Abstract
Common buckwheat (Fagopyrum esculentum Moench, CB) and Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn., TB) are used in human nutrition. The idea to screen in the haploid phase for genes affecting low amylose concentration opens the possibility for the effective search of low amylose (waxy) genotypes in CB populations. Self-pollinated homozygous plants of TB might allow us to use a part of endosperm for screening of amylose content. Phenolic substances have a significant inhibitory effect on the digestion of CB and TB proteins, thus metabolites may have impact on protein digestibility. Digestion-resistant peptides are largely responsible for the bile acid elimination. Breeding to diminish polyphenols and anti-nutritional substances might have negative effects on the resistance of plants against pests, diseases and UV-radiation. Bread and pasta are popular CB and TB dishes. During dough making most of CB or TB rutin is degraded to quercetin by rutin-degrading enzymes. The new trace-rutinosidase TB variety makes possible making TB bread with considerable amount of rutin, preserving the initial rutin from flour. Breeding CB and TB for larger embryos would make it possible to increase protein, rutin, and essential minerals concentration in CB and TB grain.
Keywords: flavonoids, low-amylose, quercetin, recessive genes, rutin, waxy starch
Introduction
Two buckwheat species, common buckwheat (Fagopyrum esculentum Moench, CB) and Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn., TB) are used to make various foods and dishes. In Japan, China and Korea, CB and TB are used mostly to prepare noodles and other pasta products. In Italy, CB flour is used to prepare pasta, and in Slovenia and Austria, traditional dishes are CB and TB porridge (žganci) (Fig. 1A, 1B), and bread (Bonafaccia et al. 2003a, Costantini et al. 2014, Kreft et al. 2007, Lukšič et al. 2016, Vogrinčič et al. 2010). French CB pancakes (galettes) and Russian blini are popular the world over (Fig. 1C, 1D). In South Korea, TB sprouts are a new vegetable, used for salads and smoothies (Kim et al. 2004, 2008, Park et al. 2000). In Korea, TB is recently used for a non-alcoholic drink, in Japan, Korea and China, CB is used for strong drinks (in Japan soba shochu), and in Luxembourg, Germany, Slovenia and Italy, attempts are underway to make a beer-like CB or TB drink. CB groats dishes are very important in Slovenia, Croatia, Poland, Ukraine, Belarus and Russia, in Slovenia groats are made also from TB.
Fig. 1.

European buckwheat dishes. (A) Žganci, with greaves, and served with a soup. (B) Tartary buckwheat pasta (left) and common buckwheat pasta (right). (C) Blini, with sour cream, and chive. (D) Galette, with butter, cheese, an egg and pepper.
Japan, China, Korea, Poland, Ukraine, Belarus, Russia, and Slovenia have a long-standing tradition of breeding CB, and many new varieties were released, chiefly those of CB, but also some new varieties of TB. The utmost attention in CB and TB breeding was given to secure high and stable yields of grain in specific environmental and agricultural conditions. Some attention was focused, especially in the case of TB, on the breeding which would facilitate husking and provide pleasant taste. Fewer efforts have been devoted to improve the nutritional quality of CB and TB by breeding.
Nutritional importance of polyphenol-protein interactions
CB and TB proteins have a very well-balanced amino acid composition (Eggum et al. 1981, Javornik et al. 1981). Differences between amino acid compositions of CB samples, and between CB and TB, are not considerable (Bonafaccia et al. 2003b). Javornik and Kreft (1984) established some differences between solubility fractions in the amino acid composition, but these differences are not great. As lysine content is higher in albumins and globulins, these fractions contribute to the well balanced amino acid composition of CB and TB, breeding to change the proportion of solubility fractions could thus somewhat improve the overall amino acid composition of proteins (Javornik and Kreft 1984). CB and TB breeding aimed to produce bigger embryos could be a promising possibility to increase the concentration of proteins and to improve their quality.
CB and TB proteins have a low digestibility (Eggum et al. 1981). Polyphenols, naturally present in CB and TB, lower the true digestibility of proteins, but do not adversely affect the biological value of proteins (Eggum et al. 1981, Skrabanja et al. 1998, 2000). As reported by Ikeda et al. (1986), phenolic substances have a significant inhibitory effect on the in vitro peptic and pancreatic digestion of globulin, thus CB and TB secondary metabolites may have impact on protein digestibility. Considerable interaction between polyphenols and proteins was observed after hydrothermal treatment (Skrabanja et al. 2000).
It was stated by Annor et al. (2017) that protein digestibility in millet is slower comparing to other cereals, may be because of binding of polyphenolic substances to proteins. Similar explanation can be suggested for CB and especially TB, having higher concentration of low molecular mass phenolic substances (for example rutin and quercetin) in comparison to cereals. The interaction between phenolic substances and proteins reduces the digestion of proteins through the small and large intestine. However, microbial processes in the colon enhance the digestibility of protein otherwise blocked by polyphenols in hydrothermally processed CB (Skrabanja et al. 1998, 2000).
