Abstract
Ecotype breeding is a key technology in common buckwheat (Fagopyrum esculentum Moench) for the breeding of highly adaptive cultivars and their introduction to other cultivation areas. However, the details of the relationship between photoperiod sensitivity and ecotype remain unclear. Here, we evaluated photoperiod sensitivity in 15 landraces from different parts of Japan, and analyzed quantitative trait loci (QTLs) for photoperiod sensitivity using two F2 segregating populations derived from the crosses between self-compatible lines (‘Kyukei SC2’ or ‘Buckwheat Norin PL1’, early days-to-flowering) and allogamous plants (intermediate or late days-to-flowering). We clarified that (1) photoperiod sensitivity and differences in ecotype are closely related; (2) photoperiod sensitivity is controlled by several QTLs common among population of different ecotypes; and (3) orthologues of GIGANTEA and EARLY FLOWERING 3 will be useful markers in future detailed elucidation of the photoperiod sensitivity mechanism in common buckwheat. This study provides the basis for genomics-assisted breeding for local adaptation and ecotype breeding in common buckwheat.
Keywords: common buckwheat, photoperiod sensitivity, difference in ecotype, genetic analysis, ecotype breeding
Introduction
Common buckwheat (Fagopyrum esculentum Moench, 2n = 16), a short-day plant and an outcrossing heterostylous pseudocereal crop, is highly adaptable and is grown widely from low to high latitudes in Asia, Europe and North America. Common buckwheat is cultivated throughout most of Japan, and is classified into autumn, intermediate autumn, intermediate summer and summer ecotypes based on the adaptability of the cultivar to the environment in each area. The characteristics of each ecotype and the hypothesis that the ecotype is determined by photoperiod sensitivity have been reviewed by Morishita et al. (2020). Because the yield of an ecotype inappropriate for an environment is low (Nagase 2001), choosing the correct ecotypes or improving the suitable ecotype is important for cultivation of common buckwheat. Thus, ecotype breeding is a key breeding technology for the development of highly adaptive cultivars and for their introduction to other cultivation areas.
In long-day cultivation, the phenotypic variation within a common buckwheat population, such as that in flowering time and maturity time, is expanded (Hara and Ohsawa 2013, Minami and Namai 1986). Adaptability to the environment differs between plants with early and late flowering (Minami and Namai 1986). In late-flowering plants, the number of malformed flowers is increased significantly by long day or high temperature (Nagatomo 1961, Nakamura and Nakayama 1950, Sugawara 1958). The ability to set seed and pollen fertility decreases after full flowering time (Nagato et al. 1951, Nagatomo 1961, Ohsawa et al. 2001, Sugawara and Sugiyama 1954). Although the difference in ecotype in common buckwheat are deeply related to those in photoperiod sensitivity, this relationship has not yet been fully clarified.
Hara et al. (2011) demonstrated that photoperiod sensitivity in common buckwheat is controlled by at least three loci, which house candidate genes orthologous to those involved in the photoperiod pathway in Arabidopsis (Putterill et al. 2004). However, Hara et al. (2011) used only a segregating population derived from a cross between two summer-ecotype self-compatible lines; in other common buckwheat ecotypes, loci different from those detected by Hara et al. (2011) may control photoperiod sensitivity.
For ecotype breeding in common buckwheat by controlling photoperiod sensitivity, we need to further elucidate the relationship between photoperiod sensitivity and difference in ecotype by genetic analysis of photoperiod sensitivity. Here, we (1) evaluated photoperiod sensitivity in landraces collected across Japan and in three F2 segregating populations, and (2) analyzed QTLs for photoperiod sensitivity using two F2 segregating populations with different photoperiod sensitivities.
