Abstract
Background
Bone disease is common in children with chronic kidney disease (CKD) and when untreated may result in bone deformities, bone pain, fractures and reduced growth rates. This is an update of a review first published in 2010.
Objectives
This review aimed to examine the benefits (improved growth rates, reduced risk of bone fractures and deformities, reduction in PTH levels) and harms (hypercalcaemia, blood vessel calcification, deterioration in kidney function) of interventions (including vitamin D preparations and phosphate binders) for the prevention and treatment of metabolic bone disease in children with CKD.
Search methods
We searched the Cochrane Kidney and Transplant Specialised Register to 8 September 2015 through contact with the Trial's Search Co‐ordinator using search terms relevant for this review.
Selection criteria
We included randomised controlled trials (RCTs) comparing different interventions used to prevent or treat bone disease in children with CKD stages 2 to 5D.
Data collection and analysis
Data were assessed for study eligibility, risk of bias and extracted independently by two authors. Results were reported as risk ratios (RR) or risk differences (RD) with 95% confidence intervals (CI) for dichotomous outcomes. For continuous outcomes the mean difference (MD) or standardised mean difference (SMD) with 95% confidence intervals (CI) was used. Statistical analyses were performed using the random‐effects model.
Main results
This review included 18 studies (576 children); three new studies were added for this update. Adequate sequence generation and allocation concealment were reported in 12 and 11 studies respectively. Only four studies reported blinding of children, investigators or outcome assessors. Nine studies were at low risk of attrition bias and 12 studies were at low risk of selective reporting bias.
Eight different interventions were compared. Two studies compared intraperitoneal (IP) with oral calcitriol. PTH levels were significantly lower with IP compared with oral calcitriol (1 study: MD ‐501.00 pg/mL, 95% CI ‐721.54 to ‐280.46) but the number of children with abnormal bone histology did not differ between treatments. Three studies compared intermittent with daily oral calcitriol. The change in mean height SDS (1 study: MD 0.13, 95% CI ‐0.22 to 0.48) and the percentage fall in parathyroid hormone (PTH) levels at eight weeks (1 study: MD ‐5.50%, 95% CI ‐32.37 to 21.37) and 12 months (1 study: MD ‐6.00% 95% CI ‐25.27 to 13.27) did not differ between treatments.
Four studies compared active vitamin D preparations (calcitriol, paricalcitol, 1α‐hydroxyvitamin D) with placebo or no specific treatment. One study reported vitamin D preparations significantly reduced PTH levels (‐55.00 pmol/L, 95% CI ‐83.03 to ‐26.97). There was no significant difference in hypercalcaemia risk with vitamin D preparations compared with placebo or no specific treatment (4 studies, 103 children: RD 0.08 mg/dL, 95% CI ‐0.08 to 0.24). However, there was heterogeneity (I2 = 55%) with one study showing a significantly greater risk of hypercalcaemia with intravenous (IV) calcitriol administration. Two studies (97 children) compared calcitriol with other vitamin D preparations and both found no significant differences in growth between preparations.
Two studies compared ergocalciferol in patients with CKD and vitamin D deficiency. Elevated PTH levels developed significantly later in ergocalciferol treated children (1 study: hazard ratio 0.30, 95% CI 0.09 to 0.93) though the number with elevated PTH levels did not differ between groups (1 study, 40 children: RR 0.33, 95% CI 0.11 to 1.05).
Two studies compared calcium carbonate with aluminium hydroxide as phosphate binders. One study (17 children: MD ‐0.86 SDS, 95% CI ‐2.24 to 0.52) reported no significant difference in mean final height SDS between treatments. Three studies compared sevelamer with calcium‐containing phosphate binders. There were no significant differences in the final calcium, phosphorus or PTH levels between binders. More episodes of hypercalcaemia occurred with calcium‐containing binders. One study reported no significant differences between calcitriol and doxercalciferol in bone histology or biochemical parameters.
Authors' conclusions
Bone disease, assessed by changes in PTH levels, is improved by all vitamin D preparations. However, no consistent differences between routes of administration, frequencies of dosing or vitamin D preparations were demonstrated. Although fewer episodes of high calcium levels occurred with the non‐calcium‐containing phosphate binder, sevelamer, compared with calcium‐containing binders, there were no differences in serum phosphorus and calcium overall and phosphorus values were reduced to similar extents. All studies were small with few data available on patient‐centred outcomes (growth, bone deformities) and limited data on biochemical parameters or bone histology resulting in considerable imprecision of results thus limiting the applicability to the care of children with CKD.
Plain language summary
Interventions for metabolic bone disease in children with chronic kidney disease
Chronic kidney disease (CKD) resulting in reduced kidney function and the need for dialysis and kidney transplant is associated with abnormalities in serum calcium and phosphorus levels leading to high levels of the parathyroid hormone (PTH) and to bone disease. This may result in bone deformities, bone pain, fractures and reduced growth rates. Commonly used treatments (vitamin D compounds and phosphate binders) aim to prevent or correct these outcomes. However, these treatments may raise levels of blood calcium, allow calcium and phosphorus deposition in blood vessels and lead to early cardiovascular disease, which is known to be a problem in adults with CKD.
This review identified 18 small randomised studies involving 576 children comparing different vitamin D compounds administered via different routes and frequencies and different phosphate binders. Only five studies reported growth rates and no differences were detected between treatments. Bone disease, as assessed by changes in PTH levels, was improved by all vitamin D preparations regardless of preparation or route or frequency of administration. Fewer episodes of high blood calcium levels occurred with the non‐calcium‐containing binder, sevelamer, compared with calcium‐containing binders. As newer treatments for renal bone disease are developed, comparisons with the current standard therapies will be required in well‐designed randomised studies in children using outcome measures including those of direct clinical relevance to children and their families such as rates of growth, reduction in bone fractures and bone pain and reduction in calcification in blood vessels.
Background
Description of the condition
Chronic kidney disease (CKD) causes disordered regulation of mineral metabolism (Wesseling‐Perry 2013). Because this disorder results in renal osteodystrophy and vascular and/or soft tissue calcification, the manifestations of the disorder are now known as chronic kidney disease mineral and bone disorder (CKD‐MBD) (Moe 2006). CKD‐MBD is defined as a systemic disorder of bone and mineral metabolism due to CKD and manifested by one or a combination of:
abnormalities of calcium. phosphorus, parathyroid hormone (PTH) or vitamin D metabolism;
abnormalities in bone turnover, mineralization, volume, linear growth or strength; and
vascular or other soft tissue calcification.
In children CKD‐MBD may be associated with increased fracture rates, reduced linear growth, bony deformities and chronic pain. In a review of 890 children on peritoneal dialysis, 5% had limb deformities, 1.4% had bone pain and 1.5% had vascular calcification (Borzych 2010). Abnormalities of bone turnover, mineralization and volume in CKD‐MBD can be quantitated using bone histomorphometry. The predominant lesion noted on bone biopsy in children on dialysis is one of high bone turnover (in 57% to 100% of patients) with low turnover bone disease much less common (4% of patients) (Bakkaloglu 2010; Hodson 1982; Salusky 2005a). Abnormal skeletal mineralization is commonly associated with both high and low turnover bone disease in dialysis patients. Among children with CKD stages 2‐4, high turnover bone disease was seen in 29% of children with CKD stage 4 but was not seen in children with stage 2 disease and was uncommon in children with stage 3 disease (Wesseling‐Perry 2012; Hodson 1982). In contrast mineralization abnormalities occurred in 43% children with stage 2 CKD and in 86% of children with stage 4 CKD (Wesseling‐Perry 2012); these findings confirm previous findings in early stages of CKD (Hodson 1982). Low turnover bone disease is rare in children not on dialysis.
Although bone disease may not be evident on bone histology in early CKD and plasma levels of calcium and phosphorus are normal, increased levels of the hormone fibroblast growth factor 23 (FGF23) (Wesseling‐Perry 2013) increase renal phosphate excretion and inhibit 1α‐hydroxylase activity thus suppressing circulating levels of 1, 25 (OH)2D leading to increased levels of parathyroid hormone (PTH).
Bone biopsy is an invasive procedure and is now generally limited to research studies so that radiological and biochemical abnormalities are used as surrogate measures of bone disease in CKD‐MBD. Radiological diagnosis is insensitive and cannot distinguish low‐turnover or adynamic bone disease from the high turnover state of secondary hyperparathyroidism. Biochemical abnormalities of parathyroid hormone, serum calcium and phosphate levels are frequently used as markers of bone disease if outside of the recommended KDOQI or European guideline parameters (KDOQI 2005; Klaus 2006). Abnormalities of these values, suggestive of histological changes, have been demonstrated in 28% to 81% of children with CKD stages 2‐5 (Blaszak 2005; Seikaly 2003). These biochemical abnormalities have also been used to specifically diagnose low turnover bone disease. In 41 dialysed children (31 peritoneal dialysis), low turnover bone disease was diagnosed in 48% based on the presence of elevated serum calcium with parathyroid hormone (PTH) values below recommended levels (Avila‐Diaz 2006). PTH levels are most commonly used to monitor the effectiveness of therapy. However optimal target ranges are unclear in part because earlier PTH assays measured active and inactive PTH fragments and newer assays still show considerable variation between PTH assays.
In the absence of clinical symptoms and signs, it has been unclear until recently what impact CKD‐MBD has on the outcome for children with CKD. Recent data have demonstrated an increased risk of coronary arterial calcification in young adults on dialysis (Goodman 2000) while elevated levels of PTH and phosphate are independent risk factors for left ventricular hypertrophy (Bakkaloglu 2011). These factors have been associated with increased mortality in children and young adults with CKD.
Description of the intervention
Treatment of CKD‐MBD aims to normalise mineral metabolism and minimise progression of extraskeletal calcification by maintaining blood levels of calcium and phosphorus close to the normal range for age and maintaining PTH levels at levels considered to be appropriate for the stage of CKD. The mainstays of treatment are with phosphate binders (calcium or non‐calcium containing) and vitamin D metabolites (calcitriol, 1α‐hydroxyvitamin D, newer vitamin D analogues). Dietary measures are instituted to reduce phosphate intake while maintaining adequate calcium and vitamin D intake. Also calcium levels in the dialysis fluid can be manipulated to maintain normocalcaemia. New agents include calcimimetic agents (cinacalcet), which control secondary hyperparathyroidism. For medically unresponsive patients, parathyroidectomy may be required.
How the intervention might work
Early in the development of CKD, circulating levels of 1,25 (OH)2 vitamin D falls following suppression of the renal enzyme 1‐ɑ‐hydoxylase by FGF23, resulting in reduced calcium absorption from the gut and increased PTH levels (Wesseling‐Perry 2013). Increased PTH levels initially maintain serum calcium levels by increasing bone resorption and by stimulating 1‐ɑ‐hydoxylase activity. With further decline in glomerular filtration rate (GFR), phosphate levels rise. These lower calcium levels and further suppress renal enzyme 1‐ɑ‐hydoxylase levels so PTH levels rise further. PTH increases bone turnover leading to renal osteodystrophy. Therefore therapies which increase gut absorption of calcium, reduce phosphate levels and increase circulating levels of 1,25 (OH)2 vitamin D will reduce PTH levels. However both calcium‐containing phosphate binders and vitamin D metabolites may cause hypercalcaemia and elevated calcium‐phosphorus product and predispose to vascular and soft tissue calcification. Calcimimetic agents modulate the calcium sensitive receptor in parathyroid glands, increase intracellular calcium and decrease PTH release.
Why it is important to do this review
There are a large number of studies reporting the efficacy of various medications and dietary manipulations to prevent and treat CKD‐MBD in children and there is extensive clinical experience confirming that current treatment of CKD‐MBD has reduced the severity of bony deformities and fractures over the past few decades. However there remains considerable uncertainty in children about the vascular outcomes related to treatment or non‐treatment of CKD‐MBD. In addition because of the recognised severity of potential side‐effects and the uncertain efficacy of some of the therapeutic agents used to treat CKD‐MBD, it is appropriate to critically review the treatment options.
Objectives
This review aimed to examine the benefits (improved growth rates, reduced risk of bone fractures and deformities, reduction in PTH levels) and harms (hypercalcaemia, blood vessel calcification, deterioration in kidney function) of interventions (including vitamin D preparations and phosphate binders) for the prevention and treatment of metabolic bone disease in children with CKD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs (studies in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) examining treatments for the prevention and treatment of CKD‐MBD in children and adolescents were included.
Types of participants
Inclusion criteria
Studies involving children with CKD stages 2 to 5D (glomerular filtration rate < 90 mL/min/1.73 m2)
Childhood was defined according to the definitions applied in the included studies, but did not exceed 21 years of age.
Exclusion criteria
Studies of children with CKD secondary to primary tubulopathies, e.g. cystinosis, or with diseases known to directly affect bones e.g. primary hyperoxaluria, and studies in children following kidney transplant were excluded. However it is possible that individual children with the above disorders might be included within an eligible study but not specifically specified. Studies of recombinant human growth hormone in children with CKD were excluded as these are included in a separate Cochrane Review (Growth Hormone in children with chronic kidney disease) (Hodson 2012).
Types of interventions
Interventions considered for inclusion were as follows.
Dietary
Pharmacological ‐ specifically vitamin D or metabolites, calcimimetic and phosphate binding agents
Surgical
Herbal or alternative treatments
Changes in dialysis prescription.
For each of these interventions the following comparisons were considered.
Intervention versus placebo
Intervention A versus intervention B
Frequency and mode of administration (e.g. oral or intravenous (IV))
Dose and duration of treatment.
Types of outcome measures
Primary outcomes
Patient‐centred outcome measures
Growth
Bone fractures
Bone deformities
Symptoms related to hypercalcaemia
Parathyroidectomy
Secondary outcomes
Patient‐centred outcome measures
Commencing dialysis treatment
Dialysis‐related clinical events
Parathyroidectomy.
Surrogate outcomes
Change in bone histology
Changes in radiological abnormalities
Changes in PTH levels
Changes in alkaline phosphatase (ALP) levels
Changes in serum calcium, phosphorus and calcium‐phosphorus product
Changes in FGF23 levels
Adverse events
Vascular or extraosseous calcifications
Deterioration of kidney function
Hyperphosphataemia
Hypercalcaemia
Elevation of calcium‐phosphorus product
Radiological deterioration of CKD‐MBD
Development of adynamic bone disease on bone histomorphometry
Hypertension or hypotension
Aggravation of anaemia.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 8 September 2015 through contact with the Trials Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Specialised Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of clinical practice guidelines review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
In the previous version of this review (Geary 2009) all electronically derived abstracts and study titles were assessed for subject relevance and methodological quality. All possible RCTs or quasi‐RCTs which were relevant were assigned specific topic keywords in Reference Manager and the full published paper was obtained for full assessment. The review was undertaken by four authors. The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. The titles and abstracts were screened independently by DG, EH and DH, who discarded studies that were not applicable. However studies and reviews that might include relevant data or information on studies were retained initially. Three authors independently assessed retrieved abstracts, and if necessary, the full text of these studies to determine which studies satisfied the inclusion criteria.
In this update, study titles and abstracts were reviewed by two authors. Full text articles of studies considered relevant were obtained and assessed for eligibility by the same authors.
Data extraction and management
A data abstraction form was devised to record details of data elements such as outcome measures, participants and intervention of each included study for the 2009 review. Only comparisons and outcomes which were pre‐specified in the protocol were included. For this review, data were abstracted by a single assessor and a sample was double checked.
For this update, data extraction and assessment of risk of bias were performed by two authors using standardised data abstraction forms. Disagreements not resolved by discussion between authors were referred to a third author. Studies reported in languages other than English were to be translated before data extraction, but no foreign language reports were identified. Where more than one report of a study was identified, data were extracted from all reports. Where there were discrepancies between reports, data from the primary source were used. Study authors were contacted for additional information about studies.
Assessment of risk of bias in included studies
Hard copies of studies were independently assessed for the methodological quality by two assessors for the 2010 review. Quality assessments were made for allocation concealment, blinding, description of withdrawals and drop‐outs, numbers lost to follow‐up, and whether intention‐to‐treat (ITT) analysis was possible.
In the 2014 update, the following terms were assessed using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias) ?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (number of children with improved growth, radiological bone changes, improved bone histology) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). For numbers of children experiencing adverse effects, risk difference (RD) with 95% CI was used. For continuous outcomes scales of measurement were used to assess the effects of treatment (levels of PTH, serum levels of calcium, phosphorus and calcium‐phosphorus product, and creatinine clearance (CrCl), the mean difference (MD) with 95% CI were calculated unless the scales were different; in this instance, standard mean difference (SMD) was used.
Unit of analysis issues
Data from the first phase of cross‐over RCTs could not be separated so results from cross‐over studies were reported qualitatively.
Dealing with missing data
We aimed to analyse available data in meta‐analyses using intention‐to‐treat (ITT) data. However, where ITT data were only available graphically or not provided and additional information could not be obtained from study authors, per‐protocol (PP) data were used in analyses.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (Higgins 2003). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity respectively.
Assessment of reporting biases
Because of the few studies available for each intervention, it was not possible to use funnel plots to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
Data were pooled using a random‐effects model for dichotomous and continuous data.
