Abstract
Minor and pseudo-cereals, which can grow with lower input and often produce specific nutrients compared to major cereal crops, are attracting worldwide attention. Since these crops generally have a large genetic diversity in a breeding population, rapid genetic improvement can be possible by the application of genomics-assisted breeding methods. In this review, we discuss studies related to biparental quantitative trait locus (QTL) mapping, genome-wide association study, and genomic selection for minor and pseudo-cereals. Especially, we focus on the current progress in a pseudo-cereal, buckwheat. Prospects for the practical utilization of genomics-assisted breeding in minor and pseudo-cereals are discussed including the issues to overcome especially for these crops.
Keywords: QTL analysis, genome-wide association study, marker-assisted selection, genomic selection, minor cereal, pseudocereal, buckwheat
Introduction
The world population is predicted to exceed 9.7 billion by 2050 (FAOSTAT 2018), and as a result, the global food crisis will be exacerbated over the next few decades. The crisis will also be worsened by the ongoing progression of global-scale climate change and the limitation of resources for agricultural production, such as water, fertilizer, and suitable land. Therefore, in order to circumvent negative consequences, multifaceted efforts that incorporate multiple strategies, such as the improvement of cultivation management technologies (e.g., smart farming systems) and the reduction of food loss during cultivation and distribution, will be needed.
The world’s current food production is mainly supported by a small number of major crops, and, thus is vulnerable to major threats, such as pests, diseases, and, now, climate change (Massawe et al. 2016). Therefore, the diversification of food crops, including underutilized crops, is an important strategy for distributing risk of major threats in food production and for stabilizing global food production. Importantly, some of the crops that are currently underutilized, like minor and pseudo-cereals, are also suitable for low-input agriculture and exhibit high resistance to a variety of environmental stressors (Zhang et al. 2018). Because minor and pseudo-cereals are at least as nutritious as major cereals and often produce special nutrients that are not produced by major cereal crop, they are also important in supporting the dietary health of human populations (Cernansky 2015, Cheng et al. 2017, Kaur et al. 2014, Mir et al. 2018, Nowak et al. 2016, Sytar et al. 2016, Vega-Gálvez et al. 2010). As a result, minor and pseudo-cereals are currently attracting the attention of various stakeholders and breeders (Chiurugwi et al. 2019, D’Amelia et al. 2018, Joshi et al. 2019, Upadhyaya and Vetriventhan 2018, Zurita-Silva et al. 2014).
However, because minor and pseudo-cereals have a short history of modern breeding, their yields are often lower than those of major cereals. For example, minor and pseudo-cereals often have characteristics, such as lodging and seed shattering, that cause low yield (Cheng et al. 2017, Hinterthuer 2017). Minor and pseudo-cereals also possess other disadvantages, such as the presence of antinutrients, poor digestibility, and low palatability, all of which greatly affect their utilization as food sources (Kaur et al. 2014, Zurita-Silva et al. 2014). Therefore, it is necessary to establish breeding programs to build upon the crops’ advantages and to compensate for their disadvantages. One of the most important means of addressing this is to improve the genetic quality of crop species through efficient breeding programs (Abberton et al. 2016, Tester and Langridge 2010).
During the last few decades, three major DNA marker-based methods aiming at efficient genomics-assisted breeding, namely biparental QTL mapping, genome-wide association studies (GWAS), and genomic selection (GS), have been developed and applied to the breeding of crops (Abberton et al. 2016, Bernardo 2016, Hickey et al. 2017). Indeed, both researchers and breeders of major crops have developed and used these methods as front runners for genomics-assisted breeding. However, recent advances in molecular genetic technology and the commoditization of such technologies have improved the availability of genomic methods, even for the breeding of minor crops, like minor and pseudo-cereals. Crucial disadvantages of minor and pseudo-cereals, such as lodging, seed shattering, and other characteristics affecting their utility, show their lack of refinement as domestic food crops. The success of genetic improvement depends on the efficient use of genetic resource. Major crops that have experienced intense modern breeding may be exposed to a loss of genetic diversity in a breeding population (Esquinas-Alcázar 2005, Fu 2015). On the other hand, breeding populations of minor crops are expected to retain genetic variation held in their founder populations due to their shorter history of modern breeding. Thus, it should be noted that both minor and pseudo-cereals are expected to have great potential to be improved through modern breeding utilizing genomics information (Armstead et al. 2009, Varshney et al. 2012).
Accordingly, the aim of the present paper was to review the progress achieved to date, in regards to the use of biparental QTL mapping, GWAS, and GS for the genomics-assisted breeding of minor and pseudo-cereals, and to discuss future prospects for the application of genomics-assisted breeding.
Biparental QTL mapping
One strategy, namely biparental QTL mapping, has become routinely performed in many crop species owing to the appearance of versatile DNA markers and the development of statistical models and methods (Kearsey and Farquhar 1998). Indeed, QTLs for a variety of traits have been reported for multiple crop species. In fact, Bernardo (2008) reported that 1,200 QTL mapping studies, which involved 10,000 QTLs and 12 major crop species, had been published, and the number of such studies has increased since then (Bernardo 2016).
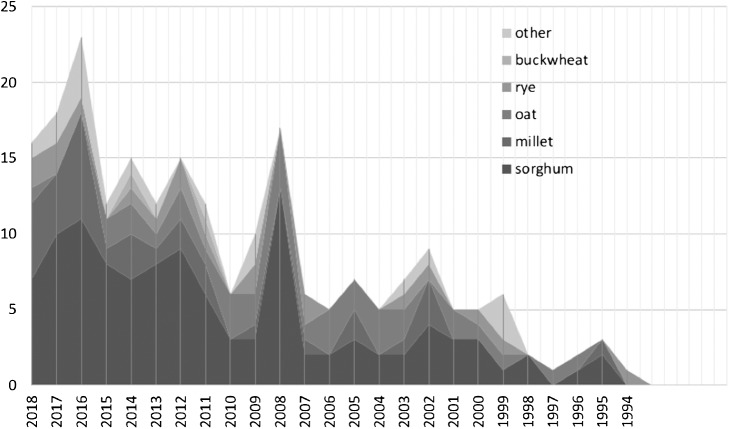
On December 26th, 2018, we searched the Web of Science database (http://www.webofknowledge.com/) for titles that contained terms “QTL”, “QTLs”, “quantitative trait locus”, “quantitative trait loci”, or “markers” + “associated”. The searching query was the same as that described by Bernardo (2008), except that we focused on different crop species (see below). As a result, 220 QTL mapping studies involving 12 words meaning minor and pseudo-cereal species, we identified: millet, spelt (Triticum spelta L.), emmer (Triticum turgidum L.), einkorn (Triticum monococcum L.), sorghum (Sorghum bicolor L. Moench), quinoa (Chenopodium quinoa Willd), amaranth (Amaranthus L.), buckwheat (common buckwheat (Fagopyrum esculentum Moench) and tartary buckwheat (Fagopyrum tataricum Gaertn.), chia (Salvia hispanica L.), oat (Avena sativa L.), rye (Secale cereale L.), and teff [Eragrostis tef (Zuccagni) Trotter]. Although the number is far smaller than that reported for major crops, the number of the studies published per year has been increasing steadily, especially during the last decade (Fig. 1).
Fig. 1.
Number of papers published about the QTL analysis of minor and pseudo-cereals. The Web of Science database (http://www.webofknowledge.com/) was searched for studies about millets, spelt wheat, emmer wheat, einkorn wheat, sorghum, quinoa, amaranth, buckwheat, chia, oat, rye, and teff, as described in the ‘Biparental QTL mapping’ section.
Most of the QTL mapping studies of minor and pseudo-cereals have focused on sorghum, which is the forth largest calorie-supplying cereals in the world (FAOSTAT 2013). Interestingly, because sorghum is important both as a food and feed crop and as a bioenergy crop, QTL mapping studies of sorghum increased most dramatically after the late 2000s (e.g., Murray et al. 2008). In addition, the development of QTL mapping in sorghum may have been easier than that in other minor and pseudo-cereals because the researchers could use DNA markers developed for other species, such as maize (Zea mays L.) (e.g., Hulbert et al. 1990, Lin et al. 1995). This was a great advantage in the 1990s, when marker development was costly, and the application of markers from other species helped to identify orthologous QTLs. Another advantage of sorghum as a target of QTL mapping is its autogamous reproductive system, which has facilitated the development of experimental populations, such as recombinant inbred lines (RILs) and near-isogenic lines (NILs) (e.g., Harris et al. 2007, Sanchez et al. 2002). Recently, a nested association mapping (NAM) population, which includes 2,214 RILs derived from 10 diverse global lines crossed with an elite reference line, was developed (Bouchet et al. 2017) and is being used by the sorghum research community.
Studies involving the QTL mapping of other minor and pseudo-cereals, however, are relatively limited and mostly include studies in rye (Erath et al. 2016, 2017, Miedaner et al. 2018, Myśków et al. 2018, Wang et al. 2015), oat (Admassu-Yimer et al. 2019, Babiker et al. 2015, Pellizzaro et al. 2016, Schneider et al. 2015, Sunstrum et al. 2018, Zimmer et al. 2018), and millet. Of the various types of millet, pearl millet (Ambawat et al. 2016, Aparna et al. 2015, Kumar et al. 2016b, 2017, 2018, Pucher et al. 2018, Punnuri et al. 2016, Taunk et al. 2018) and foxtail millet (Fang et al. 2016, Jia et al. 2017, Mauro-Herrera and Doust 2016, Ni et al. 2017, Odonkor et al. 2018, Wang et al. 2017a, 2017b, 2019) have received the majority of research attention, although one study also focused on proso millet (Rajput et al. 2016). Notably, very few QTL mapping studies have focused on pseudocereals. For wheat’s close relatives, triticale (a hybrid of wheat and rye) and progeny populations derived from crossing wheat and its wild relatives have been analyzed with QTL mapping (Dhariwal et al. 2018, Kalih et al. 2015, Miedaner et al. 2016, Wen et al. 2018). For example, emmer wheat has been used as a donner of disease resistance, drought resistance, productivity, and grain nutrient to durum wheat (Triticum turgidum ssp. durum) and bread wheat (Merchunk-Ovnat et al. 2016a, 2016b, Zhang et al. 2014).