Materials and Methods
Evaluation of photoperiod sensitivity and preparation of segregating and mapping populations
To accurately evaluate photoperiod sensitivity, we used 15 landraces (Table 1) grown under various photoperiod conditions throughout Japan and two self-compatible lines, ‘Kyukei SC2’ (KSC2) and ‘Buckwheat Norin PL1’ (BNPL1) (Matsui et al. 2008); both lines were bred at the National Agricultural Research Center for Kyushu Okinawa Region. Plants were grown and evaluated in an isolated glasshouse (25°C day, 20°C night) at the University of Tsukuba from August 2010. For each of the 15 landraces, a total of 60 seeds (6 seeds × 2 rows × 5 planters) were sown in soil (Peat Pot V, NPK = 200:1000:200 mg/L; Hokkaido Peat Moss Co., Ltd., Hokkaido, Japan) in plastic planters (19 cm × 59 cm × 16 cm; height × length × width); for KSC2 and BNPL1, 36 seeds (6 seeds × 2 rows × 3 planters) were sown. On the basis of previous studies (Hagiwara et al. 1998, Hara and Ohsawa 2013, Michiyama and Hayashi 1998, Michiyama et al. 2005, Nagatomo 1961, Onda and Takeuchi 1942), the photoperiod was 15 h, which is a long-day condition that causes the expression of differences in photoperiod sensitivity among landraces. Fluorescent lamps for growing plants (Biolux-A FL40SBR-A; NEC Lighting, Ltd., Tokyo, Japan) were used to control the photoperiod. Photoperiod sensitivity evaluation was based on Hara et al. (2011) and Hara and Ohsawa (2013) and was performed as follows. The dates of cotyledon development and of the first flower opening in each cluster on the main stem of each plant were recorded. The number of days from the expansion of cotyledons to the first flower opening was defined as days-to-flowering (DTF) and was used as a measure of the photoperiod sensitivity of each plant. Measurements were taken every day until 90 days after sowing, when flowering had ended in all plants. To evaluate differences in photoperiod sensitivity among the landraces, we compared the average DTF by analysis of variance (ANOVA) and Tukey-Kramer tests in JMP v. 6.0 software (SAS Institute Inc., Cary, NC, USA). The relationships of latitude with average DTF and coefficient of variation (C.V.) were examined with Kendall’s rank correlation coefficient (τ).
Table 1.
Plant materials
| Landrace | Abbreviation | Location perfecture | Ecotypea | JP No.a |
|---|---|---|---|---|
| ASAHI ZAIRAI | ASA | Gifu | Autumn | 41819 |
| MIYAZAKI ZAIRAI | MIZ | Miyazaki | Autumn | 48617 |
| KANOYA ZAIRAI | KAN | Kagoshima | Intermediate summer | 41846 |
| ZAIRAISHU (GONOHE) | GON | Aomori | Intermediate summer | 42885 |
| SOTOYAMA ZAIRAI | SOT | Iwate | Summer | 36192 |
| IZUMO (SHIMANE) | IZU | Shimane | Summer | 48595 |
| BOTANSOBAb | BOT | Hokkaido | Summer | 53898 |
| OONO ZAIRAI | OON | Ibaraki | Unkown | 36197 |
| KUZUU ZAIRAI | KUZ | Tochigi | Unkown | 36207 |
| KAIDA ZAIRAI | KAI | Nagano | Unkown | 41815 |
| TANNO HIUSHINAI | TAN | Hokkaido | Unkown | 72500 |
| MIYAKO ZAIRAI | MIK | Fukushima | Unkown | Tsukuba Univ.c |
| ASAHIMURA ZAIRAI | ASM | Niigata | Unkown | Tsukuba Univ.c |
| YABAKEI ZAIRAI | YAB | Oita | Unkown | Tsukuba Univ.c |
| IYA ZAIRAI | IYA | Tokushima | Unkown | Tsukuba Univ.c |
a From Genebank Project, NARO.
b Cultivar bred at the Hokkaido Agricultural Research Center, NARO.
c Population maintained at the University of Tsukuba.
To develop segregating populations, we selected three seed parents derived from three landraces with different DTF—‘SOTOYAMA ZAIRAI’ (SOT, early DTF), ‘KUZUU ZAIRAI’ (KUZ, intermediate DTF) and ‘MIYAZAKI ZAIRAI’ (MIZ, late DTF)—on the basis of the photoperiod sensitivity. BNPL1 and KSC2 (both with early DTF) were selected as pollen parents and were used for artificial pollination. The ‘Early-DTF × Early-DTF’ (SOT × BNPL1) and ‘Intermediate-DTF × Early-DTF’ (KUZ × BNPL1) F2 segregating populations were prepared by crossing SOT (38 DTF) and KUZ (64 DTF) plants, with long-styled flowers, with BNPL1 (27 DTF) (Table 2). The ‘Late-DTF × Early-DTF’ (MIZ × KSC2) F2 segregating population was prepared by crossing a MIZ plant with long-styled flower (83 DTF) with KSC2 (25 DTF) (Table 2). The MIZ × KSC2 F2 segregating population was also used for mapping because it had the largest difference in DTF between parents. The F1 seeds were sown in the isolated glass-house described above, and F2 segregating populations were produced by selfing.
