Abstract
Buckwheat contains an abundance of antioxidants such as polyphenols and is considered a functional food. Among polyphenols, flavonoids have multiple functions in various aspects of plant growth and in flower and leaf colors. Flavonoids have antioxidant properties, and are thought to prevent cancer and cardiovascular disease. Here, we summarize the flavonoids present in various organs and their synthesis in buckwheat. We discuss the use of this information to breed highly functional and high value cultivars.
Keywords: secondary metabolite, transcription factor, Buckwheat Genome Data Base, transport, high value cultivar
1. Introduction
Common buckwheat (Fagopyrum esculentum L.) is an annual crop grown widely around the world. Flour, made from the seeds, is used in foods such as noodles and pancakes, and sprouted seeds are used as a vegetable. Buckwheat accumulates several kinds of flavonoids, such as flavonols, flavones, anthocyanins and proanthocyanidins (PAs). Flavonoids are synthesized and accumulate in a variety of tissues in many plant species and can help to protect against various stresses (Winkel-Shirley 2001, 2002). As part of a balanced diet, flavonoids reduce inflammation, enhance immunity, and lower the risks of cardiovascular disease and certain cancers (Santos-Buelga and Scalbert 2000, Yildizogluari et al. 1991).
Many researchers have identified the types and chemical structures of these flavonoids in buckwheat and have tried to alter the type and concentration by changing the growing conditions (e.g. Kalinova and Vrchotova 2009, Kim et al. 2007, 2013, Kiprovski et al. 2015, Nam et al. 2015, Park et al. 2017, Watanabe et al. 1997, 1998). Improvements in technology, such as next generation sequencing, gene expression analysis, transient assay experiments, and analysis of transgenic plants, have advanced the study of their synthesis and regulation (e.g. Gupta et al. 2011, Li et al. 2010, Matsui et al. 2018a). In particular, the creation of the Buckwheat Genome Data Base (BGDB; Yasui et al. 2016) has accelerated molecular analyses.
In this review, we describe the different classes of flavonoids present in buckwheat. We also describe enzymes, both known and putative, involved in the synthesis, transport, and regulation of each flavonoid class. Finally, we discuss the use of this information in breeding high value cultivars with improved flavonoid content.
2. Flavonoids in buckwheat: types and distribution
2-1. Distribution pattern of flavonoids in buckwheat
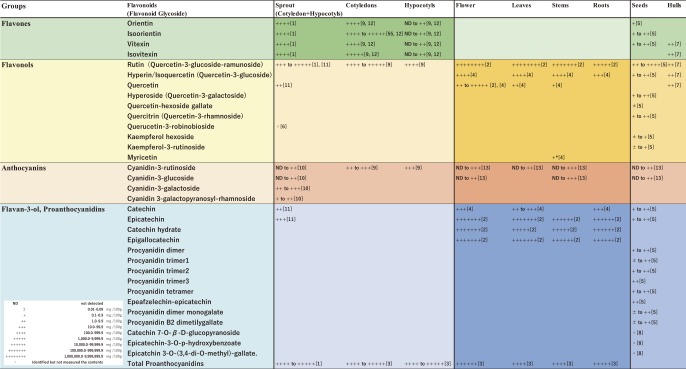
Many researchers have investigated the types and contents of flavonoids in buckwheat plants, seeds, flowers, and food products (e.g. Kalinova and Vrchotova 2009, Kim et al. 2007, 2013, Kiprovski et al. 2015, Nam et al. 2015, Park et al. 2017, Watanabe et al. 1997, 1998; Fig. 1). The flavonoids present depend on growth stage, organ, cultivar, growing area and season, and are synthesized in response to biotic and abiotic signals such as environmental conditions (e.g. Kalinova and Vrchotova 2009, Watanabe and Ito 2002).
Fig. 1.
Distribution of flavonoids in buckwheat based on previous reports. This figure is based on Supplementary Figure S1 of Matsui et al. (2018a) with added information. References: [1] Matsui et al. (2008), [2] Li et al. (2010), [3] Matsui et al. (2016), [4] Kalinova and Vrchotova (2009), [5] Kiprovski et al. (2015), [6] Nam et al. (2015), [7] Watanabe et al. (1997), [8] Watanabe (1998), [9] Watanabe (2007), [10] Kim et al. (2007), [11] Park et al. (2017), [12] Nagatomo et al. (2014), [13] Kim et al. (2013). Plant materials, growing conditions, and methods of measurement were different in each paper.
2-2. Chemical characteristics of flavonoids
2-2.1. Basic chemical structure of flavonoid groups
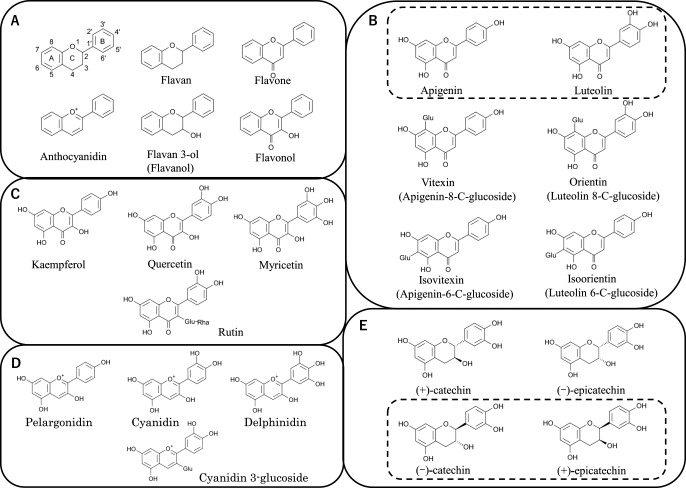
Flavonoids are divided into several groups, including flavones, flavonols, anthocyanins and PAs, by their chemical structure and the degree of saturation and oxidation of rings (Fig. 2A). They are classified as flavans when all positions in the C ring are saturated; as flavones when position 4 is a ketone and positions 2 and 3 are not saturated; and as anthocyanidins when the charge is positive and all rings are aromatic (Fig. 2A). When the C ring has an OH substitution, the suffix becomes -ol; for example, an OH at position 3 of the C ring of flavone creates flavan-3-ol, which is a flavanol (Fig. 2A). Flavonols are further classified by the substitution pattern on the B ring: position 4 and optionally positions 3 and 5 can have an OH: one at position 4 is called kaempferol, two at positions 3 and 4 is named quercetin, and three at positions 3, 4, and 5 is named myricetin (Fig. 2C).
Fig. 2.
Chemical structures of flavonoids. (A) Basic chemical structures. (B) Buckwheat flavones. Apigenin is an aglycone of vitexin and isovitexin; luteolin is an aglycone of orientin and isoorientin. However, both apigenin and luteolins themselves are not contained in buckwheat (the box with the dashed line suggests). (C) Representative flavonols and a flavonol glycoside, rutin. (D) Representative anthocyanidins. Buckwheat has only cyanidin 3-glycosides. (E) Catechins and epicatechins. Buckwheat, like most other plant species, has only (+)-catechin and (–)-epicatechin.