CB and TB proteins can reduce the concentration of cholesterol in the serum by increasing the fecal excretion of steroids, which is induced by the binding of steroids to undigested proteins (Wieslander et al. 2011, 2012). Digestion-resistant peptides are largely responsible for the bile acid elimination. CB proteins have been reported to prevent gallstone formation more strongly than soy protein isolates, and they may slow mammary carcinogenesis by lowering serum estradiol, as well as suppress colon carcinogenesis by reducing cell proliferation (Tomotake et al. 2000). These effects are most probably connected with the limited digestibility of CB proteins.
CB and TB proteins are mainly located in the embryo, this is indicated by the location of sulphur in the embryo and by the distribution of nutrients among milling fractions (Chettry et al. 2018, Pongrac et al. 2013, 2016, Skrabanja et al. 2004, Vombergar and Luthar 2018). Breeding CB and TB for larger embryos would make it possible to increase protein concentration in grain. As rutin and minerals are collocated with proteins (Skrabanja et al. 2004), breeding CB and TB for larger embryos would have an impact on the increase of rutin, protein and minerals.
It could be expected that breeding CB and TB for lower content of polyphenols could enhance the nutritional quality of proteins, this, on the other hand, could be unfavorable for the desired effects of polyphenol-protein complexes (Skrabanja et al. 2000, Wieslander et al. 2012). Here we should bear in mind that polyphenols are responsible for the protection of CB and TB plants against diseases, pests and UV radiation (Germ et al. 2013). Breeding to diminish polyphenols might have negative effects on the resistance of plants.
Starch, amylose and amylopectin
CB flour contains about 70–90% of starch, depending on the sample and milling method (Skrabanja et al. 2004). The amylose part of starch is the basis for the formation of retrograded starch during the hydrothermal processing of CB materials (Skrabanja et al. 2001). CB groats were found to be a prebiotic food because they can affect the increase of lactic acid bacteria in the intestine due to the presence of resistant starch (Skrabanja et al. 1998, 2001).
CB and TB are known to have relatively small starch granules, and the amylose content in starch granules is higher than that of cereals (Gao et al. 2016, Gregori and Kreft 2012, Skrabanja and Kreft 1998). Starch in TB has amylose content close to 39%, in addition to a high flavonoid content, indicating that TB has the potential to be exploited for the production of functional foods with a low glycemic index (Gao et al. 2016). In regard to amylose content, there are differences among varieties (Gao et al. 2016), so it is possible to alter the amylose concentration by breeding for the desired size of starch granules. CB and TB have, in comparison to cereals, much smaller starch granules, this could be a starting point for breeding aimed at even smaller size of starch granules and for the production of fat replacers (Gao et al. 2016). However, obtaining a low amylose and high amylopectin content in CB or TB could be a challenge, as it would require obtaining varieties suitable for making cakes and other products with desired eating qualities. Waxy (amylose-free) mutants are known to lack an effective starch granule-bound protein, known as waxy (Wx) protein, connected with amylose biosynthesis (Chrungoo et al. 2016). According to Hung et al. (2006), the development of waxy and high-amylose wheats with varying amylose contents is contributing to produce diverse noodle types with desired eating qualities. Similar variation in noodle qualities could be expected in the case of waxy CB, if the characteristic could be stable through generations. As waxy mutants are expected to be recessive in comparison to non-waxy alleles, a search for a waxy form was performed by investigating starch in CB pollen grains. The haploid phase of pollen grain is expected to have a full expression of recessive genes (Gregori and Kreft 2012). This type of mutants is known in rice and in several other cereals, but not yet in CB or TB. The search for the mutant endosperm has so far produced no result, as the impact of the recessive low amylose mutated gene could be in diploid CB covered by a dominant normal allele. The search for this type of mutation by screening a haploid pollen grain has resulted in finding a low amylose type in a population of Slovenian CB (Gregori and Kreft 2012). In the studied waxy material, the plants had many defective pollen grains (some slightly smaller than normal) and defective endosperm, so there were problems in propagation and maintenance of this genetic material. Crossing within the progeny of plants with the mutated gene resulted in grain with very low amylose content, but endosperm was poorly developed and in most cases not able to support the germinating embryo with enough amount of assimilates (Gregori and Kreft 2012).