Subgroup analysis and investigation of heterogeneity
We had planned subgroup analyses to examine certain between‐study differences in participants (age, stage of CKD, type of dialysis), interventions (agent, dose and duration of treatment) and risk of bias hypothesised to explain any observed heterogeneity of treatment effects but there were insufficient studies for these to be performed.
Sensitivity analysis
We wished to perform sensitivity analyses in order to explore the influence of the following factors on effect size.
Repeating the analysis excluding unpublished studies
Repeating the analysis taking account of risk of bias, as specified
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
However except for one analysis, the maximum number of studies included in any analysis was two so we were not able to carry out any sensitivity analyses.
Results
Description of studies
Results of the search
2009 review
In the 2009 review, of the 1137 titles and abstracts identified, 19 studies were identified for full text review. Of these 15 studies (26 reports) (Ardissino 2000; Eke 1983; GFRD Study 1990; Greenbaum 2005; Greenbaum 2007; Hodson 1985; Jones 1994; Klaus 1995; Mak 1985; Pieper 2006; Salusky 1991; Salusky 1998; Salusky 2005; Schmitt 2003; Watson 1988) involved the defined populations and addressed relevant interventions and were included in the review. A copy of the completed manuscript was provided before publication by Greenbaum 2007. Three studies were cross‐over studies (Jones 1994; Mak 1985; Pieper 2006), and one study (Klaus 1995) was available in abstract form only. Four studies (nine reports) were excluded for ineligible intervention (Ardissino 2000a), ineligible population (El Husseini 2004; Ferraris 2000) and uncertainty as to whether the study was an RCT (Bettinelli 1986). There was no disagreement between authors regarding inclusion of studies. The 2009 review included 15 studies (26 reports) with 472 children.
2015 update
For the 2015 update 47 new reports were identified (Figure 1). Of these, four reports were of three new studies (Gulati 2010; Rianthavorn 2013; Shroff 2012) and 38 reports were of seven previously included studies (Eke 1983; GFRD Study 1990; Greenbaum 2005; Salusky 1991; Salusky 1998; Salusky 2005; Watson 1988). The remaining five new reports were excluded. Two studies were excluded as the populations were ineligible (Choudhary 2014; Kim 2006b), and in one study (Witmer 1976) randomisation was unclear. The remaining three reports were from two previously excluded studies (Ardissino 2000a; Ferraris 2000).
1.
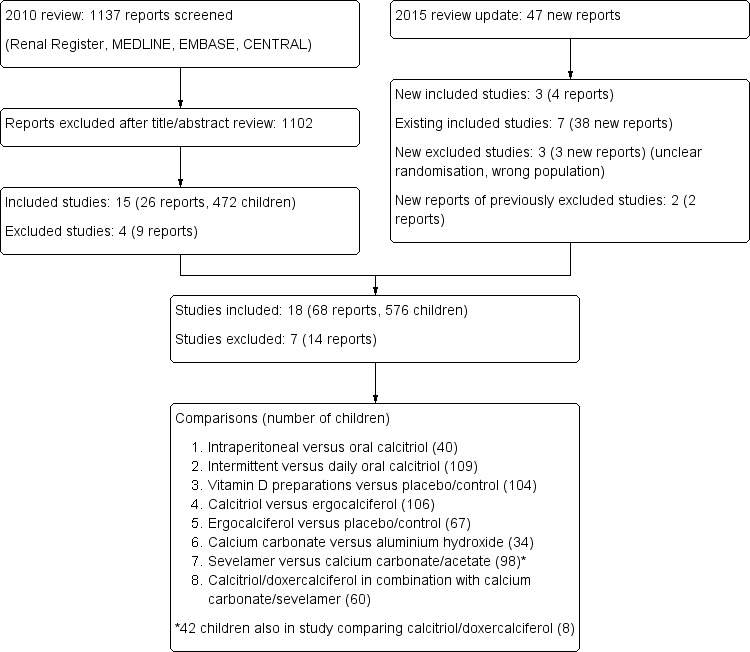
Study flow diagram
Re‐evaluation of Ardissino 2000 and Schmitt 2003 indicated that the data in Schmitt 2003 represented a 12 month follow‐up of 29 prepubertal children treated in Ardissino 2000. For ease of identification, we have continued to report these studies as two studies.
To allow analyses of comparisons between calcitriol and doxercalciferol and for separate reporting of the comparison between sevelamer and calcium carbonate in this 2 x 2 longitudinal factorial study, we have divided Salusky 2005 into three studies (Salusky 2005; Salusky 2005a; Salusky 2005b). Salusky 2005 includes the groups treated with sevelamer or calcium carbonate irrespective of vitamin D preparation used. Salusky 2005a includes the two groups treated with doxercalciferol while Salusky 2005b includes the two groups treated with calcitriol.
Therefore the 2015 update included 18 studies (68 reports) with 576 enrolled children.
Included studies
The 18 included studies were divided into eight treatment comparisons (Figure 1).
Intraperitoneal calcitriol versus oral calcitriol
Two studies (40 enrolled; 40 evaluated) compared intraperitoneal compared with oral calcitriol. Children receiving continuous cycling or continuous ambulatory peritoneal dialysis were treated for 12 months (Salusky 1998) or 3 months (Jones 1994). The study by Jones 1994 was a cross‐over study and data could not be meta‐analysed.
Intermittent oral calcitriol versus daily oral calcitriol
Three studies (109 enrolled; 104 evaluated) compared intermittent oral administration of calcitriol with daily oral administration in children with CKD stages 2 to 5 for 8 to 10 weeks (Ardissino 2000), 12 months (Schmitt 2003), or from 2 to 36 weeks (Klaus 1995).
Different vitamin D preparations versus placebo/no specific treatment
Four studies (104 enrolled; 103 evaluated) compared different vitamin D preparations administered orally or IV with placebo/no specific treatment in CKD patients stages 3 and 4 (Eke 1983) and in patients receiving peritoneal or haemodialysis (Greenbaum 2005; Greenbaum 2007; Watson 1988).
Different vitamin D preparations
Two studies (106 enrolled; 97 evaluated) compared different vitamin D preparations. GFRD Study 1990 compared oral calcitriol with oral dihydrotachysterol in children with CKD stages 3 and 4 treated for 12 months. Hodson 1985 compared oral calcitriol with oral ergocalciferol in children on dialysis or CKD stages 2 to 4.
One study (60 enrolled; 51 evaluated) compared doxercalciferol (Salusky 2005a) and calcitriol (Salusky 2005b) in combination with either sevelamer or calcium carbonate. Comparisons were reported for vitamin D preparations irrespective of the phosphate binder given.
Ergocalciferol versus placebo/control
Two studies (67 enrolled; 60 evaluated) compared ergocalciferol with placebo/no specific treatment in patients with CKD stages 2 to 5D (Rianthavorn 2013; Shroff 2012). In Rianthavorn 2013 the primary outcome was reduction in the dose of erythrocyte‐stimulating agent (ESA).
Calcium carbonate versus aluminium hydroxide
Two studies (34 enrolled; 29 evaluated) compared calcium hydroxide with calcium carbonate in pre‐dialysis children (Mak 1985) or those receiving peritoneal dialysis (Salusky 1991).
Sevelamer versus calcium‐containing phosphate binders
Three studies (98 enrolled; 66 evaluated) compared sevelamer with calcium‐containing phosphate binders in children with CKD stages 2 to 4 (Gulati 2010) or receiving dialysis (Pieper 2006; Salusky 2005) compared the non‐calcium‐containing sevelamer with calcium carbonate (Salusky 2005) or calcium acetate (Gulati 2010; Pieper 2006). In Salusky 2005, children were also randomised to receive doxercalciferol or calcitriol. Factorial analysis provided no evidence of treatment interaction between the two sterols so comparisons were reported for phosphate binders irrespective of vitamin D sterol given.
No RCTs examining interventions with dietary changes, surgery, alterations in dialysis prescription or calcimimetic agents were identified.
Excluded studies
Seven studies were excluded (Ardissino 2000a; Bettinelli 1986; Choudhary 2014; El Husseini 2004; Ferraris 2000; Kim 2006b; Witmer 1976). One cross‐over study (Ardissino 2000a) in children with CKD examined calcium absorption only after oral or IV calcitriol. Two studies examined bone mineral density in kidney transplant patients treated with calcitonin, alendronate or 1α‐hydroxyvitamin D (El Husseini 2004) or with methylprednisolone or deflazacort (Ferraris 2000). Randomisation was unclear and data was no longer available in Witmer 1976.
Risk of bias in included studies
2.
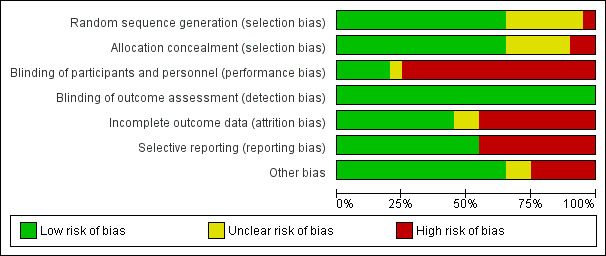
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
3.
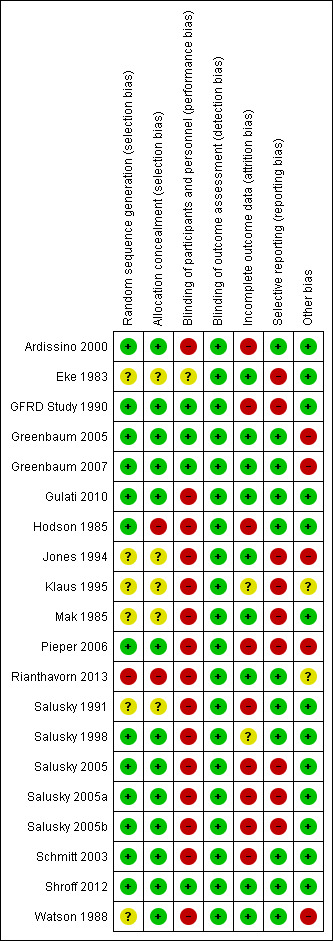
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation was deemed to be at low risk of bias in 12 studies (Ardissino 2000; GFRD Study 1990; Greenbaum 2005; Greenbaum 2007; Gulati 2010; Hodson 1985; Pieper 2006; Salusky 1998; Salusky 2005; Schmitt 2003; Shroff 2012; Watson 1988). One study was at high risk of bias as patients were randomised sequentially (Rianthavorn 2013). In the remaining five studies, the sequence generation methodology was unclear.
Allocation concealment was at low risk of bias in 11 studies (Ardissino 2000; GFRD Study 1990; Greenbaum 2005; Greenbaum 2007; Gulati 2010; Pieper 2006; Salusky 1998; Salusky 2005; Schmitt 2003; Shroff 2012; Watson 1988). Two studies (Hodson 1985; Rianthavorn 2013) were considered at high risk of bias. Allocation concealment methodology was unclear in the remaining five studies (Eke 1983; Jones 1994; Klaus 1995; Mak 1985; Salusky 1991).
Blinding
Four studies were blinded and considered to be at low risk of bias for performance bias ( GFRD Study 1990; Greenbaum 2005; Greenbaum 2007; Shroff 2012). Blinding was unclear in one study, which was reported to be double‐blinded but did not clarify how this was achieved (Eke 1983). The remaining thirteen studies were not blinded and were considered at high risk of performance bias.
Since the primary outcomes (PTH level, bone mineralization) in all studies were based on laboratory assessment, and unlikely to be influenced by blinding, all included studies were considered to be at low risk of detection bias.
Incomplete outcome data
Nine studies were considered at low risk of attrition bias (Eke 1983; Greenbaum 2005; Greenbaum 2007; Gulati 2010; Jones 1994; Mak 1985; Rianthavorn 2013; Shroff 2012; Watson 1988). Seven studies were considered at high risk of attrition bias with more than 15% loss to follow‐up or exclusion from analysis (Ardissino 2000; GFRD Study 1990; Hodson 1985; Pieper 2006; Salusky 1991; Salusky 2005; Schmitt 2003). In the remaining two studies attrition bias was considered unclear (Klaus 1995; Salusky 1998). Loss to follow‐up or exclusion resulted commonly when children on dialysis underwent kidney transplant. Other reasons for exclusion were non‐adherence to treatment, protocol violation or withdrawal by families or physicians.
Selective reporting
Studies were considered to be at high risk of reporting bias if they did not provide data on final or change in PTH, calcium, phosphorus, calcium‐phosphorus product or ALP levels, bone histology, patient centred outcomes such as fractures or growth, adverse events such as hypercalcaemia or all‐cause mortality. Seven studies were considered at high risk of reporting bias (Eke 1983; GFRD Study 1990; Jones 1994; Klaus 1995; Mak 1985; Pieper 2006; Salusky 2005). The remaining 11 studies were considered to be at low risk of reporting bias.
Other potential sources of bias
Eleven studies appeared to be free of other potential sources of bias (Ardissino 2000; Eke 1983; GFRD Study 1990; Gulati 2010; Hodson 1985; Mak 1985; Salusky 1991; Salusky 1998; Salusky 2005; Schmitt 2003; Shroff 2012). Five studies were funded by industry and considered at high risk of bias (Greenbaum 2005; Greenbaum 2007; Jones 1994; Pieper 2006; Watson 1988). In the remaining two studies, it was unclear whether the study was free of other potential sources of bias (Klaus 1995; Rianthavorn 2013).
Effects of interventions
Intraperitoneal versus oral calcitriol
Two studies investigated this comparison (Salusky 1998; Jones 1994) (Table 1). The first study (Jones 1994) compared IP with oral calcitriol in a cross‐over study including seven children and reported that mean height standard deviation score (SDS) did not differ between groups at the end of the study. The data for each part of the cross‐over could not be separated.
1. Intraperitoneal versus oral calcitriol in children on peritoneal dialysis.
| Outcome | Jones 1994a | Salusky 1998 | Summary estimateb |
| Abnormal bone biopsy | Not reported | 12/16c versus 12/17d | RR 1.06 (0.70 to 1.61) |
| Adynamic bone disease | Not reported | 7/16 versus 5/17 | RR 1.49 (0.59 to 3.74) |
| Osteitis fibrosa | Not reported | 2/16 versus 6/17 | RR 0.35 (0.08 to 1.51) |
| Mixed/mild disease | Not reported | 3/16 versus 7/17 | RR 0.46 (0.14 to 1.46) |
| Normal/reduced bone formation rate | Not reported | 11/16 versus 11/17 | RR 1.06 (0.66 to 1.72) |
| Bone formation rate (µm2/mm2/d) | Not reported | 297 ± 472 versus 586 ± 973 | MD ‐289.00 (‐806.13 to 228.13) |
| PTH (pg/mL) | No significant difference | 169 ± 228 versus 670 ± 400 | MD ‐501.00 (‐721.54 to ‐280.46) |
| Maximum serum calcium (mg/dL) | Not reported | 10.4 ± 2 versus 9.7 ± 1.64 | MD 0.70 (‐0.55 to 1.95) |
| Hypercalcaemic patients | 0/7 versus 0/7 | 8/16 versus 5/17 | RD 0.21 (‐0.12 to 0.53) |
| Hypophosphataemic patients | Not reported | 2/16 versus 3/17 | RD ‐0.05 (‐0.29 to 0.19) |
| Peritonitis episodes/patient‐month | 1/ 11 versus 1/11 | 1/12 versus 1/10 | RD 0.01 (‐0.24 to 0.26) |
| Height SDS | ‐2.26 (‐4.5 to ‐1.61) versus ‐2.33 (‐4.27 to ‐1.43) (end) | Not reported | Not calculated |
a Cross‐over study
b Summary estimate (RR, RD, MD) and 95% CI; cross‐over studies excluded
c Experimental intervention
d Comparative intervention
PTH ‐ parathyroid hormone; SDS ‐ standard deviation score
Salusky 1998 reported no significant differences in the number of children overall with abnormal bone histology (Analysis 1.1.1 (1 study, 33 children): RR 1.06, 95% CI 0.70 to 1.61), the number with adynamic bone disease (Analysis 1.1.2 (1 study, 33 children): RR 1.49, 95% CI 0.59 to 3.74), osteitis fibrosa (Analysis 1.1.3 (1 study, 33 children): RR 0.35, 95% CI 0.08 to 1.51), mixed or mild disease (Analysis 1.1.4 (1 study, 33 children): RR 0.46, 95% CI 0.14 to 1.46) or normal/reduced bone formation rates (Analysis 1.1.5 (1 study, 33 children): RR 1.06, 95% CI 0.66 to 1.72).
1.1. Analysis.
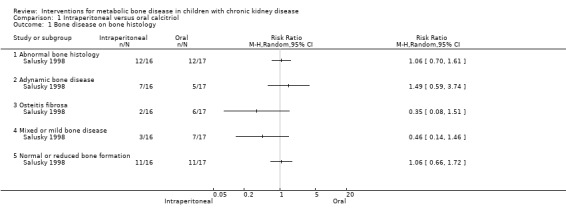
Comparison 1 Intraperitoneal versus oral calcitriol, Outcome 1 Bone disease on bone histology.
Salusky 1998 reported bone formation rates did not differ significantly between treatment groups (Analysis 1.2 (1 study, 33 children): MD ‐289.00 μm2/mm2/d, 95% CI ‐806.13 to 228.13).