In terms of materials and methods, RILs are most commonly used for QTL mapping, followed by F2 populations. Therefore, even in minor cereals, RIL populations have been developed for QTL mapping and are shared among researchers. In triticale, a doubled haploid (DH) population have been created and used (Dhariwal et al. 2018, Kalih et al. 2015, Miedaner et al. 2016). In rye, a three-way cross progeny of two F3:4 populations crossed to a common cytoplasmic male sterility (CMS) tester was developed (Miedaner et al. 2012) and used in several studies (e.g., Miedaner et al. 2018, Wang et al. 2015). This is because rye is commonly improved by hybrid breeding based on a CMS system (Miedaner et al. 2012).
In contrast, there are few reports of shared materials for the QTL mapping of pseudocereals. This is partly due to the fact that the major pseudocereals, such as common buckwheat, quinoa, and amaranth, are all allogamous. In common buckwheat, an inbred population (F4) have been derived from two interspecific hybrids between common buckwheat Fagopyrum esculentum Moench and its autogamous wild relative Fagopyrum homotropicum (Hara et al. 2011). The development of immortal populations, such as RILs and DH populations, will facilitate collaborative phenotyping and the aggregation of phenotype data from multiple institutions.
Among the strategies used for QTL mapping, the use of single nucleotide polymorphism (SNP) markers is the most common. Such markers can be investigated using either DNA array technologies (e.g., Babiker et al. 2015, Dhariwal et al. 2018, Erath et al. 2017, Schneider et al. 2015) or next-generation sequencing (NGS) technologies, such as genotyping-by-sequencing (GBS; Elshire et al. 2011) and restriction site-associated DNA sequencing (RAD-Seq; Baird et al. 2008, Miller et al. 2007), (e.g., Punnuri et al. 2016, Rajput et al. 2016, Sunstrum et al. 2018, Wang et al. 2017a, Wen et al. 2018). Not a few studies have used simple sequence repeats (SSRs) as markers (e.g., Aparna et al. 2015, Fang et al. 2016, Taunk et al. 2018).
Furthermore, QTL mapping has been used to investigate a variety of target traits. Studies frequently focused on biotic and abiotic stress resistance, including disease resistance, drought tolerance, frost tolerance, aluminum tolerance, etc. (e.g., Aparna et al. 2015, Erath et al. 2017, Schneider et al. 2015, Sunstrum et al. 2018), as well as a variety of general agronomical and morphological traits. QTL mapping has also been performed in minor cereals to identify loci associated with nutrient content (e.g., Kumar et al. 2018). Many traits, such as disease resistance and seed shattering, are often controlled by primary genes, so the strategy of identifying QTLs and performing subsequent marker-assisted selection (MAS) is promising for the improvement of crop traits.
The major disadvantage of using QTL mapping in minor and pseudo-cereals is that experimental populations are needed. Indeed, when compared to the resources available for major cereals, relatively few experimental populations of minor and pseudo-cereals have been developed, and even though genotyping of materials in populations is no longer a bottleneck, the construction of experimental populations will require both time and money. It is worth noting that other strategies, such as GWAS, do not require the construction of experimental populations. However, QTL mapping is still superior in its estimation of allele location and effect, especially for low-frequency alleles (Bernardo 2016). Alleles that are useful but underutilized in breeding history may be rare in a population of breeding materials or a population of germplasm collections.
Genome-wide association studies
More recently, the advent of NGS technology encouraged researchers to conduct GWAS. The purpose of GWAS is to detect markers associated with target traits from a huge number of genome-wide markers. GWAS can be performed using natural genetic resources or breeding populations because the use of high-density marker sets permits the utilization of linkage disequilibrium (LD) between QTLs and markers without creating linkage blocks, as in biparental QTL mapping. Therefore, GWAS can capture a substantial proportion of the variation found in a species-wide population (Hamblin et al. 2011).
Another advantage of GWAS is that they could solve some of the disadvantages of biparental QTL analysis. For example, GWAS does not require the construction of mapping populations, thereby shortening the time necessary for analysis. Because mapping populations of less-studied minor or pseudo-cereals would sometimes need to be constructed from scratch, the possibility of using natural or breeding populations is a major advantage. Another disadvantage of QTL analysis is the bias in detectable QTLs, which depend on parents used to generate the mapping population, potentially identifying useless markers or QTLs in the target breeding population. For example, when one of the QTL mapping parents is even a wildtype of the target crop species (i.e., Garvin et al. 2009, Keller et al. 1999a, 1999b), the detected QTLs may not be useful in some cases, e.g., when an allele is fixed in the cultivated breeding population. In contrast, GWAS is not biased by the selection of parents. For the genomics-assisted breeding of minor or pseudo-cereals, in which the use of the breeding populations’ high heterogeneity is expected, GWAS may be a more powerful method than biparental QTL mapping.
To date, GWAS has been conducted for many members of the Poaceae, even minor ones. Morris et al. (2013a), for example, conducted a GWAS of plant height components and inflorescence architecture in sorghum. The authors used 971 worldwide accessions and characterized 265,487 SNPs using genotyping-by-sequencing (GBS), based on a previously published reference genome (Paterson et al. 2009). Morris et al. (2013a), thereby, demonstrated the usefulness of NGS technology by obtaining hundreds of thousands of DNA markers, with some GWAS peaks even located in or near candidate genes, which were homologous to genes known in maize, rice, or Arabidopsis.
The usefulness of the GWAS approach has also been demonstrated in other Poaceae minor cereals. Jia et al. (2013) conducted GWAS of 916 diverse varieties of foxtail millet and 2.58 million SNPs. Upadhyaya et al. (2015) also conducted GWAS using 190 foxtail millet germplasm accessions. In addition, Sharma et al. (2018) performed GWAS of finger millet (Eleusine coracana L.) using SNP markers developed by Kumar et al. (2016a). Varshney et al. (2017) successfully constructed a reference genome, and then conducted GWAS and genomic prediction in pearl millet. These reports demonstrate the ability of GWAS to detect QTLs in species-wide sample populations and encourage the use of such genomics-assisted breeding for less-studied or underutilized crops even from scratch (e.g., from constructing reference genome).
Recently, GWAS of minor cereals have focused on practical traits, whereas earlier studies tended to focus on the evaluation and potential of using GWAS in each target species. Disease resistance is one of the most commonly targeted traits for GWAS of oat (Montilla-Bascón et al. 2015), emmer wheat (Liu et al. 2017), and sorghum (Prom et al. 2019). To utilize the rich nutrients of minor cereals (e.g., reviewed by Muthamilarasan et al. (2016)), several GWAS results for grain ingredients have been reported in sorghum (Rhodes et al. 2014), oat (Newell et al. 2012), and foxtail millet (Jaiswal et al. 2019). It is likely that GWAS will be suitable to improve traits relating to the advantages of individual crops, especially for minor crops, by immediately contribute to MAS.
It is important to note that several aspects are crucial for conducting GWAS efficiently. The biggest concern may be the strong population structure (i.e., the samples are randomly selected from a homogeneous population, or not), which is known to disturb GWAS and should be adjusted (Patterson et al. 2006, Wen et al. 2014, Yu et al. 2006). This problem occurs especially when using a large number of genotypes as the experimental population or when the population harbors high levels of genetic diversity. Morris et al. (2013a), for example, found that their global sorghum accessions exhibited strong population structure, according to both morphological type and geographic origin. Additionally, evaluation of the GWAS accuracy for a known sorghum gene Tannin1 revealed difficulty in treating strong population structure (Morris et al. 2013b). Strong population structure has also been reported in foxtail millet (Jia et al. 2013). However, Newell et al. (2011) analyzed the LD and population structure of an oat population to evaluate the feasibility of GWAS and found relatively weak population structure, despite high diversity. In GWAS of plant species, especially for species with large genetic diversity, the existence of strong population structure sometimes worries researchers. It is recommended that researchers evaluate the population structure of candidate populations and select GWAS methods that are optimal for the situation.
Several methods have been proposed for circumventing the effect of population structure in GWAS. Using principal components or eigenanalysis (implemented in the R package rrBLUP, one of the familiar GWAS tools; Endelman 2011, Endelman and Jannink 2012) is one of the typical ways. On the other hand, when the total number of genotypes in an experimental population is quite large, it may also be possible to conduct separate GWAS (Crowell et al. 2016). Bayesian methods or other variable selection methods that are used for genomic prediction can have a possibility of GWAS with circumventing the effect of population structure. For the application of GWAS to genomics-assisted breeding, the analysis of population genetics and choice of optimal method would work be key to identifying truly useful QTLs.
