract
Objectives:
Sodium, a vital micronutrient that is often in scarce supply for tropical herbivores, is sometimes found at high concentration in decaying wood. We tested two hypotheses for chimpanzees: first, that wood-eating facilitates acquisition of sodium; second, that wood-eating occurs in response to the low availability of sodium from other dietary sources.
Materials and Methods:
We studied the behavior of more than 50 chimpanzees of all age-sex classes in the Kanyawara community of Kibale National Park, Uganda. We quantified the sodium content of dietary items, including wood samples from tree species that chimpanzees consumed or did not consume. To assess variation in sodium intake, we used 7 years of data on time spent feeding on plant foods, 18 months of data on rates of food intake by adult females, and 20 years of data on meat-eating. Results: Major dietary sources of sodium were wood, fruits and meat. Chimpanzees consumed wood primarily from decaying trees of Neoboutonia macrocalyx (Euphorbiaceae), which had substantially higher sodium content than all other dietary items tested. Wood-eating was negatively correlated with fruit-eating. Females ate wood more often than males, while males had a greater probability of consuming meat at predation events.
Discussion:
We propose that females ate wood more often than males because females had reduced access to meat, their preferred source of sodium. This hypothesis suggests that the need for sodium is a motivating reason for chimpanzees to consume both meat and wood.
Keywords: dietary salt, Kibale National Park, meat-eating, nutritional ecology, sex differences
1 |. INTRODUCTION
Sodium is necessary for osmoregulation, pH homeostasis, neuronal communication and macronutrient uptake in animals, with deficits resulting in both short-term and long-term fitness consequences (Geerling & Loewy, 2008). Consequently, sodium occurs at predictably high concentration in all animal species (Denton, 1982). While carnivores therefore readily obtain sodium from their prey, herbivores must secure it elsewhere. The availability of environmental ions, like sodium, varies widely with a pattern of reduced levels further away from oceans (Stallard & Edmond, 1981). In these reduced mineral, inland environments, herbivores, and carnivores exhibit different nutrient seeking behaviors (Kaspari, Yanoviak, & Dudley, 2008), including herbivores visiting mineral licks to supply ions like sodium (Denton, 1982). Since most plant materials have low-sodium concentration (Kaspari et al., 2008; Kaspari, Yanoviak, Dudley, Yuan, & Clay, 2009), it is often difficult for noncarnivores living far from the coast to satisfy their sodium needs (Kaspari et al., 2008; Koops et al., 2019).
Various inland primate species illustrate this problem by consuming plant diets in which most food items have little sodium (Rode, Chapman, Chapman, & McDowell, 2003; Rothman, Van Soest, & Pell, 2006). Several species are known to address this sodium shortage by supplementing their energy-rich foods with the wood of dead trees (Stanford & Nkurunungi, 2003; Rothman et al., 2006; Chaves, Stoner, Ángeles-Cam-pos, & Arroyo-Rodríguez, 2011; Reynolds, Lloyd, Babweteera, English, & Vitzthum, 2009; Rode et al., 2003; Iwata et al., 2015; D. Watts, personal communication). There are no significant macronutrients in these decayed woods, which consist mainly of insoluble fiber and only trivial concentrations of soluble fiber, fats, proteins, and sugars (Chaves et al., 2011; Reynolds et al., 2009; Rothman et al., 2006). However, the woods that primates selectively consume are always high in sodium (Chaves et al., 2011; Iwata et al., 2015; Reynolds et al., 2009; Rothman et al., 2006).
Kibale National Park, Uganda, is a low-sodium environment (Mahaney et al., 1997; 2005; Reynolds et al., 2015). Chimpanzees of the Kanyawara community in Kibale National Park have few obvious sources of sodium, since they hardly ever eat insects and hunt mammals relatively rarely (Gilby et al., 2017). However, they regularly consume wood, primarily from decaying trees of Neoboutonia macrocalyx (Euphorbiaceae) located in or close to swamps. In this study, we test the hypotheses that the wood of dead N. macrocalyx in swamps has sufficiently higher concentrations of sodium than both the wood of N. macrocalyx upland and other tree species in swamps to explain why chimpanzees eat it.
The likelihood that wood-eating serves to obtain sodium provides an opportunity to test a prediction from the Meat-Scrap Hypothesis (Tennie, Gilby, & Mundry, 2009). According to the Meat-Scrap Hypothesis, the primary goal of chimpanzee hunting is not macronutrient gain. Instead, hunting maximizes each individual’s odds of obtaining enough meat to fulfill demands for micronutrients, such as minerals, which occur at high concentration in meat (Eaton & Konner, 1985; Liebermann, 1987; Lukmanji et al., 2008; O’Malley & Power, 2012). In Kanyawara, as is typical of chimpanzees, males are the main hunters, and they have greater access to meat than females (Gilby et al., 2015; 2017; Gilby, Eberly, & Wrangham, 2008). Since this means that females obtain less sodium from meat than males do, we examine whether females compensate for their lower meat intake by consuming relatively more wood. Further, because there are major shifts in feeding behavior at different ages in the two sexes (Bray, Emery Thompson, Muller, Wrangham, & Machanda, 2018), we also examine an effect of age category on meat and wood eating.
2 |. MATERIALS AND METHODS
2.1 |. Chimpanzee demographics and data collection
The Kanyawara chimpanzee community lives in the northwestern sector of Kibale National Park in southwestern Uganda. The park contains 795 km2 of varied vegetation including forests, swamps, and grassland. An estimated 1,450 chimpanzees occupy the park’s evergreen and semideciduous forest environments (Uganda Wildlife Authority, 2012). During the study period (2009–2016), the Kanyawara community consisted of 47–52 individuals. We considered chimpanzees under 4 years old as “infants,” between 4.0 and 9.9 years old as “juveniles,” male chimpanzees between 10 and 14.9 years old and female chimpanzees between 10 years and age of first swelling as “adolescent,” male chimpanzees between 15.0 and 34.9 years and female chimpanzees from the age of first observed swelling to 34.9 years as “adult” individuals, and over 35.0 years as “past prime” individuals. These age categories reflect major shifts in chimpanzee feeding patterns (Bray et al., 2018).