1.2. Analysis.
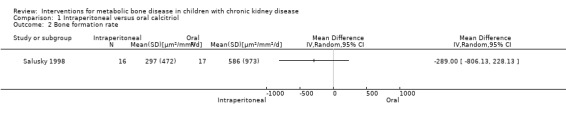
Comparison 1 Intraperitoneal versus oral calcitriol, Outcome 2 Bone formation rate.
Jones 1994 (cross‐over RCT) reported that no significant differences were found in PTH levels, the number of children with hypercalcaemia and the number of peritonitis episodes between groups (Table 1). Salusky 1998. reported mean PTH levels were significantly lower with IP calcitriol compared with oral (Analysis 1.3 (1 study, 33 children): MD ‐501.00 pg/mL, 95% CI ‐721.54 to ‐280.46).
1.3. Analysis.
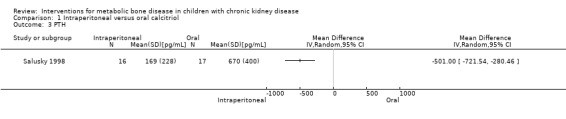
Comparison 1 Intraperitoneal versus oral calcitriol, Outcome 3 PTH.
Salusky 1998 reported maximum serum calcium levels (Analysis 1.4 (1 study, 33 children): MD 0.70 mg/dL, 95% CI ‐0.55 to 1.95) and the number of children with hypercalcaemia (Analysis 1.5.1 (1 study, 33 children): RD 0.21, 95% CI ‐0.12 to 0.53) or hyperphosphataemia (Analysis 1.5.2 (1 study, 33 children): RD ‐0.05, 95% CI ‐0.29 to 0.19) did not differ between treatment groups. The number of peritonitis episodes/patient‐month did not differ between treatment groups (Analysis 1.6 (1 study, 33 children): RD 0.01, 95% CI ‐0.24 to 0.26).
1.4. Analysis.
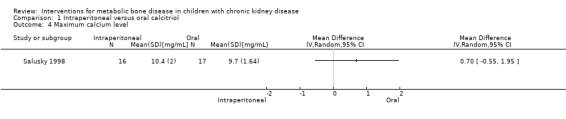
Comparison 1 Intraperitoneal versus oral calcitriol, Outcome 4 Maximum calcium level.
1.5. Analysis.
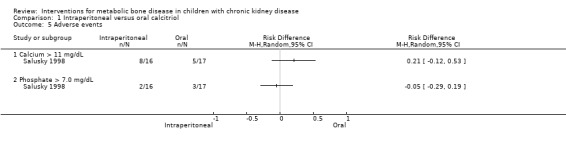
Comparison 1 Intraperitoneal versus oral calcitriol, Outcome 5 Adverse events.
1.6. Analysis.
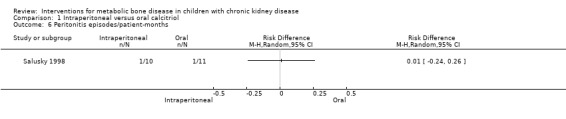
Comparison 1 Intraperitoneal versus oral calcitriol, Outcome 6 Peritonitis episodes/patient‐months.
Intermittent oral versus daily oral calcitriol
Three parallel studies (104 evaluated children) compared intermittent oral with daily oral calcitriol (Ardissino 2000; Klaus 1995; Schmitt 2003) (Table 2).
2. Intermittent oral versus daily oral calcitriol.
| Outcome | Ardissino 2000 | Klaus 1995 | Schmitt 2003 | Summary estimate a |
| % fall PTH | 13.7 ± 46.7b versus 19.2 ± 57.8c | Not reported | 58 ± 26 versus 64 ± 22 | MD ‐5.83 (‐21.49 to 9.83) |
| Number with fall in PTH | 21/30 versus 23/29 | "median PTH fell in both groups" | Not reported | RR 0.88 (0.65 to 1.19) |
| Mean integrated PTH (pg/mL) | Not reported | Not reported | 285 ± 213 versus 343 ± 171 | MD ‐58.00 (‐212.55 to 96.55) |
| CrCl (mL/min/1.73 m2) | 22 ± 13 versus 22 ± 11(end) | Not reported | ‐1.6 ± 4.0 versus 3.1± 4.8 (change) | MD 0.50 (‐5.72 to 6.72) |
| Change in height SDS | Not reported | Not reported | ‐0.05 ± 0.52 versus ‐0.18 ± 0.34 | MD 0.13 (‐0.22 to 0.48) |
| Hypercalcaemic patients | 1/30 versus 1/29 | 2/12 versus 3/9 | No difference in number of episodes | RD ‐0.02 (‐0.17 to 0.13) |
| Hypophosphataemic patients | 0/30 versus 1/29 | Not reported | No difference in number of episodes | RD 0.03 (‐0.06 to 0.12) |
a Summary estimate (RR, RD, MD) and 95%
b Experimental intervention
c Comparative intervention
CrCl ‐ creatinine clearance; PTH ‐ parathyroid hormone; SDS ‐ standard deviation score
Schmitt 2003 reported no significant difference in change in mean height SDS (Analysis 2.1 (1 study, 24 children): MD 0.13, 95% CI ‐0.22 to 0.48). No significant differences between treatment routes were found for any surrogate biochemical outcome.
2.1. Analysis.
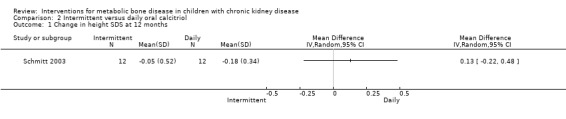
Comparison 2 Intermittent versus daily oral calcitriol, Outcome 1 Change in height SDS at 12 months.
Ardissino 2000 and Schmitt 2003 reported no significant differences in the fall in PTH levels at 8 weeks (Analysis 2.2.1 (Ardissino 2000, 59 children): MD ‐5.50%, 95% CI ‐32.37 to 21.37) and 12 months (Analysis 2.2,2 (Schmitt 2003, 48 children): MD ‐6.00%, 95% CI ‐25.27 to 13.27), the number with reduction in PTH (Analysis 2.3 (Ardissino 2000, 59 children): RR 0.88, 95% CI 0.65 to 1.19) and the mean integrated PTH levels (Analysis 2.4 (Schmitt 2003, 24 children): MD ‐58.00 pg/mL, 95% CI ‐212.55 to 96.55). In Klaus 1995, median (range) PTH levels fell significantly in both treatment groups with no differences between treatment groups (Table 2).
2.2. Analysis.
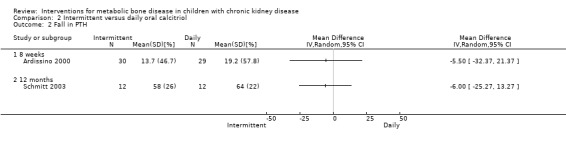
Comparison 2 Intermittent versus daily oral calcitriol, Outcome 2 Fall in PTH.
2.3. Analysis.
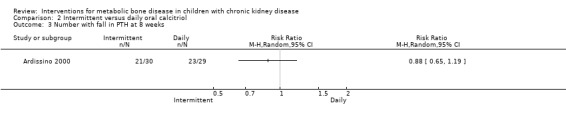
Comparison 2 Intermittent versus daily oral calcitriol, Outcome 3 Number with fall in PTH at 8 weeks.
2.4. Analysis.
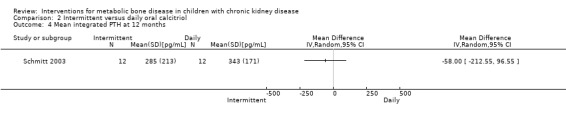
Comparison 2 Intermittent versus daily oral calcitriol, Outcome 4 Mean integrated PTH at 12 months.
There were no significant differences in CrCl at eight weeks (Analysis 2.5.1 (Ardissino 2000, 59 children): MD 0.50 mL/min/1.73 m2, 95% CI ‐5.72 to 6.72) or 12 months (Analysis 2.5.2 (Schmitt 2003, 24 children): MD 1.50 mL/min/1.73 m2, 95% CI ‐2.04 to 5.04,), or in numbers with hypercalcaemia (Analysis 2.6.1 (2 studies, 80 children): RD ‐0.02, 95% CI ‐0.17 to 0.13; I2 = 19%) or hyperphosphataemia (Analysis 2.6.2 (Ardissino 2000, 59 children): RD 0.03, 95% CI ‐0.06 to 0.12). Schmitt 2003 reported no difference in the number of episodes of hypercalcaemia or hyperphosphataemia between the treatment groups.
2.5. Analysis.
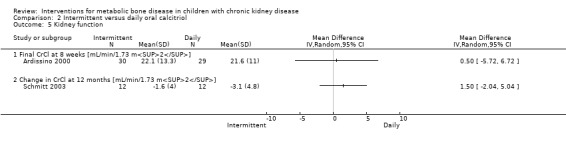
Comparison 2 Intermittent versus daily oral calcitriol, Outcome 5 Kidney function.
2.6. Analysis.
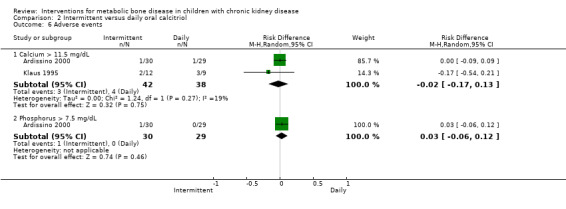
Comparison 2 Intermittent versus daily oral calcitriol, Outcome 6 Adverse events.
Active vitamin D preparations versus placebo or no specific treatment
Four parallel studies compared active vitamin D preparations (calcitriol, paricalcitol, 1α‐hydroxyvitamin D) with placebo or no specific treatment (Table 3) (Eke 1983; Greenbaum 2005; Greenbaum 2007; Watson 1988). None of the studies reported any data for patient‐centred outcomes.
3. Vitamin D preparations versus placebo/no treatment.
| Outcomes | Eke 1983 | Greenbaum 2005 | Greenbaum 2007 | Watson 1988 | Summary estimatea |
| Number with abnormal bone histology | 7/8b versus 2/7c | Not reported | Not reported | Reduced osteoid, bone formation and resorption in 1αOHD versus control | RR 0.17 (0.03 to 1.16) |
| PTH (pmol/L) | No difference | Not reported | Not reported | 18 ± 15 versus 73 ± 32 | MD ‐55.00 (‐83.03 to ‐26.97) |
| Elevated PTH | Not reported | Not reported | Not reported | 1/6 versus 6/6 | RR 0.17 (0.03 to 1.00) |
| Number with 30% fall in PTH | Not reported | 11/21 versus 5/26 | 9/15 versus 3/14 | Not reported | RR 2.75 (1.39 to 5.47) |
| Change in PTH (pg/mL) | Not reported | ‐193 ± 637 versus 10 ± 347 | ‐164 versus +238 | Not reported | MD ‐203.00 (‐506.34 to 100.34) |
| Hypercalcaemic patients | 0/8 versus 0/7 | 5/21 versus 0/26 | 0/15 versus 0/14 | 3/6 versus 2/6 | RD 0.08 (‐0.08 to 0.24) |
| Ca x P > 7.5 mg2/dL2 | Not reported | 8/21 versus 1/26 | Not reported | Not reported | RD 0.34 (0.12 to 0.56) |
| Phosphorus > 6.5 mg/dL x 2 | Not reported | 15/21 versus 12/26 | Not reported | Not reported | RD 0.25 (‐0.02 to 0.52) |
| Change in serum calcium (mg/dL) | No difference | 0.38 ± 0.6 versus 0.08 ± 0.66 | 0.00 ± 1.01 versus 0.29 ± 1.01 | No difference | MD ‐0.10 (‐0.45 to 0.65) |
| Change in Ca x P (mg2/dL2) | Not reported | 4.7 ± 12.4 versus 0.6 ± 14.8 | ‐1.35 ± 14.1 versus 3.21 ± 14.1 | Not reported | MD ‐0.45 (‐7.94 to 8.83) |
| Change in serum phosphorus (mg/dL) | No difference | 0.23 ± 1.33 versus 0.00 ± 1.68 | ‐0.15 ± 1.35 versus 0.18 ± 1.35 | No difference | MD ‐0.01 (‐0.66 to 0.63) |
| Change in bone ALP (U/L) | No difference | ‐4.5 ± 67 versus 43.2 ± 66 | Not reported | Not reported | MD ‐47.70 (‐88.54 to ‐6.86) |
a Summary estimate (RR, RD, MD) and 95% CI
b Experimental intervention
c Comparative intervention
ALP ‐ alkaline phosphatase; Ca x P ‐ calcium‐phosphorus product; PTH ‐ parathyroid hormone
Two parallel studies (28 children) compared 1α‐hydroxyvitamin D with no specific treatment (Eke 1983; Watson 1988). Though only 1/8 children treated with 1α‐hydroxyvitamin D versus 5/7 not treated had abnormal bone histology at the end of treatment, the difference was not significant due to small numbers (Analysis 3.1 (Eke 1983, 15 patients): RR 0.17, 95% CI 0.03 to 1.16). This study reported no significant difference in PTH levels at the end of the study (Table 3). Watson 1988 reported children treated with 1α‐hydroxyvitamin D showed reduced osteoid volume and both the number of children with PTH levels above the normal range of 3 to 25 pmol/L (Analysis 3.2 (12 children): RR 0.23, 95% CI 0.06 to 0.97) and the mean PTH levels (Analysis 3.3 (12 children): MD ‐55.00 pmol/L, 95% CI ‐83.03 to ‐26.97) were significantly lower in treated children compared with controls.
3.1. Analysis.
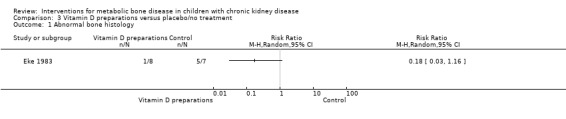
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 1 Abnormal bone histology.
3.2. Analysis.
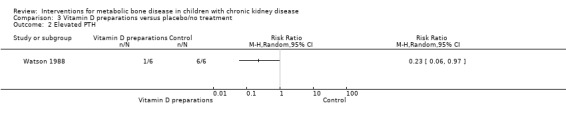
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 2 Elevated PTH.
3.3. Analysis.
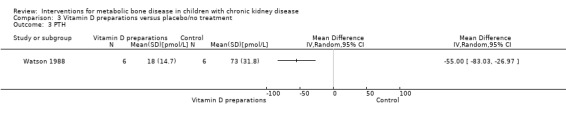
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 3 PTH.
Two parallel studies (57 children) compared IV calcitriol (Greenbaum 2005) or IV paricalcitol (Greenbaum 2007) given three times/week with placebo. IV vitamin D preparations (calcitriol or paricalcitol) significantly increased the number of children who achieved a 30% fall in PTH levels on at least two occasions during the study (Analysis 3.4 (2 studies, 76 children): RR 2.75, 95% CI 1.39 to 5.47; I2 = 0%). However changes in mean PTH levels during treatment were not significantly different in children treated with IV calcitriol compared with placebo (Analysis 3.5 (1 study, 47 children): MD ‐203.00 pg/mL, 95% CI ‐506.34 to 100.34). An analysis of mean PTH levels following paricalcitol therapy was not possible as standard deviations were not provided.
3.4. Analysis.
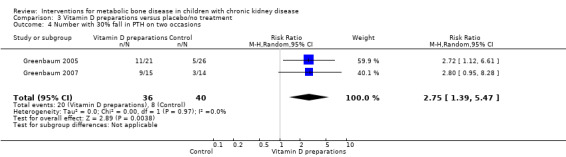
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 4 Number with 30% fall in PTH on two occasions.
3.5. Analysis.
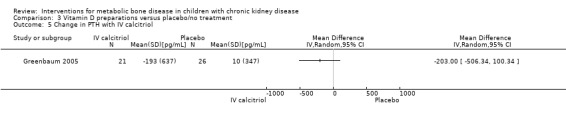
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 5 Change in PTH with IV calcitriol.
Overall there was no significant difference in the risk of hypercalcaemia with vitamin D preparations compared with placebo/no specific treatment (Analysis 3.6 (4 studies, 103 children): RD 0.08, 95% CI ‐0.08 to 0.24; I2 = 55%). However there was heterogeneity with one study showing a significantly greater risk of hypercalcaemia in children treated with IV calcitriol. Following IV calcitriol, the number of children with elevated serum calcium‐phosphorus products (Analysis 3.7 (Greenbaum 2005, 47 children): RD 0.34, 95% CI 0.12 to 0.56) was increased compared with placebo while there was no significant difference in number with hyperphosphataemia (Analysis 3.8 (Greenbaum 2005, 47 children): RD 0.25, 95% CI ‐0.02 to 0.52). Mean changes in levels of serum calcium (Analysis 3.9.1 (2 studies, 76 children): MD 0.10 mg/dL, 95% CI ‐0.45 to 0.65; I2 = 50%), serum calcium‐phosphorus product (Analysis 3.9.2 (2 studies. 76 children): MD 0.45 mg2/dL2, 95% CI ‐7.94 to 8.83; I2 = 42%) and serum phosphorus (Analysis 3.9.3 (2 studies, 76 children): MD ‐0.01 mg/dL, 95% CI ‐0.66 to 0.63; I2 = 0%) did not differ between children treated with IV calcitriol or paricalcitol and placebo. Bone ALP was significantly reduced following IV calcitriol (Analysis 3.9.4 (Greenbaum 2005, 41 children): MD ‐47.70 µg/L, 95% CI ‐88.54 to ‐6.86). In the studies of 1α‐hydroxyvitamin D no differences were reported in mean serum calcium or phosphorus levels at the end of treatment but only graphical data or data without standard deviations were provided (Table 3).