Another disadvantage of GWAS is that rare and low-frequency alleles can sometimes have relatively large effects on target traits (Manolio et al. 2009). Indeed in the field of human genetics, researchers have tried to solve this issue by collecting data from hundreds of thousands of people (e.g., Marouli et al. 2017). In GWAS of plant species, however, collecting and evaluating such a large number of genotypes may be impossible. Recently developed high-throughput phenotyping systems will help us collect phenotype data from large sample populations (e.g., Spindel et al. 2018). For example, it is reported that image analysis can be used for phenotyping in GWAS of sorghum (Zhou et al. 2019). If the problems of size of experimental field, cost, and phenotyping can be solved, the collection and utilization of genotypes that encompass the diversity of the population or species is a key factor in GWAS, in order to incorporate minor allele into the population. Therefore, the collaboration of researchers, gene banks, and breeders will be necessary.
The use of GWAS is expected to promote the genomics-assisted breeding of less-studied minor and pseudo-cereals. However, it is important to note that GWAS can only detect major genes, as in QTL analysis. MAS may be one strategy for using the QTL information produced by GWAS, as mentioned in the section of biparental QTL mapping. Spindel et al. (2016) used markers detected by GWAS as fixed effects in a genomic prediction model for rice, which may be another useful strategy.
Genomic selection
Meuwissen et al. (2001) proposed the idea of GS for accelerating the genetic improvement of quantitative traits controlled by a number of genes. When using this strategy, a prediction model is built based on the relationship between phenotypic values and genome-wide marker genotypes in a training population and then used to calculate the genotypic values of selection candidates. GS models utilize the LD between QTLs and high-density markers, as in GWAS. Because the purpose of GS is to predict the breeding values of selection candidates, causal loci are not necessarily identified (Hamblin et al. 2011). By combining GS with accelerating generations using off-season nursery, like conventional MAS based on QTL mapping, breeders can conduct more than one cycle of GS per cycle of phenotypic selection, thereby achieving high-speed breeding.
The advantage of GS over QTL-based MAS is the involvement of a large number of QTLs with small effects at the selection step (Hayes et al. 2013, Heffner et al. 2010). In biparental QTL analysis and GWAS, only major genes can be detected, so it is difficult to improve the traits controlled by a number of genes. In addition, GS could potentially solve some of the other disadvantages of MAS based on known QTLs, such as the overestimation of QTL effects (Strauss et al. 1992) and disagreement of effective QTLs between mapping and breeding population (Hoeschele and VanRaden 1993, Lande and Thompson 1990, Melchinger et al. 1998). This also mentioned in the ‘Genome-wide association studies’ section as the bias of detectable QTLs in biparental QTL mapping. Because of the advantages of GS over the conventional MAS, GS is a promising alternative for improving the efficiency of plant breeding for both major and minor crops.
In addition, GS should work especially well for underutilized minor crops with high genetic diversity in a breeding population. Indeed, GS requires neither marker position information nor QTL information. Therefore, GS can even be used for less-studied minor or orphan crops if genotyping and phenotyping systems are built. Moreover, the genetic diversity harbored by breeding populations of minor crops is ideal for GS, which can include a number of QTLs with small effects. Together, both the possibility of immediate application and the high genetic diversity of breeding population give reason to apply GS to minor and pseudo-cereals.
Several attempts for performing GS in minor and pseudo-cereals have been reported. Previous studies have reported empirical GS for the selection of β-glucan concentrations in oat (Asoro et al. 2013). Asoro et al. (2013) conducted GS and compared its efficiency with phenotypic selection based on BLUP (Henderson 1984) and MAS based on GWAS in the GS training population, in which the advantage of GS (i.e., acceleration of breeding cycles and avoiding bias in detectable QTLs) were not hold. Even though they did not demonstrate the advantage of GS over other methods in the level of genotypic improvement, they suggested that greater genetic variation was maintained by GS than by BLUP-based phenotypic selection by accounting for Mendelian segregation and thus avoiding the selection of related individuals. In the ‘Genomics-assisted breeding of buckwheat’ section, we will present studies relating to GS breeding in common buckwheat, as an example of successful GS in an allogamous pseudo-cereal (Yabe et al. 2018).
To conduct GS breeding, breeding strategy must be optimized. GS breeding includes marker genotyping and generally the acceleration of generations, thereby requiring more cost and labor than ordinary phenotypic selection. Especially when the GS of minor or pseudo-cereals is performed from scratch, strategy optimization is crucial. Several studies have addressed GS breeding in minor cereals. In many cases, prediction accuracy has been evaluated to determine the suitability of using GS, optimal prediction method, optimal training population set, and necessary number of DNA markers (e.g., in rye (Auinger et al. 2016, Bernal-Vasquez et al. 2014, 2017, Wang et al. 2014), oat (Asoro et al. 2011), pearl millet (Liang et al. 2018) and sorghum (Fernandes et al. 2018, Hunt et al. 2018)). To optimize breeding strategy for several members of the Poaceae, both the prediction accuracy and estimated response to selection have been calculated (Marulanda et al. 2016). On the other hand, Yabe et al. (2013, 2014b) conducted simulation studies to optimize breeding strategy for the GS breeding of common buckwheat. Such preparation for actual breeding programs may enable researchers to utilize the genetic variation of breeding populations more efficiently and is necessary for GS programs, especially in minor crop populations with high genetic variation.
Recently developed genotyping systems can help us perform GS breeding for minor and pseudo-cereals, for which the allowable cost or scale of breeding is sometimes limited. The utilization of high-throughput phenotyping technology, such as unmanned aerial vehicles (UAVs), could also be used for genomic prediction modeling, even in large-scale of the field of sorghum plants (Watanabe et al. 2017). The rapidly developing technology will also help GS breeding in minor cereals with great diversity in a large field.
Genomics-assisted breeding of buckwheat
It is likely that genomics-assisted breeding will be used to help meet the growing demand for minor and pseudo-cereals, especially for underutilized pseudo-cereals with high genetic diversity in breeding populations. However, the situation is more serious for pseudo-cereals than minor graminaceous cereals, owing to the difficulty of applying genetic information or markers from major graminaceous cereals, as indicated by the small number of studies that have reported performing genetic analysis in pseudo-cereals. The goal of this section is to summarize previous reports of genetic analysis and genomics-assisted breeding in common buckwheat, as an example of an allogamous pseudo-cereal.
Common buckwheat is an annual crop that is grown widely in temperate zones and that is used in both of bakery products (e.g., bread, biscuits, and noodles) and non-bakery products (e.g., honey and tea), as a great source of nutrients (Giménez-Bastida et al. 2015). In addition, the perception of common buckwheat as a healthy and functional food is growing (Giménez-Bastida and Zieliñski 2015), and efforts have been made to incorporate buckwheat’s beneficial nutrients into our everyday eating habits (e.g., Baljeet et al. 2010).
The breeding of common buckwheat, however, has had a difficult history. Most types of common buckwheat are characterized by complete outcrossing, owing to heteromorphic self-incompatibility (Campbell 1997) controlled by the S-locus (Lewis and Jones 1992), and as in breeding programs of other cross-pollinated species, mass selection for population improvement is performed conventionally, in which the lack of pollen control and inbreeding depression hinder efficient genetic improvement (Acquaah 2009). The difficulty of evaluating single plants reduces the efficiency of mass selection, as well (Yano et al. 2002). Therefore, genomics-assisted breeding is expected to provide more rapid results than conventional phenotypic selection methods, especially for crops like common buckwheat, for which selection has been hindered by the disadvantages of phenotype-based mass selection.
Biparental QTL mapping has also been reported in common buckwheat, for several growth and morphological traits. However, common buckwheat’s allogamous reproductive system works as a barrier because the allogamous reproductive system has hindered the development of a suitable mapping population (e.g., RIL, NIL, or NAM population). Hara et al. (2011) used QTL analysis to investigate the photoperiod sensitivity of common buckwheat using an F4 population that was derived from a cross between autogamous lines, in order to circumvent the complex genetic patterns caused by the cross-pollinating reproductive system. In their study, Hara et al. (2011) constructed a linkage map, performed QTL analysis using 63 expressed sequence tag (EST) markers and three candidate genes, which were homologs of Arabidopsis genes, and identified a single candidate gene and two ESTs that were associated with the target trait. Besides, linkage maps for allogamous common buckwheat populations can also be constructed using pseudo-testcross mapping strategy (Grattapaglia and Sederoff 1994), in which a map was constructed for each parent and then two maps were bridged (Konishi and Ohnishi 2006, Yasui et al. 2004). Yabe et al. (2014a), for example, used this strategy and high-density markers, with NGS technology, to construct a high-density linkage map for a common buckwheat population, and, then conducted QTL analysis for main stem length. This study verified the usefulness of linkage maps, even for allogamous populations. Such studies suggested that QTL analysis and other QTL detection methods (e.g., GWAS) will contribute to allogamous crop breeding via conventional MAS for major genes.
Meanwhile, GS is one of the most promising breeding methods for common buckwheat populations with high genetic diversity, partly owing to the crop’s allogamous reproductive system, which can create new combinations of beneficial genes. Moreover, GS, through evaluating genetic ability based on marker genotype and phenotypic information, can realize a higher accuracy than the relatively low accuracy of phenotypic selection with single plant evaluation, thereby solving the problem of allogamous crop breeding. Yabe et al. (2018) performed empirical GS breeding in common buckwheat and verified that GS was useful for mass selection in allogamous populations with low LD. The authors also demonstrated the importance of both breeding cycle acceleration and frequent model updating, the latter of which may be a unique requirement of GS breeding in allogamous crop populations with low LD. In addition, the marker genotyping system used for the study was created from scratch using NGS technology (Yabe et al. 2014a), simulation studies were used to evaluate the efficiency of GS mass selection and to optimize GS breeding strategy before empirical breeding was attempted (Yabe et al. 2013, 2014b). It is important to note, however, that, even though common buckwheat population possesses high genetic diversity, which is beneficial for GS, the low LD of allogamous common buckwheat populations might actually hinder GS. Such attempt of the combination of simulations and empirical GS breeding suggests that GS breeding can be used to achieve rapid genetic improvement in common buckwheat and confirm the importance of using optimized breeding strategies.