2.2 |. Wood samples
We collected wood samples from 26 decaying trees that varied in species and location. Trees in swamps had roots in still water whereas nonswamp (upland) trees were at higher elevations without access to still water. Sampled trees included 11 swamp N. macrocalyx trees, three upland N. macrocalyx trees, eight swamp trees of other species, and four upland trees of other species. In total, we took 41 samples from swamp N. macrocalyx trees, including three root samples, seven upland N. macrocalyx samples, three swamp Mitragyna sp. samples, two swamp Milletia dura samples, three swamp Myrianthus arboreus samples, one swamp Ficus sur (capensis) sample, five swamp Diospyros abyssinica samples, four swamp samples from an unknown species, 1 upland Milletia dura 1 sample, 1 upland Olea welwitschii sample, 1 upland Celtis durandii sample, and 3 upland Markhamia platycalyx samples. Observers saw chimpanzees eat wood from all 11 of the swamp N. macrocalyx trees and the swamp Mitragyna spp. that we sampled.
When the chimpanzees consume decaying N. macrocalyx wood, they remove and discard any bark before extracting handfuls of wood from the tree (videos available with Supporting Information). We therefore used machetes to remove bark from trees prior to sample collection. We dried the samples to constant weight in a NESCO food dehydrator and milled them in a Thomas Wiley mill in preparation for sodium analysis. We analyzed dried wood samples for sodium content at DairyOne’s Feed and Forage Lab and/or in the Primate Nutritional Ecology Laboratory of Hunter College (Supporting Information).
2.3 |. Focal feeding observations
Between 2009 and 2016, teams of 2–3 trained Ugandan field assistants conducted full-day focal observations, aiming to record at least one full day observation of every individual in the chimpanzee community each month. Field assistants recorded the behavior of the focal chimpanzee, including the species and part of any food consumed. Field assistants also noted party composition and behavior of other individuals present with the focal chimpanzee every 15 min. Wood eating was a relatively rare feeding behavior, and estimating wood eating by percent of feeding minutes per focal generated zero-inflated data. We therefore scored wood-eating by the number of days an individual consumed wood divided by total days of observation, and we henceforth refer to this score as frequency of wood eating.
2.4 |. Statistical analysis
We developed a generalized linear mixed model to analyze how sodium concentrations differed between wood samples from N. macrocalyx and other species as well as those from swamp or upland areas. We used sodium concentration as our response variable and tree species (N. macrocalyx or “other”) and location (swamp or upland) as predictors. We also included an interaction between tree species and location. Since we had multiple samples of wood from individual trees, we used tree identity as a random effect. We also performed a generalized linear mixed model to determine if sodium concentrations of the wood samples differed between wood observed to be eaten or not while controlling for species (N. macrocalyx or “other”).
To test if male and female chimpanzees differed in the frequency that they consumed N. macrocalyx wood, we implemented a generalized estimating equation with percent of days observed eating wood as the response variable and chimpanzee identity as a subject variable. We restricted the individuals included in the model to those with at least 10 days of focal observation. Since some individuals were represented in more than one age category over our dataset, we used age category (infant, juvenile, adolescent, adult, past prime adult) as a within-subject variable. We set sex and age category as predictor variables and included an interaction between sex and age-category. Statistical analyses were carried out in SPSS version 25 (IBM Corp., 2017).
2.5 |. Meat eating
To assess whether access to meat differed between males and females, we extracted start and end times from 160 successful hunting events that included meat-eating between 1996 and 2015. We compiled a list of all chimpanzees present in the group during each meat-eating event and noted which chimpanzees were recorded consuming meat. Observers did not record the quantity of meat consumed, so we could not assess rates of consumption. Instead, we executed a logistic regression to determine whether sex and age predicted the probability of an individual consuming meat when present in a given meat-eating bout. In our model, we set chimpanzee identity as a random factor to account for repeated presence of individuals. We restricted this analysis to adult and past prime individuals.
2.6 |. Estimated rates of food intake
To estimate the amount of sodium ingested from different types of plant foods, MU estimated food consumption rates by recording the food item consumed, the duration of observed feeding bouts (to the nearest minute), and the intake rate of each food per minute by female chimpanzees. Feeding included reaching for, picking, handling, and chewing a food as well as short intervals (<5 s) of searching for the next food item. We estimated intake rate by measuring the mass of a discrete food unit, which mimicked a chimpanzee’s handful of the food item, and the rate of food unit consumption. The mass of a food unit varied by food type, as did the rate at which chimpanzees consumed units per time interval. To quantify the mass of each food unit, MU collected 30 unit samples per food type from the plant fed upon or a nearby comparable plant of the same species. Food samples were dried and milled as described for wood and sent to the Feed and Forage Lab at DairyOne in Ithaca, New York for sodium content analysis. We obtained dry grams of food consumed per minute by dividing the dry weight of each food unit by the number of units consumed fresh per minute. We used the mean dry-weight sodium content of each food type, the dry-weight intake rate (in grams per minute), and the mean total annual minutes consuming each food type across adult females to compare the relative annual dietary sodium contributions of the food items.
When we determined that a food type contributed substantially to annual sodium consumption, we implemented a generalized linear mixed model to investigate if probability of wood consumption varied with the proportion of this food type in the daily diet. We estimated the proportion of food type by dividing the minutes spent consuming the food type by the total minutes spent feeding per focal. We limited our focal observations to full focal days with at least 8 hours of observation. In our model, we used sex and proportion of food consumed as predictor variables and wood consumption as the response variable. Since individuals vary in feeding behavior (Bray et al., 2018), we used chimpanzee identity as a random effect in the model
2.7 |. Ethics statement
This research was conducted in compliance with the American Society of Primatologists’ Principles for the Ethical Treatment of non-Human Primates, and was approved by the IACUC of Harvard University (Harvard: protocol number 96–03). All research was noninvasive. Permission to conduct the research was given by the Uganda Wildlife Authority and the Uganda National Council for Science and Technology (UNCST).