3.6. Analysis.
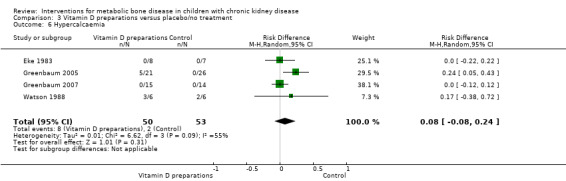
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 6 Hypercalcaemia.
3.7. Analysis.
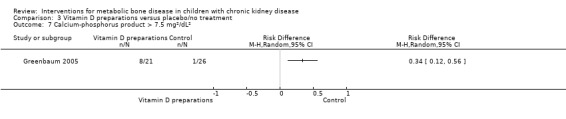
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 7 Calcium‐phosphorus product > 7.5 mg²/dL².
3.8. Analysis.
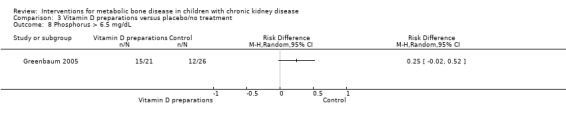
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 8 Phosphorus > 6.5 mg/dL.
3.9. Analysis.
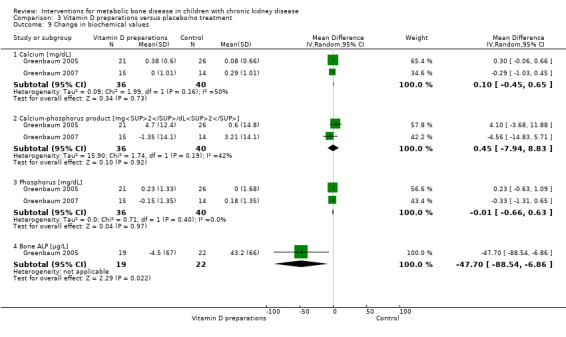
Comparison 3 Vitamin D preparations versus placebo/no treatment, Outcome 9 Change in biochemical values.
Calcitriol versus dihydrotachysterol or ergocalciferol
One study (82 children) compared the effect of calcitriol and dihydrotachysterol on growth, GFR and the number of episodes of hypercalcaemia (GFRD Study 1990). Data on growth and GFR were reported as changes in slopes of growth rates so were not amenable to meta‐analysis. Growth rates did not differ between treatment groups. GFR fell during treatment in both groups but there was no difference between groups. There was no significant difference in the number of episodes of hypercalcaemia between groups (Table 4).
4. Calcitriol versus other active vitamin D preparations.
| Outcome | GFRD Study 1990 | Hodson 1985 |
Salusky 2005a Doxercalciferol treatment |
Salusky 2005b Calcitriol treatment |
Summary estimatea |
| PTH (pg/mL) | Not reported | 0.87 ± 0.44b versus 1.35 ± 0.89c | Graphical data only | Graphical data only | MD ‐0.48 (‐1.23 to 0.27) |
| Number with Improved histology | Not reported | 7/8 versus 4/7 | Not reported | Not reported | RR 1.53 (0.77 to 3.06) |
| Height velocity ≥ expected | Positive growth scores with both treatments | 1/8 versus 4/7 | Not reported | Not reported | RR 0.22 (0.03 to 1.53) |
| Hypercalcaemic patients | No difference in number of episodes | 6/8 versus 3/7 | 17 in sevelamer treatedb versus 48 episodes in CaCO3 treatedc | 17 in sevelamer treatedb versus 48 episodes in CaCO3 treatedc | RR 1.75 (0.68 to 4.50) |
| GFR (mL/min/1.73 m2) | Fell in both groups | Not reported | Peritoneal dialysis patients | Peritoneal dialysis patients | Not calculated |
| Calcium (mmol/L) | Not reported | 2.63 ± 0.10 versus 2.45 ± 0.20 | Graphical data only | Graphical data only | MD 0.18 (0.01 to 0.35) |
| Phosphorus (mmol/L) | Not reported | 1.59 ± 0.30 versus 1.93 ± 0.47 | Graphical data only | Graphical data only | MD ‐0.34 (‐0.76 to 0.08) |
| ALP (U/L) | Not reported | 169 ± 58 versus 208 ± 84 | Graphical data only | Graphical data only | MD ‐39.00 (‐116.63 to 38.63) |
| Bone formation rate (µm3/µm2/d) | Not reported | Not reported | 60 ± 97 versus 53 ± 100 | 62 ± 201 versus 45 ± 73 | MD 10.29 (‐53.07 to 73.65) |
| Osteoid volume (%) | Not reported | Not reported | 11.1 ± 19.8 versus 10.8 ± 10.4 | 8.2 ± 20.1 versus 8.9 ± 14.3 | MD ‐0.16 (‐9.16 to 8.83) |
| Eroded bone surface (%) | Not reported | Not reported | 7.9 ± 9.7 versus 7.3 ± 15.6 | 8.6 ± 12.8 versus 7.7 ± 11.6 | MD 0.76 (‐6.16 to 7.71) |
| Bone volume (%) | Not reported | Not reported | 29.9 ± 17.3 versus 28.2 ± 15.9 | 30.3 ± 44.5 versus 26 ± 24.9 | MD 2.19 (‐9.12 to 13.91) |
a Summary estimate (RR, RD, MD) and 95% CI
b Experimental intervention
c Comparative intervention
ALP ‐ alkaline phosphatase; GFR ‐ glomerular filtration rate; PTH ‐ parathyroid hormone
Hodson 1985 compared calcitriol with ergocalciferol and found no significant differences between treatments in the number with height velocity ≥ expected (Analysis 4.1 (15 children): RR 0.22, 95% CI 0.03 to 1.53), in the number with improved bone histology (Analysis 4.2 (15 children): RR 1.53, 95% CI 0.77 to 3.06) and in final PTH levels (Analysis 4.3 (15 children): MD ‐0.48 ng/mL, 95% CI ‐1.23 to 0.27). The number of children with hypercalcaemia did not differ between groups (Analysis 4.4 (15 children): RR 1.75, 95% CI 0.68 to 4.50). The mean levels of serum calcium (Analysis 4.5.1 (15 children): MD 0.18 mmol/L, 95% CI 0.01 to 0.35), serum phosphorus (Analysis 4.5.2 (15 children): MD ‐0.34 mmol/L, 95% CI ‐0.76 to 0.08) and serum ALP (Analysis 4.5.3 (15 children): MD ‐39.00 U/L, 95% CI ‐116.63 to 38.63) at the end of the study did not differ between groups.
4.1. Analysis.
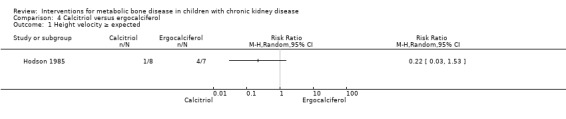
Comparison 4 Calcitriol versus ergocalciferol, Outcome 1 Height velocity ≥ expected.
4.2. Analysis.
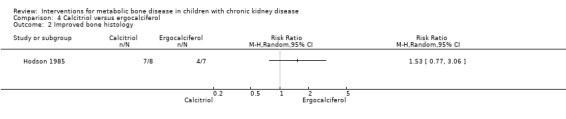
Comparison 4 Calcitriol versus ergocalciferol, Outcome 2 Improved bone histology.
4.3. Analysis.
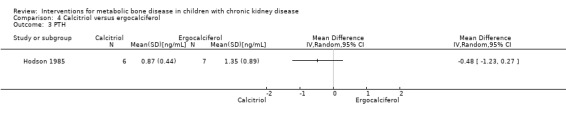
Comparison 4 Calcitriol versus ergocalciferol, Outcome 3 PTH.
4.4. Analysis.
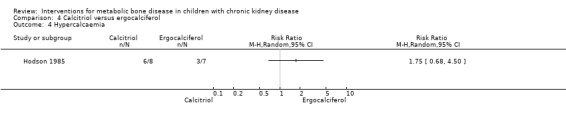
Comparison 4 Calcitriol versus ergocalciferol, Outcome 4 Hypercalcaemia.
4.5. Analysis.
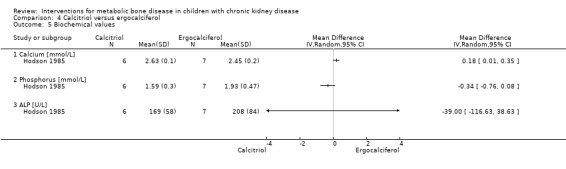
Comparison 4 Calcitriol versus ergocalciferol, Outcome 5 Biochemical values.
Ergocalciferol (replacement doses) versus placebo or no treatment
Two studies (Rianthavorn 2013; Shroff 2012) compared ergocalciferol in patients with CKD and vitamin D deficiency. Fewer children treated with ergocalciferol developed secondary hyperparathyroidism but the difference was not significant due to small patient numbers (Analysis 5.1 (Shroff 2012, 40 children): RR 0.33, 95% CI 0.11 to 1.05). However the time to development of hyperparathyroidism was significantly longer in children treated with ergocalciferol compared with placebo (hazard ratio 0.30, 95% CI 0.09‐0.93) (Shroff 2012). There were no significant differences between treatment groups in final PTH (Analysis 5.2 (Rianthavorn 2013, 20 children): MD ‐1.16 pg/mL, 95% CI ‐1.04 to 0.71), phosphorus levels (Analysis 5.4 (2 studies, 60 children): MD ‐0.29 mg/dL, 95% CI ‐0.96 to 0.39; I2 = 0%) and final calcium (Analysis 5.3 (2 studies, 60 children): MD 0.26 mg/dL, 95% CI ‐0.28 to 0.81; I2 = 42%). Vitamin D (1,25 (OH)) levels (Analysis 5.5 (Shroff 2012, 40 children): MD 27.00 pmol/L, 95%CI 17.35 to 36.65) were significantly higher in the treatment group compared to control group though the differences were not clinically important. Both studies reported no adverse effects related to ergocalciferol and no child developed hypercalcaemia.
5.1. Analysis.
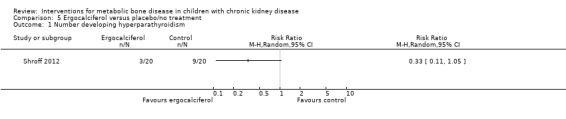
Comparison 5 Ergocalciferol versus placebo/no treatment, Outcome 1 Number developing hyperparathyroidism.
5.2. Analysis.
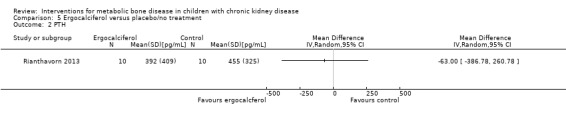
Comparison 5 Ergocalciferol versus placebo/no treatment, Outcome 2 PTH.
5.4. Analysis.
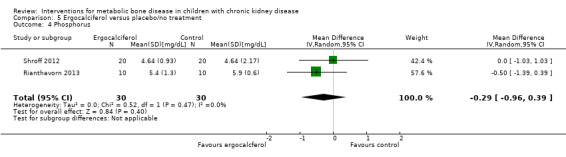
Comparison 5 Ergocalciferol versus placebo/no treatment, Outcome 4 Phosphorus.
5.3. Analysis.
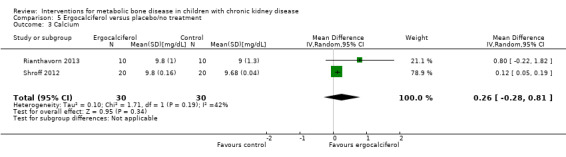
Comparison 5 Ergocalciferol versus placebo/no treatment, Outcome 3 Calcium.
5.5. Analysis.
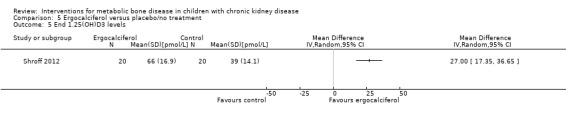
Comparison 5 Ergocalciferol versus placebo/no treatment, Outcome 5 End 1.25(OH)D3 levels.
Phosphate binders: calcium carbonate versus aluminium hydroxide
Two studies (Salusky 1991 (parallel study); Mak 1985 (cross‐over study) compared calcium carbonate with aluminium hydroxide as phosphate binders (Table 5). Salusky 1991 reported no significant difference in mean final height SDS between treatments (Analysis 6.1 (17 children): MD ‐0.86 SDS, 95% CI ‐2.24 to 0.52). The number with abnormal bone biopsies at the end of treatment was significantly lower in children treated with calcium carbonate compared with aluminium hydroxide (Analysis 6.2 (17 children): RR 0.35, 95% CI 0.13 to 0.95). Bone aluminium levels (Analysis 6.3 (17 children): MD ‐1.00 mg/kg dry weight, 95% CI ‐12.29 to 10.29), final PTH levels (Analysis 6.4 (17 children): MD ‐187.00 mL‐eq/L, 95% CI ‐1089.25 to 715.25) and final ALP (Analysis 6.5 (17 children): MD 21.00 U/L, 95% CI ‐216.62 to 258.62) did not differ significantly between groups. The number with hypercalcaemia did not differ between groups (Analysis 6.6 (17 children): RD 0.31, 95% CI ‐0.14 to 0.77). In the cross‐over study by Mak 1985 (12 children), results were not reported separately for each group. PTH levels normalised in both treatment groups. Serum calcium and phosphorus levels did not differ between groups. Plasma aluminium levels were significantly higher at the end of aluminium treatment compared with calcium carbonate treatment. Results of bone histology (overall no change), GFR (improved) and growth velocity SDS (improved) were not reported separately for treatment groups.
5. Calcium carbonate versus aluminium hydroxide.
| Outcome | Mak 1985a | Salusky 1991 | Summary estimateb |
| Abnormal bone biopsy | 4 improved, 5 no change, 2 worse | 3/10 versus 6/7 | RR 0.35 (0.13 to 0.95) |
| Bone aluminium (mg/kg) | Not reported | 9 ± 9.5 versus 10 ± 13 | MD ‐1.00 (‐12.29 to 10.29) |
| PTH levels (mL‐eq/L) | Normalised with both treatments | 731 ± 1062c versus 918 ± 833d | MD ‐‐187.00 (‐1089.25 to 715.25) |
| ALP (IU/L) | Not reported | 259 ± 231 versus 328 ± 256 | MD 21.00 (‐216.62 to 258.62) |
| Hypercalcaemic patients | Not reported | 6/10 versus 2/7 | RD 0.31 (‐0.14 to 0.77) |
| GFR (mL/min/1.73 m2) | 21 (SE 4; beginning) versus 23 (SE 5; end) | Peritoneal dialysis patients | Not calculated |
| Height SDS | Not reported | ‐2.73 ± 1.55 versus ‐1.92 ± 1.33 (end) | MD ‐0.86 (‐2.44 to 0.52) |
| Growth velocity SDS for chronological age | ‐0.82 (SE 0.31) increased to +0.31 (SE 0.39) P < 0.01 | Not reported | Not calculated |
| Plasma aluminium (µ/dL) | 3.0 ± 2.1 versus 9.0 ± 4.5 | Not reported | Not calculated |
a Cross‐over study
b Summary estimate (RR, RD, MD) and 95% CI; cross‐over studies excluded
c Experimental intervention
d Comparative intervention
ALP ‐ alkaline phosphatase; GFR ‐ glomerular filtration rate; PTH ‐ parathyroid hormone; SDS ‐ standard deviation score
6.1. Analysis.
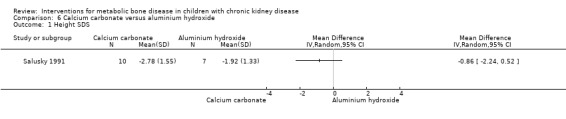
Comparison 6 Calcium carbonate versus aluminium hydroxide, Outcome 1 Height SDS.
6.2. Analysis.
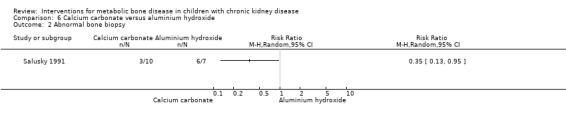
Comparison 6 Calcium carbonate versus aluminium hydroxide, Outcome 2 Abnormal bone biopsy.
6.3. Analysis.
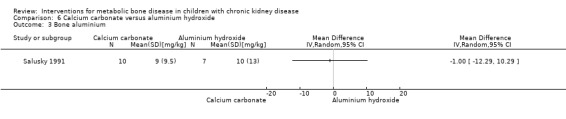
Comparison 6 Calcium carbonate versus aluminium hydroxide, Outcome 3 Bone aluminium.