It is essential to figure out an optimal breeding program in advance, especially for common buckwheat breeding, with few research results to date due to the short history of genomics-assisted breeding. In the above-mentioned GS for common buckwheat, the GS model only incorporated additive effects, possibly accumulated via mass selection in a population and, on average, maintained, even during seed multiplication after GS. Due to the species’ completely outcrossing reproductive process, it is difficult to utilize the dominance (or overdominance) and epistasis, including dominance, in mass selection of a common buckwheat population, due to heterogeneity. Meanwhile, it may be possible to fix epistasis that consists of additive effects only, even in an outcrossing population, if the effects could be precisely estimated through a large-scale dataset or bi-parental QTL mapping. When the hybrid breeding performed in maize breeding is applied to buckwheat breeding in the future, the utilization of dominance effects in genomics-assisted breeding will be required. The GS model should not only be chosen based on selection accuracy, but also on the assumed type of the population to be released as a variety. In addition, optimal genomics-assisted breeding methods should be employed. MAS, based on biparental QTL analysis or GWAS, can drastically improve genetic ability in a common buckwheat population, which has not experienced intense selection, especially when the target trait is controlled by a small number of genes or the detected QTL considerably explains the amount of variation in the population. On the other hand, GS is also promising for the genetic improvement of polygenic traits where a single method could be used to build a GS model incorporating detected QTLs, or MAS and GS could be conducted in series. To create an optimal breeding program for common buckwheat, genetic knowledge of the target traits and population as well as breeding simulations will be needed.
Studies mentioned above were performed without using any reference genome information for common buckwheat, because there were no available resources. However, the recently published draft genome of common buckwheat (1.18 Gbp; Yasui et al. 2016b) enables us to make progress. Using the genome information of common buckwheat, the knowledge of agronomically useful genes in other plant species facilitated the identification of potentially useful homologs (Matsui et al. 2018, Yasui et al. 2016b), which can then be analyzed to estimate function and evolutionary history (Che et al. 2018, Mizuno and Yasui 2019). This rapid growth of genomics technology will enhance the genomics-assisted breeding in common buckwheat.
Prospects for genetic improvement in minor and pseudo-cereals
Genomics-assisted breeding methods, such as GS and MAS, will be useful for the genetic improvement needed to meet the growing demand for minor and pseudo-cereals, as well as for the development of new varieties that can adapt to future climates (Varshney et al. 2018). Genomics-assisted breeding of minor and pseudo-cereals is also expected to contribute to global food safety, by simultaneously increasing crop diversification and improving crop yield and quality.
Genetic variation is essential for efficient genetic improvement. Because of their relatively short or non-intensive history of breeding, the current collections of cultivars of minor and pseudo-cereals are expected to harbor high genetic diversity (Zhang et al. 2018). However, when compared to the resources available for major crop species, the numbers of minor and pseudo-cereal accessions in gene banks are inferior and insufficient (FAO 2010) and DNA polymorphism information of their genetic resources has not been prepared. For these reasons and to promote the use of current accessions for breeding programs, it is crucial that useful genetic resources be collected before they are lost and that the available genetic resources be characterized and evaluated, possibly including DNA polymorphism information (FAO 2010).
One of the main advantages of GS, QTL mapping, and GWAS is that no reference genome is required. For example, no reference genome information was used in the GS breeding experiment of buckwheat (Yabe et al. 2018). However, the availability of reference genomes could further increase the usefulness of genomics-assisted breeding. To detect candidate genes that cause associations between markers and phenotypes, for example, GWAS requires marker position information and an annotate genome. Therefore, draft genomes have recently been developed for common buckwheat (Yasui et al. 2016b), pearl millet (Varshney et al. 2017), grain amaranth (Amaranthus hypochondriacus L.; Clouse et al. 2016), tef (Cannarozzi et al. 2014), and quinoa (Yasui et al. 2016a, Zou et al. 2017), and Varshney et al. (2017) verified the usefulness of the draft pearl millet genome for GWAS and genomic prediction. In addition, databases of genomic-resources have also been developed for a variety of minor and pseudo-cereals, e.g., buckwheat (Yasui et al. 2016b) and pearl millet (Jaiswal et al. 2018). The cost efficiency and high-throughput nature of whole-genome sequence analysis are improved rapidly by the improvement of sequencing technology.
Because both GS and MAS accelerate selection cycles and improve genetic gain per unit time (Bernardo 2008, Heffner et al. 2010), the development of generation acceleration methods and protocols becomes very important (Ghosh et al. 2018). However, the development of generation acceleration techniques and protocols for minor and pseudo-cereals is generally lacking (Chiurugwi et al. 2019), with few exceptions (e.g., Ghosh et al. 2018, Stetter et al. 2016). In a recent review, Chiurugwi et al. (2019) reviewed the advantages of speed-breeding capsules and speed-breeding centers. Whereas the equipment quired to control cultivation environments (e.g., large growth chambers and greenhouses) is usually expensive, speed-breeding capsules, which consist of shipping containers that have been equipped with hydroponic systems, lighting, air conditioning, and greenhouse benches, can be used as more cost-effective generation acceleration facilities. Meanwhile, speed-breeding centers are used for the intensive improvement of target crops and include both generation acceleration facilities and the integration of the technology needed for genomics-assisted breeding. The construction of such facilities and centers is considered important for effective genomics-assisted breeding technologies in minor and pseudo-cereals.
Both marker selection and efficient phenotyping are important factors for the acceleration of breeding. This is because selection accuracy and selection intensity can be increased by improving the efficiency of trait phenotyping (Araus et al. 2018). In genomics-assisted breeding, it is likely that the improvement of trait phenotyping would improve the power of QTL mapping and GWAS, as well as the prediction accuracy of GS. Furthermore, because high-throughput phenotyping technologies, such as drone remote sensing, are generally versatile and applicable to a wide variety of crop species, it should be easy to apply the technologies developed and used in major crops directly to minor and pseudo-cereals. New technologies for phenotyping enable us to perform QTL mapping/GWAS-based breeding, or GS for characteristics that have been difficult to evaluate so far (e.g., Sivasakthi et al. 2018, Spindel et al. 2018). High-throughput phenotyping will be indispensable in genomics-assisted breeding in the future.
Genome editing, which is one of the most advanced technologies to be developed in recent years, is also likely to be useful for crop development in the near future. The present review focused on the genomics-assisted breeding of minor and pseudo-cereals, mainly based on marker selection. The genetic improvement of these crops can be further accelerated by the use of genome editing, in combination with QTL mapping, GWAS, and GS. In minor and pseudo-cereals, an ancestral state may still remain in agronomic traits (e.g., shattering habit) that have been improved in major crops. Although it is possible to utilize the natural variations present in minor and pseudo-cereals for trait improvement, it might also be possible to edit important genes using genome editing (Østerberg et al. 2017, Varshney et al. 2018). In such cases, biparental QTL mapping and GWAS may play important roles in narrowing down candidate genes.
The advantage of developing breeding strategies for minor and pseudo-cereals is that there is no need to re-create the methodological research required for the advancement of major crops. During the last decade, for example, it has become unnecessary to conduct DNA marker development in order to conduct marker-assisted breeding, and a large number of markers are currently available without special development. In addition, the analysis of whole genome sequences, which has been carried out in global collaborative research, can also be carried out by one research team in some cases. Furthermore, the pros and cons of each method have become apparent in major crop studies. As Bernardo (2016) states, researchers and breeders should choose “waves” to ride on as “surfers”, and the researchers and breeders of minor and pseudo-cereals can assess the potential of “waves” that have previously influenced research and breeding in major crops. This is a major advantage of minor cereal- and pseudocereal-breeding programs that implementing genomics-assisted breeding later than it has been performed in major crops. Currently, the genomic information is available and methodologies are mostly refined, which is owing to the trial-and-error that major crop breeding has experienced over a long period, which means genetic improvements in minor and pseudo-cereals can be realized more expediently than they have previously been in major crops.
Author Contribution Statement
S.Y. and H.I. contributed to the planning the work, to the writing of the manuscript, and to designing the figure.
Acknowledgments
The preparation of this review was partly supported by JST CREST, Japan (grant no. JPMJCR16O2).