3 |. RESULTS
3.1 |. Sodium concentration in wood
We sampled decaying wood from 14 N. macrocalyx trees (11 from swamps and three upland) as well as decaying wood from 12 other species of trees (eight from swamps and four upland). Samples from decaying N. macrocalyx had more sodium (model estimate: 831.5 mg/kg) than decaying wood samples from other tree species located in the swamp (model estimate: 54.7 mg/kg, p < .0001; Figure 1). Samples from decaying N. macrocalyx in the swamp also had more sodium than decaying N. macrocalyx trees found outside a swamp (model estimate: 176.4 mg/kg, p = .024; Figure 1). There was no significant difference in sodium content between other tree species in or out of the swamp (p = .933) or between upland N. macrocalyx trees and other upland trees (model estimate: 31.8, p = .668). The three N. macrocalyx root samples’ sodium content was among the highest measured of the N. macrocalyx samples (mean = 1,580.0 mg/kg).
FIGURE 1.
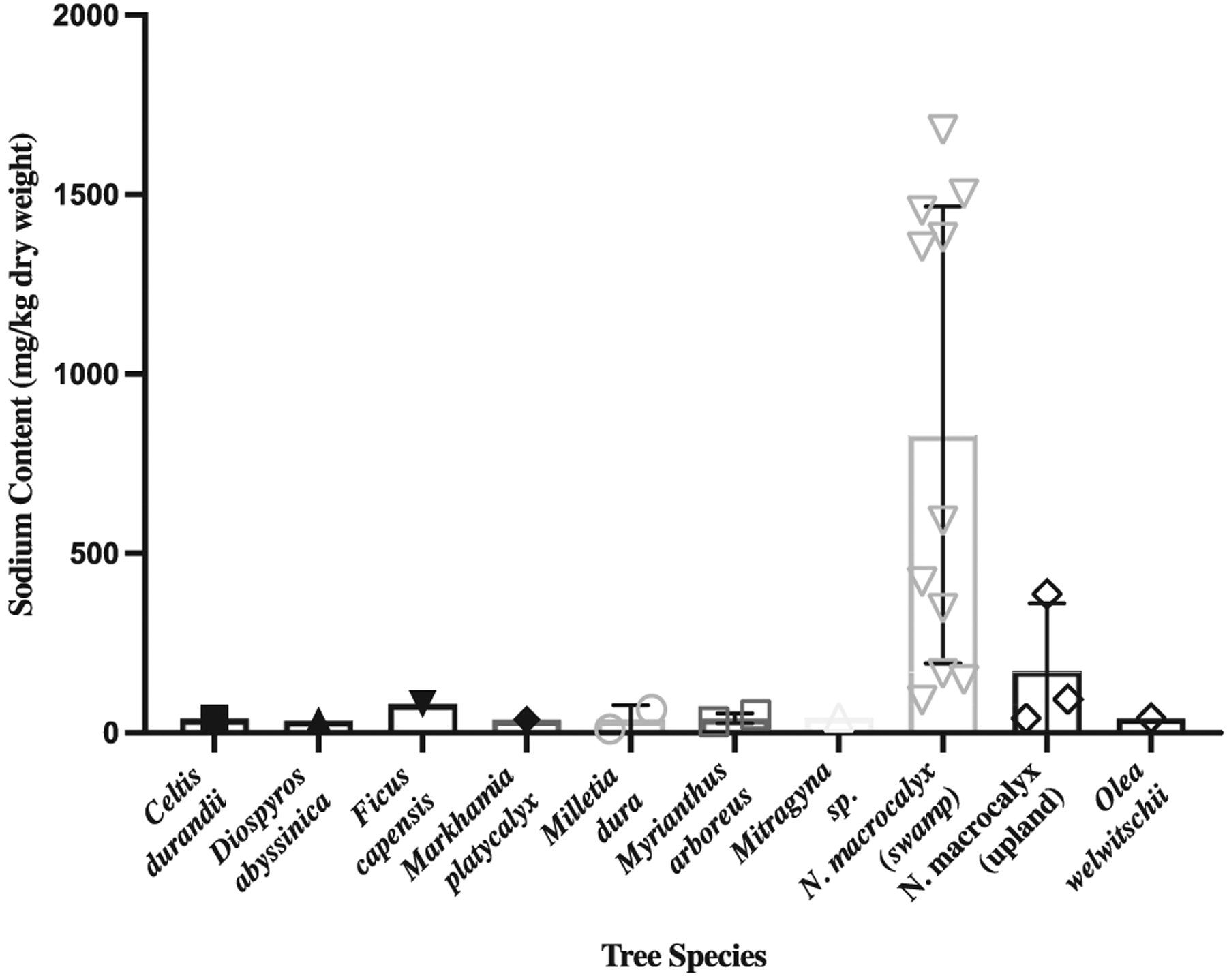
Wood sodium concentration of decaying Kibale trees across species. Bars show the mean measured dry-weight sodium content in mg/kg of (left to right) Celtis durandii (n = 1 tree), Diospyros abyssinica (n = 1 tree), Ficus sur (capensis) (n = 1 tree), Markhamia platycalyx (n = 1 tree), Milletia dura (n = 2 trees), Myrianthus arboreus (n = 2 trees), Mitragyna sp. (n = 1 tree), Neoboutonia macrocalyx swamp (n = 14 trees), Neoboutonia macrocalyx upland (n = 3 trees), and Olea welwitschii (n = 1 tree) trees. We calculated the mean within-tree sodium content across samples prior to finding the mean across trees. Error bars represent standard deviation
3.2 |. Feeding observations
Over seven years, we observed sixty-one individuals, including 21 infants, 17 juveniles, 11 adolescents, 25 adults, and 11 past prime. Individuals observed across multiple age categories were counted in each age category. We recorded wood-eating on 305 days during 4,526 days of focal observations, or 6.7% of days. Since N. macrocalyx wood was the only tree species with notably high-sodium concentrations and N. macrocalyx wood accounted for 93.7% of total wood feeding minutes, we restricted our analysis of the frequency of wood-eating to N. macrocalyx. Females ate decaying N. macrocalyx wood on significantly more days than males (females 7.0% of observed days (n = 35 individuals), males 4.6% (n = 26 individuals), Wald X2 = 4.589, p = .032; Figure 2). Age category alone did not significantly explain the frequency of wood eating (Wald X2 = 6.842, p = .144). However, the interaction between age and sex predicting the percent of days observed eating wood was significant (Wald X2 = 13.291, p = .010), driven by the sex difference found among adults (Figure 2). Adult females consumed wood on significantly more days than adolescent (p = .005), adult (p < .000), and past prime males (p < .000), but did not consume wood on significantly different days than juvenile (p = .667), adolescent (p = 3.91), or past prime (p = .244) females as well as infant (p = 2.60) or juvenile (p = .404) males. Similarly, adolescent females consumed wood on significantly more days than adult (p = .005) and past prime (p = .001) males, as did juvenile females (p = .001 and p < .00, respectively), but not significantly more than any other age-sex category. Infant females did not follow the trend of other females, consuming wood on fewer days than juvenile (p = .019), adolescent (p = .041), and adult (p < .000) females. Within age categories, infant (p = .212) juvenile (p = .690), adolescent (p = .147), and past prime (p = .133) individuals did not consume wood on different percent of days by sex. The percent of days that adult and past prime females consumed decaying N. macrocalyx wood was not related to reproductive state (Supporting Information; Table S1).