Table 2.
F2 segregating and mapping populations
| F2 segregating population | Abbreviation | Seed parent | Pollen parent | ||
|---|---|---|---|---|---|
| Original landrace | DTF | Original line | DTF | ||
| Early-DTF × Early-DTF | SOT × BNPL1 | SOT | 38 | BNPL1 | 27 |
| Intermediate-DTF × Early-DTF | KUZ × BNPL1 | KUZ | 64 | BNPL1 | 27 |
| Late-DTF × Early-DTFa | MIZ × KSC2 | MIZ | 83 | KSC2 | 25 |
a This population was used as the mapping population.
Search for candidate gene regions for photoperiod sensitivity
Genes related to the photoperiod pathways have been identified in many plant species (Hayama and Coupland 2004, Kojima et al. 2002, Liu et al. 2001a, Nemoto et al. 2003, Yano et al. 2000). Hara et al. (2011) suggested the existence of a similar photoperiod pathway in common buckwheat. To test this suggestion and to determine the genetic relations between these orthologous genes and QTLs, we selected 16 genes that are related to flowering time in Arabidopsis (Putterill et al. 2004) (Supplemental Table 1). The sequences were obtained from the National Center for Biotechnology Information database and used as queries in BLASTP searches in the Buckwheat Genome Database (BGDB; Yasui et al. 2016). Matches were considered to be significant when the smallest sum probability (P) was <0.0001 and the bit scores was >100. The retrieved orthologous regions were considered candidate photoperiod sensitivity gene regions, and single nucleotide polymorphism (SNP)-based markers for the candidate genes were generated as in Hara et al. (2011).
Marker development and linkage map construction
We developed new expressed sequence tag (EST)-based markers using the 863 cDNA clones (139 inflorescence-derived clones, designated the Fest_F group, and 724 leaf-derived clones, designated the Fest_L group) which were generated by Hara et al. (2011). For developing new EST-based markers (cleaved amplified polymorphic sequence markers, CAPS; derived cleaved amplified polymorphic sequence markers, dCAPS; and insertion/deletion [Indel] markers, which produced large differences in the length of polymerase chain reaction [PCR] products), primer design and searches for polymorphism (SNPs, insertions and deletions) between mapping population parents were done as in Hara et al. (2011). To compare the linkage map developed in this study and a buckwheat high-density linkage map developed Yabe et al. (2014), which includes many DNA micro-array markers, neighbor markers were developed near the micro-array markers located at both ends of each linkage group (LG) in the high-density linkage map. Neighbor markers (CAPS, dCAPS and Indel markers) were also developed for large intervals between markers on the linkage map after integration. Candidate gene markers (including 3 described in Hara et al. 2011), EST-based markers (including 76 described in Hara et al. 2011), neighbor markers and 180 microsatellite markers (Konishi and Ohnishi 2006) were assayed in the MIZ × KSC2 F2 mapping population (n = 384). A framework linkage map for QTL analysis was constructed as in Hara et al. (2011) in JoinMap v. 4.0 software (Van Ooijen 2006). Total DNA of both parental lines and of each of the F2 segregating plants was extracted as follows: ~200 mg milled leaf tissue was mixed with 750 μl lysis buffer (0.3% sodium dodecyl sulfate, 20 mM Tris-HCl [pH 8.0 at 25°C], 5 mM EDTA, 400 mM NaCl), incubated at 65°C for 10 min and centrifuged at 17,800 × g for 2 min. The supernatant was diluted 1:500 with ultrapure water (Milli-Q) and was used for PCR. PCR amplification, sequencing, restriction enzyme treatment, electrophoresis and gel imaging were carried out as described in Hara et al. (2011). Integration with the high-density linkage map (Yabe et al. 2014) was performed by using the neighbor markers as anchor markers.