The idea to screen in the haploid phase for genes affecting low amylose concentration opens the possibility for the effective search of low amylose (waxy) CB genotypes in CB populations. If so, it would be suitable to use one of the best local varieties as a starting material, to avoid too many backcrossing generations afterwards, for getting the desired gene in a suitable high yielding locally adapted variety. On the other hand in self-pollinated homozygous plants of TB, we would utilize a part of endosperm for screening of amylose content, and directly use the rest of the seed for further propagation in vitro, or by embryo rescue method. This would be a suitable method of breeding for waxy TB.
Rutin, rutinosidase and quercetin
CB and TB are important sources of antioxidant activity in functional foods (Gaberščik et al. 2002, Holasova et al. 2002, Kreft 2016, Matsui et al. 2018, Pexová Kalinová et al. 2019, Zhang et al. 2018). CB and TB products decrease the level of cholesterol, reduce fatigue symptoms and improve lung capacity in humans (Sikder et al. 2014, Wieslander et al. 2011, 2012, Yang et al. 2014). CB and TB extracts can also protect DNA from damage caused by hydroxyl radicals (Vogrinčič et al. 2010). DNA protecting effects of CB and TB extracts are not attributed solely to rutin or quercetin, but also to a spectrum of CB and TB grain constituents; flavonoids, however, are one of the main factors (Vogrinčič et al. 2013).
Flavonoids (rutin, quercetin) are thus of special interest when it comes to CB and TB grain and products. Rutin tends to prevent flour deterioration in CB and TB (Suzuki et al. 2005a). According to Suzuki (2016), rutin is involved in the reactions, important in generating volatile compounds of boiled CB noodles (soba). Some volatile compounds found in his experiment are important contributors to the unique flavor of CB noodles (soba). Enzymatic activity in flour is important for flavor generation of boiled CB noodles whereas rutin does not have important function. Suzuki (2016) suggests that it is a need to develop the variety with enhanced flavor, but not easily exposed to the deterioration.
Rutin is important for protecting CB and TB plants from solar UV radiation, cold, desiccation and pests (Gaberščik et al. 2002, Kreft et al. 2002, Suzuki et al. 2005b). The combination of high levels of rutin content and the activity of the rutin-degrading enzyme rutinosidase produces a strong bitterness after grazing, which protects CB and especially TB, from being eaten by animals (Suzuki et al. 2015a). Chitarrini et al. (2014) suggest that rutin-derived quercetin is more efficient in inhibiting aflatoxin biosynthesis by Aspergillus flavus than rutin.
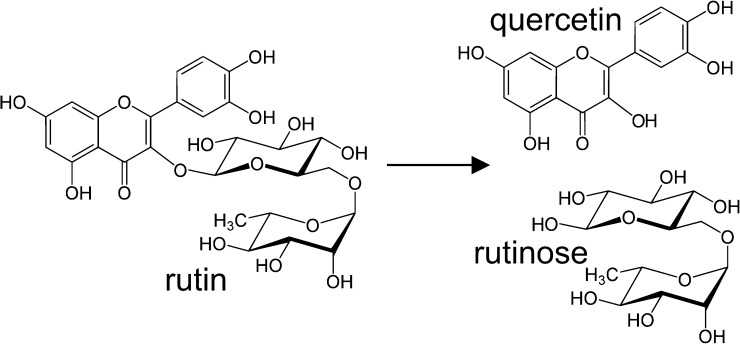
During bread making most of rutin is degraded to quercetin by rutin-degrading enzymes (Yasuda and Nakagawa 1994), thus no rutin passes untransformed during the procedure and none remains in TB bread (Fig. 2) (Germ et al. 2019, Vogrinčič et al. 2010). By scalding TB flour with hot water, a considerable amount of rutin is conserved from flour through the process to the final bread product (Lukšič et al. 2016). Suzuki et al. (2002, 2014, 2015a, 2015b, 2015c) used new trace-rutinosidase TB variety to produce wheat-TB combined bread with considerable amount of rutin, preserving about 50% of the initial rutin from TB flour in the bread. Trace-rutinosidase TB may become more popular in Japan. Among Japanese consumers, TB dishes are generally less popular since they expect “sweet” taste of soba noodles. For the markets where customers prefer gentle taste of CB and TB dishes, TB could be transferred to improve the taste of TB varieties by backcrossing the new trace-rutinosidase TB.
Fig. 2.
Rutin transformation to quercetin and rutinose.
In breeding CB and TB it is possible the use in vitro tissue culture methods to regenerate for example from one cotyledon several genetically identical regenerated plants (Luthar and Marchetti 1994). However, the possibility of regenerating plants from tissue cultures is in CB and TB limited by a high concentration of phenolic substances.