FIGURE 2.
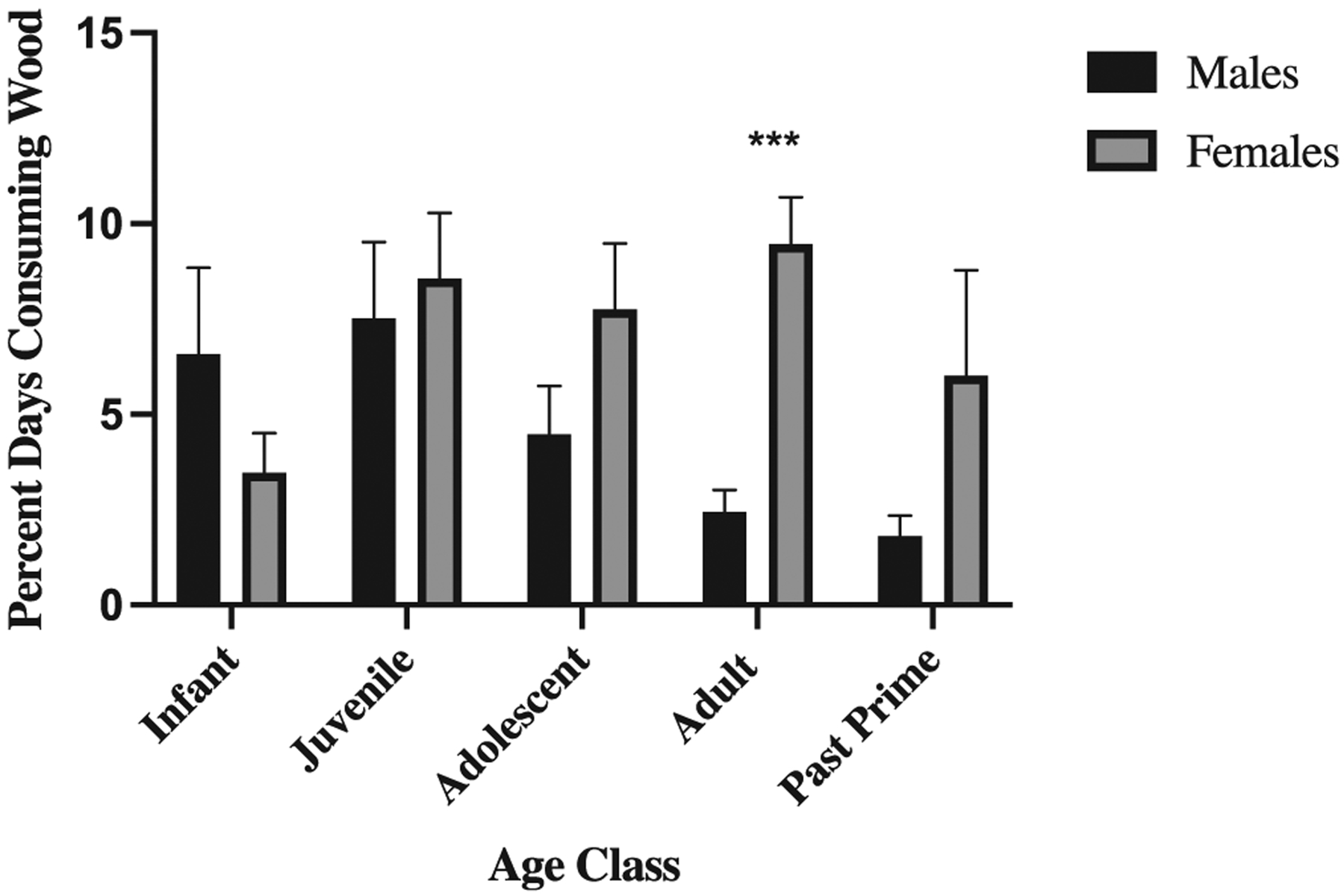
Sex difference in frequency of eating sodium-rich wood. Bars represent the percent days of observed days that individuals consume wood across sex and age classes, including infant males (n = 7), infant females (n = 14), juvenile males (n = 9), juvenile females (n = 8), adolescent males (n = 8), adolescent females (n = 3), adult males (n = 10), adult females (n = 15), past-prime males (n = 5), and past-prime females (n = 5). Error bars represent standard deviation. In total, 4,526 days of focal observation are included between 2009 and 2016. See text for statistics
3.3 |. Meat eating
Sex predicted the probability of eating meat during a meat-eating bout (F = 13.582, p < .001) with males exhibiting a higher probability of meat eating than females (model estimates: male = 0.246 ± 0.044, female = 0.092 ± 0.019). The sex difference in frequency of eating meat was thus in the opposite direction to the sex difference in eating wood (Figure 3). Age category was not a significant predictor of meat eating (F = 0.471, p = .493).
FIGURE 3.