2-2.2. Flavones
Four flavone glycosides have been identified in buckwheat: vitexin (apigenin-8-C-glucoside), isovitexin (apigenin-6-C-glucoside), orientin (luteolin 8-C-glucoside), and isoorientin (luteolin 6-C-glucoside) (Fig. 2B). These flavone C-glycosides accumulate in cotyledons and seeds of common buckwheat (Fig. 1). In other cultivated buckwheat species, such as F. tataricum and F. cymosum, these flavones are not present (Margna et al. 1990, Watanabe and Ito 2002).
Plants synthesize C-glycosides via two known pathways (Brazier-Hicks et al. 2009, Du et al. 2010, Yonekura-Sakakibara and Hanada 2011) in which C-glycosylation is performed either before or after the flavone skeleton is completed (Sasaki et al. 2015). In buckwheat the former pathway is the major method, but it is unclear whether the latter is also utilized.
2-2.3. Flavonols (flavonol glycosides)
Flavonols and their glycosides, as well as other flavonoids, protect plants against biotic and abiotic stresses, such as UV light. They also have important roles in auxin transport (Besseau et al. 2007, Buer and Muday 2004, Peer et al. 2004, Santelia et al. 2008) and the formation of functional pollen tubes in maize (Mo et al. 1992) and tobacco (Ylstra et al. 1992). Flavonols, such as kaempferol, quercetin, and myricetin, and their glycosides are important for coloring of many flowers and fruits via copigmentation with anthocyanins (Baranac et al. 1996), (Fig. 2C). Rutin (quercetin 3-glucoside-rhamnoside also called quercetin 3-rutinoside) is the main flavonol glycoside in buckwheat and is present in most organs (Fig. 1). It is thought to be a major allelochemical by which buckwheat inhibits the growth of other plants (Golisz et al. 2007). Several other flavonol glycosides, such as isoquercetin (quercetin 3-glucoside) and quercetin, are found in buckwheat (Fig. 1).
2-2.4. Anthocyanins
Anthocyanins are glycosides of anthocyanidins. Many kinds of anthocyanins are recognized in plants and appear red, purple, or blue depending on structure and pH. Anthocyanins color many organs of buckwheat red. The part excluding sugar and organic acid bound to anthocyanin is also called “aglycones”. By the difference in the number of OH groups in the B ring, three aglycones are recognized: cyanidin, delphinidin, and pelargonidin (Fig. 2D). Buckwheat contains only cyanidin, in the forms of cyanidin-3-rutinoside (cyanidin-3-glucoside-rhamnoside), cyanidin-3-glucoside, cyanidin-3-galactoside, and cyanidin 3-galactopyranosyl-rhamnoside (Kim et al. 2007, Watanabe 2007). This specificity is related to the substrate preference of dihydroflavonol 4-reductase (DFR) of buckwheat (see 3-2.9; Katsu et al. 2017).
Anthocyanins in buckwheat accumulate mainly at the base of the stem, which fades in color toward the top, and their composition differs depending on the location in the stem (Eguchi et al. 2008). Some anthocyanins may be transported from roots or the base of the buckwheat stem to the top, since genes involved in anthocyanin synthesis are expressed in roots (Katsu et al. 2017), but further study is needed to clarify this.
2-2.5. Flavan-3-ol (flavanols)/PAs
Catechins occur as the (+) isomer and epicatechins as the (–) isomer (Fig. 2E). Buckwheat accumulates monomeric (+)-catechin and (–)-epicatechin, as well as epigallocatechin and oligomeric flavan-3-ols (Figs. 1, 2E). The latter are called PAs or condensed tannins, which form by the polymerization of flavan-3-ols and other flavonoids. They are a major group of flavonoids synthesized via the phenylpropanoid biosynthesis pathway, which has not yet been fully characterized in buckwheat or other plants.
3. Synthesis and regulation of flavonoids in buckwheat
3-1. Flavonoid biosynthesis pathway in buckwheat
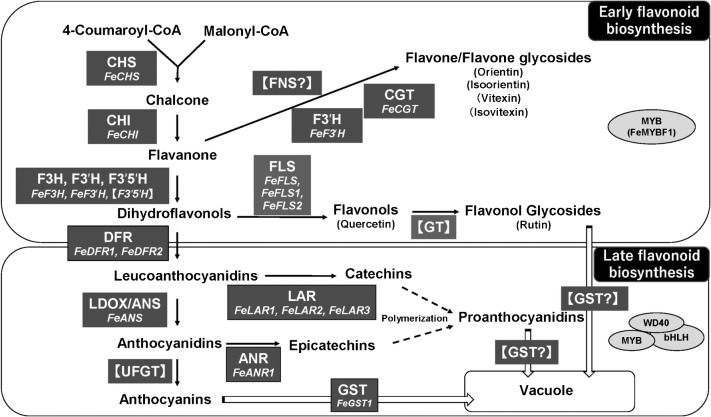
Flavonoids are synthesized via the flavonoid biosynthesis pathway in several steps which is part of the phenylpropanoid biosynthesis pathway, and is similar among plant species, although enzymes and the genes encoding them can differ. Many enzymes in the buckwheat flavonoid biosynthetic pathway remain unidentified and the flow of components through the pathway is unknown (Fig. 3). With the development of the BGDB, the identification of some genes has been accelerated.
Fig. 3.
Putative flavonoid biosynthesis pathway in buckwheat. Enzymes are indicated in upper-case letters. Identified genes are written under the enzymes. Postulated genes are indicated with [ ]. Dashed arrows indicate uncertain steps. White arrows indicate putative transportation pathways. CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′5′-hydroxylase; FLS, flavonol synthase; FNS, flavone synthase, GT, flavonol glycosyltransferase; DFR, dihydroflavonol 4-reductase; ANS/LDOX, anthocyanidin synthase/leucoanthocyanidin dioxygenase; UFGT, UDP glucose-flavonoid 3-O-glycosyltransferase; ANR, anthocyanidin reductase; LAR, leucoanthocyanidin reductase.
The flavonoid biosynthetic pathway in Arabidopsis is broadly divided into two phases, early and late (Gonzalez et al. 2008, Pelletier et al. 1997, Quattrocchio et al. 2006). Early flavonoid biosynthesis involves a major group of flavones and flavonols and is regulated by transcription factors (TFs) of the R2R3 MYB family. Late flavonoid biosynthesis includes anthocyanins and PAs and is regulated mainly by TFs of the R2R3 MYB, basic helix–loop–helix (bHLH), and WD40 repeat (WDR) protein families in many species. This model for the pathway could be a reasonable model for the pathway in buckwheat (Fig. 3). However, there seem to be some differences. In this section, we discuss key genes encoding enzymes and transcription factors in the flavonoid biosynthesis pathway in buckwheat and also discuss the differences to other plant species. We also added information of the results of the search with BGDB in each key enzyme. However, the draft genome of buckwheat in the BGDB still includes gaps between contigs because short reads were used (Yasui et al. 2016), so confirmation of the sequence of these genes will be required.