The possibility for breeding for high content of micro-elements
Differences in micro-elements distribution between CB and TB were studied by Bonafaccia et al. (2003a) and Ikeda et al. (2006). Research by Breznik et al. (2005) and Golob et al. (2015) suggested CB and TB, enriched with Se, as an important source of nutritionally Se. Pongrac et al. (2013, 2016) established that valuable essential elements, like Mg, P, S, K, Mn, Fe and Zn are located mainly in the embryo. To enhance their concentration by CB and TB breeding efforts, it could be feasible to breed for larger embryos. Cross sections of CB and TB grain are suitable to estimate the size of embryos (Kreft and Kreft 2000, Pongrac et al. 2013). Ca is concentrated in husk, allocation of Cu, which could be toxic in high concentrations due to polluted soil, is more evenly dispersed among tissues of TB grain (Pongrac et al. 2013).
Anti-nutritional factors concerning CB and TB breeding
CB and TB plants contain some fagopyrin, a phototoxic substance. It is mainly concentrated in green parts (sprouts, leaves) and less in grain (Kočevar Glavač et al. 2017, Kreft et al. 2013, Stojilkovski et al. 2013). The possibilities to avoid fagopyrin by breeding have not been studied yet. Fagopyrin could play a role in protection of plants against UV radiation, pests or diseases, so breeding for low concentration of fagopyrin may bring lateral, undesired effects.
CB and TB, like their relatives from the Polygonaceae family (like rhubarb, Rheum rhabarbarum L.), contains Ca oxalate druses and oxalic acid involved in the protection against solar radiation and against aluminum toxicity respectively (Golob et al. 2018, Klug and Horst 2010). According to Peng et al. (2002), there is a genotypic difference in aluminum resistance and oxalate exudation in CB. Genotypic differences could be the starting points for breeding for low content of oxalates in CB and TB. However, breeding aimed to reduce the concentration of oxalic acid may result in sensitivity of CB and TB plants to solar radiation or aluminum toxicity. Among anti-nutritional factors are also some metabolites with inhibitory potency against enzymes, like dietary fiber, tannins, phytates, and a protein protease inhibitor (Ikeda et al. 1986). In a study of impact on the in vitro pepsin-pancreatin digestibility of proteins, the protein inhibitor exhibited the highest inhibitory capacity among the substances examined, while that of phytate was the lowest (Ikeda et al. 1986).
Standard TB variety flour exhibits a high level of α-glucosidase inhibitory activity, whereas the newly developed cultivar ‘Manten-Kirari’ with a low level of a rutin-degrading enzyme exhibits no α-glucosidase inhibitory activity. Quercetin, present in TB grain, may be a factor, responsible for α-glucosidase inhibitory activity (Ikeda et al. 2017). These effects are interesting in regard to diabetes mellitus prevention (Ikeda et al. 2017). Quercetin may be a factor responsible for the ability of TB to lower blood sugar in patients suffering from diabetes mellitus. This research is important in the development of more powerful antidiabetes drugs and wider utilization of TB, already used in the diet of diabetic patients’ prevention (Ikeda et al. 2017, Li et al. 2009, Ren et al. 2018). TB sprouts, leaves and even TB roots extracts have a potential to be utilized as a functional food for preventing diabetes due to α-glucosidase inhibitory activity. Glucosidase inhibitors are used as drugs to treat diabetes (Li et al. 2009, Ren et al. 2018, Sharma et al. 2012).
Enzyme inhibitors lower digestibility of proteins or carbohydrates. Removal of these “anti-nutrients” by breeding would improve digestibility of metabolites in CB and TB grain, on the other hand, it could harm other, desired nutritional effects of CB and TB flour (like suppressing gallstone formation, lowering plasma cholesterol, preventing diabetes mellitus).
Conclusions, challenges and prospects for the future
It is very important to collect CB and TB samples from around the world, in countries and places where domestic varieties and wild relatives still exist. Cross-fertilized populations, like CB, are genetically and phenotypically very heterogenous. In screening CB genetic resources for valuable recessive genes, it is important to take into account that recessive genes could be hidden in the population as a part of heterozygotes. CB and TB breeding was in many places not very intense, so there could still exist in populations the genes involved in synthesis of valuable nutrients. It is necessary to evaluate populations and find valuable genes for breeding CB and TB, including breeding for improved nutritional value of CB and TB. This holds true for TB, though it is expected that plants are homozygous because of selfing and that valuable properties are expressed. However, due to less rigorous breeding of TB, many varieties are a group of self-pollinating lines. In such a mixture, it is difficult to find nutritionally valuable properties with bulk analyses, it is better to isolate lines from the populations and screen them for valuable genes individually.
It is expected that in future more attention will be focused on breeding for nutritional quality of CB and TB. Nutritional quality of CB and TB is a result of complex interactions between genes, and as well between primary and secondary plant metabolites.