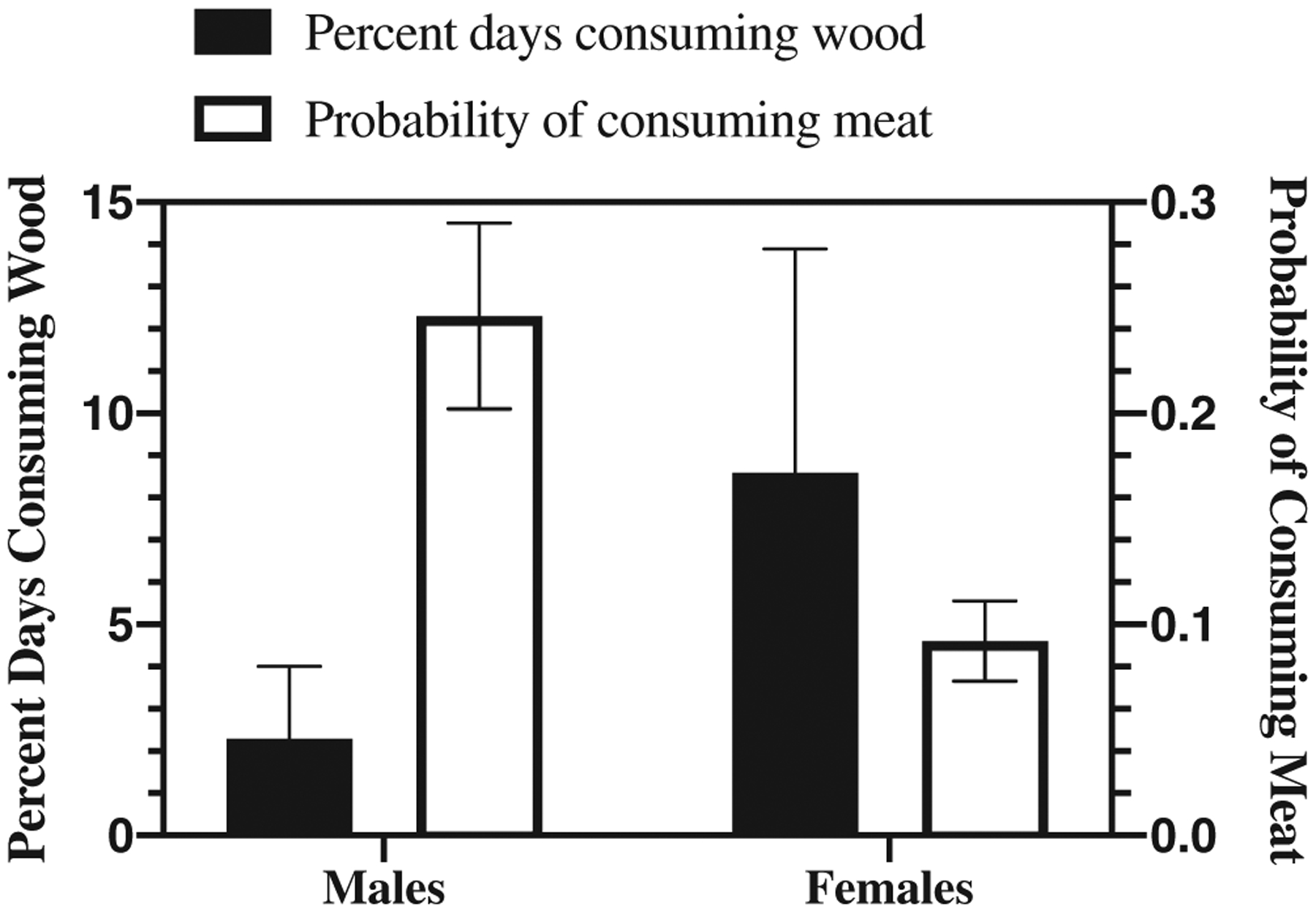
Dietary sodium sources for adult male vs. female chimpanzees. For wood-eating, the bars show the percent of observed days that a focal individual consumed wood. Adult and past prime females consumed wood on 8.2% days (SD = 5.2) whereas adult and past prime males consumed wood on 2.3% days (SD = 1.7). For meat-eating, bars show the probability that an individual present during a group meat-eating bout was observed to consume meat and ranges are SEs. Adult and past prime males had a 0.25 (SE = 0.04) probability of consuming meat during a meat eating bout, whereas adult females had a 0.092 (SE = 0.02) probability (n = 160 meat-eating bouts between 1996 and 2015)
3.4 |. Annual intake estimation
We estimated how much sodium adult female chimpanzees ingested annually from different plant food sources from 2014 to 2016. We found that wood was the greatest source of sodium (708.0 mg/year; Table 1), followed closely by ripe fruits (653.1 mg/year; Table 1). The contribution from fruits was high because although their sodium concentrations were consistently low, they were eaten in greater bulk than any other food type (Table 1). To test whether chimpanzees ate more wood when their diet contained less fruit, we analyzed 1,032 focal observation days for females (N = 18 individuals) and 772 focal observation days for males (N = 16 individuals). We found that the proportion of ripe fruit consumed was negatively correlated with the probability that wood was eaten that day (F = 5.905, p = .015; Figure 4).
TABLE 1.
Estimated annual sodium intake by adult females from plant sources
| Food item | Dry-weight sodium content (mg/kg) | Dry-weight intake rate (g/min) | Mg sodium per minute | Minutes eaten per year | Mg sodium intake per year | Intake of food item (% min) |
|---|---|---|---|---|---|---|
| Ripe fruits | 35.5 | 3.4 | 0.119 | 5,488 | 653.1 | 60.0 |
| Young leaves | 10.0 | 2.1 | 0.024 | 2,072 | 49.7 | 22.6 |
| Pith | 15.0 | 1.8 | 0.023 | 973 | 22.4 | 10.6 |
| Bark | 30.0 | 2.9 | 0.086 | 137 | 11.9 | 1.5 |
| Wood | 1,763.6 | 0.8 | 1.478 | 479 | 708.0 | 5.2 |
Note: See text for methods. Data from 2014–2016.
FIGURE 4.