6.4. Analysis.
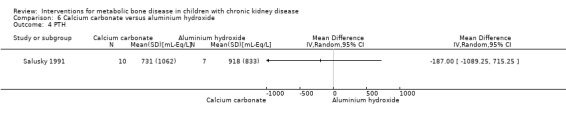
Comparison 6 Calcium carbonate versus aluminium hydroxide, Outcome 4 PTH.
6.5. Analysis.
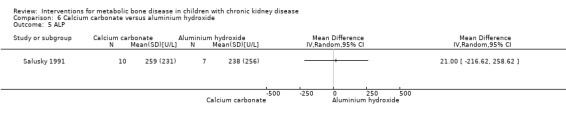
Comparison 6 Calcium carbonate versus aluminium hydroxide, Outcome 5 ALP.
6.6. Analysis.
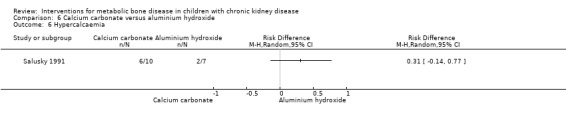
Comparison 6 Calcium carbonate versus aluminium hydroxide, Outcome 6 Hypercalcaemia.
Phosphate binders: sevelamer compared with calcium carbonate or calcium acetate
Two parallel group (Gulati 2010; Salusky 2005) and one cross‐over study (Pieper 2006) compared sevelamer with calcium carbonate or calcium acetate (Table 6). No study reported any patient‐centred outcomes. There were no significant differences in the final PTH levels (Analysis 7.1 ( 2 studies, 48 children): MD 51.92 pg/mL, 95% CI ‐77.53 to 181.36; I2 = 34%), final ALP levels (Analysis 7.2 (2 studies, 48 children): MD 90.48 IU/L, 95% CI ‐139.38 to 320.35; I2 = 30%), mean serum calcium‐phosphorus product (Analysis 7.3 (2 studies, 48 children): MD ‐1.12 mg2/dL2, 95% CI, ‐5.88 to 3.64; I2 = 0%), mean serum calcium levels (Analysis 7.4 (2 studies, 48 children): MD ‐0.40 mg/dL, 95% CI ‐1.16 to 0.36; I2 = 59%) or mean serum phosphorus levels (Analysis 7.5 (2 studies, 48 children): MD 0.17 mg/dL, 95% CI 0.37 to 0.71; I2 = 0%) between groups.
6. Sevelamer versus calcium carbonate or calcium acetate.
| Outcome | Gulati 2010 | Pieper 2006a | Salusky 2005 | Summary estimateb |
| PTH (pg/mL) | 165 ± 45 versus 144 ± 34 (final) | ‐7 ± 196 versus ‐43 ± 270 (change) | 562 ± 403c versus 369 ± 344d (final) | MD 51.92 (‐77.53 to 181.36) |
| ALP (U/L) | 1352 ± 500 versus1018 ± 557 (final) | 70 ± 108 versus 34 ± 127 (change) | 254 ± 155 versus 220 ± 157 (final) | MD 90.48 (‐139.38 to 320.35) |
| Hypercalcaemic patients | 0/10 versus 0/9 | 1/32 versus 6/30 periods of calcitriol | 5 episodes versus 22 episodes | Not calculated |
| Ca x P (mg2/dL2) | 51.2 ± 8.4 versus 49.4 ± 12.9 | ‐1.37 ± 1.4 versus ‐1.12 ± 1.25 (change) | 51 ± 7.4 versus 53 ± 7.5 | MD ‐1.12 (‐5.9 to 3.64) |
| Calcium (mg/dL) | 8.6 ± 1 versus 8.5 ± 1 | ‐0.2 ± 0.7 versus 0.4 ± 1.0 (change) | 8.9 ± 0.8 versus 9.6 ± 0.4 | MD ‐0.4 (‐1.16 to ‐0.36) |
| Phosphorus (mg/dL) | 6.2 ± 0.4 versus 5.8 ± 1.7 | ‐1.5 ± 1.6 versus ‐1.7 ± 1.7 (change) | 5.6 ± 1.2 versus 5.5 ± 0.4 | MD 0.17 (‐0.37 to 0.71) |
a Cross‐over study
b Summary estimate (RR, RD, MD) and 95% CI; cross‐over studies excluded
c Experimental intervention
d Comparative intervention
ALP ‐ alkaline phosphatase; Ca x P ‐ calcium‐phosphorus product; PTH ‐ parathyroid hormone
7.1. Analysis.
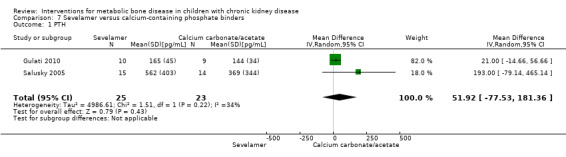
Comparison 7 Sevelamer versus calcium‐containing phosphate binders, Outcome 1 PTH.
7.2. Analysis.
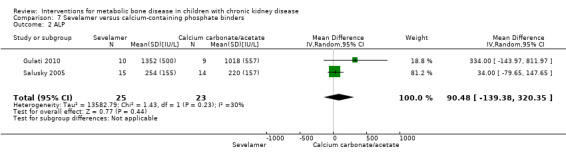
Comparison 7 Sevelamer versus calcium‐containing phosphate binders, Outcome 2 ALP.
7.3. Analysis.
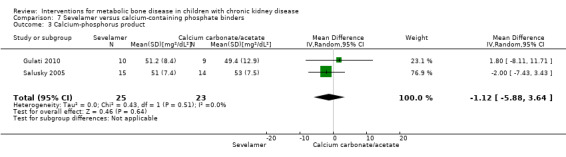
Comparison 7 Sevelamer versus calcium‐containing phosphate binders, Outcome 3 Calcium‐phosphorus product.
7.4. Analysis.
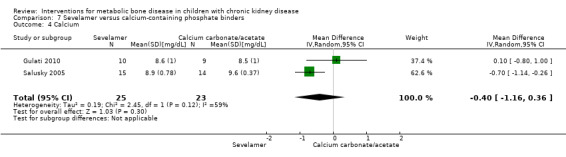
Comparison 7 Sevelamer versus calcium‐containing phosphate binders, Outcome 4 Calcium.
7.5. Analysis.
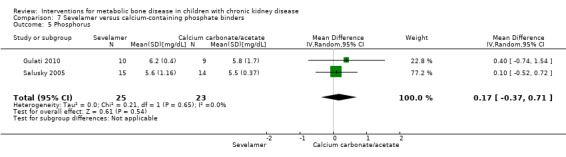
Comparison 7 Sevelamer versus calcium‐containing phosphate binders, Outcome 5 Phosphorus.
Salusky 2005 reported bone histology parameters of bone formation rates, % fall in bone formation rates, eroded perimeter, osteoid seam width, and bone area did not differ between treatments (Analysis 7.6). Osteoid area (Analysis 7.6.4 (1 study, 29 children); MD 4.20%, 95% CI 0.99 to 7.41) and osteoid perimeter (Analysis 7.6.5 (1 study, 29 children): MD 13.00%, 95% CI 3.81 to 22.19) were significantly higher in sevelamer treated children but the differences were of no clinical significance. In the cross‐over study by Pieper 2006 the change in PTH levels and serum ALP did not differ between groups. Similarly the change in mean serum calcium‐phosphorus product, serum calcium and serum phosphorus levels did not differ between therapy groups. Salusky 2005 reported 22 episodes of hypercalcaemia with calcium carbonate compared with five in children receiving sevelamer while Pieper 2006 reported six episodes of hypercalcaemia with calcium carbonate compared with one in children receiving sevelamer. No hypercalcaemic episodes were reported by Gulati 2010.
7.6. Analysis.
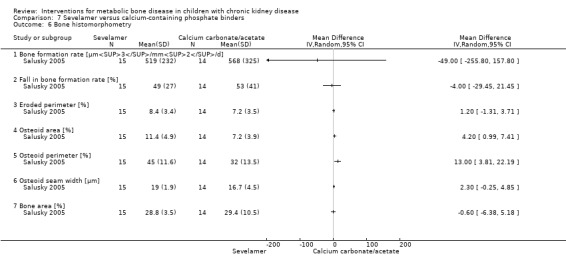
Comparison 7 Sevelamer versus calcium‐containing phosphate binders, Outcome 6 Bone histomorphometry.
Calcitriol versus doxercalciferol
Salusky 2005a and Salusky 2005b compared doxercalciferol plus calcium carbonate or sevelamer to calcitriol plus calcium carbonate or sevelamer (Table 4). Bone histology parameters of bone formation rate (Analysis 8.1.1 (51 children): MD 10.29 µm3/µm2/d, 95% CI ‐53.07 to 73.65), percentage eroded bone (Analysis 8.1.2 (51 children): MD 0.76%, 95% CI ‐6.19 to 7.71), percentage osteoid volume (Analysis 8.1.3 ( 51 children): MD ‐0.16%, 95% CI ‐9.16 to 8.83), percentage osteoid surface (Analysis 8.1.4 (51 children): MD 1.04%, 95% CI ‐29.63 to 31.71), osteoid maturation time (Analysis 8.1.5 (51 children): MD 0.82 days, 95% CI ‐14.41 to 16.05) and percentage bone volume (Analysis 8.1.6 (51 children): MD 2.19%, 95% CI ‐9.82 to 13.91) did not differ significantly between treatment groups. Final levels of PTH, calcium, phosphorus, serum alkaline phosphatase (ALP) and FGF23 did not differ between groups (data only shown graphically in study reports). Values of PTH and ALP fell significantly while values of FGF23 rose significantly with either vitamin D preparation. No differences in episodes of hypercalcaemia were seen between the two vitamin D therapies.
8.1. Analysis.
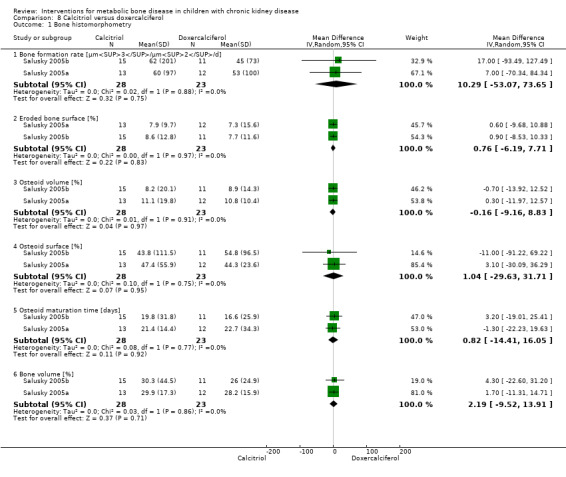
Comparison 8 Calcitriol versus doxercalciferol, Outcome 1 Bone histomorphometry.
Outcomes not reported
No studies reported fractures, bone deformities, need for parathyroidectomy, dialysis‐related events, symptoms related to hypercalcaemia or vascular/extra osseous calcification.
Discussion
Summary of main results
We were only able to identify 18 RCTs of all interventions used for CKD‐MBD in children over a period of 30 years. All identified studies examined phosphate binders or vitamin D sterols. No studies specifically examining dietary or surgical interventions, changes in dialysis prescription or calcimimetics were identified. Six studies of treatment of CKD‐MBD involved phosphate binders (aluminium hydroxide) or vitamin D sterols (ergocalciferol, dihydrotachysterol) or routes of administration (intraperitoneal) which are no longer used or are uncommonly used in current clinical practice except for the small doses of ergocalciferol recommended for children with CKD and low 25 hydroxyvitamin D levels (KDOQI 2009). There were few data to assist clinicians with the prevention of complications of renal bone disease since the only patient‐centred‐outcome reported was growth. This was reported in five studies (GFRD Study 1990; Hodson 1985; Jones 1994; Salusky 1991; Schmitt 2003) with no significant differences identified between treatments.
Treatment with calcitriol by both intraperitoneal and oral routes was effective in improving bone histology (Salusky 1998) but growth rates did not differ between routes (Jones 1994). The number of hypercalcaemic episodes did not differ between treatment routes although intraperitoneal calcitriol lowered PTH levels significantly more than oral calcitriol (Salusky 1998). However both treatments used intermittently and in high dose increased the number of children with adynamic bone disease (Salusky 1998). Intraperitoneal calcitriol is no longer recommended.
No differences in height SDS, PTH levels and frequency of hypercalcaemia were found between oral daily or oral intermittent calcitriol therapy (Ardissino 2000; Klaus 1995; Schmitt 2003). Oral intermittent therapy is no longer recommended.
Vitamin D sterols given orally or IV resulted in reduced PTH levels compared with placebo or no specific treatment. Hypercalcaemic episodes were more common with IV calcitriol in one study (Greenbaum 2005). Increased risk of hypercalcaemia was not reported with 1α‐hydroxyvitamin D or paricalcitol. Qualitative description of bone histology indicated improvement in children treated with vitamin D sterols (Eke 1983; Watson 1988).
No significant differences in growth rates (GFRD Study 1990; Hodson 1985) or bone histology (Salusky 2005a; Salusky 2005b) were detected in studies comparing different vitamin D sterols.
Two studies (Rianthavorn 2013; Shroff 2012) compared ergocalciferol in patients with CKD and vitamin D deficiency. Although there was no significant difference in the number of children, who developed secondary hyperparathyroidism, the development of secondary hyperparathyroidism was significantly delayed while calcium levels were significantly increased with ergocalciferol compared with placebo.
Overall we found that phosphate binders (aluminium hydroxide, calcium carbonate or acetate and sevelamer) had indistinguishable effects in lowering serum phosphorus, reducing PTH and on mean height SDS but that hypercalcaemia was more common with calcium‐containing binders (Gulati 2010; Mak 1985; Pieper 2006; Salusky 1991; Salusky 2005). One study suggested that bone histology remained abnormal less commonly in calcium carbonate treated children compared with those treated with aluminium hydroxide (Salusky 1991).
Overall completeness and applicability of evidence
There were significant gaps between the interventions and outcomes that we had planned to study in this systematic review and the available data. In particular, no study provided data on patient‐centred outcomes such as fractures, deformities and bone pain with only three studies providing numerical data on changes in height. The majority of studies only provided surrogate biochemical outcomes of PTH, serum ALP, calcium and phosphorus levels with a few early studies also reporting on radiological changes. We did not identify any studies, which considered non‐pharmacological or surgical interventions or any studies, which evaluated calcimimetic agents in children.
There were many limitations in the available data which precluded combining results across studies in meta‐analyses in many cases. Criteria for diagnosis of CKD‐MBD varied between studies. As well as variation in the interventions examined, there was variation on outcomes reported and in how the outcomes were measured. Many studies reported the point estimate of the results but not the SD or 95% CI. The cross‐over studies only presented the combined data for both arms, rather than each arm separately, and so could not be included in the meta‐analyses. Some inconsistencies in outcome reporting are inevitable when comparing studies published in different eras. Early studies tended to focus only on the incidence of hypercalcaemia as an adverse consequence of both vitamin D and phosphate binders whereas more recent studies included hyperphosphataemia and elevated serum calcium‐phosphorus product, since recognition of the adverse consequences of these parameters. Similarly, reporting of radiological abnormalities was a common outcome measure historically which has now largely been discarded. The relevance of certain outcome measures has changed over time. For example, measurements of plasma or bone aluminium levels, which were of relevance when aluminium hydroxide was used as a phosphate binder is no longer relevant.
Bone histomorphometry has been considered the reference standard to assess treatment efficacy in this setting, but only two of 18 included studies (Salusky 1998; Salusky 2005; Salusky 2005a; Salusky 2005b) provided adequate and comparable bone biopsy data making the value of bone histomorphometry in assessing treatment response difficult to assess in this systematic review. In addition, bone histomorphometry of trabecular bone does not reflect the effects of CKD on cortical bone. CKD reduces cortical bone volume and alters its architecture increasing the risk of fractures in long bones.
Though a surrogate measure, reduction in PTH levels is the most commonly used measure of efficacy of therapies in CKD‐MBD. However in the reported studies there was considerable variation in the way in which PTH levels were measured. PTH values were variably reported as end of study mean or median values, percentage fall in PTH, the number of children with a fall in PTH levels, the mean integrated PTH value, the mean change in PTH levels during the study and the number of children with two consecutive falls of ≥ 30% in PTH values. The potential for outcomes reporting bias is high when children are reported as having a successful outcome if their PTH value has fallen by an apparently arbitrary proportion at any time during the study period, rather than reporting whether the benefit was transient, or sustained, or what the primary outcome measure was. Also, the comparison of PTH values between studies is limited because different PTH assays have been used in different studies reflecting the variations in PTH assays over the past 30 years (Wesseling‐Perry 2013).
Comparisons of new therapeutic agents or a new method for their administration against placebo are of little clinical relevance if alternative agents are already recognised as successfully treating the disorder. Such a comparison was described in four studies. Two of these studies (Eke 1983; Watson 1988) were published in the 1980s when alternative successful treatments for renal bone disease were not confirmed. However two recently published studies reported that IV calcitriol (Greenbaum 2005) or IV paricalcitol (Greenbaum 2007) reduce PTH values more effectively than placebo. These results are not remarkable because it is generally agreed that vitamin D analogues are beneficial for biochemical abnormalities associated with CKD‐MBD. Of more relevance to the clinician would be knowing whether IV calcitriol or IV paricalcitol are associated with improvements in patient‐centred outcomes such as improved growth rates as well as fewer episodes of hypercalcaemia and reduction in PTH levels compared with oral calcitriol. In studies evaluating newer agents, or alternative modes of administration, it is important to compare a new agent, or its mode of administration with agents considered to represent the current standard of care using patient‐centred outcomes as the primary outcomes.