Author Contribution Statement
I.K. initiated and coordinated the writing, wrote part on polyphenol-protein interactions, and conclusions, prepared the final version of the manuscript. M.Z. wrote the parts on micro-elements, anti-nutritional factors, breeding, and contributed remarks to other parts. A.G., M.G., M.L and K.D. wrote the abstract, introduction, part on rutin, rutinosidase, quercetin, and coordinated cited literature. Z.L. was leading the writing, wrote the part on starch, amylose, amylopectin, recessive genes, and contributed the figures.
Acknowledgments
This study was financed by the Slovenian Research Agency, through the programs P1-0143 “Biology of Plants” (P1-0212) and P3-0395 “Nutrition and Public Health”, and the applied project L4-9305, co-financed by the Ministry of Agriculture, Forestry and Food, Republic of Slovenia, and by applied research project “Optimisation of barley and buckwheat processing for sustainable use in high quality functional foods” (L4-7552), funded by the Slovenian Research Agency, and Valens Int d.o.o., and supported by EUFORINNO 7th FP EU Infrastructure Program (RegPot No. 315982). M.Z. acknowledges the grants received from National Key R&D program of China (2017YFE0117600). The funding organizations had no role in the design, analysis or writing of this article.
Literature Cited
- Annor G.A., Tyl C., Marcone M., Ragaee S. and Marti A. (2017) Why do millets have slower starch and protein digestibility than other cereals? Trends Food Sci. Technol. 66: 73–83. [Google Scholar]
- Bonafaccia G., Gambelli L., Fabjan N. and Kreft I. (2003a) Trace elements in flour and bran from common and Tartary buckwheat. Food Chem. 83: 1–5. [Google Scholar]
- Bonafaccia G., Marocchini M. and Kreft I. (2003b) Composition and technological properties of the flour and bran from common and tartary buckwheat. Food Chem. 80: 9–15. [Google Scholar]
- Breznik B., Germ M., Gaberščik A. and Kreft I. (2005) Combined effects of elevated UV-B radiation and the addition of selenium on common (Fagopyrum esculentum Moench) and tartary [Fagopyrum tataricum (L.) Gaertn.] buckwheat. Photosynthetica 43: 583–589. [Google Scholar]
- Chettry U., Dohtdong L. and Chrungoo N.K. (2018) Analysing structural diversity of seed storage protein gene promoters: Buckwheat a case study. Fagopyrum 35: 5–17. [Google Scholar]
- Chitarrini G., Nobili C., Pinzari F., Antonini A., De Rossi P., Del Fiore A., Procacci S., Tolaini V., Scala V., Scarpari M. et al. (2014) Buckwheat achenes antioxidant profile modulates Aspergillus flavus growth and aflatoxin production. Int. J. Food Microbiol. 189: 1–10. [DOI] [PubMed] [Google Scholar]
- Chrungoo, N., N. Devadasan and I. Kreft (2016) Waxy locus in buckwheat: implications for designer starches. In: Zhou, M., I. Kreft, S.-H. Woo, N. Chrungoo and G. Wieslander (eds.) Molecular breeding and nutritional aspects of buckwheat, Elsevier, Amsterdam, pp. 401–410. [Google Scholar]
- Costantini L., Lukšič L., Molinari R., Kreft I., Bonafaccia G., Manzi L. and Merendino N. (2014) Development of gluten-free bread using tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 165: 232–240. [DOI] [PubMed] [Google Scholar]
- Eggum B.O., Kreft I. and Javornik B. (1981) Chemical composition and protein quality of buckwheat (Fagopyrum esculentum Moench). Plant Foods Hum. Nutr. 30: 175–179. [Google Scholar]
- Gaberščik A., Vončina M., Trošt T., Germ M. and Björn L.O. (2002) Growth and production of buckwheat (Fagopyrum esculentum) treated with reduced, ambient, and enhanced UV-B radiation. J. Photochem. Photobiol. B, Biol. 66: 30–36. [DOI] [PubMed] [Google Scholar]
- Gao J., Kreft I., Chao G., Wang Y., Liu X., Wang L., Wang P., Gao X. and Feng B. (2016) Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch, a side product in functional food production, as a potential source of retrograded starch. Food Chem. 190: 552–558. [DOI] [PubMed] [Google Scholar]
- Germ M., Breznik B., Dolinar N., Kreft I. and Gaberščik A. (2013) The combined effect of water limitation and UV-B radiation on common and tartary buckwheat. Cereal Res. Commun. 41: 97–105. [Google Scholar]