QTL analysis for photoperiod sensitivity in segregating populations
Plants were grown in an isolated glasshouse at Tsukuba University as described above. Photoperiod sensitivity under short-day (12 h) conditions was surveyed from January 2015, and that under long-day conditions from August 2014. The photoperiod sensitivity of each plant of the three F2 segregating populations was measured as described above. DNA markers were used to screen for polymorphisms among parents. The genotype of each plant of the F2 segregating population was determined using polymorphic markers. PCR amplification, restriction enzyme treatment, electrophoresis and gel imaging were carried out as described in Hara et al. (2011). Interval mapping, cofactor analysis and multiple-QTL method (MQM) analysis were performed using the photoperiod sensitivity of each plant of the KUZ × BNPL1 and MIZ × KSC2 F2 segregating populations (the SOT × BNPL1 was not analyzed), the genotype at each marker and the created linkage map as described in Hara et al. (2011) in JoinMap software and MapQTL v. 5.0 software (Van Ooijen 2004).
Results
Photoperiod sensitivity of landraces collected across Japan
Under long-day conditions, the average DTF varied among landraces and showed a continuous distribution from 26.2 days (SOT) to 60.2 days (MIZ) (Table 3). A negative correlation was found between latitude and average DTF (τ = –0.4519, P < 0.01). The distribution of DTF within each landrace was also continuous; among individual plants, the earliest flowering (16 DTF) was found in ‘BOTANSOBA’ (BOT) and the latest flowering (86 DTF) in ‘ASAHIMURA ZAIRAI’(ASM) (Supplemental Fig. 1). Since the average DTF of landraces was distributed continuously and the seed set ability was not measured in this study, it was difficult to determine the ecotype of each landrace. No significant correlation was found between latitude and C.V. (τ = –0.1635, P > 0.05); the within-landrace diversity of photoperiod sensitivity was highest in the middle-latitude regions of Japan, followed by the low- and high-latitude regions (Table 3).
Table 3.
Days-to-flowering of each population under long-day conditions
| Landrace | Latitude of prefectural office location | Average DTF (mean ± SD) | C.V.a (%) | Tukey-Kramer test |
|---|---|---|---|---|
| SOT | 39° 42ʹ | 26.2 ± 3.96 | 15.1 | a |
| TAN | 43° 03ʹ | 28.8 ± 4.46 | 15.5 | a |
| GON | 40° 49ʹ | 29.2 ± 5.40 | 18.5 | a |
| BOT | 43° 03ʹ | 30.7 ± 4.72 | 15.4 | ab |
| IZU | 35° 28ʹ | 36.6 ± 8.16 | 22.3 | bc |
| OON | 36° 20ʹ | 38.0 ± 10.18 | 26.8 | c |
| KUZ | 36° 33ʹ | 38.4 ± 13.09 | 34.1 | c |
| IYA | 34° 03ʹ | 41.3 ± 10.94 | 26.5 | cd |
| MIK | 37° 45ʹ | 42.4 ± 11.53 | 27.2 | cde |
| KAN | 31° 33ʹ | 44.9 ± 10.46 | 23.3 | de |
| KAI | 36° 39ʹ | 45.6 ± 12.04 | 26.4 | de |
| ASA | 35° 23ʹ | 47.7 ± 10.92 | 22.9 | de |
| YAB | 33° 14ʹ | 48.7 ± 12.32 | 25.3 | e |
| ASM | 37° 54ʹ | 55.9 ± 17.16 | 30.7 | f |
| MIZ | 31° 54ʹ | 60.2 ± 14.15 | 23.5 | f |
a Coefficient of variation = standard deviation/average value × 100.
Search for candidate photoperiod sensitivity gene regions in the buckwheat genome database
We detected a total of 38 scaffolds with high sequence identity to the selected 16 Arabidopsis photoperiod pathway genes (Supplemental Table 1). Multiple orthologous regions other than the TIMING OF CAB EXPRESSION 1 (TOC1), EARLY FLOWERING 3 (ELF3) and GIGANTEA (GI) genes were identified, clearly indicating that buckwheat contains a set of orthologous genes associated with the photoperiod pathway from light perception to floral induction. A total of 15 candidate gene markers for photoperiod sensitivity genes were developed and tested for linkage map construction (Supplemental Tables 1, 2).