3-2. Genes of key enzymes in flavonoid biosynthesis pathway
3-2.1. CHS, chalcone synthase
CHS catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone. Two full length genes encoding CHS are registered in the database of the International Nucleotide Sequence Database Collaboration (INSDC; DDBJ/EMBL-EBI/NCBI) (HM149787 and GU172166), but it is not yet known whether these genes occur at the same locus or not. A key word search for “chalcone synthase” in the BGDB (v. 1.1) returned nine scaffolds.
3-2.2. CHI, chalcone isomerase
CHI catalyzes the conversion from chalcones to flavanones. A locus (HM149788) encoding buckwheat CHI is registered in the INSDC database. A search for “chalcone isomerase” in the BGDB identified six scaffolds with full or partial sequence similarity to CHI.
3-2.3. FNS, flavone synthase
FNS catalyzes the conversion from flavanones to flavones. No buckwheat FNS gene is registered in the INSDC database. Two types of FNS occur in plants: FNS I and FNS II; FNSI class belongs to the superfamily of soluble Fe2+/2-oxoglutarate-dependent dioxygenases (2-ODDs) and FNSII correspond to oxygen- and NADPH-dependent cytochrome P450 (CYPs) membrane-bound monooxygenases (Jiang et al. 2016, Martens and Mithofer 2005). Flavone formation in many plant species is catalyzed by FNS II (Martens and Mithofer 2005) but recently FNSI enzymes have been characterized from maize and Arabidopsis (Jiang et al. 2016). A key word search for “flavone synthase” with the BGDB returned two scaffolds with full or partial sequence similarity to FNS II. Flavones of buckwheat are all C-glycosylated: orientin, isoorientin, vitexin, and isovitexin (Nagatomo et al. 2014, Watanabe and Ito 2002). In buckwheat, these flavone glycosides are C-glycosylated before the skeleton of apigenin or luteolin flavones is completed (Nagatomo et al. 2014) indicating FNS is not required for the synthesis of flavones, so whether these genes encode actual FNS enzymes involved in flavone synthesis will need to be confirmed.
3-2.4. CGT, UDP-glucose: 2-hydroxyflavanone C-glucosyltransferase: FeCGTa and FeCGTb
C-glucosyltransferase (CGT) of buckwheat was first partially purified by Kerscher and Franz (1988). FeCGTa (UGT708C1, AB909375) and FeCGTb (UGT708C2, AB909376) were identified by Nagatomo et al. (2014) and thought to be allelic due to the high similarity of deduced amino acid sequence and observation of hybrid genes arising through a possible recombination event between FeCGTa and FeCGTb in the cDNA library (Nagatomo et al. 2014).
Buckwheat synthesizes vitexin (apigenin-8-C-glucoside), isovitexin (apigenin-6-C-glucoside), orientin (luteolin 8-C-glucoside), and isoorientin (luteolin 6-C-glucoside). FeCGTs do not perform C-glycosylation for apigenin and luteorin, but 2-hydroxyflavanones and 2-phenyl-2′,4′,6′-trihydroxyacetophenone (Nagatomo et al. 2014). Thus, flavone glycoside is C-glycosylated before the flavone skeleton is completed (Nagatomo et al. 2014). Nagatomo et al. (2014) reported differences in molecular mass, peak activity, and sugar donor between the C-glucosyltransferase reported by Kerscher and Franz (1988) and the FeCGTs that they identified. Some species, such as Gentiana, have a CGT which can catalyze direct C-glycosylation of the flavone skeleton with a C-linkage (Sasaki et al. 2015). The presence of such CGTs in buckwheat will need to be investigated.
3-2.5. F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase and F3′5′H, flavonoid 3′5′-hydroxylase
Flavanone 3-hydroxylase (HM149789) catalyzes the conversion of flavanones to dihydroflavonols. F3′H (HM149790, partial) catalyzes hydroxylation at the 3′ position of the B-ring of the flavonoid while F3′5′H catalyzes hydroxylation at the 3′ and 5′ positions of the B-ring of flavonoids. No buckwheat F3′5′H gene is registered in the INSDC database. Key word searches for these genes in the BGDB identified sixteen, two and three scaffolds with full or partial sequences respectively.
3-2.6. FLS, flavonol synthase: FeFLS FeFLS1 and FeFLS2
Flavonol synthase (FLS) converts dihydroflavonols to flavonol aglycones (kaempferol, quercetin, and myricetin) (Fig. 3). Three buckwheat loci encoding FLS (JF274261), are registered in the INSDC database. The full-length sequences of FeFLS1 (HM357804), and FeFLS2 (HM357805) are still to be identified (Li et al. 2010). FeFLS1 is expressed mainly in buds and flowers whereas FeFLS2 is expressed in roots, leaves, and stems of seedlings, and in seeds (Li et al. 2010, Matsui et al. 2018a). FeFLS is strongly expressed in flowers (Matsui et al. 2018a).
3-2.7. DFR, dihydroflavonol 4-reductase: FeDFR (ACZ48698), FeDFR1a (BBC48377), and FeDFR2 (BBC48378)
DFR is the key enzyme committed to the formation of anthocyanins and PAs in the flavonoid biosynthesis pathway (Fig. 3). It catalyzes the NADPH-dependent reduction of dihydroflavonols to leucoanthocyanidins, including dihydrokaempferol, dihydroquercetin, and dihydromyricetin to leucopelargonidin, leucocyanidin, and leucodelphinidin, respectively, in many species (Fig. 1). Although DFR proteins in many plants can catalyze these three substrates, some species cannot produce some leucoanthocyanidins, so some forms of DFR may have substrate preferences (Forkmann and Ruhnau 1987, Gerats et al. 1982, Johnson et al. 1999, 2001). The relationships between substrate preference and amino acids in the region responsible for substrate specificity have been investigated in several plant species (Johnson et al. 1999, Leonard et al. 2008, Shimada et al. 2005, Xie et al. 2004).
In buckwheat, anthocyanins accumulate in several organs, notably stems and petioles, coloring them red. On the other hand, PAs accumulate in most organs. In buckwheat seeds, cyanidin and pelargonidin were identified by acid treatment of PAs (Durkee 1977, Ölschläger et al. 2008). Furthermore, (–)-epigallocatechin, which is synthesized by anthocyanidin reductase from delphinidin, was detected in stems, leaves, and flowers (Li et al. 2010). However, only cyanidin is recognized as an aglycone of buckwheat anthocyanins (Kim et al. 2007, Troyer 1964).
Two gene loci, FeDFR1 and FeDFR2, and one allele of FeDFR1, FeDFR1a, have been reported (Katsu et al. 2017). FeDFR2 has sequence similarity to FeDFR1a but with a different exon–intron structure. The two loci are genetically linked but not allelic. Unlike common DFR proteins in other plant species, FeDFR2 has a valine instead of the typical asparagine at the position 3 and an extra glycine between position 6 and 7 in the region that determines substrate specificity (Johnson et al. 2001), and has less activity using dihydrokaempferol as a substrate than FeDFR1a, which has an asparagine at the position 3 (Katsu et al. 2017). The substrate preferences of buckwheat DFR would be determined not only by the amino acid at the third position of the 26-amino-acid region identified to determine substrate specificity, but also by its neighboring residues that are involved in the formation of the substrate-binding pocket (Katsu et al. 2017).