- Germ M., Árvay J., Vollmannová A., Tóth T., Golob A., Luthar Z. and Kreft I. (2019) The temperature threshold for the transformation of rutin to quercetin in Tartary buckwheat dough. Food Chem. 283: 28–31. [DOI] [PubMed] [Google Scholar]
- Golob A., Stibilj V., Kreft I. and Germ M. (2015) The feasibility of using Tartary buckwheat as a Se-containing food material. J. Chem. 2015: 1–4. [Google Scholar]
- Golob A., Stibilj V., Nečemer M., Kump P., Kreft I., Hočevar A., Gaberščik A. and Germ M. (2018) Calcium oxalate druses affect leaf optical properties in selenium-treated Fagopyrum tataricum. J. Photochem. Photobiol. B, Biol. 180: 51–55. [DOI] [PubMed] [Google Scholar]
- Gregori M. and Kreft I. (2012) Breakable starch granules in a low-amylose buckwheat (Fagopyrum esculentum Moench) mutant. J. Food Agric. Environ. 10: 258–262. [Google Scholar]
- Holasova M., Fiedlerova V., Smrcinova H., Orsak M., Lachman J. and Vavreinova S. (2002) Buckwheat—the source of antioxidant activity in functional foods. Food Res. Int. 35: 207–211. [Google Scholar]
- Hung P.V., Maeda T. and Morita N. (2006) Waxy and high-amylose wheat starches and flours—characteristics, functionality and application. Trends Food Sci. Technol. 17: 448–456. [Google Scholar]
- Ikeda K., Oku M., Kusano T. and Yasumoto K. (1986) Inhibitory potency of plant antinutrients towards the in vitro digestibility of buckwheat protein. J. Food Sci. 51: 1527–1530. [Google Scholar]
- Ikeda K., Ishida Y., Ikeda S., Asami Y. and Lin R. (2017) Tartary, but not common, buckwheat inhibits α-glucosidase activity: its nutritional implications. Fagopyrum 34: 13–18. [Google Scholar]
- Ikeda S., Yamashita Y., Tomura K. and Kreft I. (2006) Nutritional comparison in mineral characteristics between buckwheat and cereals. Fagopyrum 23: 61–65. [Google Scholar]
- Javornik B., Eggum B.O. and Kreft I. (1981) Studies on protein fractions and protein quality of buckwheat. Genetika 13: 115–121. [Google Scholar]
- Javornik B. and Kreft I. (1984) Characterization of buckwheat proteins. Fagopyrum 4: 30–38. [Google Scholar]
- Kim S-L., Kim S.-K. and Park C.-H. (2004) Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Res. Int. 37: 319–327. [Google Scholar]
- Kim S.-J., Zaidul I.S.M., Suzuki T., Mukasa Y., Hashimoto N., Takigawa S., Noda T., Matsuura-Endo C. and Yamauchi H. (2008) Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 110: 814–820. [DOI] [PubMed] [Google Scholar]
- Klug B. and Horst W.J. (2010) Oxalate exudation into the root-tip water free space confers protection from aluminum toxicity and allows aluminum accumulation in the symplast in buckwheat (Fagopyrum esculentum). New Phytol. 187: 380–391. [DOI] [PubMed] [Google Scholar]
- Kočevar Glavač N., Stojilkovski K., Kreft S., Park C.H. and Kreft I. (2017) Determination of fagopyrins, rutin, and quercetin in Tartary buckwheat products. LWT - Food Sci. Technol. 79: 423–427. [Google Scholar]
- Kreft, I., C. Ries and C. Zewen (2007) Das Buchweizen Buch: mit Rezepten aus aller Welt. 2nd edn. Islek ohne Grenzen EWIV, Arzfeld, p. 269. [Google Scholar]
- Kreft M. (2016) Buckwheat phenolic metabolites in health and disease. Nutr. Res. Rev. 29: 30–39. [DOI] [PubMed] [Google Scholar]
- Kreft S. and Kreft M. (2000) Localization and morphology of the buckwheat embryo. Fagopyrum 17: 15–19. [Google Scholar]
- Kreft S., Štrukelj B., Gaberščik A. and Kreft I. (2002) Rutin in buckwheat herbs grown at different UV-B radiation levels: comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 53: 1801–1804. [DOI] [PubMed] [Google Scholar]
- Kreft S., Janes D. and Kreft I. (2013) The content of fagopyrin and polyphenols in common and tartary buckwheat sprouts. Acta Pharm. 63: 553–560. [DOI] [PubMed] [Google Scholar]
- Li Y.Q., Zhou F.C., Gao F., Bian J.S. and Shan F. (2009) Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J. Agric. Food Chem. 57: 11463–11468. [DOI] [PubMed] [Google Scholar]