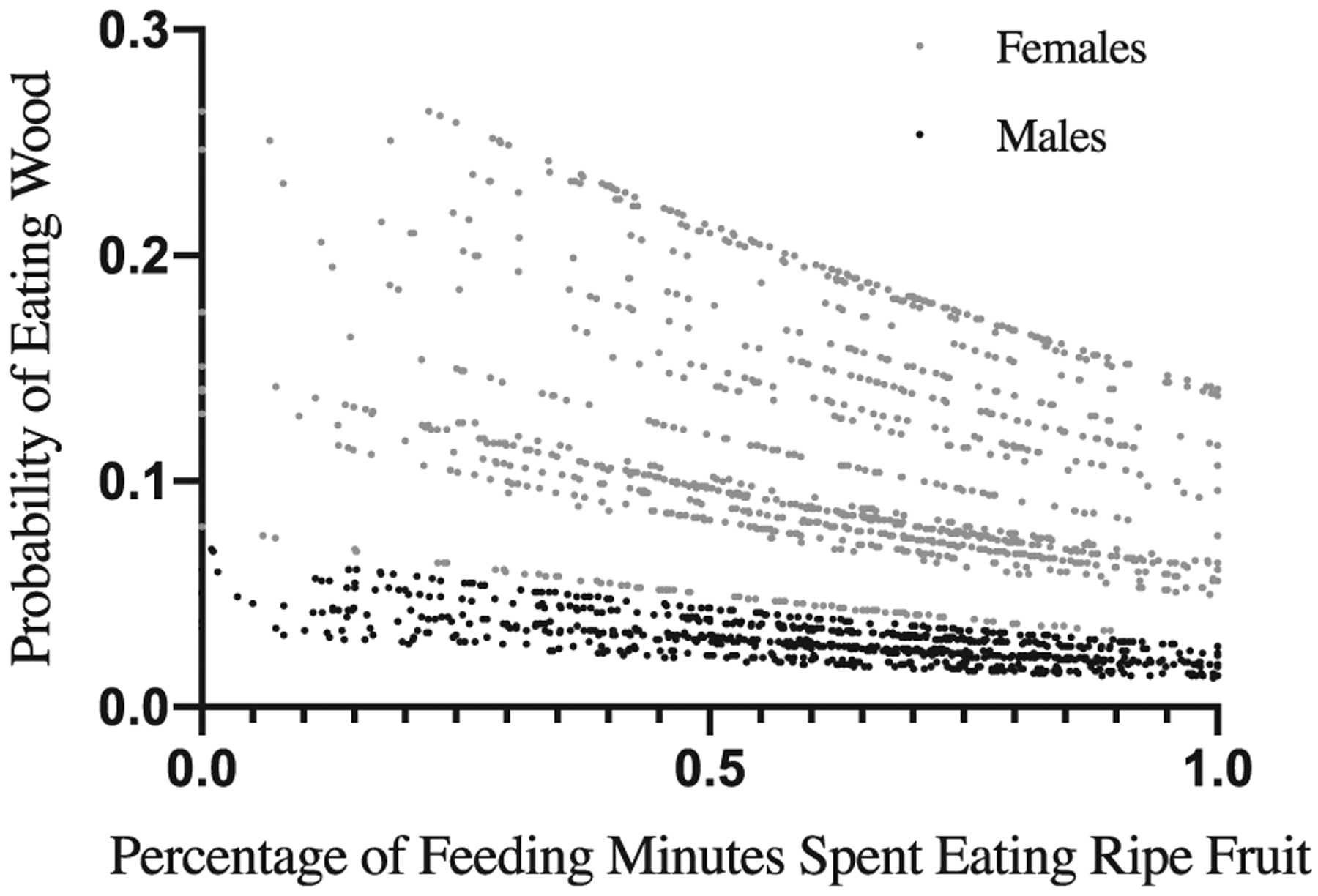
Wood-eating in relation to fruit-eating. Figure shows the probability that chimpanzees, when observed as a focal for at least 8 hours, consumed wood in relation to percent of feeding minutes spent consuming ripe fruit on the same day. Each linear trajectory is an individual chimpanzee; each data point represents 1 day of observation (n = 1,804 days). There was a consistent negative relationship between the proportion of ripe fruit consumed in a day and the probability that wood was eaten that day (F = 5.904, p-value = .015). Sex significantly affected the probability of consuming wood at a given proportion of ripe fruit in the diet: for a given proportion of feeding minutes spent eating ripe fruit, all females ate more wood than any male did. (F-value = 22.07, p-value < .001). Note also that when fruit intake is controlled, all adult females are found to eat more wood than any adult male
4 |. DISCUSSION
To understand why Kanyawara chimpanzees regularly eat decaying wood in swamps, we analyzed the sodium content in diverse plants foods, including the species whose wood was most frequently eaten (N. macrocalyx). We found that decaying N. macrocalyx wood in swamps had higher sodium concentration than any other swamp plants or than N. marocalyx outside of swamps. Chimpanzees were more likely to consume N. macrocalyx wood on days when they consumed fewer ripe fruits. Although fruits had a low-sodium concentration, they constituted such a high proportion of food in the chimpanzees’ diet that we estimated their total contribution to annual sodium intake as being about the same as the total from decaying wood.
4.1 |. Sodium content of wood
Our results support the hypothesis that decaying N. macrocalyx wood from trees located in the swamp is a source of dietary sodium for the Kanyawara chimpanzees. These trees had higher sodium content than the wood of upland N. macrocalyx trees. A previous study in Kibale National Park noted that the mean sodium content of plants consumed by black-and-white colobus monkeys (Colobus guereza) was 5.8 times greater in eight species of swamp-living plant than in eight species of dry-land plants (data from table 3 in Oates, 1978). Other sites echo this pattern, including Bwindi and the Budongo Forest in Uganda, Moukalaba-Doudou National Park in Gabon, and Maya Nord Bai in Odzala-Kokoua National Park in Republic of Congo, where plants located in swamps or marshland had higher overall mineral concentrations than plants not located in the swamps (Iwata et al., 2015; Magliocca & Gautier-Hion, 2002; Reynolds et al., 2009; Rothman et al., 2006).