Quality of the evidence
Included studies were commonly reported incompletely and were of poor methodological quality, although this may reflect pre‐2001 CONSORT (Consolidated Standards of Reporting Trials) practices (www.consort‐statement.org). Sequence generation and allocation concealment was adequate in 12 and 11 of 18 studies respectively. Four studies reported blinding of participants, investigators or outcome assessors. All studies were considered at low risk of detection bias because they measured laboratory‐based outcomes unlikely to be influenced by lack of blinding. Seven studies reported loss of follow‐up or exclusion from data analysis (attrition bias) exceeding 10% and six studies were at high risk of selective reporting bias. Absence of allocation concealment, blinding and intention‐to‐treat analysis tends to lead to an over‐estimate of the observed treatment effects (Schulz 1995; Wood 2008). Many studies were too small to detect any differences between treatments even if differences did exist. Several studies provided outcome data qualitatively as normal or not statistically different without providing the numeric results. Although this under‐reporting of data was more common in the earlier studies, it was still evident in the most recent studies (Figure 3).
Studies included small numbers of patients. Few studies used the same interventions and/or reported outcomes in the same way so therefore they could not be combined in the meta‐analyses. Therefore there were insufficient data to create summary of findings tables.
Potential biases in the review process
Since the study was commenced, the literature search has been run several times up to September 2015 making it unlikely that any studies have been missed. However 40% of study reports in the Cochrane Kidney and Transplant Specialised Register have been identified by handsearching of conference proceedings so it remains possible that further studies of therapies for CKD‐MBD in children will be identified as conference proceedings from different congresses are searched.
The inability to include any data from cross‐over studies in meta‐analyses may have resulted in bias towards the results from parallel studies. However results from cross‐over studies have been included in the additional tables as well as being referred to in the text (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6).
Agreements and disagreements with other studies or reviews
Systematic reviews evaluating the use of vitamin D compounds in adults identified similar limitations to their review as we did (Palmer 2009a; Palmer 2009b). In particular few studies reported patient‐centred outcomes, few studies compared the newer vitamin D preparations with established ones and for each comparison there were limited numbers of studies and patients limiting the conclusions that could be drawn. While established vitamin D preparations (calcitriol,1α‐hydroxyvitamin D) were not demonstrated to reduce PTH levels significantly, there was considerable heterogeneity in the analyses. Newer vitamin D preparations including paricalcitol significantly reduced mean PTH levels. All vitamin D preparations increased the risk of hypercalcaemia compared with placebo. The authors concluded that the value of vitamin D therapy on important clinical outcomes in patients with CKD remains uncertain.
In a systematic review of nutritional vitamin D compounds of four RCTs (90 participants), which included both dialysis and non‐dialysis CKD patients, the PTH levels decreased significantly with vitamin D therapy (Kandula 2011).
Two systematic reviews (Navaneethan 2011; Tonelli 2007), comparing sevelamer with calcium‐containing phosphate binders, identified no differences between binders for all‐cause mortality or cardiovascular mortality. Following sevelamer treatment the risk of hypercalcaemia was reduced and serum calcium levels were lower. However serum phosphate levels were higher and levels of serum calcium‐phosphorus product did not differ. End of treatment PTH levels were significantly higher with sevelamer compared with calcium salts (Navaneethan 2011).
As in our review, the primary outcomes reported in these systematic reviews were surrogate biochemical markers rather than patient‐centred outcomes so that the clinical value of vitamin D compounds or non‐calcium‐containing phosphate binders in patients with CKD remains uncertain.
Authors' conclusions
Implications for practice.
In conclusion, this review confirms that renal bone disease, assessed by changes in PTH levels, is improved by all vitamin D preparations. However we do not know whether a reduction in PTH levels translates to a an improvement in clinical outcomes such as improved growth rate, reduction in fracture rates or reduced risk of cardiovascular calcification. No consistent differences between different routes of administration, different frequencies of dosing or different vitamin D preparations have been demonstrated in existing RCTs. Though fewer episodes of high serum calcium levels occurred with the non‐calcium‐containing binder, sevelamer, compared with calcium‐containing binders, both were effective in lowering serum phosphorus levels and there were no differences in serum phosphorus though calcium levels were lower in sevelamer treated children. Six existing studies evaluated agents that are no longer in general clinical use. Studies evaluating new agents, such as the phosphate binder lanthanum carbonate, new vitamin D preparations or calcimimetic agents, are required in children. However recently a sponsored study assessing efficacy and safety of cinacalcet in children with CKD and secondary hyperparathyroidism receiving dialysis was terminated by the US Food and Drug Administration because of adverse effects (NCT01277510).
Implications for research.
Existing RCTs provide limited data on the efficacy of interventions for the prevention and treatment of CKD‐MBD in children other than for surrogate biochemical outcomes so there remains considerable uncertainty about the benefits and harms of interventions. As newer vitamin D sterols, calcimimetic agents and phosphate binders are developed, head‐to‐head comparisons with the current standard therapies will be required in well‐designed adequately powered paediatric RCTs using standardised outcome measures including those of direct clinical relevance to children and their families such as growth, fractures, bone deformities and measures of bone health as well as surrogate biochemical markers.
Ideally efficacy studies should utilise an accurate non‐invasive quantitative assessment of bone health that includes assessment of both cortical and trabecular bone and correlates with patient‐centred outcomes such as fractures. Peripheral quantitative computed tomography may be more beneficial in determining fracture risk in kidney failure as it provides a more accurate estimate of volumetric bone mineral density (g/cm³) with improved differentiation between cortical and trabecular bone (Sanchez 2008). It is known kidney failure affects cortical bone more significantly than trabecular bone. Paediatric data is however limited and therefore these investigations are not established in the paediatric setting (Bacchetta 2011). MicroMRI could also be investigated as a marker of bone health.
The value of new surrogate markers such as serum FGF23 should also be evaluated (Wesseling‐Perry 2013).
What's new
| Date | Event | Description |
|---|---|---|
| 2 November 2015 | New citation required but conclusions have not changed | No change to conclusions |
| 2 November 2015 | New search has been performed | New search; 3 new studies included |
| 8 September 2015 | Amended | Search strategies revised |
Acknowledgements
We wish to than Denis Geary who contributed to the original concept and design, data extraction, analysis and interpretation, writing the manuscript
The authors wish to thank the following people.
Cochrane Kidney and Transplant for their help at all stages of this review
Dr Kate Wesseling‐Perry and Dr Pornpimol Rianthovorn who provided additional information about their studies
The referees for their comments and feedback during the preparation of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
| Was there adequate sequence generation? | Yes (low risk of bias): Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| No (high risk of bias): Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
| Was allocation adequately concealed? | Yes (low risk of bias): Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| No (high risk of bias): Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
| Was knowledge of the allocated interventions adequately prevented during the study? | Yes (low risk of bias): No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken; either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias. |
| No (high risk of bias): No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken; either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No' | |
| Were incomplete outcome data adequately addressed? | Yes (low risk of bias): No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| No (high risk of bias): Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. | |
| Are reports of the study free of suggestion of selective outcome reporting? | Yes (low risk of bias): The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| No (high risk of bias): Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. | |
| Was the study apparently free of other problems that could put it at a risk of bias? | Yes (low risk of bias): The study appears to be free of other sources of bias. |
| No (high risk of bias): Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to permit judgement of ‘Yes’ or ‘No'. |
Data and analyses
Comparison 1. Intraperitoneal versus oral calcitriol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone disease on bone histology | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Abnormal bone histology | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adynamic bone disease | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Osteitis fibrosa | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Mixed or mild bone disease | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Normal or reduced bone formation | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Bone formation rate | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 PTH | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Maximum calcium level | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 Calcium > 11 mg/dL | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Phosphate > 7.0 mg/dL | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Peritonitis episodes/patient‐months | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Intermittent versus daily oral calcitriol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in height SDS at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Fall in PTH | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 8 weeks | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 12 months | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number with fall in PTH at 8 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Mean integrated PTH at 12 months | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Kidney function | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Final CrCl at 8 weeks [mL/min/1.73 m2] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Change in CrCl at 12 months [mL/min/1.73 m2] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse events | 2 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Calcium > 11.5 mg/dL | 2 | 80 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.17, 0.13] |
| 6.2 Phosphorus > 7.5 mg/dL | 1 | 59 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
Comparison 3. Vitamin D preparations versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Abnormal bone histology | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Elevated PTH | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 PTH | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Number with 30% fall in PTH on two occasions | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 2.75 [1.39, 5.47] |
| 5 Change in PTH with IV calcitriol | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Hypercalcaemia | 4 | 103 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.08, 0.24] |
| 7 Calcium‐phosphorus product > 7.5 mg²/dL² | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Phosphorus > 6.5 mg/dL | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Change in biochemical values | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 Calcium [mg/dL] | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.45, 0.65] |
| 9.2 Calcium‐phosphorus product [mg2/dL2] | 2 | 76 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐7.94, 8.83] |
| 9.3 Phosphorus [mg/dL] | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.66, 0.63] |
| 9.4 Bone ALP [µg/L] | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐47.7 [‐88.54, ‐6.86] |
Comparison 4. Calcitriol versus ergocalciferol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Height velocity ≥ expected | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Improved bone histology | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 PTH | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Hypercalcaemia | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Biochemical values | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Calcium [mmol/L] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Phosphorus [mmol/L] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 ALP [U/L] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Ergocalciferol versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number developing hyperparathyroidism | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 PTH | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Calcium | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.28, 0.81] |
| 4 Phosphorus | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.96, 0.39] |
| 5 End 1.25(OH)D3 levels | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Calcium carbonate versus aluminium hydroxide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Height SDS | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Abnormal bone biopsy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Bone aluminium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 PTH | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 ALP | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Hypercalcaemia | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Sevelamer versus calcium‐containing phosphate binders.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PTH | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 51.92 [‐77.53, 181.36] |
| 2 ALP | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 90.48 [‐139.38, 320.35] |
| 3 Calcium‐phosphorus product | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐1.12 [‐5.88, 3.64] |
| 4 Calcium | 2 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.16, 0.36] |
| 5 Phosphorus | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.37, 0.71] |
| 6 Bone histomorphometry | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Bone formation rate [µm3/mm2/d] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Fall in bone formation rate [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Eroded perimeter [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Osteoid area [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Osteoid perimeter [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 Osteoid seam width [µm] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 Bone area [%] | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Calcitriol versus doxercalciferol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bone histomorphometry | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Bone formation rate [µm3/µm2/d] | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 10.29 [‐53.07, 73.65] |
| 1.2 Eroded bone surface [%] | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 0.76 [‐6.19, 7.71] |
| 1.3 Osteoid volume [%] | 2 | 51 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐9.16, 8.83] |
| 1.4 Osteoid surface [%] | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 1.04 [‐29.63, 31.71] |
| 1.5 Osteoid maturation time [days] | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐14.41, 16.05] |
| 1.6 Bone volume [%] | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐9.52, 13.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ardissino 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation stratified for PTH levels 70 to 399 ng/mL and > 399 ng/mL |
| Allocation concealment (selection bias) | Low risk | Central randomisation stratified for PTH levels 70 to 399 ng/mL and > 399 ng/mL |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. Lack of blinding could influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the full publication, all 59 reported patients completed study in full publication. However as abstract reports that 85 patients were enrolled in the study. |
| Selective reporting (reporting bias) | Low risk | Data available on PTH, GFR & hypercalcaemia for meta‐analysis |
| Other bias | Low risk | No evidence of other bias |
Eke 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be "double‐blind" study but no other information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Said to be double‐blind but no other information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are laboratory based and unlikely to be influenced by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient excluded when commenced RRT but this unlikely to influence results |
| Selective reporting (reporting bias) | High risk | No reporting of actual numbers for outcomes of GFR, PTH and other biochemistry |
| Other bias | Low risk | Leo Laboratories provided medications |
GFRD Study 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central sequence of random treatment assignments |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants received identical regimens by appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants received identical regimens by appearance. Primary outcome was growth. All investigators trained to do measurements. Laboratory results measured in central laboratory |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12 (13%) patients excluded after randomisation and unclear from which group |
| Selective reporting (reporting bias) | High risk | No outcomes reported in format that can be entered in meta‐analyses. No results of PTH levels provided |
| Other bias | Low risk | NIH and other grants. Medications provided by Hoffmann‐La Roche |
Greenbaum 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation stratified by age. Information obtained from authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled study using IV medications |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled study using IV medications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 (38%) did not complete study but data from these patients included in results. Data on primary outcome available in all patients |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes are included |
| Other bias | High risk | Supported by Abbott Laboratories |
Greenbaum 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and active medication given IV after dialysis |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo and active medication given IV after dialysis; Primary outcome was laboratory based |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 (41%) prematurely withdrawn (5 treatment; 10 placebo) but these patients included in evaluation of primary outcome |
| Selective reporting (reporting bias) | Low risk | All relevant laboratory outcomes are included |
| Other bias | High risk | Supported by Abbott Laboratories |
Gulati 2010.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Computer generated randomisation and opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding may influence management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open label but primary outcome was laboratory based |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 (14%) of 22 lost to follow‐up after randomisation |
| Selective reporting (reporting bias) | Low risk | All relevant laboratory outcomes and adverse events included |
| Other bias | Low risk | Emcure Pharmaceuticals Limited provided medication |
Hodson 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Systematic ranking design in blocks of 4 based on severity of bone histology changes |
| Allocation concealment (selection bias) | High risk | Investigators aware of blocks so could influence next entry |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No Blinding. Lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory based outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/24 (38%) excluded from final analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Jones 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment/control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Said to be random assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed study |
| Selective reporting (reporting bias) | High risk | No data available in form that can be included in meta‐analyses as cross over study |
| Other bias | High risk | Supported by Abbott Laboratories |
Klaus 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract only. "Patients were randomised to..." No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Abstract only. "Patients were randomised to..." No other information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Duration of treatment unclear so unclear whether any children left the study |
| Selective reporting (reporting bias) | High risk | No report on adverse effects. Biochemical data could not be included in meta‐analyses |
| Other bias | Unclear risk | No information provided |
Mak 1985.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment/control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | Patients said to be randomised |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients appear to be included |
| Selective reporting (reporting bias) | High risk | Only combined data provided for both treatment groups so no data available for meta‐analyses |
| Other bias | Low risk | National Medical Research Fund |
Pieper 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers with prior allocation to each centre |
| Allocation concealment (selection bias) | Low risk | Computer generated random numbers with prior allocation to each centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label study. Lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 18/40 (45%) completed both parts of the cross‐over study. 30 completed first part. Exclusions could have influenced overall result |
| Selective reporting (reporting bias) | High risk | Outcomes reported incompletely and cannot be included in meta‐analyses |
| Other bias | High risk | Chief investigator and study supported by Genzyme Europe |
Rianthavorn 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Simple randomisation, randomisation was sequentially done (information from authors) |
| Allocation concealment (selection bias) | High risk | Simple randomisation, randomisation was sequentially done (information from authors) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding could affect patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory outcome and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed 12 week therapy |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes mentioned |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Salusky 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Said to be randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Said to be randomly assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding could affect patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes are laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 withdrawn for transplant, i.e. 23% did not complete study |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | Grants from US Public Health Service and Department of Veterans Affairs |
Salusky 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 3 randomisation schedules according to bone histology. Blocks of 4, 6, 8 with block size determined at random |
| Allocation concealment (selection bias) | Low risk | Independent biostatistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13 (28%) of 46 did not complete study and unclear whether these excluded pre‐ or post‐randomisation |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | Supported by grants from USPHS & Casey Lee Ball Foundation |
Salusky 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
|
| Outcomes | Outcomes for comparison between phosphate binders
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised longitudinal factorial study. Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Computer generated, allocated by statistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, care givers, investigators not blinded to interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based and unlikely to be influenced by lack of blinding. Bone histology performed by scientist, who was blinded to treatment group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/42 (31%) did not complete study. Lack of data on these patients could have influenced results |
| Selective reporting (reporting bias) | High risk | Primary outcome was bone histology; ; secondary outcomes only reported graphically |
| Other bias | Low risk | USPH grants and Casey Lee Ball Foundation but Bone Care International provided medications and other unrestricted support for the study |
Salusky 2005a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 2
Treatment group 4
Co‐interventions
|
|
| Outcomes | Outcomes for comparison between vitamin D preparations
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised longitudinal factorial study. Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Computer generated, allocated by statistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, care givers, investigators not blinded to interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/60 (15%) did not complete study. Lack of data on these patients could have influenced results |
| Selective reporting (reporting bias) | High risk | Primary outcome was bone histology; secondary outcomes only reported graphically |
| Other bias | Low risk | USPH grants and Casey Lee Ball Foundation but Bone Care International provided medications and other unrestricted support for the study |
Salusky 2005b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 3
Co‐interventions
|
|
| Outcomes | Outcomes for comparison between vitamin D preparations
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised longitudinal factorial study. Computer generated randomisation |
| Allocation concealment (selection bias) | Low risk | Computer generated, allocated by statistician |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants, care givers, investigators not blinded to interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes laboratory based and unlikely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/60 (15%) did not complete study. Lack of data on these patients could have influenced results |
| Selective reporting (reporting bias) | High risk | Primary outcome was bone histology; secondary outcomes only reported graphically |
| Other bias | Low risk | USPH grants and Casey Lee Ball Foundation but Bone Care International provided medications and other unrestricted support for the study |
Schmitt 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised according to PTH levels |
| Allocation concealment (selection bias) | Low risk | Centrally randomised according to PTH levels |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | not blinded but lack of blinding unlikely to influence patient management |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/29 (17%) left study (RRT 4, rhGH 1). Could have influenced results |
| Selective reporting (reporting bias) | Low risk | Data reported on all expected outcomes |
| Other bias | Low risk | No evidence of other bias |
Shroff 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised 1:1 using blocks of 10. Random number generator |
| Allocation concealment (selection bias) | Low risk | Random number generator. Randomisation by trial pharmacist |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinician and participants blinded. Identical bottles and contents matched for colour, odour and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians/participants blinded until all data collected and evaluated. Outcomes were laboratory based |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All given medication completed the study |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Medications supplied by Specials Laboratory |
Watson 1988.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Randomly allocated by sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and lack of blinding could influence patient management |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory results and bone histology assessed by investigator unaware of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed study |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Other bias | High risk | Supported by Leo laboratories |
AKI ‐ acute kidney injury; ALP ‐ alkaline phosphatase; AKI ‐ acute kidney injury; BMD ‐ bone mineral density; Ca ‐ serum calcium; Ca x P ‐ calcium‐phosphorus product; CAPD ‐ continuous ambulatory peritoneal dialysis; CCPD ‐ continuous cycling peritoneal dialysis; CKD ‐ chronic kidney disease; CrCl ‐ creatinine clearance; DHT ‐ dihydrotachysterol; eGFR ‐ estimated glomerular filtration rate; HD ‐ haemodialysis; HVSDS ‐ height velocity standard deviation score; IP ‐ intraperitoneal; IV ‐ intravenous; K ‐ serum potassium; M/F ‐ male/female; PD ‐ peritoneal dialysis; PTH ‐ parathyroid hormone; SLE ‐ systemic lupus erythrocytosis; RCT ‐ randomised controlled trial; rhGH ‐ growth hormone; RRT ‐ renal replacement therapy; SDS ‐ standard deviation score
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ardissino 2000a | Examining calcium absorption only |
| Bettinelli 1986 | Ten children included and 5 ceased calcitriol for second 6 months of study. Unclear as to how patients were chosen for cessation of calcitriol |
| Choudhary 2014 | Ineligible population; children with nephrotic syndrome and normal kidney function |
| El Husseini 2004 | Examining osteopenia in transplant patients |
| Ferraris 2000 | Examining corticosteroids in kidney transplant patients |
| Kim 2006b | Wrong population |
| Witmer 1976 | Randomisation unclear |
Contributions of authors
Elisabeth M. Hodson: concept and design, data extraction, analysis and interpretation, writing the manuscript
Deirdre Hahn: data extraction, analysis and interpretation, writing the manuscript
Jonathan C. Craig: concept and design, interpretation of the data, writing the manuscript
Declarations of interest
Elisabeth Hodson: I was the lead author on a study published in 1985 (Hodson 1985), which was eligible for and included in this review. Data extraction was carried out independently by Dr Geary and myself and all data included in the review was agreed on by both authors.