3-2.8. ANS, anthocyanidin synthase/leucoanthocyanidin dioxygenase
ANS catalyzes the conversion of leucoanthocyanidins to anthocyanidins. Buckwheat ANS (ADT63066) was first reported by Li et al. (2010) and is the only the locus encoding buckwheat ANS that is registered in the INSDC database. A search for “anthocyanidin synthase” in the BGDB also returned one scaffold validating the hypothesis of a single gene.
3-2.9. ANR, anthocyanidin reductase, FeANR1
ANR catalyzes the conversion of anthocyanidins to (–)-epicatechins. A buckwheat ANR gene (BAV56574) was identified by degenerate PCR and RACE (Matsui et al. 2016). Only the locus encoding buckwheat ANR is registered in the INSDC database and a search for “anthocyanidin reductase” in the BGDB identified one scaffold. Matsui et al. (2016) reported that the expression of FeANR1 was high in roots and cotyledons and resembled the pattern of PA accumulation.
3-2.10. LAR, leucoanthocyanidin reductase: FeLAR1 FeLAR2 FeLAR3
LAR can convert leucocyanidins to (+)-catechins. In buckwheat, three loci, FeLAR1–FeLAR3, (BAV56575, BAV56576, BAV56577), have been characterized (Matsui et al. 2016). These genes were identified by sequence homology with other plant species and searches in the BGDB. FeLAR1–FeLAR3 are expressed in most organs but have different expression patterns in different organs at the flowering stage and in seeds at different maturation stages. The expression of FeLAR1 is high in cotyledons and low in roots and hypocotyls, and FeLAR2 and FeLAR3 are expressed mainly in roots. The unique expression patterns of FeLAR1–FeLAR3 suggest that all three genes play a role in PA synthesis in a distinct spatiotemporal manner (Matsui et al. 2016).
3-3. Regulation of flavonoid biosynthesis by transcription factors
The expression of genes encoding enzymes of the flavonoid biosynthesis pathway is regulated by many TFs, several of which have been well studied in various plant species (e.g. Bogs et al. 2007, Czemmel et al. 2009, Kobayashi et al. 2004, Lloyd et al. 1992, Mehrtens et al. 2005, Takos et al. 2006, Walker et al. 2007). Most of them belong to the two largest families of TFs in plants: the R2R3 MYB family and the bHLH family. As yet there is no report of a bHLH related to flavonoid biosynthesis in buckwheat.
3-3.1. MYB transcription factors
MYB proteins are believed to be key factors because of the specific expression patterns of their encoding genes (Stracke et al. 2001). The MBW complex (MYB factor– bHLH factor–WD40 protein) is required for regulation of the expression of genes encoding enzymes in the late flavonoid biosynthesis pathway (Fig. 3) in many species (e.g. Baudry et al. 2004, Gonzalez et al. 2008, Quattrocchio et al. 1998, Walker et al. 1999), but not those in the early pathway (flavonol synthesis) in Arabidopsis (Mehrtens et al. 2005, Stracke et al. 2007, Zimmermann et al. 2004).
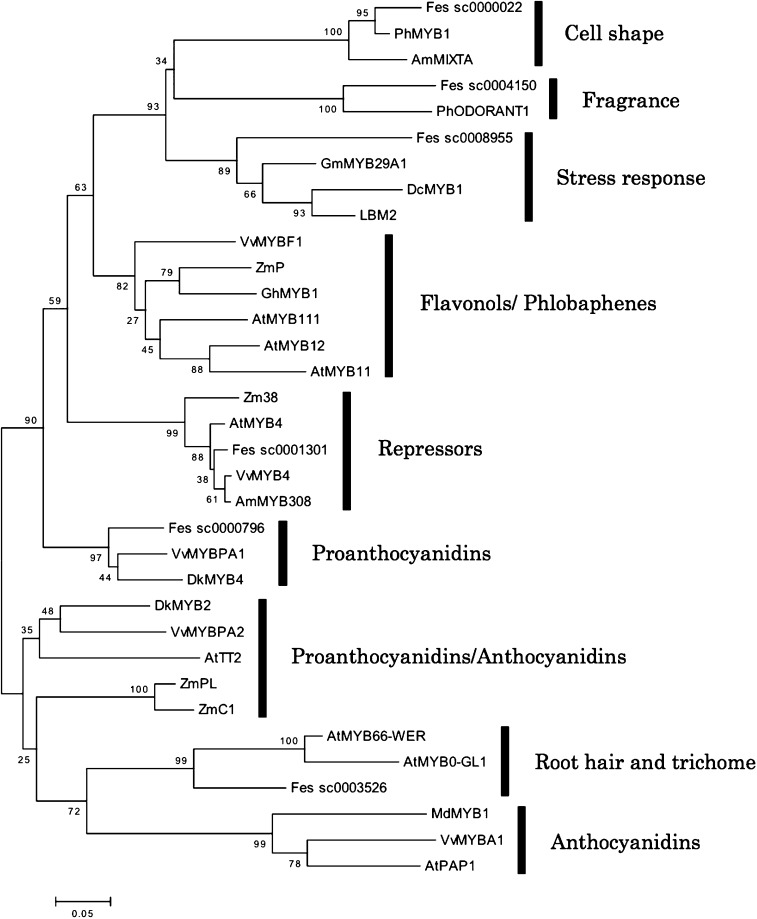
Yasui et al. (2016) identified 274 potential MYB TFs in the BGDB using “MYB” in a key word search. After exclusion of partial sequences, pseudogenes, and genes that did not contain fully conserved R2R3 regions, 71 putative R2R3 MYB TFs remained. Six putative R2R3 MYBs were assigned within known functional groups consisting of representatives from other plant species (Yasui et al. 2016; Fig. 4). Matsui et al. (2018a) reported a flavonol-specific MYB TF, FeMYBF1 (see 3-3.2), but a gene encoding FeMYBF1 was not found among the 71 candidate genes. The buckwheat genome has 16 chromosomes (2n = 2x = 16), but the draft genome in the BGDB (FES_r1.0) includes many gaps between contigs due to the use of short reads (Yasui et al. 2016), and still includes 387 594 scaffolds. Improvement of BGDB and functional studies would be needed to identify more MYB TFs in buckwheat.
Fig. 4.
A phylogenetic tree for six putative R2R3 MYB transcription factors with representatives from other plants. Bootstrap values (500 replicates) are shown next to the branches. The scale bar corresponds to 0.05 substitutions per site. The root was assumed at the midpoint of the tree. This figure was derived from Supplementary Figure S2 of Yasui et al. (2016).