Literature Cited
- Abberton M., Batley J., Bentley A., Bryant J., Cai H., Cockram J., de Oliveira A.C., Cseke L.J., Dempewolf H., De Pace C. et al. (2016) Global agricultural intensification during climate change: a role for genomics. Plant Biotechnol. J. 14: 1095–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquaah, G. (2009) Principles of plant genetics and breeding. John Wiley & Sons, West Sussex, UK. [Google Scholar]
- Admassu-Yimer B., Gordon T., Bonman J.M. and Esvelt Klos K. (2019) Development and validation of a quantitative PCR assay method of assessing relative resistance of oat (Avena sativa) to crown rust (Puccinia coronata f. sp. avenae). Plant Pathol. 68: 669–677. [Google Scholar]
- Ambawat S., Senthilvel S., Hash C.T., Nepolean T., Rajaram V., Eshwar K., Sharma R., Thakur R.P., Rao V. P., Yadav R.C. et al. (2016) QTL mapping of pearl millet rust resistance using an integrated DArT-and SSR-based linkage map. Euphytica 209: 461–476. [Google Scholar]
- Aparna K., Nepolean T., Srivastsava R.K., Kholova J., Rajaram V., Kumar S., Rekha B., Senthilvel S., Hash C.T. and Vadez V. (2015) Quantitative trait loci associated with constitutive traits control water use in pearl millet [Pennisetum glacum (L.) R. Br.]. Plant Biol. (Stuttg) 17: 1073–1084. [DOI] [PubMed] [Google Scholar]
- Araus J.L., Kefauver S.C., Zaman-Allah M., Olsen M.S. and Cairns J.E. (2018) Translating high-throughput phenotyping into genetic gain. Trends Plant Sci. 23: 451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstead I., Huang L., Ravagnani A., Robson P. and Ougham H. (2009) Bioinformatics in the orphan crops. Brief. Bioinformatics 10: 645–653. [DOI] [PubMed] [Google Scholar]
- Asoro F.G., Newell M.A., Beavis W.D., Scott M.P. and Jannink J.-L. (2011) Accuracy and training population design for genomic selection on quantitative traits in elite North American oats. Plant Genome 4: 132–144. [Google Scholar]
- Asoro F.G., Newell M.A., Beavis W.D., Scott M.P., Tinker N.A. and Jannink J.-L. (2013) Genomic, marker-assisted, and predigree-BLUP selection methods for β-glucan concentration in elite oat. Crop Sci. 53: 1894–1906. [Google Scholar]
- Auinger H.-J., Schonleben M., Lehermeier C., Schmidt M., Korzun V., Geiger H.H., Piepho H.-P., Gordillo A., Wilde P., Bauer E. et al. (2016) Model training across multiple breeding cycles significantly improves genomic prediction accuracy in rye (Secale cereale L.). Theor. Appl. Genet. 129: 2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker E.M., Gordon T.C., Jackson E.W., Chao S., Harrison S.A., Carson M.L., Obert D.E. and Bonman J.M. (2015) Quantitative trait loci form two genotypes of oat (Avena sativa) conditioning resistance to Puccinia coronata. Phytopathology 105: 239–245. [DOI] [PubMed] [Google Scholar]
- Baird N.A., Etter P.D., Atwood T.S., Currey M.C., Shiver A.L., Lewis Z.A., Selker E.U., Cresko W.A. and Johnson E.A. (2008) Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 3: e3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baljeet S.Y., Ritika B.Y. and Roshan L.Y. (2010) Studies on functional properties and incorporation of buckwheat flour for biscuit making. Int. Food Res. J. 17: 1067–1076. [Google Scholar]
- Bernal-Vasquez A.-M., Mohring J., Schmidt M., Schonleben M., Schon C.-C. and Piepho H.-P. (2014) The importance of phenotypic data analysis for genomic prediction—a case study comparing different spatial models in rye. BMC Genomics 15: 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Vasquez A., Gordillo A., Schmidt M. and Piepho H.-P. (2017) Genomic prediction in early selection stages using multi-year data in a hybrid rye breeding program. BMC Genet. 18: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo R. (2008) Molecular markers and selection for complex traits in plants: learning from the last 20 years. Crop Sci. 48: 1649–1664. [Google Scholar]
- Bernardo R. (2016) Bandwagons I, too, have known. Theor. Appl. Genet. 129: 2323–2332. [DOI] [PubMed] [Google Scholar]
- Bouchet S., Olatoye M.O., Marla S.R., Perumal R., Tesso T., Yu J., Tuinstra M. and Morris G.P. (2017) Increased power to dissect adaptive traits in global sorghum diversity using a nested association mapping population. Genetics 206: 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, C.G. (1997) Buckwheat, Fagopyrum esculentum Moench. Vol. 19. Bioversity International. [Google Scholar]
- Cannarozzi G., Plaza-Wuthrich S., Esfeld K., Larti S., Wilson Y.S., Girma D., de Castro E., Chanyalew S., Blösch R., Farinelli L. et al. (2014) Genome and transcriptome sequencing identifies breeding targets in the orphan crop tef (Eragrostis tef). BMC Genomics 15: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernansky R. (2015) The rise of Africa’s super vegetables. Nature 522: 146–148. [DOI] [PubMed] [Google Scholar]
- Che J., Yamaji N., Yokosho K., Shen R.F. and Ma J.F. (2018) Two genes encoding a bacterial-type ATP-binding cassette transporter are implicated in aluminum tolerance in buckwheat. Plant Cell Physiol. 59: 2502–2511. [DOI] [PubMed] [Google Scholar]
- Cheng A., Mayes S., Dalle G., Demissew S. and Massawe F. (2017) Divesifying crops for food and nutrition security—a case of teff. Biol. Rev. Camb. Philos. Soc. 92: 188–198. [DOI] [PubMed] [Google Scholar]
- Chiurugwi T., Kemp S., Powell W. and Hickey L.T. (2019) Speed breeding orphan crops. Theor. Appl. Genet. 132: 607–616. [DOI] [PubMed] [Google Scholar]
- Clouse J.W., Adhikary D., Page J.T., Ramaraj T., Deyholos M.K., Udall J.A., Fairbanks D.J., Jellen E.N. and Maughan P.J. (2016) The amaranth genome: genome, transcriptome, and physical map assemply. Plant Genome 9. doi: 10.3835/plantgenome2015.07.0062. [DOI] [PubMed] [Google Scholar]
- Crowell S., Korniliev P., Falcao A., Ismail A., Gregorio G., Mezey J. and McCouch S. (2016) Genome-wide association and high-resolution phenotyping link Oryza sativa panicle traits to numerous trait-specific QTL clusters. Nat. Commun. 7: 10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelia V., Aversano R., Chiaiese P. and Carputo D. (2018) The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem. Rev. 17: 611–625. [Google Scholar]
- Dhariwal R., Fedak G., Dion Y., Pozniak C., Laroche A., Eudes F. and Randhawa H.S. (2018) High density single nucleotide polymorphism (SNP) mapping and quantitative trait loci (QTL) analysis in a biparental spring triticale population localized major and minor effect Fusarium head blight resistance and associated traits QTL. Genes (Basel) 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshire R.J., Glaubitz J.C., Sun Q., Poland J.A., Kawamoto K., Buckler E.S. and Mitchell S.E. (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6: e19379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endelman J.B. (2011) Ridge regression and other kernels for genomic selection with R package rrBLUP. Plant Genome 4: 250–255. [Google Scholar]
- Endelman J.B. and Jannink J.-L. (2012) Shrinkage estimation of the realized relationship matrix. G3 (Bethesda) 2: 1405–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erath W., Beuer E., Kastirr U., Schmidt M., Korzun V., Schmiedchen B., Wilde P. and Schön C.-C. (2016) Oligogenic control of resistance to soil-borne viruses SBCMV and WSSMV in rye (Secale cereale L.). Plant Breed. 135: 552–559. [Google Scholar]
- Erath W., Bauer E., Fowler D.B., Gardillo A., Korzun V., Ponomareva M., Schmidt M., Schmiedchen B., Wilde P. and Schön C.-C. (2017) Exploring new alleles for frost tolerance in winter rye. Theor. Appl. Genet. 130: 2151–2164. [DOI] [PubMed] [Google Scholar]
- Esquinas-Alcázar J. (2005) Protecting crop genetic diversity for food security: political, ethical and technical challenges. Nat. Rev. Genet. 6: 946 – 953. [DOI] [PubMed] [Google Scholar]
- Fang X., Dong K., Wang X., Liu T., He J., Ren R., Zhang L., Liu R., Liu X., Li M. et al. (2016) A high density genetic map and QTL for agronomic and yield traits in Foxtail millet [Setaria italica (L.) P. Beauv.]. BMC Genomics 17: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO (2010) The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture. Rome. [Google Scholar]
- FAOSTAT (2013) http://www.fao.org/faostat/en/#data/FBS. [Google Scholar]
- FAOSTAT (2018) http://www.fao.org/faostat/en/#data/OA. [Google Scholar]
- Fernandes S.B., Dias K.O.G., Ferreira D.F. and Brown P.J. (2018) Efficiency of multi-trait, indirect, and trait-assisted genomic selection for improvement of biomass sorghum. Theor. Appl. Genet. 131: 747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.B. (2015) Understanding crop genetic diversity under modern plant breeding. Theor. Appl. Genet. 128: 2131–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin D.F., Stack R.W. and Hansen J.M. (2009) Quantitative trait locus mapping of increased Fusarium head blight susceptibility associated with a wild emmer wheat chromosome. Phytopathology 99: 447–452. [DOI] [PubMed] [Google Scholar]
- Giménez-Bastida J.A. and Zieliñski H. (2015) Buckwheat as a functional food and its effects on health. J. Agric. Food Chem. 63: 7896–7913. [DOI] [PubMed] [Google Scholar]