Deirdre Hahn: none declared
Jonathan Craig: none declared
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ardissino 2000 {published data only}
- Ardissino G, Schmitt CP, Testa S, Claris‐Appiani A, Mehls O. Calcitriol pulse therapy is not more effective than daily calcitriol therapy in controlling secondary hyperparathyroidism in children with chronic renal failure. European Study Group on Vitamin D in Children with Renal Failure. Pediatric Nephrology 2000;14(7):664‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ardissino G, Schmitt CP, Testa S, Claris‐Appiani A, Mehls O, European Study Group on Vitamin D in Children with (Chronic) Renal Failure. Growth and PTH in children treated with daily vs intermittent oral calcitriol for secondary hyperparathyroidism. Preliminary results [abstract]. Journal of the American Society of Nephrology 1998;9(Program & Abstracts):562A. [CENTRAL: CN‐00444220] [Google Scholar]
Eke 1983 {published data only}
- Eke FU, Winterborn MH. Effect of 1a‐hydroxycholecalciferol on glomerular filtration rate in children with moderate renal failure [abstract]. Kidney International 1982;22(5):581. [CENTRAL: CN‐00583351] [Google Scholar]
- Eke FU, Winterborn MH. Effect of low dose 1 alpha‐hydroxycholecalciferol on glomerular filtration rate in moderate renal failure. Archives of Disease in Childhood 1983;58(10):810‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GFRD Study 1990 {published data only}
- Abitbol CL, Warady BA, Massie MD, Baluarte HJ, Fleischman LE, Geary DF, et al. Linear growth and anthropometric and nutritional measurements in children with mild to moderate renal insufficiency: a report of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S46‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Anonymous. Growth failure in children with renal diseases. A clinical trial in pediatric nephrology: Growth Failure in Renal Diseases (GFRD) Study. Journal of Pediatrics 1990;116(2):S1‐62. [MEDLINE: ] [PubMed] [Google Scholar]
- Boyle RM, Chinchilli VM, Shasky DA. Masking of physicians in the Growth Failure in Children with Renal Diseases Clinical Trial. Pediatric Nephrology 1993;7(2):204‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Boyle RM, Fortner CA, Chinchilli VM, Chan JC. Data coordination and management in the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S28‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Brouhard BH, Chan JC, Carter WH Jr. Final report of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1996;129(2):S1‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan JC, Boineau FG, Ruley EJ, Lum GM, Weiss RA, Waldo FB, et al. Descriptions of the participating centers and patient population in the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S24‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan JC, Greifer I, Boineau FG, Mendoza SA, McEnery PT, Strife CF, et al. Rationale of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S11‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan JC, McEnery PT, Brouhard BH, Chinchilli VM, Greifer I. History and organization of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S3‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan JC, McEnery PT, Chinchilli VM, Abitbol CL, Boineau FG, Friedman AL, et al. A prospective, double‐blind study of growth failure in children with chronic renal insufficiency and the effectiveness of treatment with calcitriol versus dihydrotachysterol. The Growth Failure in Children with Renal Diseases Investigators. Journal of Pediatrics 1994;124(4):520‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chan JC, McEnery PT, Chinchilli VM, Abitbol CL, Boineau FG, Friedman AL, et al. Growth failure renal diseases (GFRD) study: calcitriol (1, 25‐d) vs dihydrotachysterol (DHT) [abstract no: 11P]. Journal of the American Society of Nephrology 1992;3(3):280. [CENTRAL: CN‐00460522] [Google Scholar]
- Chan JC, McEnery PT, Chinchilli VM, Boyle RM, Massie MD, Jennings SS, et al. Protocol of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S17‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chinchilli VM, McEnery PT, Chan JC. Statistical methods and determination of sample size in the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S32‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Foreman JW, Abitbol CL, Trachtman H, Garin EH, Feld LG, Strife CF, et al. Nutritional intake in children with renal insufficiency: a report of the Growth Failure in Children with Renal Diseases Study. Journal of the American College of Nutrition 1996;15(6):579‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Friedman AL, Heiliczer JD, Gundberg CM, Mak RH, Boineau FG, McEnery PT, et al. Serum osteocalcin concentrations in children with chronic renal insufficiency who are not undergoing dialysis. Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S55‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hirschman GH, Striker GE, Vernier RL, Chesney RW, Holliday MA, Ingelfinger JR, et al. Growth Failure in Children with Renal Diseases Study: an overview from the National Institutes of Health and the Advisory Committee. Journal of Pediatrics 1990;116(2):S8‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Massie MD, Strife CF, Foreman JW, Chan JC. Quality control of the nutritional component of the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S40‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mendoza SA, Moore ES, Boyle FS, Wellons MD, Chan JC. Laboratory quality control for a multicenter clinical trial: a report from the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S37‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shasky DA, Coppedge SH, Boyle RM, Chan JC. Masking procedure, randomization and stratification, and compliance monitoring in the Growth Failure in Children with Renal Diseases Study. Journal of Pediatrics 1990;116(2):S22‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trachtman H, Chan JC, Boyle R, Farina D, Baluarte HJ, Chinchilli VM, et al. The relationship between calcium, phosphorus, and sodium intake, race, and blood pressure in children with renal insufficiency: a report of the Growth Failure in Children with Renal Diseases (GFRD) Study. Journal of the American Society of Nephrology 1995;6(1):126‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Trachtman H, Chan JC, Boyle R, Farina D, Baluarte J, Chinchilli VM, et al. Dietary calcium (C) and phosphorus (P) intake, nutritional status, and blood pressure (BP) in black vs. white children: report of GFRD clinical trial [abstract]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):259. [CENTRAL: CN‐00486184] [Google Scholar]
Greenbaum 2005 {published data only}
- Greenbaum LA, Grenda R, Qiu P, Restaino I, Wojtak A, Paredes A, et al. Intravenous calcitriol for treatment of hyperparathyroidism in children on hemodialysis. Pediatric Nephrology 2005;20(5):622‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salusky IB, Greenbaum L, Grenda R, Qiu P, Langman CB. IV calcitriol safely and effectively reduces iPTH levels in pediatric patients [abstract no: A4031]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):771A‐2AS. [CENTRAL: CN‐00765519] [Google Scholar]
Greenbaum 2007 {published data only}
- Greenbaum LA, Benador N, Goldstein SL, Paredes A, Melnick JZ, Mattingly S, et al. Intravenous paricalcitol for treatment of secondary hyperparathyroidism in children on hemodialysis. American Journal of Kidney Diseases 2007;49(6):814‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Kommala DR, Benador N, Goldstein S, Pardes A, Mattingly S, Amdahl M, et al. Paricalcitol (Zemplar®) injection for the treatment of secondary hyperparathyroidism in pediatric hemodialysis patients [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):199A. [CENTRAL: CN‐00583154] [Google Scholar]
Gulati 2010 {published data only}
- Gulati A, Sridhar V, Bose T, Hari P, Bagga A. Short‐term efficacy of sevelamer versus calcium acetate in patients with chronic kidney disease stage 3‐4. International Urology & Nephrology 2010;42(4):1055‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hodson 1985 {published data only}
- Hodson EM, Evans RA, Dunstan CR, Hills E, Wong SY, Rosenberg AR, et al. Treatment of childhood renal osteodystrophy with calcitriol or ergocalciferol. Clinical Nephrology 1985;24(4):192‐200. [MEDLINE: ] [PubMed] [Google Scholar]
Jones 1994 {published data only}
- Jones CL, Vieth R, Spino M, Ledermann S, Kooh SW, Balfe J, et al. Comparisons between oral and intraperitoneal 1,25‐dihydroxyvitamin D3 therapy in children treated with peritoneal dialysis. Clinical Nephrology 1994;42(1):44‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Klaus 1995 {published data only}
- Klaus G, Hinderer J, Lingens B, Keuth B, Querfield U, Mehls O. Comparison of intermittent and continuous (daily) oral calcitriol for treatment of renal hyperparathyroidism in children on dialysis [abstract]. Pediatric Nephrology 1995;9(6):C75. [CENTRAL: CN‐00550467] [Google Scholar]
Mak 1985 {published data only}
- Mak RH, Turner C, Thompson T, Powell H, Haycock GB, Chantler C. Suppression of secondary hyperparathyroidism in children with chronic renal failure by high dose phosphate binders: calcium carbonate versus aluminium hydroxide. British Medical Journal Clinical Research Ed 1985;291(6496):623‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pieper 2006 {published data only}
- Pieper AK, Haffner D, Hoppe B, Dittrich K, Offner G, Bonzel KE, et al. A randomized crossover trial comparing sevelamer with calcium acetate in children with CKD. American Journal of Kidney Diseases 2006;47(4):625‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pieper AK, Haffner D, Muller‐Honger R, Hoppe B, Plank C, Offner G, et al. Sevelamer vs. calcium acetate in children with chronic renal failure [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):681‐2. [CENTRAL: CN‐00447215] [Google Scholar]
Rianthavorn 2013 {published data only}
- Rianthavorn P, Boonyapapong P. Ergocalciferol decreases erythropoietin resistance in children with chronic kidney disease stage 5. Pediatric Nephrology 2013;28(8):1261‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salusky 1991 {published data only}
- Goodman WG, Coburn JW, Foley J, Salusky IB. Prospective assessment of aluminum retention in dialyzed patients given calcium carbonate or aluminum hydroxide [abstract]. Kidney International 1990;37(1):328. [CENTRAL: CN‐00626083] [Google Scholar]
- Salusky IB, Coburn JW, Foley J, Slatopolsky E, Goodman WG. Prospective trial of calcium carbonate versus aluminum hydroxide with oral calcitriol therapy in renal osteodystrophy [abstract]. Kidney International 1990;37(1):451. [Google Scholar]
- Salusky IB, Foley J, Goodman WG. Calcium carbonate versus aluminum hydroxide with oral calcitriol therapy in pediatric renal osteodystrophy. [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:416A.