Why swamp plants tend to contain relatively high levels of minerals is not currently established. One possibility is that the decaying process is responsible. According to this hypothesis, evaporation in decaying wood promotes capillary uptake of swamp water, leading to an increasing intensity of minerals (Battie-Laclau et al., 2014; De Schepper, De Swaef, Bauweraerts, & Steppe, 2013). The wood of swamp trees that have been dead for longer is therefore expected to have higher concentrations of sodium. This hypothesis has not been tested directly. However, in support, in Gabon, Iwata et al. (2015) found that sodium content was higher in decaying wood than in living wood, and in the Republic of the Congo Magliocca and Gautier-Hion (2002) found that mineral concentration in swamp plants increased as the proportion of days of flooding increased. Another possibility is that the relatively high-sodium concentrations found in decaying wood results from greater decomposer activity (Kaspari et al., 2009). It should be noted that the decaying process may also affect other micronutrients, as in the case of Licania platypus wood eaten by spider monkeys in Mexico. Here, decaying wood shows an increase in calcium rather than sodium (Chaves et al., 2011). Thus, we cannot exclude the possibility that the Kanyawara chimpanzees ingest N. macrocalyx wood for minerals besides sodium, and further micronutrient quantification of N. macrocalyx wood is warranted.
4.2 |. Sex differences in sodium acquisition
Female chimpanzees consumed wood more frequently than males, whereas male chimpanzees had a higher probability of consuming meat, which contains a high concentration of sodium. Therefore, to explain why chimpanzees eat wood and why females eat wood more often than males, we propose that Kanyawara chimpanzees selectively consume high-sodium N. macrocalyx wood when other dietary sources of sodium, namely meat and ripe fruits, are inadequate. This idea conforms to the Meat-Scrap Hypothesis by suggesting that a major function of meat eating for chimpanzees is the acquisition of micronutrients such as sodium (Tennie et al., 2009).
Sex differences in total meat consumption by Kanyawara chimpanzees are admittedly not well documented (Gilby et al., 2017). In other sites, however, adult males are known both to have primary access to prey and to consume more meat than females (Fahy, Richards, Riedel, Hublin, & Boesch, 2013; Wrangham & van Zinnicq Bergmann Riss, 1990). In Kanyawara, adult male chimpanzees hunt much more frequently than adult females: although females averaged about 65% of the total number of adults (54) in the Kanyawara community between 1996 and 2015, female hunters accounted for only 8.8% of 261 kills (Gilby et al., 2017). Furthermore, females, when they did possess meat, were likely to have that meat stolen from them by adult males (Gilby et al., 2017). The conclusion that adult males consume meat more frequently than adult females is therefore unsurprising.
Our proposal that wood-eating serves to provide extra sodium for individuals who do not get enough sodium from meat is supported by age changes in male diets. Juvenile, adolescent, adult, and past prime females all consumed wood at similar frequencies. The percent of days that males consumed wood was at female levels until adolescence. At that point males start to participate in hunts (Gilby et al., 2015), and their wood-eating declined. We suggest that the decline in wood-eating by males as they entered adulthood occurred because from that age onwards, males ate more meat than they did as juveniles (Gilby et al., 2015). The similarity in the percent of days that adult females and infant and juvenile males consumed N. macrocalyx is unsurprising given that infants and juveniles forage with their mothers (Bray et al., 2018).
In sum, we conclude that the Kanyawara chimpanzees ingest the wood of decaying N. macrocalyx as a major source of sodium that complements sodium intake from meat and ripe fruits. Our data indicate that the search for sodium by primarily herbivorous species can lead to specific dietary items being included even when they have little or no caloric significance. Inclusion of dietary items for their sodium content alone is expected to be more common in inland environments, given that the availability of environmental sodium falls with distance from the ocean (Kaspari et al., 2009; Stallard & Edmond, 1981). In such low-sodium environments, the dietary items that make special contributions to sodium acquisition will likely be consumed by noncarnivores as a micronutrient supplement.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Uganda Wildlife Authority and the Makerere University Biological Field Station for permission to work in Kibale National Park. Research was funded by the Harvard College Research Program, the Harvard University Museum of Comparative Zoology, Hunter College of CUNY, the National Science Foundation (grant ## 9807448, 0416125, 1355014, 1521528), and NIH-National Institute of Aging (grant 1R01AG049395). We thank Melissa Emery Thompson for reproductive data and advice, Steve Worthington for statistical help, David Watts for insights into N. macrocalyx root consumption in Ngogo, and David Watts and anonymous reviewers for their valuable commentary. Fred Baguma, Richard Karamagi, James Kyomuhendo, Sunday John, Seezi Atwijuze, Wilberforce Tweheyo, and Daniel Akaruhanga assisted in collecting data and wood samples.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Battie-Laclau P, Laclau J, Domec J, Christina M, Bouillet J, Cassia Piccolo M, … Nouvellon Y (2014). Effects of potassium and sodium supply on drought-adaptive mechanisms in Eucalyptus grandis plantations. New Phytologist, 203(2), 401–413. [DOI] [PubMed] [Google Scholar]
- Bray J, Emery Thompson M, Muller M, Wrangham R, & Machanda Z (2018). The development of feeding behavior in wild chimpanzees (Pan troglodytes schweinfurthii). American Journal of Physical Anthropology, 165(1), 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves OM, Stoner KE, Angeles-Campos S, & Arroyo-Rodríguez V (2011). Wood consumption by Geoffroyi’s spider monkeys and its role in mineral supplementation. PLoS One, 6(9), e25070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schepper V, De Swaef T, Bauweraerts I, & Steppe K (2013). Phloem transport: A review of mechanisms and controls. Journal of Experimental Botany, 64(16), 4839–4850. [DOI] [PubMed] [Google Scholar]