- Giménez-Bastida J.A., Piskula M.K. and Zieliñski H. (2015) Recent advances in processing and development of buckwheat derived bakery and non-bakery products-a review. Pol. J. Food Nutr. Sci. 65: 9–20. [Google Scholar]
- Ghosh S., Watson A., Gonzalez-Navarro O.E., Ramirez-Gonzalez R.H., Yanes L., Mendoza-Suárez M., Simmonds J., Wells R., Rayner T., Green P. et al. (2018) Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 13: 2944–2963. [DOI] [PubMed] [Google Scholar]
- Grattapaglia D. and Sederoff R. (1994) Genetic linkage maps of Eucalyptus grandis and Eucalyptus urophylla using a pseudo-testcross: mapping strategy and RAPD markers. Genetics 137: 1121–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin M.T., Buckler E.S. and Jannink J.L. (2011) Population genetics of genomics-based crop improvement methods. Trends Genet. 27: 98–106. [DOI] [PubMed] [Google Scholar]
- Hara T., Iwata H., Okuno K., Matsui K. and Ohsawa R. (2011) QTL analysis of photoperiod sensitivity in common buckwheat by using markers for expressed sequence tags and photoperiod-sensitivity candidate genes. Breed. Sci. 61: 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K., Subudhi P.K., Borrell A., Jordan D., Rosenow D., Nguyen H., Klein P., Klein R. and Mullet J. (2007) Sorghum stay-green QTL individually reduce post-flowering drought-induced leaf senescence. J. Exp. Bot. 58: 327–338. [DOI] [PubMed] [Google Scholar]
- Hayes B.J., Cogan N.O.I., Pembleton L.W., Goddard M.E., Wang J., Spangenberg G.C. and Foster J.W. (2013) Prospects for genomic selection in forage plant species. Plant Breed. 132: 133–143. [Google Scholar]
- Heffner E.L., Lorenz A.J., Jannink J.L. and Sorrells M.E. (2010) Plant breeding with genomic selection: gain per unit time and cost. Crop Sci. 50: 1681–1690. [Google Scholar]
- Henderson, C.R. (1984) Applications of linear models in animal breeding. Univ. of Guelph, Guelph, Ontario. [Google Scholar]
- Hickey J.M., Chiurugwi T., Mackay I., Powell W. and Implementing Genomic Selection in CGIAR Breeding Programs Workshop Participants (2017) Genomic prediction unifies animal and plant breeding programs to form platforms for biological discovery. Nat. Genet. 49: 1297–1303. [DOI] [PubMed] [Google Scholar]
- Hinterthuer A. (2017) Can Ancient Grains find their way in modern agriculture? Crops, Soils Agron. News 62: 4–9. [Google Scholar]
- Hoeschele I. and VanRaden P.M. (1993) Bayesian analysis of linkage between genetic markers and quantitative trait loci. I. Prior knowledge. Theor. Appl. Genet. 85: 953–960. [DOI] [PubMed] [Google Scholar]
- Hulbert S.H., Richter T.E., Axtell J.D. and Bennetzen J.L. (1990) Genetic mapping and characterization of sorghum and related crops by means of maize DNA probes. Proc. Natl. Acad. Sci. USA 87: 4251–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C.H., van Eeuwijk F.A., Mace E.S., Hayes B.J. and Jordan D.R. (2018) Development of genomic prediction in sorghum. Crop Sci. 58: 690–700. [Google Scholar]
- Jaiswal S., Antala T.J., Mandavia M.K., Chopra M., Jasrotia R.S., Tomar R.S., Kheni J., Angadi U.B., Iquebal M.A., Golakia B.A. et al. (2018) Transcriptomic signature of drought response in pearl millet (Pennisetum glaucum L.) and development of web-genomic resources. Sci. Rep. 8: 3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal V., Bandyopadhyay T., Gahlaut V., Gupta S., Dhaka A., Ramchiary N. and Prasad M. (2019) Genome-wide association study (GWAS) delineates genomic loci for ten nutritional elements in foxtail millet (Setaria italica L.). J. Cereal Sci. 85: 48–55. [Google Scholar]
- Jia G., Huang X., Zhi H., Zhao Y., Zhao Q., Li W., Chai Y., Yang L., Liu K., Lu H. et al. (2013) A haplotype map of genomic variations and genome-wide association studies of agronomic traits in foxtail millet (Setaria italica). Nat. Genet. 45: 957–961. [DOI] [PubMed] [Google Scholar]
- Jia G., Wang H., Tang S., Zhi H., Liu S., Wen Q., Qiao Z. and Diao X. (2017) Detection of genomic loci associated with chromosomal recombination using high-density linkage mapping in Setaria. Sci. Rep. 7: 15180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D.C., Chaudhari G.V., Sood S., Kant L., Pattanayak A., Zhang K., Fan Y., Janovská D., Meglič V. and Zhou M. (2019) Revisiting the versatile buckwheat: reinvigorating genetic gains through integrated breeding and genomics approach. Planta 250: 783–801. [DOI] [PubMed] [Google Scholar]
- Kalih R., Maurer H.P. and Miedaner T. (2015) Genetic architecture of Fusarium head blight resistance in four winter triticale populations. Phytopathology 105: 334–341. [DOI] [PubMed] [Google Scholar]
- Kaur K.D., Jha A., Sabikhi L. and Singh A.K. (2014) Significance of coarse cereals in health and nutrition: a review. J. Food Sci. Technol. 51: 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearsey M.J. and Farquhar A.G.L. (1998) QTL analysis in plants; where are we now? Heredity (Edinb) 80: 137–142. [DOI] [PubMed] [Google Scholar]
- Keller M., Karutz Ch., Schmid J.E., Stamp P., Winzeler M., Keller B. and Messmer M.M. (1999a) Quantitative trait loci for lodging resistance in a segregating wheat × spelt population. Theor. Appl. Genet. 98: 1171–1182. [Google Scholar]
- Keller M., Keller B., Schachermayr G., Winzeler M., Schmid J.E., Stamp P. and Messmer M.M. (1999b) Quantitative trait loci for resistance against powdery mildew in a segregating wheat × spelt population. Theor. Appl. Genet. 98: 903–912. [Google Scholar]
- Konishi T. and Ohnishi O. (2006) A linkage map for common buckwheat based on microsatellite and AFLP markers. Fagopyrum 23: 1–6. [Google Scholar]
- Kumar A., Sharma D., Tiwari A., Jaiswal J.P., Singh N.K. and Sood S. (2016a) Genotyping-by-sequencing analysis for determining population structure of finger millet germplasm of diverse origins. Plant Genome 9. doi: 10.3835/plantgenome2015.07.0058. [DOI] [PubMed] [Google Scholar]
- Kumar S., Hash C.T., Thirunavukkarasu N., Singh G., Rajaram V., Rathore A., Senapathy S., Mahendrakar M.D., Yadav R.S. and Srivastava R.K. (2016b) Mapping quantitative trait loci controlling high iron and zinc content in self and open pollinated grains of pearl millet [Pennisetum glaucum (L.) R. Br.]. Front. Plant Sci. 7: 1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Hash C.T., Nepolean T., Satyavathi C.T., Singh G., Mahendrakar M.D., Yadav R.S. and Srivastava R.K. (2017) Mapping QTLs controlling flowering time and important agronomic traits in pearl millet. Front. Plant Sci. 8: 1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Hash C.T., Nepolean T., Mahendrakar M.D., Satyavathi C.T., Singh G., Rathore A., Yadav R.S., Gupta R. and Srivastava R.K. (2018) Mapping grain iron and zinc content quantitative trait loci in an Iniadi-derived immortal population of pearl millet. Genes (Basel) 9: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. and Thompson R. (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124: 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, D. and D.A. Jones (1992) The genetics of heterostyly. In: Barrett, S.C.H. (ed.) Evolution and function of heterostyly. Springer, Berlin. pp. 129–150. [Google Scholar]
- Liang Z., Gupta S.K., Yeh C.-T., Zhang Y., Ngu D.W., Kumar R., Patil H.T., Mungra K.D., Yadav D.V., Rathore A. et al. (2018) Phenotypic data from inbred parents can improve genomic prediction in pearl millet hybrids. G3 (Bethesda) 8: 2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-R., Schertz K.F. and Paterson A.H. (1995) Comparative analysis of QTLs affecting plant height and maturity across the Poaceae, in reference to an interspecific sorghum population. Genetics 141: 391–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Maccaferri M., Chen X., Laghetti G., Pignone D., Pumphrey M. and Tuberosa R. (2017) Genome-wide association mapping reveals a rich genetic architecture of stripe rust resistance loci in emmer wheat (Triticum turgidum ssp. dicoccum). Theor. Appl. Genet. 130: 2249–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio T.A., Collins F.S., Cox N.J., Goldstein D.B., Hindorff L.A., Hunter D.J., McCarthy M.I., Ramos E.M., Cardon L.R., Chakravarti A. et al. (2009) Finding the missing heritability of complex diseases. Nature 461: 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marouli E., Graff M., Medina-Gomez C., Lo K.S., Wood A.R., Kjaer T.R., Fine R.S., Lu Y., Schurmann C., Highland H.M. et al. (2017) Rare and low-frequency coding variants alter human adult height. Nature 542: 186–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marulanda J.J., Mi X., Melchinger A.E., Xu J.-L., Wurschum T. and Longin C.F.H. (2016) Optimum breeding strategies using genomic selection for hybrid breeding in wheat, maize, rye, barley, rice and triticale. Theor. Appl. Genet. 129: 1901–1913. [DOI] [PubMed] [Google Scholar]
- Massawe F., Mayes S. and Cheng A. (2016) Crop diversity: an unexploited treasure trove for food security. Trends Plant Sci. 21: 365–368. [DOI] [PubMed] [Google Scholar]
- Matsui K., Tomatsu T., Kinouchi S., Suzuki T. and Sato T. (2018) Identification of a gene encoding glutathione S-transferase that is related to anthocyanin accumulation in buckwheat (Fagopyrum esculentum). J. Plant Physiol. 231: 291–296. [DOI] [PubMed] [Google Scholar]