- Salusky IB, Foley J, Nelson P, Goodman WG. Aluminum accumulation during treatment with aluminum hydroxide and dialysis in children and young adults with chronic renal disease. New England Journal of Medicine 1991;324(8):527‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salusky 1998 {published data only}
- Goodman WG, Belin T, Gales B, Juppner H, Sgre GV, Salusky IB. Skeletal response to intermittent oral or intraperitoneal calcitriol in patients with secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 1994;5(3):850. [CENTRAL: CN‐00626086] [Google Scholar]
- Goodman WG, Ramirez JA, Gales B, Segre GV, Salusky IB. Skeletal response to one year of intermittent calcitriol therapy in dialyzed children [abstract no: 23P]. Journal of the American Society of Nephrology 1992;3(3):672. [CENTRAL: CN‐00460833] [Google Scholar]
- Greenbaum LA, Goodman WG, Holloway M, Chon Y, Gales B, Salusky IB. A prospective evaluation of the peritonitis rates between oral & intraperitoneal calcitriol [abstract]. Journal of the American Society of Nephrology 1995;6(3):531. [CENTRAL: CN‐00484167] [Google Scholar]
- Holloway MS, Ramirez JA, Goodman WG, Salusky IB. Peritonitis rates in children on CCPD treated with intermittent calcitriol therapy: oral vs intraperitoneal [abstract]. Journal of the American Society of Nephrology 1992;3(3):412. [CENTRAL: CN‐00460953] [Google Scholar]
- Kuizon BD, Goodman WG, Juppner H, Boechat I, Nelson P, Gales B, et al. Diminished linear growth during intermittent calcitriol therapy in children undergoing CCPD. Kidney International 1998;53(1):205‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ramirez JA, Goodman WG, Gales B, Segre GV, Salusky IB. Intermittent oral vs intraperitoneal calcitriol therapy in children on CCPD [abstract]. Journal of the American Society of Nephrology 1992;3(3):676. [CENTRAL: CN‐00461558] [Google Scholar]
- Salusky I, Ramirez J, Belin T, Gales B, Juppner H, Segre G, et al. Pulse calcitriol therapy: a prospective randomized trial [abstract no: FC037]. Pediatric Nephrology 1995;9(6):C45. [CENTRAL: CN‐00583473] [Google Scholar]
- Salusky IB, Goodman WG, Kuizon BD. Consequences of intermittent calcitriol therapy in pediatric patients with secondary hyperparathyroidism. Peritoneal Dialysis International 1999;19 Suppl 2:S441‐4. [MEDLINE: ] [PubMed] [Google Scholar]
- Salusky IB, Goodman WG, Kuizon BD. Implications of intermittent calcitriol therapy on growth and secondary hyperparathyroidism. Pediatric Nephrology 2000;14(7):641‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salusky IB, Kuizon BD, Belin TR, Ramirez JA, Gales B, Segre GV, et al. Intermittent calcitriol therapy in secondary hyperparathyroidism: a comparison between oral and intraperitoneal administration. Kidney International 1998;54(3):907‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salusky IB, Ramirez J, Goodman WG. Present strategies for the management of renal osteodystrophy [abstract no: S‐15.3]. Pediatric Nephrology 1992;6(5):C68. [CENTRAL: CN‐00485705] [DOI] [PubMed] [Google Scholar]
- Salusky IB, Ramirez JA, Belin T, Gales B, Segre GV, Juppner H, et al. Pulse calcitriol therapy: a prospective randomized trial [abstract]. Journal of the American Society of Nephrology 1994;5(3):855. [Google Scholar]
Salusky 2005 {published data only}
- Hernandez J, Wesseling K, Jueppner H, Goodman WG, Gales B, Elashoff R, et al. Skeletal response to phosphate binders and intermittent oral vitamin D in dialyzed children with secondary hyperparathyroidism [abstract no: F‐FC044]. Journal of the American Society of Nephrology 2005;16:47A. [Google Scholar]
- Pereira RC, Hernandez JD, Wesseling K, Gales B, Jueppner HW, Salusky IB. Differential effect of doxercalciferol and calcitriol on skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO133]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740590] [Google Scholar]
- Salusky I, Jueppner H, Gales B, Elashoff R, Goodman WG. Skeletal response to the treatment of secondary hyperparathyroidism: a comparison between calcitriol and 1‐Alpha‐D2 [abstract no: F‐PO643]. Journal of the American Society of Nephrology 2003;14:202A. [Google Scholar]
- Salusky IB, Goodman WG, Elashoff R, Gales B, Shaney S, Jueppner H. Prospective comparison of calcium‐based binder versus sevelamer HCL on the control of the skeletal lesions of secondary hyperparathyroidism [abstract no: F‐PO989]. Journal of the American Society of Nephrology 2004;15:281A. [Google Scholar]
- Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, et al. Sevelamer controls parathyroid hormone‐induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. Journal of the American Society of Nephrology 2005;16(8):2501‐08. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salusky IB, Jueppner H, Gales B, Elashoff R, Goodman WG. A prospective assessment of sevelamer hydrochloride versus calcium carbonate in dialyzed children. [abstract no: F‐PO661]. Journal of the American Society of Nephrology 2003;14:206A. [Google Scholar]
- Salusky IB, Wesseling K, Hernandez J, Lavigne JR, Zahranik RJ, Gales B, et al. Comparison between first and second generation PTH assays as predictors of bone turnover during treatment of secondary hyperparathyroidism in dialyzed children [abstract no: SA‐PO833]. Journal of the American Society of Nephrology 2005;16:739A. [Google Scholar]
- Wesseling‐Perry K, Harkins GC, Wang HJ, Sahney S, Gales B, Elashoff RM, et al. Response of different PTH assays to therapy with sevelamer or CaCO3 and active vitamin D sterols. Pediatric Nephrology 2009;24(7):1355‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling‐Perry K, Jueppner H, Goodman WG, Gales B, Elasoff R, Wang H, et al. Similar predictive value of bone turnover by first and second generation PTH assays during treatment of secondary hyperparthyroidism in dialyzed children [abstract no: F‐PO980]. Journal of the American Society of Nephrology 2004;15(Oct):279A. [Google Scholar]
- Wesseling‐Perry K, Juppner H, Gales B, Wang H, Elashoff R, Goodman WG, et al. Effects of vitamin D on FGF‐23, PTH and the skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO132]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740591] [Google Scholar]
- Wesseling‐Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor‐23 in secondary hyperparathyroidism. Kidney International 2011;79(1):112‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salusky 2005a {published data only}
- Pereira RC, Hernandez JD, Wesseling K, Gales B, Jueppner HW, Salusky IB. Differential effect of doxercalciferol and calcitriol on skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO133]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740590] [Google Scholar]
- Salusky I, Jueppner H, Gales B, Elashoff R, Goodman WG. Skeletal response to the treatment of secondary hyperparathyroidism: a comparison between calcitriol and 1‐Alpha‐D2 [abstract no: F‐PO643]. Journal of the American Society of Nephrology 2003;14:202A. [Google Scholar]
- Salusky IB, Wesseling K, Hernandez J, Lavigne JR, Zahranik RJ, Gales B, et al. Comparison between first and second generation PTH assays as predictors of bone turnover during treatment of secondary hyperparathyroidism in dialyzed children [abstract no: SA‐PO833]. Journal of the American Society of Nephrology 2005;16:739A. [Google Scholar]
- Wesseling‐Perry K, Harkins GC, Wang HJ, Sahney S, Gales B, Elashoff RM, et al. Response of different PTH assays to therapy with sevelamer or CaCO3 and active vitamin D sterols. Pediatric Nephrology 2009;24(7):1355‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling‐Perry K, Jueppner H, Goodman WG, Gales B, Elasoff R, Wang H, et al. Similar predictive value of bone turnover by first and second generation PTH assays during treatment of secondary hyperparthyroidism in dialyzed children [abstract no: F‐PO980]. Journal of the American Society of Nephrology 2004;15(Oct):279A. [Google Scholar]
- Wesseling‐Perry K, Juppner H, Gales B, Wang H, Elashoff R, Goodman WG, et al. Effects of vitamin D on FGF‐23, PTH and the skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO132]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740591] [Google Scholar]
- Wesseling‐Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor‐23 in secondary hyperparathyroidism. Kidney International 2011;79(1):112‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salusky 2005b {published and unpublished data}
- Pereira RC, Hernandez JD, Wesseling K, Gales B, Jueppner HW, Salusky IB. Differential effect of doxercalciferol and calcitriol on skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO133]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740590] [Google Scholar]
- Salusky I, Jueppner H, Gales B, Elashoff R, Goodman WG. Skeletal response to the treatment of secondary hyperparathyroidism: a comparison between calcitriol and 1‐Alpha‐D2 [abstract no: F‐PO643]. Journal of the American Society of Nephrology 2003;14:202A. [Google Scholar]
- Salusky IB, Wesseling K, Hernandez J, Lavigne JR, Zahranik RJ, Gales B, et al. Comparison between first and second generation PTH assays as predictors of bone turnover during treatment of secondary hyperparathyroidism in dialyzed children [abstract no: SA‐PO833]. Journal of the American Society of Nephrology 2005;16:739A. [Google Scholar]
- Wesseling‐Perry K, Harkins GC, Wang HJ, Sahney S, Gales B, Elashoff RM, et al. Response of different PTH assays to therapy with sevelamer or CaCO3 and active vitamin D sterols. Pediatric Nephrology 2009;24(7):1355‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesseling‐Perry K, Jueppner H, Goodman WG, Gales B, Elasoff R, Wang H, et al. Similar predictive value of bone turnover by first and second generation PTH assays during treatment of secondary hyperparthyroidism in dialyzed children [abstract no: F‐PO980]. Journal of the American Society of Nephrology 2004;15(Oct):279A. [Google Scholar]
- Wesseling‐Perry K, Juppner H, Gales B, Wang H, Elashoff R, Goodman WG, et al. Effects of vitamin D on FGF‐23, PTH and the skeletal lesions of secondary hyperparathyroidism [abstract no: TH‐PO132]. Journal of the American Society of Nephrology 2006;17(Abstracts):134A. [CENTRAL: CN‐00740591] [Google Scholar]
- Wesseling‐Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor‐23 in secondary hyperparathyroidism. Kidney International 2011;79(1):112‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmitt 2003 {published data only}
- Schmitt CP, Ardissino G, Testa S, Claris‐Appiani A, Mehls O. Growth in children with chronic renal failure on intermittent versus daily calcitriol. Pediatric Nephrology 2003;18(5):440‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shroff 2012 {published data only}
- Shroff R, Wan M, Gullett A, Lederman S, Shute R, Knott C, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trial. Clinical Journal of The American Society of Nephrology: CJASN 2012;7(2):216‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, et al. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism‐a randomised, double‐blinded placebo controlled study [abstract no: PS1‐THU‐506]. Pediatric Nephrology 2011;26(9):1634. [DOI] [PubMed] [Google Scholar]
Watson 1988 {published data only}
- Watson AR, Kooh SW, Tam C, Reilly B, Farine M, Balfe JW. The prevention of renal osteodystrophy (ROD) in children under going CAPD: a prospective randomised trial using 1 a hydroxycholecalciferol (1a) [abstract]. Pediatric Nephrology 1987;1(1):C38. [CENTRAL: CN‐00448294] [Google Scholar]
- Watson AR, Kooh SW, Tam CS, Reilly BJ, Balfe JW, Vieth R. Renal osteodystrophy in children on CAPD: a prospective trial of 1‐alpha‐hydroxycholecalciferol therapy. Child Nephrology & Urology 1988;9(4):220‐7. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Ardissino 2000a {published data only}
- Ardissino G, Schmitt CP, Bianchi ML, Dacco V, Claris‐Appiani A, Mehls O. No difference in intestinal strontium absorption after oral or IV calcitriol in children with secondary hyperparathyroidism. The European Study Group on Vitamin D in Children with Renal Failure. Kidney International 2000;58(3):981‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ardissino G, Schmitt CP, Claris‐Appiani A, Bianchi ML, Dacco V, Mehls O, et al. Equal intestinal calcium absorption and greater PTH suppression with oral vs IV calcitriol bolus in children with secondary hyperparathyroidism [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):571A. [CENTRAL: CN‐00444219] [Google Scholar]
- Ardissino GL, Schmitt CP, Dacco V, Bianchi ML, Claris‐Appiani A, Mehls O, et al. Oral and IV calcitriol pulse are equally effective on control of serum PTH and in stimulating intestinal calcium absorption [abstract no: A2821]. Journal of the American Society of Nephrology 1996;7(9):1810. [CENTRAL: CN‐00615905] [Google Scholar]
- Schmitt C, Ardissino G, Bianchi M, Mehls O, European Study Group on Vit D in children with CRF. Higher intestinal calcium absorption with iv calcitriol compared to oral in children with 2º hyperparathyroidism (HPT) [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A115. [CENTRAL: CN‐00677748] [Google Scholar]
Bettinelli 1986 {published data only}
- Bettinelli A, Bianchi ML, Aimini E, Ortolani S, Soldati L, Edefonti A. Effects of 1,25‐dihydroxyvitamin‐D3 treatment on mineral balance in children with end stage renal disease undergoing chronic hemofiltration. Pediatric Research 1986;20(1):5‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Choudhary 2014 {published data only}
- Choudhary S, Agarwal I, Seshadri MS. Calcium and vitamin D for osteoprotection in children with new‐onset nephrotic syndrome treated with steroids: a prospective, randomized, controlled, interventional study. Pediatric Nephrology 2014;29(6):1025‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
El Husseini 2004 {published data only}
- Husseini A, El‐Agroudy A, El‐Sayed M, Sobh M, Ghoneim M. Treatment of bone loss in renal transplant children and adolescents [abstract]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):544. [CENTRAL: CN‐00445216] [Google Scholar]
- Husseini A, Elagroudy A, Elsayed M, Sobh M, Ghoneim M. Treatment of bone loss with vitamin D in live related kidney transplant children and adolescents: a prospective randomized study. 41st Congress of ERA‐EDTA; 2004 May 15‐18; Lisbon (Portugal). 2004:93. [CENTRAL: CN‐00509176]
- Husseini AA, Agroudy AE, Sayed MF, Sobh MA, Ghoneim MA. Treatment of osteopenia and osteoporosis in renal transplant children and adolescents. Pediatric Transplantation 2004;8(4):357‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- El‐Husseini AA, El‐Agroudy AE, El‐Sayed M, Sobh MA, Ghoneim MA. A prospective randomized study for the treatment of bone loss with vitamin D during kidney transplantation in children and adolescents. American Journal of Transplantation 2004;4(12):2052‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ferraris 2000 {published data only}
- Ferraris JR, Pasqualini T, Alonso G, Legal S, Sorroche P, Galich AM, et al. Effects of deflazacort vs. methylprednisone: a randomized study in kidney transplant patients. Pediatric Nephrology 2007;22(5):734‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferraris JR, Pasqualini T, Legal S, Sorroche P, Galich AM, Pennisi P, et al. Effect of deflazacort versus methylprednisone on growth, body composition, lipid profile, and bone mass after renal transplantation. The Deflazacort Study Group. Pediatric Nephrology 2000;14(7):682‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kim 2006b {published data only}
- Cho B, Kim S. Pamidronate therapy for preventing steroid‐induced bone loss in children receiving corticosteroid therapy [abstract no: F‐PO346]. Journal of the American Society of Nephrology 2004;15(Oct):142A. [CENTRAL: CN‐00583366] [Google Scholar]
- Kim SD, Cho BS. Pamidronate therapy for preventing steroid‐induced osteoporosis in children with nephropathy. Nephron 2006;102(3‐4):c81‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Witmer 1976 {published data only}
- Witmer G, Margolis A, Fontaine O, Fritsch J, Lenoir G, Broyer M, et al. Effects of 25‐ hydroxycholecalciferol on bone lesions of children with terminal renal failure. Kidney International 1976;10(5):395‐408. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Avila‐Diaz 2006
- Avila‐Diaz M, Matos M, Garcia‐Lopez E, Prado MD, Castro‐Vazquez F, Ventura MD, et al. Serum markers of low‐turnover bone disease in Mexican children with chronic kidney disease undergoing dialysis. Peritoneal Dialysis International 2006;26(1):78‐84. [MEDLINE: ] [PubMed] [Google Scholar]
Bacchetta 2011
- Bacchetta J, Boutroy S, Vilayphiou N, Ranchin B, Fouque‐Aubert A, Basmaison O, et al. Bone assessment in children with chronic kidney disease: data from two new bone imaging techniques in a single‐center pilot study. Pediatric Nephrology 2011;26(4):587‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bakkaloglu 2010
- Bakkaloglu SA, Wesseling‐Perry K, Pereira RC, Gales B, Wang HJ, Elashoff RM, et al. Value of the new bone classification system in pediatric renal osteodystrophy. Clinical Journal of The American Society of Nephrology: CJASN 2010;5(10):1860‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakkaloglu 2011
- Bakkaloglu SA, Borzych D, Ha IL, Serdaroglu E, Buscher R, Salas P, et al. Cardiac geometry in children receiving chronic peritoneal dialysis: findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(8):1926‐33. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blaszak 2005
- Blaszak RT, Mitsnefes MM, Ilyas M, Salman SD, Belcher SM, Brady DR. Hyperphosphatemia in children receiving peritoneal dialysis‐‐an educational program. Pediatric Nephrology 2005;20(7):967‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borzych 2010
- Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, et al. The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney International 2010;78(12):1295‐304. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodman 2000
- Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronary‐artery calcification in young adults with end‐stage renal disease who are undergoing dialysis. New England Journal of Medicine 2000;342(20):1478‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodson 1982
- Hodson EM, Evans RA, Dunstan CR, Hills EE, Shaw PF. Quantitative bone histology in children with chronic renal failure. Kidney International 1982;21(6):833‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hodson 2012
- Hodson EM, Willis NS, Craig JC. Growth hormone for children with chronic kidney disease. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003264.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandula 2011
- Kandula P, Dobre M, Schold JD, Schreiber MJ Jr, Mehrotra R, Naveethan SD. Vitamin D supplementation in chronic kidney disease: a systematic review and meta‐analysis of observational studies and randomized controlled trials. Clinical Journal of The American Society of Nephrology: CJASN 2011;6(1):50‐62. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
KDOQI 2005
- National Kidney Foundation. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Children With Chronic Kidney Disease. www2.kidney.org/professionals/KDOQI/guidelines_pedbone/ (accessed 7 October 2015).
KDOQI 2009
- KDOQI Work Group. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 Update. American Journal of Kidney Diseases 2009;53(3 Suppl 2):S1‐S123. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klaus 2006
- Klaus G, Watson A, Edefonti A, Fischbach M, Ronnholm K, Schaefer F, et al. Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatric Nephrology 2006;21(2):151‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moe 2006
- Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney International 2006;69(11):1945‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Navaneethan 2011
- Navaneethan SD, Palmer SC, Vecchio M, Craig JC, Elder GJ, Strippoli GF. Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD006023.pub2] [DOI] [PubMed] [Google Scholar]
NCT01277510
- NCT01277510. A randomized, double‐blind, placebo controlled study to assess the efficacy and safety of cinacalcet HCl in pediatric subjects with chronic kidney disease and secondary hyperparathyroidism receiving dialysis. clinicaltrials.gov/ct2/show/NCT01277510 (accessed 29 October 2015).
Palmer 2009a
- Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008175] [DOI] [PubMed] [Google Scholar]
Palmer 2009b
- Palmer SC, MacGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005633.pub2] [DOI] [PubMed] [Google Scholar]
Sanchez 2008
- Sanchez CP. Mineral metabolism and bone abnormalities in children with chronic renal failure. Reviews in Endocrine & Metabolic Disorders 2008;9(2):131‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Seikaly 2003
- Seikaly MG, Ho PL, Emmett L, Fine RN, Tejani A. Chronic renal insufficiency in children: the 2001 Annual Report of the NAPRTCS. Pediatric Nephrology 2003;18(8):796‐804. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tonelli 2007
- Tonelli M, Wiebe N, Culleton B, Lee H, Klarenbach S, Shrive F, et al. Systematic review of the clinical efficacy and safety of sevelamer in dialysis patients. Nephrology Dialysis Transplantation 2007;22(10):2856‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wesseling‐Perry 2012
- Wesseling‐Perry K, Pereira RC, Tseng CH, Elashoff R, Zaritsky JJ, Yadin O, et al. Early skeletal and biochemical alterations in pediatric chronic kidney disease. Clinical Journal of The American Society of Nephrology: CJASN 2012;7(1):146‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wesseling‐Perry 2013
- Wesseling‐Perry K. Bone disease in pediatric chronic kidney disease. Pediatric Nephrology 2013;28(4):569‐76. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Geary 2009
- Geary DF, Hodson EM, Craig JC. Interventions for bone disease in children with chronic kidney disease. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD008327] [DOI] [PubMed] [Google Scholar]