3-3.2. Flavonol-specific R2R3 MYB transcription factor in buckwheat
As rutin is a major flavonoid in buckwheat, it will be important to clarify the mechanisms of rutin synthesis and regulation. FeMYBF1, an R2R3 MYB TF, was identified as a regulator of flavonol synthesis (Matsui et al. 2018a). It was isolated using degenerate primers and its full-length sequence was determined by RACE and genome walking. By phylogenetic analysis with the deduced amino acid sequences of the R2R3 MYB domains including Arabidopsis regulators of flavonol synthesis, AtMYB11, AtMYB12 and AtMYB111, FeMYBF1 was inferred to be a MYB protein with the ability to regulate flavonol synthesis (Matsui et al. 2018a).
The function of FeMYBF1 was demonstrated by introducing FeMYBF1 under the control of the AtMYB111 promoter into the flavonol-deficient Arabidopsis triple mutant myb11 myb12 myb111 where it was shown to complement these mutations. Matsui et al. (2018a) investigated whether FeMYBF1 can complement PA synthesis by introducing FeMYBF1 under the control of the AtTT2 promoter into a PA-deficient Arabidopsis tt2 mutant, but the failure to restore PA synthesis suggests that FeMYBF1 does not regulate this process.
The WDR protein complex regulates late flavonoid biosynthesis in many species. But FeMYBF1 activated the promoter of one of the DFRs committed to anthocyanin and PA in the flavonoid biosynthesis pathway without the WDR complex (Matsui et al. 2018a). Maize DFR, which functions in the synthesis of reddish or brownish water-insoluble pigments called phlobaphenes, is activated by a MYB TF, P, alone (Grotewold et al. 1994, Pooma et al. 2002). In buckwheat, FeDFR1a could probably be activated by FeMYBF1 and may contributes to flavonol synthesis through an alternate pathway, and FeDFR2 could be activated by different TFs that may participate in the formation of the MBW complex and contribute to anthocyanin synthesis (Matsui et al. 2018a). Furthermore, FeMYBF1 activated the promoter of ANS/LDOX, which is involved in catalyzing the conversion of leucoanthocyanidins to anthocyanidins. Arabidopsis LDOX has FLS-like side activity (Stracke et al. 2009), so buckwheat LDOX may contribute to the synthesis of flavonols in addition to anthocyanidins (Matsui et al. 2018a).
4. Transport of flavonoids
4-1. Transport of flavonoids to vacuole
Several mechanisms for flavonoid transport have been proposed and demonstrated, such as vesicle trafficking, involvement of membrane transporters, and glutathione S-transferase (GST)-mediated transport (Zhao et al. 2010). In buckwheat, a gene related to transport of anthocyanins has been reported (Matsui et al. 2018b).
The basal stem color in wild-type buckwheat is red owing to the accumulation of anthocyanins. Matsui et al. (2008) reported a green-stem mutant line that cannot accumulate anthocyanins but does synthesise rutin and PAs. The glucosyltransferase (GT) activity in the green stem was similar to that in red-stem plants, indicating that the green-stem phenotype is not caused by non-functional GT, but instead seems to be caused by dysfunction of the system that transports anthocyanins to the vacuole.
In phylogenetic analysis of the 56 buckwheat candidate GST genes from the BGDB with six GST genes known to play a role in anthocyanin accumulation, five of the genes (the exception was Bz2) were classified together with a candidate gene, Fes_sc0002828.1.g000005.aua.1 (named FeGST1) (Matsui et al. 2018b). FeGST1 transcripts were present in the stem of wild-type cultivars but not in that of the green-stem lines. Interestingly, the genomic region around the FeGST1 locus was deleted in the mutant, indicating that the null allele of FeGST1 is caused by deletion of the FeGST1 locus. Linkage analysis with a self-compatible line showed that the green-stem locus is homozygous for the deletion of FeGST1 (Matsui et al. 2018b).
4-2. Movement of flavonoids between organs
Reports that the expression of genes related to flavonoid synthesis in buckwheat is not correlated with the amount of flavonoids (Li et al. 2010, Matsui et al. 2016) suggest the involvement of other, non-cell-autonomous mechanisms for the accumulation of flavonoids, such as transport from other tissues. Buer et al. (2007) reported that exogenously applied naringenin, dihydrokaempferol, and dihydroquercetin were taken up at the root tip, midroot, or cotyledons and traveled long distances via cell-to-cell movement to distal tissues. However, quercetin and kaempferol were taken up only at the root tip and were not transported. Some flavonoids are released from roots (Hungria et al. 1991, Pueppke et al. 1998, Yoo et al. 2013), but there are no reports that flavonols move between cells and travel long distances. Although buckwheat uses rutin as an allelochemical (Golisz et al. 2007, 2008), it is still unclear whether rutin can be directly released from the roots.
One possible mechanism buckwheat might use to transport flavonoids long distances via cell-to-cell movement relies on aluminum. Unlike other crops, buckwheat is resistant to aluminum: it takes up aluminum in the roots and transfers it to leaves and stems where it accumulates in vacuoles (Ma et al. 1997). Aluminum can bind with flavonoids, including flavonols, altering flower color (Tanikawa et al. 2008). Because there is no report of the relationship between the movement of flavonols and aluminum, further study is desirable.
5. Breeding for high-value buckwheat cultivars using information on flavonoid biosynthesis pathway and key genes
5-1. Development of non-discoloring line by stopping biosynthesis of PAs
PAs have important roles in the mouth feel and astringency of many fruits and in the quality of wine and tea (Aron and Kennedy 2008). They can also cause undesirable colloidal haze in beer (Gramshaw 1970) and discoloration in cooked barley grains (Tonooka et al. 2010). Buckwheat kernels also turn brown by oxidation of PAs after the hull is shed.
PA-free mutants of barley have been detected (von Wettstein et al. 1977), and a PA-free barley cultivar has been developed from a PA-free line recessive-homozygous for ant28 (Himi et al. 2012, Tonooka et al. 2010). To produce a PA-free buckwheat cultivar would be valuable, because Japanese consumers prefer pale buckwheat.
The first step toward this goal is to identify genes related to PA synthesis and regulation in buckwheat. Many genes related to PA synthesis have been reported, but not all can be used for this purpose: because consumers expect to get functional substances, especially rutin, in buckwheat, it will be necessary to breed lines that can synthesize high levels of rutin but not PAs. To produce such lines, genes for DFRs and TFs which regulate PA synthesis would be candidate targets for manipulating flavonoid composition. Two genes for DFR have been identified and could be used in breeding if mutant versions can be discovered. On the other hand, the TFs that regulate PA synthesis in buckwheat are not yet known. Yasui et al. (2016) reported a gene which was clustered with known regulators of PAs, such as the grapevine VvMYBPA1 (Fig. 4), but its function will need to be determined if it is to be used as a target gene.
Finding lines with nonfunctional genes caused by mutations would be the next step. The Targeting Induced Local Lesions In Genomes (TILLING) method, which is based on reverse genetics, is a powerful method of producing lines with mutated genes of interest. Using TILLING, valuable lines such as waxy wheat have been produced (Slade et al. 2005). But genes which govern traits of interest must first be found.