- Mauro-Herrera M. and Doust A.N. (2016) Development and genetic control of plant architecture and biomass in the Panicoid grass Setaria. PLoS ONE 11: e0151346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchinger A.E., Utz H.F. and Schon C.C. (1998) Quantitative trait locus (QTL) mapping using different testers and independent population samples in maize reveals low power of QTL detection and large bias in estimates of QTL effects. Genetics 149: 383–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchunk-Ovnat L., Barak V., Fahima T., Ordon F., Lidzbarsky G.A., Krugman T. and Saranga Y. (2016a) Ancestral QTL alleles from wild emmer wheat improve drought resistance and productivity in modern wheat cultivars. Front. Plant Sci. 7: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchunk-Ovnat L., Fahima T., Krugman T. and Saranga Y. (2016b) Ancestral QTL alleles from wild emmer wheat improve grain yield, biomass and photosynthesis across environments in modern wheat. Plant Sci. 251: 23–34. [DOI] [PubMed] [Google Scholar]
- Meuwissen T.H.E., Hayes B.J. and Goddard M.E. (2001) Prediction of total genetic value using genome-wide dense marker maps. Genetics 157: 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedaner T., Hübner M., Korzun V., Schmiedchen B., Bauer E., Haseneyer G., Wilde P. and Reif J.C. (2012) Genetic architecture of complex agronomic traits examined in two testcross populations of rye (Secale cereale L.). BMC Genomics 13: 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedaner T., Kalih R., Großmann M.S. and Mauper H.P. (2016) Correlation between Fusarium head blight severity and DON content in triticale as revealed by phenotypic and molecular data. Plant Breed. 135: 31–37. [Google Scholar]
- Miedaner T., Haffke S., Siekmann D., Fromme F.J., Roux S.R. and Hackauf B. (2018) Dynamic quantitative trait loci (QTL) for plant height predict biomass yield in hybrid rye (Secale cereale L.). Biomass Bioenergy 115: 10–18. [Google Scholar]
- Miller M.R., Dunham J.P., Amores A., Cresko W.A. and Johnson E.A. (2007) Rapid and cost-effective polymorphism identification and genotyping using restriction site associated DNA (RAD) markers. Genome Res. 17: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir N.A., Riar C.S. and Singh S. (2018) Nutritional constituents of pseudo cereals and their potential use in food systems: A review. Trends Food Sci. Technol. 75: 170–180. [Google Scholar]
- Mizuno N. and Yasui Y. (2019) Gene flow signature in the S-allele region of cultivated buckwheat. BMC Plant Biol. 19: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montilla-Bascón G., Rispail N., Sánchez-Martín J., Rubiales D., Mur L.A., Langdon T., Howarth C.J. and Prats E. (2015) Genome-wide association study for crown rust (Puccinia coronata f. sp. avenae) and powdery mildew (Blumeria graminis f. sp. avenae) resistance in an oat (Avena sativa) collection of commercial varieties and landraces. Front. Plant Sci. 6: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.P., Ramu P., Deshpande S.P., Hash C.T., Shah T., Upadhyaya H.D., Riera-Lizarazu O., Brown P.J., Acharya C.B., Mitchell S.E. et al. (2013a) Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. USA 110: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G.P., Rhodes D.H., Brenton Z., Ramu P., Thayil V.M., Deshpande S., Hash C.T., Acharya C., Mitchell S.E., Buckler E.S. et al. (2013b) Dissecting genome-wide association signals for loss-of-function phenotypes in sorghum flavonoid pigmentation traits. G3 (Bethesda) 3: 2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S.C., Sharma A., Rooney W.L., Klein P.E., Mullet J.E., Mitchell S.E. and Kresovich S. (2008) Genetic improvement of sorghum as a biofuel feedstock: I. QTL for stem sugar and grain nonstructural carbohydrates. Crop Sci. 48: 2165–2179. [Google Scholar]
- Muthamilarasan M., Dhaka A., Yadav R. and Prasad M. (2016) Exploration of millet models for developing nutrient rich graminaceous crops. Plant Sci. 242: 89–97. [DOI] [PubMed] [Google Scholar]
- Myśków B., Góralska M., Lenarczyk N., Czyczyło-Mysza I. and Stojałowski S. (2018) Putative candidate genes responsible for leaf rolling in rye (Secale cereale L.). BMC Genet. 19: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell M.A., Cook D., Tinker N.A. and Jannink J.L. (2011) Population structure and linkage disequilibrium in oat (Avena sativa L.): implications for genome-wide association studies. Theor. Appl. Genet. 122: 623–632. [DOI] [PubMed] [Google Scholar]
- Newell M.A., Asoro F.G., Scott M.P., White P.J., Beavis W.D. and Jannink J.L. (2012) Genome-wide association study for oat (Avena sativa L.) beta-glucan concentration using germplasm of worldwide origin. Theor. Appl. Genet. 125: 1687–1696. [DOI] [PubMed] [Google Scholar]
- Ni X., Xia Q., Zhang H., Cheng S., Li H., Fan G., Guo T., Huang P., Xiang H., Chen Q. et al. (2017) Updated foxtail millet genome assembly and gene mapping of nine key agronomic traits by resequencing a RIL population. Gigascience 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak V., Du J. and Charrondière U.R. (2016) Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.). Food Chem. 193: 47–54. [DOI] [PubMed] [Google Scholar]
- Odonkor S., Choi S., Charkraborty D., Martinez-Bello L., Wang X., Bahri B.A., Tenaillon M.I., Panaud O. and Devos K.M. (2018) QTL mapping combined with comparative analyses identified candidate genes for reduced shattering in Setaria italica. Front. Plant Sci. 9: 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Østerberg J.T., Xiang W., Olsen L.I., Edenbrandt A.K., Vedel S.E., Christiansen A., Landes X., Andersen M.M., Pagh P., Sandøe P. et al. (2017) Accelerating the domestication of new crops: feasibility and approaches. Trends Plant Sci. 22: 373–384. [DOI] [PubMed] [Google Scholar]
- Patterson N., Price A.L. and Reich D. (2006) Population structure and eigenanalysis. PLoS Genet. 2: e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A.H., Bowers J.E., Bruggmann R., Dubchak I., Grimwood J., Gundlach H., Haberer G., Hellsten U., Mitros T., Poliakov A. et al. (2009) The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556. [DOI] [PubMed] [Google Scholar]
- Pellizzaro K., Nava I.C., Chao S., Pacheco M.T. and Federizzi L.C. (2016) Genetics and identification of markers linked to multiflorous spikelet in hexaploid oat. Crop Breed. Appl. Biotechnol. 16: 62–70. [Google Scholar]
- Prom L.K., Ahn E., Isakeit T. and Magill C. (2019) GWAS analysis of sorghum association panel lines identifies SNPs associated with disease response to Texas isolates of Colletotrichum sublineola. Theor. Appl. Genet. 132: 1389–1396. [DOI] [PubMed] [Google Scholar]
- Pucher A., Hash C.T., Wallace J.G., Han S., Leiser W.L. and Haussmann B.I.G. (2018) Mapping a male-fertility restoration locus for the A4 cytoplasmic-genic male-sterility system in pearl millet using a genotyping-by-sequencing-based linkage map. BMC Plant Biol. 18: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnuri S.M., Wallace J.G., Knoll J.E., Hyma K.E., Mitchell S.E., Buckler E.S., Varshney R.K. and Singh B.P. (2016) Development of a high-density linkage map and tagging leaf spot resistance in pearl millet using genotyping-by-sequencing markers. Plant Genome 9. doi: 10.3835/plantgenome2015.10.0106. [DOI] [PubMed] [Google Scholar]
- Rajput S.G., Santra D.K. and Schnable J. (2016) Mapping QTLs for morpho-agronomic traits in proso millet (Panicum miliaceum L.). Mol. Breed. 36: 37. [Google Scholar]
- Rhodes D.H., Hoffmann L. Jr., Rooney W.L., Ramu P., Morris G.P. and Kresovich S. (2014) Genome-wide association study of grain polyphenol concentrations in global sorghum [Sorghum bicolor (L.) Moench] germplasm. J. Agric. Food Chem. 62: 10916–10927. [DOI] [PubMed] [Google Scholar]
- Sanchez A.C., Subudhi P.K., Rosenow D.T. and Nguyen H.T. (2002) Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Mol. Biol. 48: 713–726. [DOI] [PubMed] [Google Scholar]
- Schneider A.D.B., Nava I.C., Herve C.B., Islamovic E., Limberger E., Jackson E.W. and Delatorre C.A. (2015) Chromosome-anchored QTL conferring aluminum tolerance in hexaploid oat. Mol. Breed. 35: 121. [Google Scholar]
- Sharma D., Tiwari A., Sood S., Jamra G., Singh N.K., Meher P.K. and Kumar A. (2018) Genome wide association mapping of agro-morphological traits among a diverse collection of finger millet (Eleusine coracana L.) genotypes using SNP markers. PLoS ONE 13: e0199444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasakthi K., Thudi M., Tharanya M., Kale S.M., Kholová J., Halime M.H., Jaganathan D., Baddam R., Thirunalasundari T., Gaur P.M. et al. (2018) Plant vigour QTLs co-map with an earlier reported QTL hotspot for drought tolerance while water saving QTLs map in other regions of the chickpea genome. BMC Plant Biol. 18: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel J.E., Begum H., Akdemir D., Collard B., Redona E., Jannink J.-L. and McCouch S. (2016) Genome-wide prediction models that incorporate de novo GWAS are a powerful new tool for tropical rice improvement. Heredity (Edinb) 116: 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel J.E., Dahlberg J., Colgan M., Hollingsworth J., Sievert J., Staggenborg S.H., Hutmacher R., Jansson C. and Vogel J.P. (2018) Association mapping by aerial drone reveals 213 genetic associations for Sorghum bicolor biomass traits under drought. BMC Genomics 19: 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetter M.G., Zeitler L., Steinhaus A., Kroener K., Biljecki M. and Schmid K.J. (2016) Crossing methods and cultivation conditions for rapid production of segregating population in three grain amaranth species. Front. Plant Sci. 7: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S.H., Lande R. and Namkoong G. (1992) Limitations of molecular-marker-aided selection in forest tree breeding. Can. J. For. Res. 22: 1050–1061. [Google Scholar]