- Denton D (1982). The hunger for salt. Berlin, Heidelberg, New York: Springer-Verlag. [Google Scholar]
- Eaton SB, & Konner M (1985). Paleolithic nutrition. A consideration of its nature and current implications. The New England Journal of Medicine, 312(5), 283. [DOI] [PubMed] [Google Scholar]
- Fahy GE, Richards M, Riedel J, Hublin JJ, & Boesch C (2013). Stable isotope evidence of meat eating and hunting specialization in adult male chimpanzees. Proceedings of the National Academy of Sciences, 110(15), 5829–5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerling J, & Loewy A (2008). Central regulation of sodium appetite. Experimental Physiology, 93(2), 177–209. [DOI] [PubMed] [Google Scholar]
- Gilby I, Eberly L, & Wrangham RW (2008). Economic profitability of social predation among wild chimpanzees: Individual variation promotes cooperation. Animal Behaviour, 75, 351–360. [Google Scholar]
- Gilby IC, Machanda ZP, Mjungu DC, Rosen J, Muller MN, Pusey AE, & Wrangham RW (2015). ‘Impact hunters’ catalyse cooperative hunting in two wild chimpanzee communities. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 370(1683), 20150005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilby IC, Machanda ZP, O’Malley RC, Murray CM, Lonsdorf EV, Walker K, … Wrangham RW (2017). Predation by female chimpanzees: Toward an understanding of sex differences in meat acquisition in the last common ancestor of pan and homo. Journal of Human Evolution, 110, 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (Released 2017). IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. [Google Scholar]
- Iwata Y, Nakashima Y, Tsuchida S, Nguema P, Ando C, Ushida K, & Yamagiwa J (2015). Decaying toxic wood as sodium supplement for herbivorous mammals in Gabon. Journal of Veterinary Medical Science, 77(10), 1247–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspari M, Yanoviak SP, & Dudley R (2008). On the biogeography of salt limitation: A study of ant communities. Proceedings of the National Academy of Sciences, 105(46), 17848–17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspari M, Yanoviak SP, Dudley R, Yuan M, & Clay NA (2009). Sodium shortage as a constraint on the carbon cycle in an inland tropical rainforest. Proceedings of the National Academy of Sciences, 106(46), 19405–19409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops K, Wrangham R, Cumberlidge N, Fitzgerald M, Van Leeuwen K, Rothman J, & Matsuzawa T (2019). Crab-fishing by chimpanzees in the Nimba Mountains, Guinea. Journal of Human Evolution, 133, 230–241. [DOI] [PubMed] [Google Scholar]
- Liebermann LS (1987). Biological consequences of animals versus plants as sources of fats, protein and other nutrients In Harris M & Ross EB (Eds.), Diet and human evolution (pp. 225–260). Philadelphia, PA: Temple University Press. [Google Scholar]
- Lukmanji Z, Hertzmark E, Mlingi N, Assey V, Ndossi G, & Fawzi W (2008). Tanzania food composition tables (1st ed., pp. 77–89). Dar Es Salaam, Tanzania: Muhimbili University of Health and Allied Sciences (MUHAS), Tanzania Food and Nutrition Centre (TFNC), and Harvard School of Public Health (HSPH). [Google Scholar]
- Magliocca F, & Gautier-Hion A (2002). Mineral content as a basis for food selection by western lowland gorillas in a forest clearing. American Journal of Primatology, 57(2), 67–77. [DOI] [PubMed] [Google Scholar]
- Mahaney W, Milner C, Sanmugadas M, Hancock W, Aufreiter K, Wrangham R, & Pier G (1997). Analysis of geophagy soils in Kibale Forest, Uganda. Primates, 38(2), 159–176. [Google Scholar]
- Mahaney W, Milner M, Aufreiter S, Hancock R, Wrangham R, & Campbell S (2005). Soils consumed by chimpanzees of the Kanyawara community in the Kibale Forest, Uganda. International Journal of Primatology, 26(6), 1375–1398. [Google Scholar]
- Oates JF (1978). Biotropica; water-plant and soil consumption by guereza monkeys (Colobus guereza): A relationship with minerals and toxins in the diet? Biotropica, 10(4), 241–253. [Google Scholar]
- O’Malley R, & Power M (2012). Nutritional composition of actual and potential insect prey for the Kasekela chimpanzees of Gombe National Park, Tanzania. American Journal of Physical Anthropology, 149(4), 493–503. [DOI] [PubMed] [Google Scholar]
- Reynolds V, Lloyd AW, Babweteera F, English CJ, & Vitzthum VJ (2009). Decaying Raphia farinifera palm trees provide a source of sodium for wild chimpanzees in the Budongo Forest, Uganda. PLoS One, 4(7), e6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds V, Lloyd AW, English CJ, Lyons P, Dodd H, Hobaiter C, … Fallon B (2015). Mineral acquisition from clay by Budongo Forest chimpanzees. PLoS One, 10(7), e0134075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode K, Chapman C, Chapman L, & McDowell L (2003). Mineral resource availability and consumption by colobus in Kibale National Park, Uganda. International Journal of Primatology, 24(3), 541–573. [Google Scholar]
- Rothman JM, Van Soest PJ, & Pell AN (2006). Decaying wood is a sodium source for mountain gorillas. Biology Letters, 2(3), 321–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallard RF, & Edmond JM (1981). Geochemistry of the Amazon, 1: Precipitation chemistry and the marine contribution to the dissolved load at the time of peak discharge. Journal of Geophysical Research: Oceans, 86(C10), 9844–9858. [Google Scholar]
- Stanford C, & Nkurunungi J (2003). Behavioral ecology of sympatric chimpanzees and gorillas in Bwindi Impenetrable National Park, Uganda: Diet. International Journal of Primatology, 24(4), 901–918. [Google Scholar]
- Tennie C, Gilby I, & Mundry R (2009).The meat-scrap hypothesis: Small quantities of meat may promote cooperative hunting in wild chimpanzees (Pan troglodytes). Behavioral Ecology and Sociobiology, 63(3), 421–431. [Google Scholar]
- Uganda Wildlife Authority. (2012). Kibale National Park: Wildlife and birding. Retrieved from http://www.ugandawildlife.org/explore-ourparks/parks-by-name-a-z/kibale-national-park
- Wrangham RW, & van Zinnicq Bergmann Riss W (1990). Rates of predation on mammals by Gombe chimpanzees, 1972–1975. Primates, 31 (2), 157–170. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