5-2. Development of a high-antioxidant line by increasing flavonoids
Matsui et al. (2018a) demonstrated the ability of FeMYBF1 to regulate flavonol synthesis in Arabidopsis, and to efficiently upregulate it in transgenic Arabidopsis plants. By altering the flux through the flavonoid biosynthesis pathway, it might be possible to produce cultivars with higher level of flavonoids, especially rutin. Unlike Tartary buckwheat, common buckwheat has a low level of rutinosidase, which catalyzes rutin to quercetin (Suzuki et al. 2002), so the bitterness which is thought to be due to quercetin would not be present in common buckwheat with its high rutin content.
However, FeMYBF1 may regulate genes in other parts of the phenylpropanoid biosynthetic pathway (Matsui et al. 2018a), because Arabidopsis MYB TFs AtMYB11 and AtMYB12, which regulate flavonol synthesis, also regulate the expression of genes related to caffeoylquinic acid in tomato (Li et al. 2015, Luo et al. 2008) and tobacco (Li et al. 2015). To use FeMYBF1 in breeding, further study would be needed to clarify whether it can regulate the expression of other genes involved in other metabolic pathways.
FeMYBF1 could be used to increase flavonoid content and to improve flavonoid composition by using plant transformation techniques, including genome editing or a cisgenic approach, although it is still difficult to transform buckwheat. Molecular techniques such as marker-assisted selection and TILLING combined with conventional breeding would also accelerate the breeding of high-antioxidant lines by increasing the content of rutin in buckwheat.
Author Contribution Statement
K.M. and M.W. wrote the manuscript. Both authors have read and approved the final version of the manuscript.
Literature Cited
- Aron P.M. and Kennedy J.A. (2008) Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 52: 79–104. [DOI] [PubMed] [Google Scholar]
- Baranac J.M., Petranovic N.A. and Dimitric Markovic J.M. (1996) Spectrophotometric study of anthocyan copigmentation reactions. J. Agric. Food Chem. 44: 1333–1336. [DOI] [PubMed] [Google Scholar]
- Baudry A., Heim M.A., Dubreucq B., Caboche M., Weisshaar B. and Lepiniec L. (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 39: 366–380. [DOI] [PubMed] [Google Scholar]
- Besseau S., Hoffmann L., Geoffroy P., Lapierre C., Pollet B. and Legrand M. (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19: 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogs J., Jaffe F.W., Takos A.M., Walker A.R. and Robinson S.P. (2007) The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 143: 1347–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazier-Hicks M., Evans K.M., Gershater M.C., Puschmann H., Steel P.G. and Edwards R. (2009) The C-glycosylation of flavonoids in cereals. J. Biol. Chem. 284: 17926–17934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer C.S. and Muday G.K. (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16: 1191–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer C.S., Muday G.K. and Djordjevic M.A. (2007) Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol. 145: 478–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czemmel S., Stracke R., Weisshaar B., Cordon N., Harris N.N., Walker A.R., Robinson S.P. and Bogs J. (2009) The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 151: 1513–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.Z., Yang X.W., Han H., Cai X.H. and Luo X.D. (2010) A new flavone C-glycoside from Clematis rehderiana. Molecules 15: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee A.B. (1977) Polyphenols of bran-aleurone fraction of buckwheat seed (Fagopyrum sagittatum Gilib.). J. Agric. Food Chem. 25: 286–287. [Google Scholar]
- Eguchi K., Matsui K., Oki T. and Sato T. (2008) Variation of anthocyanin types at different nodal positions and growth stages in common buckwheat (Fagopyrum esculentum Moench). Fagopyrum 25: 9–14. [Google Scholar]
- Forkmann G. and Ruhnau B. (1987) Distinct substrate-specificity of dihydroflavonol 4-reductase from flowers of Petunia hybrida. Z. Naturforsch., C, J. Biosci. 42: 1146–1148. [Google Scholar]
- Gerats A.G.M., De Vlaming P., Doodeman M., Al B. and Schram A.W. (1982) Genetic-control of the conversion of dihydroflavonols into flavonols and anthocyanins in flowers of Petunia hybrida. Planta 155: 364–368. [DOI] [PubMed] [Google Scholar]
- Golisz A., Lata B., Gawronski S.W. and Fujii Y. (2007) Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol. Manag. 7: 164–171. [Google Scholar]
- Golisz A., Sugano M. and Fujii Y. (2008) Microarray expression profiling of Arabidopsis thaliana L. in response to allelochemicals identified in buckwheat. J. Exp. Bot. 59: 3099–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Zhao M., Leavitt J.M. and Lloyd A.M. (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 53: 814–827. [DOI] [PubMed] [Google Scholar]
- Gramshaw J.W. (1970) Beer polyphenols and the chemical basis of haze formation. II. Changes in polyphenols during the brewing and storage of beer—The composition of hazes. Master Brew. Assoc. Am. Tech. Q. 7: 122–133. [Google Scholar]
- Grotewold E., Drummond B.J., Bowen B. and Peterson T. (1994) The myb-homologous P gene controls phlobaphene pigmentation in maize floral organs by directly activating a flavonoid biosynthesis gene subset. Cell 76: 543–553. [DOI] [PubMed] [Google Scholar]
- Gupta N., Sharma S.K., Rana J.C. and Chauhan R.S. (2011) Expression of flavonoid biosynthesis genes vis-a-vis rutin content variation in different growth stages of Fagopyrum species. J. Plant Physiol. 168: 2117–2123. [DOI] [PubMed] [Google Scholar]
- Himi E., Yamashita Y., Haruyama N., Yanagisawa T., Maekawa M. and Taketa S. (2012) Ant28 gene for proanthocyanidin synthesis encoding the R2R3 MYB domain protein (Hvmyb10) highly affects grain dormancy in barley. Euphytica 188: 141–151. [Google Scholar]
- Hungria M., Joseph C.M. and Phillips D.A. (1991) Rhizobium nod gene inducers exuded naturally from roots of common bean (Phaseolus vulgaris L.). Plant Physiol. 97: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.T., Yi H.K., Shin B.C., Oh B.J., Cheong H.S. and Choi G. (1999) Cymbidium hybrida dihydroflavonol 4-reductase does not efficiently reduce dihydrokaempferol to produce orange pelargonidin-type anthocyanins. Plant J. 19: 81–85. [DOI] [PubMed] [Google Scholar]
- Johnson E.T., Ryu S., Yi H.K., Shin B., Cheong H. and Choi G. (2001) Alteration of a single amino acid changes the substrate specificity of dihydroflavonol 4-reductase. Plant J. 25: 325–333. [DOI] [PubMed] [Google Scholar]