- Sunstrum F., Bekele W.A., Wight C.P., Yan W., Chen Y. and Tinker N.A. (2018) A genetic linkage map in southern-by-spring oat identifies multiple quantitative trait loci for adaptation and rust resistance. Plant Breed. 138: 82–94. [Google Scholar]
- Sytar O., Brestic M., Zivcak M. and Tran L.-S.P. (2016) The contribution of buckwheat genetic resources to health and dietary diversity. Curr. Genomics 17: 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunk J., Sehgal D., Yadav N.R., Howarth C., Yadav R.C. and Yadav R.S. (2018) Mapping of easy to screen SSR markers for selection of RFLP markers-bracketed downy mildew resistance QTLs in pearl millet. Eur. J. Plant Pathol. 151: 401–411. [Google Scholar]
- Tester M. and Langridge P. (2010) Breeding technologies to increase crop production in a changing world. Science 327: 818–822. [DOI] [PubMed] [Google Scholar]
- Upadhyaya H.D., Vetriventhan M., Deshpande S.P., Sivasubramani S., Wallace J.G., Buckler E.S., Hash C.T. and Ramu P. (2015) Population genetics and structure of a global foxtail millet germplasm collection. Plant Genome 8. doi: 10.3835/plantgenome2015.07.0054. [DOI] [PubMed] [Google Scholar]
- Upadhyaya, H.D. and M. Vetriventhan (2018) Underutilized Climate-Smart Nutrient Rich Small Millets for Food and Nutritional Security. In: Regional Expert Consultation on Underutilized Crops for Food and Nutritional Security in Asia and the Pacific—Thematic, Strategic Papers and Country Status Reports. Asia-Pacific Association of Agricultural Research Institutions (APAARI), Thailand, pp. 109–120. [Google Scholar]
- Varshney R.K., Ribaut J.M., Buckler E.S., Tuberosa R., Rafalski J.A. and Langridge P. (2012) Can genomics boost productivity of orphan crops? Nat. Biotechnol. 30: 1172–1176. [DOI] [PubMed] [Google Scholar]
- Varshney R.K., Shi C., Thudi M., Mariac C., Wallace J., Qi P., Zhang H., Zhao Y., Wang X., Rathore A. et al. (2017) Pearl millet genome sequence provides a resource to improve agronomic traits in arid environments. Nat. Biotechnol. 35: 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney R.K., Singh V.K., Kumar A., Powell W. and Sorrells M.E. (2018) Can genomics deliver climate-change ready crops? Curr. Opin. Plant Biol. 45: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Gálvez A., Miranda M., Vergara J., Uribe E., Puente L. and Martínez E.A. (2010) Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: a review. J. Sci. Food Agric. 90: 2541–2547. [DOI] [PubMed] [Google Scholar]
- Wang J., Wang Z., Du X., Yang H., Han F., Han Y., Yuan F., Zhang L., Peng S. and Guo E. (2017a) A high-density genetic map and QTL analysis of agronomic traits in foxtail millet [Setaria italica (L.) P. Beauv.] using RAD-seq. PLoS ONE 12: e0179717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang H., Du G., Wang Z., Zou H., Du X., Li Y., Peng J., Guo E., Yong J. et al. (2017b) Mapping of Sihc1, which controls hull color, using a high-density genetic map based on restriction site-associated DNA sequencing in foxtail millet [Setaria italica (L.) P. Beauv.]. Mol. Breed. 37: 128. [Google Scholar]
- Wang Y., Mette M.F., Miedaner T., Gottwald M., Wilde P., Reif J.C. and Zhao Y. (2014) The accuracy of prediction of genomic selection in elite hybrid rye populations surpasses the accuracy of marker-assisted selection and is equally augmented by multiple field evaluation locations and test years. BMC Genomics 15: 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mette M.F., Miedaner T., Wilde P., Reif J.C. and Zhao Y. (2015) First insights into the genotype-phenotype map of phenotypic stability in rye. J. Exp. Bot. 66: 3275–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Wang J., Peng J., Du X., Jiang M., Li Y., Han F., Du G., Yang H., Lian S. et al. (2019) QTL mapping for 11 agronomic traits based on a genome-wide Bin-map in a large F2 population of foxtail millet (Setaria italica (L.) P. Beauv). Mol. Breed. 39: 18. [Google Scholar]
- Watanabe K., Guo W., Arai K., Takanashi H., Kajiya-Kanegae H., Kobayashi M., Yano K., Tokunaga T., Fujiwara T., Tsutsumi N. et al. (2017) High-throughput phenotyping of sorghum plant height using an unmanned aerial vehicle and its application to genomic prediction modeling. Front. Plant Sci. 8: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen A., Jayawardana M., Fiedler J., Sapkota S., Shi G., Peng Z., Liu S., White F.F., Bogdanove A.J., Li X. et al. (2018) Genetic mapping of a major gene in triticale conferring resistance to bacterial leaf streak. Theor. Appl. Genet. 131: 649–658. [DOI] [PubMed] [Google Scholar]
- Wen Z., Tan R., Yuan J., Bales C., Du W., Zhang S., Chilvers M.I., Schmidt C., Song Q., Cregan P.B. et al. (2014) Genome-wide association mapping of quantitative resistance to sudden death syndrome in soybean. BMC Genomics 15: 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe S., Ohsawa R. and Iwata H. (2013) Potential of genomic selection for mass selection breeding in annual allogamous crops. Crop Sci. 53: 95–105. [Google Scholar]
- Yabe S., Hara T., Ueno M., Enoki H., Kimura T., Nishimura S., Yasui Y., Ohsawa R. and Iwata H. (2014a) Rapid genotyping with DNA micro-arrays for high-density linkage mapping and QTL mapping in common buckwheat (Fagopyrum esculentum Moench). Breed. Sci. 64: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe S., Ohsawa R. and Iwata H. (2014b) Genomic selection for the traits expressed after pollination in allogamous plants. Crop Sci. 54: 1448–1457. [Google Scholar]
- Yabe S., Hara T., Ueno M., Enoki H., Kimura T., Nishimura S., Yasui Y., Ohsawa R. and Iwata H. (2018) Potential of genomic selection in mass selection breeding of an allogamous crop: an empirical study to increase yield of common buckwheat. Front. Plant Sci. 9: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K., Ohsawa R. and Yonezawa K. (2002) Cost efficiency of spatial error control in single plant selection. Breed. Sci. 52: 177–184. [Google Scholar]
- Yasui Y., Wang Y., Ohnishi O. and Campbell C.G. (2004) Amplified fragment length polymorphism linkage analysis of common buckwheat (Fagopyrum esculentum) and its wild self-pollinated relative Fagopyrum homotropicum. Genome 47: 345–351. [DOI] [PubMed] [Google Scholar]
- Yasui Y., Hirakawa H., Okikawa T., Toyoshima M., Matsuzaki C., Ueno M., Mizuno N., Nagatoshi Y., Imamura T., Miyago M. et al. (2016a) Draft genome sequence of an inbred line of Chenopodium quinoa, an allotetraploid crop with great environmental adaptability and outstanding nutritional properties. DNA Res. 23: 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y., Hirakawa H., Ueno M., Matsui K., Katsube-Tanaka T., Yang S.J., Aii J., Sato S. and Mori M. (2016b) Assembly of the draft genome of buckwheat and its applications in identifying agronomically useful genes. DNA Res. 23: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Pressoir G., Briggs W.H., Bi I.V., Yamasaki M., Doebley J.F., McMullen M.D., Gaut B.S., Nielsen D.M., Holland J.B. et al. (2006) A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38: 203–208. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li Y. and Zhu J.-K. (2018) Developing naturally stress-resistant crops for a sustainable agriculture. Nat. Plants 4: 989–996. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Axtman J.E., Faris J.D., Chao S., Zhang Z., Friesen T.L., Zhong S., Cai X., Elias E.M. and Xu S.S. (2014) Identification and molecular mapping of quantitative trait loci for Fusarium head blight resistance in emmer and durum wheat using a single nucleotide polymorphism-based linkage map. Mol. Breed. 34: 1677–1687. [Google Scholar]
- Zhou Y., Srinivasan S., Mirnezami S.V., Kusmec A., Fu Q., Attigala L., Fernandez M.G.S., Ganapathysubramanian B. and Schnable P.S. (2019) Semiautomated feature extraction from RGB images for sorghum panicle architecture GWAS. Plant Physiol. 179: 24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C.M., Ubert I.P., Pacheco M.T. and Federizzi L.C. (2018) Molecular and comparative mapping for heading date and plant height in oat. Euphytica 214: 101. [Google Scholar]
- Zou C., Chen A., Xiao L., Muller H.M., Ache P., Haberer G., Zhang M., Jia W., Deng P., Huang R. et al. (2017) A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 27: 1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita-Silva A., Fuentes F., Zamora P., Jacobson S.-E. and Schwember A.R. (2014) Breeding quinoa (Chenopodium quinoa Willd.): potential and perspectives. Mol. Breed. 34: 13–30. [Google Scholar]