- Lukšič L., Bonafaccia G., Timoracká M., Vollmannová A., Trček J., Koželj Nyambe T., Melini V., Acquistucci R., Germ M. and Kreft I. (2016) Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 69: 71–76. [Google Scholar]
- Luthar Z. and Marchetti S. (1994) Plant regeneration from mature cotyledons in a buckwheat (Fagopyrum esculentum Moench) germplasm collection. Fagopyrum 14: 65–69. [Google Scholar]
- Matsui K., Oshima Y., Mitsuda N., Sakamoto S., Nishiba Y., Walker A.R., Ohme-Takagi M., Robinson S.P., Yasui Y., Mori M. et al. (2018) Buckwheat R2R3 MYB transcription factor FeMYBF1 regulates flavonol biosynthesis. Plant Sci. 274: 466–475. [DOI] [PubMed] [Google Scholar]
- Park C.H., Kim Y.B., Choi Y.S., Heo K., Kim S.L., Lee K.C., Chang K.J. and Lee H.B. (2000) Rutin content in food products processed from groats, leaves, and flowers of buckwheat. Fagopyrum 17: 63–66. [Google Scholar]
- Peng X.X., Yu L., Yang C. and Liu Y.H. (2002) Genotypic difference in aluminum resistance and oxalate exudation of buckwheat. J. Plant Nutr. 26: 1767–1777. [Google Scholar]
- Pexová Kalinová J., Vrchotová N. and Tříska J. (2019) Phenolics levels in different parts of common buckwheat (Fagopyrum esculentum) achenes. J. Cereal Sci. 85: 243–248. [Google Scholar]
- Pongrac P., Vogel-Mikuš K., Jeromel L., Vavpetič P., Pelicon P., Kaulich B., Gianoncelli A., Eichert D., Regvar M. and Kreft I. (2013) Spatially resolved distributions of the mineral elements in the grain of Tartary buckwheat (Fagopyrum tataricum). Food Res. Int. 54: 125–131. [Google Scholar]
- Pongrac P., Kump P., Budič B. and Vogel-Mikuš K. (2016) Magnesium and phosphorus distributions in developing Tartary buckwheat cotyledons. Folia Biologica et Geologica 57: 45–56. [Google Scholar]
- Ren Q., Liu W., Zhao M., Sai C. and Wang J. (2018) Changes in α-glucosidase inhibition, antioxidant, and phytochemical profiles during the growth of Tartary buckwheat (Fagopyrum tataricum Gaertn). Int. J. Food Prop. 21: 2689–2699. [Google Scholar]
- Sharma P., Ghimeray A.K., Gurung A., Jin C.W., Rho H.S. and Cho D.H. (2012) Phenolic contents, antioxidant and α-glucosidase inhibition properties of Nepalese strain buckwheat vegetables. Afr. J. Biotechnol. 11: 184–190. [Google Scholar]
- Sikder K., Kesh S.B., Das N., Manna K. and Dey S. (2014) The high antioxidative power of quercetin (aglycone flavonoid) and its glycone (rutin) avert high cholesterol diet induced hepatotoxicity and inflammation in Swiss albino mice. Food Funct. 5: 1294–1303. [DOI] [PubMed] [Google Scholar]
- Skrabanja V. and Kreft I. (1998) Resistant starch formation following autoclaving of buckwheat (Fagopyrum esculentum Moench) groats. An in vitro study. J. Agric. Food Chem. 46: 2020–2023. [Google Scholar]
- Skrabanja V., Laerke H.N. and Kreft I. (1998) Effect of hydrothermal processing of buckwheat (Fagopyrum esculentum Moench) groats on starch enzymatic availability in vitro and in vivo in rats. J. Cereal Sci. 28: 209–221. [Google Scholar]
- Skrabanja V., Laerke H.N. and Kreft I. (2000) Protein-polyphenol interactions and in vivo digestibility of buckwheat groat proteins. Pflugers Arch. 440: R129–R131. [PubMed] [Google Scholar]
- Skrabanja V., Liljeberg Elmståhl H.G.M., Kreft I. and Björck I.M.E. (2001) Nutritional properties of starch in buckwheat products: studies in vitro and in vivo. J. Agric. Food Chem. 49: 490–496. [DOI] [PubMed] [Google Scholar]
- Skrabanja V., Kreft I., Golob T., Modic M., Ikeda S., Ikeda K., Kreft S., Bonafaccia G., Knapp M. and Kosmelj K. (2004) Nutrient content in buckwheat milling fractions. Cereal Chem. 81: 172–176. [Google Scholar]
- Stojilkovski K., Kočevar Glavač N., Kreft S. and Kreft I. (2013) Fagopyrin and flavonoid contents in common, Tartary, and cymosum buckwheat. J. Food Compost. Anal. 32: 126–130. [Google Scholar]
- Suzuki T., Honda Y., Funatsuki W. and Nakatsuka K. (2002) Purification and characterization of flavonol 3-glucosidase, and its activity during ripening in tartary buckwheat seeds. Plant Sci. 163: 417–423. [Google Scholar]