- Kalinova J. and Vrchotova N. (2009) Level of catechin, myricetin, quercetin and isoquercitrin in buckwheat (Fagopyrum esculentum Moench), changes of their levels during vegetation and their effect on the growth of selected weeds. J. Agric. Food Chem. 57: 2719–2725. [DOI] [PubMed] [Google Scholar]
- Katsu K., Suzuki R., Tsuchiya W., Inagaki N., Yamazaki T., Hisano T., Yasui Y., Komori T., Koshio M., Kubota S. et al. (2017) A new buckwheat dihydroflavonol 4-reductase (DFR), with a unique substrate binding structure, has altered substrate specificity. BMC Plant Biol. 17: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher F. and Franz G. (1988) Isolation and some properties of an UDP-glucose: 2-hydroxyflavanone-6(or 8)-C-glucosyltransferase from Fagopyrum esculentum M. cotyledons. J. Plant Physiol. 132: 110–115. [Google Scholar]
- Kim S.J., Maeda T., Sarker M.Z.I., Takigawa S., Matsuura-Endo C., Yamauchi H., Mukasa Y., Saito K., Hashimoto N., Noda T. et al. (2007) Identification of anthocyanins in the sprouts of buckwheat. J. Agric. Food Chem. 55: 6314–6318. [DOI] [PubMed] [Google Scholar]
- Kim Y.B., Park S.Y., Thwe A.A., Seo J.M., Suzuki T., Kim S.J., Kim J.K. and Park S.U. (2013) Metabolomic analysis and differential expression of anthocyanin biosynthetic genes in white- and red-flowered buckwheat cultivars (Fagopyrum esculentum). J. Agric. Food Chem. 61: 10525–10533. [DOI] [PubMed] [Google Scholar]
- Kiprovski B., Mikulic-Petkovsek M., Slatnar A., Veberic R., Stampar F., Malencic D. and Latkovic D. (2015) Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 185: 41–47. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Goto-Yamamoto N. and Hirochika H. (2004) Retrotransposon-induced mutations in grape skin color. Science 304: 982. [DOI] [PubMed] [Google Scholar]
- Leonard E., Yan Y., Chemler J., Matern U., Martens S. and Koffas M.A.G. (2008) Characterization of dihydroflavonol 4-reductases for recombinant plant pigment biosynthesis applications. Biocatal. Biotransformation 26: 243–251. [Google Scholar]
- Li X.H., Il Park N., Xu H., Woo S.H., Park C.H. and Park S.U. (2010) Differential expression of flavonoid biosynthesis genes and accumulation of phenolic compounds in common buckwheat (Fagopyrum esculentum). J. Agric. Food Chem. 58: 12176–12181. [DOI] [PubMed] [Google Scholar]
- Li Y., Chen M., Wang S.L., Ning J., Ding X.H. and Chu Z.H. (2015) AtMYB11 regulates caffeoylquinic acid and flavonol synthesis in tomato and tobacco. Plant Cell Tissue Organ Cult. 122: 309–319. [Google Scholar]
- Lloyd A.M., Walbot V. and Davis R.W. (1992) Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1. Science 258: 1773–1775. [DOI] [PubMed] [Google Scholar]
- Luo J., Butelli E., Hill L., Parr A., Niggeweg R., Bailey P., Weisshaar B. and Martin C. (2008) AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: expression in fruit results in very high levels of both types of polyphenol. Plant J. 56: 316–326. [DOI] [PubMed] [Google Scholar]
- Ma J.F., Zheng S.J., Matsumoto H. and Hiradate S. (1997) Detoxifying aluminium with buckwheat. Nature 390: 569–570.9403684 [Google Scholar]
- Margna U., Margna E. and Paluteder A. (1990) Localization and distribution of flavonoids in buckwheat seedling cotyledons. J. Plant Physiol. 136: 166–171. [Google Scholar]
- Martens S. and Mithofer A. (2005) Flavones and flavone synthases. Phytochemistry 66: 2399–2407. [DOI] [PubMed] [Google Scholar]
- Matsui K., Eguchi K. and Tetsuka T. (2008) A novel gene that diverts the anthocyanin biosynthetic pathway towards the production of proanthocyanidins in common buckwheat (Fagopyrum esculentum). Breed. Sci. 58: 143–148. [Google Scholar]
- Matsui K., Hisano T., Yasui Y., Mori M., Walker A.R., Morishita T. and Katsu K. (2016) Isolation and characterization of genes encoding leucoanthocyanidin reductase (FeLAR) and anthocyanidin reductase (FeANR) in buckwheat (Fagopyrum esculentum). J. Plant Physiol. 205: 41–47. [DOI] [PubMed] [Google Scholar]
- Matsui K., Oshima Y., Mitsuda N., Sakamoto S., Nishiba Y., Walker A.R., Ohme-Takagi M., Robinson S.P., Yasui Y., Mori M. et al. (2018a) Buckwheat R2R3 MYB transcription factor FeMYBF1 regulates flavonol biosynthesis. Plant Sci. 274: 466–475. [DOI] [PubMed] [Google Scholar]
- Matsui K., Tomatsu T., Kinouchi S., Suzuki T. and Sato T. (2018b) Identification of a gene encoding glutathione S-transferase that is related to anthocyanin accumulation in buckwheat (Fagopyrum esculentum). J. Plant Physiol. 231: 291–296. [DOI] [PubMed] [Google Scholar]
- Mehrtens F., Kranz H., Bednarek P. and Weisshaar B. (2005) The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol. 138: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Y.Y., Nagel C. and Taylor L.P. (1992) Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc. Natl. Acad. Sci. USA 89: 7213–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatomo Y., Usui S., Ito T., Kato A., Shimosaka M. and Taguchi G. (2014) Purification, molecular cloning and functional characterization of flavonoid C-glucosyltransferases from Fagopyrum esculentum M. (buckwheat) cotyledon. Plant J. 80: 437–448. [DOI] [PubMed] [Google Scholar]
- Nam T.-G., Lee S.M., Park J.-H., Kim D.-O., Baek N.-I. and Eom S.H. (2015) Flavonoid analysis of buckwheat sprouts. Food Chem. 170: 97–101. [DOI] [PubMed] [Google Scholar]
- Ölschläger C., Regos I., Zeller F.J. and Treutter D. (2008) Identification of galloylated propelargonidins and procyanidins in buckwheat grain and quantification of rutin and flavanols from homostylous hybrids originating from F. esculentum × F. homotropicum. Phytochemistry 69: 1389–1397. [DOI] [PubMed] [Google Scholar]
- Park, C.H., H.J. Yeo, Y.J. Park, A.M.A. Morgan, M.V. Arasu, N.A. Al-Dhabi and S.U. Park (2017) Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer W.A., Bandyopadhyay A., Blakeslee J.J., Makam S.N., Chen R.J., Masson P.H. and Murphy A.S. (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16: 1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier M.K., Murrell J.R. and Shirley B.W. (1997) Characterization of flavonol synthase and leucoanthocyanidin dioxygenase genes in Arabidopsis. Further evidence for differential regulation of “early” and “late” genes. Plant Physiol. 113: 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooma W., Gersos C. and Grotewold E. (2002) Transposon insertions in the promoter of the Zea mays a1 gene differentially affect transcription by the Myb factors P and C1. Genetics 161: 793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pueppke S.G., Bolanos-Vasquez M.C., Werner D., Bec-Ferte M.P., Prome J.C. and Krishnan H.B. (1998) Release of flavonoids by the soybean cultivars McCall and Peking and their perception as signals by the nitrogen-fixing symbiont Sinorhizobium fredii. Plant Physiol. 117: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrocchio F., Wing J.F., van der Woude K., Mol J.N.M. and Koes R. (1998) Analysis of bHLH and MYB domain proteins: species-specific regulatory differences are caused by divergent evolution of target anthocyanin genes. Plant J. 13: 475–488. [DOI] [PubMed] [Google Scholar]
- Quattrocchio F., Verweij W., Kroon A., Spelt C., Mol J. and Koes R. (2006) PH4 of petunia is an R2R3 MYB protein that activates vacuolar acidification through interactions with basic-helix-loop-helix transcription factors of the anthocyanin pathway. Plant Cell 18: 1274–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D., Henrichs S., Vincenzetti V., Sauer M., Bigler L., Klein M., Bailly A., Lee Y., Friml J., Geisler M. et al. (2008) Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J. Biol. Chem. 283: 31218–31226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Buelga C. and Scalbert A. (2000) Proanthocyanidins and tannin-like compounds—nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 80: 1094–1117. [Google Scholar]
- Sasaki N., Nishizaki Y., Yamada E., Tatsuzawa F., Nakatsuka T., Takahashi H. and Nishihara M. (2015) Identification of the glucosyltransferase that mediates direct flavone C-glucosylation in Gentiana triflora. FEBS Lett. 589: 182–187. [DOI] [PubMed] [Google Scholar]
- Shimada N., Sasaki R., Sato S., Kaneko T., Tabata S., Aoki T. and Ayabe S. (2005) A comprehensive analysis of six dihydroflavonol 4-reductases encoded by a gene cluster of the Lotus japonicus genome. J. Exp. Bot. 56: 2573–2585. [DOI] [PubMed] [Google Scholar]
- Slade A.J., Fuerstenberg S.I., Loeffler D., Steine M.N. and Facciotti D. (2005) A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat. Biotechnol. 23: 75–81. [DOI] [PubMed] [Google Scholar]
- Stracke R., Werber M. and Weisshaar B. (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 4: 447–456. [DOI] [PubMed] [Google Scholar]
- Stracke R., Ishihara H., Barsch G.H.A., Mehrtens F., Niehaus K. and Weisshaar B. (2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 50: 660–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R., De Vos R.C.H., Bartelniewoehner L., Ishihara H., Sagasser M., Martens S. and Weisshaar B. (2009) Metabolomic and genetic analyses of flavonol synthesis in Arabidopsis thaliana support the in vivo involvement of leucoanthocyanidin dioxygenase. Planta 229: 427–445. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Honda Y., Funatsuki W. and Nakatsuka K. (2002) Purification and characterization of flavonol 3-glucosidase, and its activity during ripening in Tartary buckwheat seeds. Plant Sci. 163: 417–423. [Google Scholar]
- Takos A.M., Jaffe F.W., Jacob S.R., Bogs J., Robinson S.P. and Walker A.R. (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol. 142: 1216–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanikawa N., Kashiwabara T., Hokura A., Abe T., Shibata M. and Nakayama M. (2008) A peculiar yellow flower coloration of camellia using aluminum-flavonoid interaction. J. Japan. Soc. Hort. Sci. 77: 402–407. [Google Scholar]
- Tonooka T., Kawada N., Yoshida M., Yoshioka T., Oda S., Hatta K., Hatano T., Fujita M. and Kubo K. (2010) Breeding of a new food barley cultivar ‘Shiratae Nijo’ exhibiting no after-cooking discoloration. Breed. Sci. 60: 172–176. [Google Scholar]
- Troyer J.R. (1964) Anthocyanin formation in excised segments of buckwheat-seedling hypocotyls. Plant Physiol. 39: 907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein D., Jende-Strid B., Ahrenst-Larsen B. and Sørensen J.A. (1977) Biochemical mutant in barley renders chemical stabilization of beer superfluous. Carlsberg Res. Commun. 42: 341–351. [Google Scholar]
- Walker A.R., Davison P.A., Bolognesi-Winfield A.C., James C.M., Srinivasan N., Blundell T.L., Esch J.J., Marks M.D. and Gray J.C. (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11: 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.R., Lee E., Bogs J., McDavid D.A.J., Thomas M.R. and Robinson S.P. (2007) White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 49: 772–785. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Ohshita Y. and Tsushida T. (1997) Antioxidant compounds from buckwheat (Fagopyrum esculentum Moench) hulls. J. Agric. Food Chem. 45: 1039–1044. [Google Scholar]
- Watanabe M. (1998) Catechins as antioxidants from buckwheat (Fagopyrum esculentum Moench) groats. J. Agric. Food Chem. 46: 839–845. [Google Scholar]
- Watanabe M. and Ito M. (2002) Changes in antioxidative activity and flavonoid composition of the extracts from aerial parts of buckwheat during growth period. J. Jpn. Soc. Food Sci. Technol. 49: 119–125. [Google Scholar]
- Watanabe M. (2007) An anthocyanin compound in buckwheat sprouts and its contribution to antioxidant capacity. Biosci. Biotechnol. Biochem. 71: 579–582. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 126: 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B. (2002) Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 5: 218–223. [DOI] [PubMed] [Google Scholar]
- Xie D.Y., Jackson L.A., Cooper J.D., Ferreira D. and Paiva N.L. (2004) Molecular and biochemical analysis of two cDNA clones encoding dihydroflavonol-4-reductase from Medicago truncatula. Plant Physiol. 134: 979–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y., Hirakawa H., Ueno M., Matsui K., Katsube-Tanaka T., Yang S.J., Aii J., Sato S. and Mori M. (2016) Assembly of the draft genome of buckwheat and its applications in identifying agronomically useful genes. DNA Res. 23: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildizogluari N., Altan V.M., Altinkurt O. and Ozturk Y. (1991) Pharmacological effects of rutin. Phytother. Res. 5: 19–23. [Google Scholar]
- Ylstra B., Touraev A., Moreno R.M.B., Stoger E., Van tunen A.J., Vicente O., Mol J.N.M. and Heberle-Bors E. (1992) Flavonols stimulate development, germination, and tube growth of tobacco pollen. Plant Physiol. 100: 902–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K. and Hanada K. (2011) An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 66: 182–193. [DOI] [PubMed] [Google Scholar]
- Yoo D., Hara T., Fujita N., Waki T., Noguchi A., Takahashi S. and Nakayama T. (2013) Transcription analyses of GmICHG, a gene coding for a β-glucosidase that catalyzes the specific hydrolysis of isoflavone conjugates in Glycine max (L.) Merr. Plant Sci. 208: 10–19. [DOI] [PubMed] [Google Scholar]
- Zhao J., Pang Y.Z. and Dixon R.A. (2010) The mysteries of proanthocyanidin transport and polymerization. Plant Physiol. 153: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann I.M., Heim M.A., Weisshaar B. and Uhrig J.F. (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. 40: 22–34. [DOI] [PubMed] [Google Scholar]