- Suzuki T., Honda Y., Mukasa Y. and Kim S. (2005a) Effects of lipase, lipoxygenase, peroxidase, and rutin on quality deteriorations in buckwheat flour. J. Agric. Food Chem. 53: 8400–8405. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Honda Y. and Mukasa Y. (2005b) Effects of UV-B radiation, cold and desiccation stress on rutin concentration and rutin glucosidase activity in tartary buckwheat (Fagopyrum tataricum) leaves. Plant Sci. 168: 1303–1307. [Google Scholar]
- Suzuki T., Morishita T., Mukasa Y., Takigawa S., Yokota S., Ishiguro K. and Noda T. (2014) Breeding of “Manten-Kirari”, a non-bitter and trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.). Breed. Sci. 64: 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Morishita T., Kim S.-J., Park S.U., Woo S.H., Noda T. and Takigawa S. (2015a) Physiological roles of rutin in the buckwheat plant. Jpn. Agric. Res. Q. 49: 37–43. [Google Scholar]
- Suzuki T., Morishita T., Noda T. and Ishiguro K. (2015b) Acute and subacute toxicity studies on rutin-rich Tartary buckwheat dough in experimental animals. J. Nutr. Sci. Vitaminol. 61: 175–181. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Morishita T., Takigawa S., Noda T. and Ishiguro K. (2015c) Characterization of rutin-rich bread made with ‘Manten-Kirari’, a trace-rutinosidase variety of Tartary buckwheat (Fagopyrum tataricum Gaertn.). Food Sci. Technol. Res. 21: 733–738. [Google Scholar]
- Suzuki, T. (2016) Flavor and lipid deterioration in buckwheat flour related to lipoxygenase pathway enzymes. In: Zhou, M., I. Kreft, S.-H. Woo, N. Chrungoo and G. Wieslander (eds.) Molecular breeding and nutritional aspects of buckwheat, Elsevier, Amsterdam, pp. 335–343. [Google Scholar]
- Tomotake H., Shimaoka I., Kayashita J., Yokoyama F., Nakajoh M. and Kato N. (2000) A buckwheat protein product suppresses gallstone formation and plasma cholesterol more strongly than soy protein isolate in hamsters. J. Nutr. 130: 1670–1674. [DOI] [PubMed] [Google Scholar]
- Vogrinčič M., Timoracka M., Melichacova S., Vollmannova A. and Kreft I. (2010) Degradation of rutin and polyphenols during the preparation of Tartary buckwheat bread. J. Agric. Food Chem. 58: 4883–4887. [DOI] [PubMed] [Google Scholar]
- Vogrinčič M., Kreft I., Filipič M. and Žegura B. (2013) Antigenotoxic effect of Tartary (Fagopyrum tataricum) and common (Fagopyrum esculentum) buckwheat flour. J. Med. Food 16: 944–952. [DOI] [PubMed] [Google Scholar]
- Vombergar B. and Luthar Z. (2018) The concentration of flavonoids, tannins and crude proteins in grain fractions of common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tataricum Gaertn.). Folia Biologica et Geologica 59: 101–157. [Google Scholar]
- Wieslander G., Fabjan N., Vogrinčič M., Kreft I., Janson C., Spetz-Nyström U., Vombergar B., Tagesson C., Leanderson P. and Norbäck D. (2011) Eating buckwheat cookies is associated with the reduction in serum levels of myeloperoxidase and cholesterol: A double blind crossover study in day-care centre staffs. Tohoku J. Exp. Med. 225: 123–130. [DOI] [PubMed] [Google Scholar]
- Wieslander G., Fabjan N., Vogrinčič M., Kreft I., Vombergar B. and Norbäck D. (2012) Effects of common and Tartary buckwheat consumption on mucosal symptoms, headache and tiredness: A double-blind crossover intervention study. J. Food Agric. Environ. 10: 107–110. [Google Scholar]
- Yang N., Li Y.M., Zhang K., Jiao R., Ma K.Y., Zhang R., Ren G. and Chen Z.-Y. (2014) Hypocholesterolemic activity of buckwheat flour is mediated by increasing sterol excretion and down-regulation of intestinal NPC1L1 and ACAT2. J. Funct. Foods 6: 311–318. [Google Scholar]
- Yasuda T. and Nakagawa H. (1994) Purification and characterization of rutin-degrading enzymes in tartary buckwheat seeds. Phytochemistry 37: 133–136. [Google Scholar]
- Zhang K., Logacheva M.D., Meng Y., Hu J., Wan D., Li L., Janovská D., Wang Z., Georgiev M.I., Yu Z. et al. (2018) Jasmonate-responsive MYB factors spatially repress rutin biosynthesis in Fagopyrum tataricum. J. Exp. Bot. 69: 1955–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]