Marker development and linkage map construction
We developed 116 novel EST-based markers (13 from the Fest_F group, 103 from the Fest_L group) from among the original 863 EST regions, and 73 neighbor markers (Ne_FE group) based on DNA micro-array markers developed by Yabe et al. (2014). Among these, we confirmed polymorphisms between parents of the mapping population (MIZ × KSC2, n = 384) at 17 candidate genes (including 2 described in Hara et al. 2011), 192 EST-based markers (including 76 described in Hara et al. 2011), the 73 above neighbor markers and 180 simple sequence repeat (SSR) markers (Konishi and Ohnishi 2006). Out of the total 462 markers, polymorphisms were confirmed at 275 markers (15 candidate genes, 141 EST-based, 73 neighbor and 46 SSR markers). Of these, 229 were SNP-based markers (including 155 CAPS and 57 dCAPS markers) and 17 were Indel markers. Because genotype classification was difficult for 26 markers, they were used as dominant markers; the other markers were co-dominant. Information on the 229 markers with polymorphisms (excluding 46 SSR markers) is shown in Supplemental Table 2.
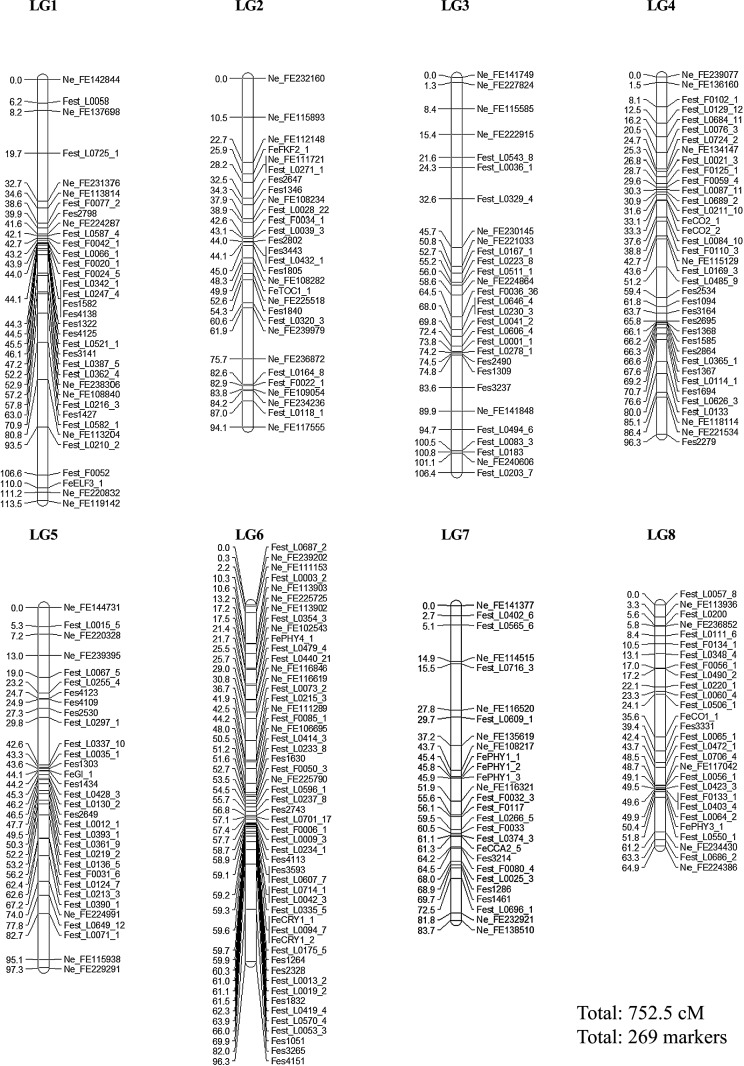
Through the linkage mapping of these markers, the positions of 269 of them (15 candidate genes, 141 EST-based, 67 neighbor and 46 SSR markers) were estimated; 6 other markers showed a departure from Mendelian segregation ratios (Fig. 1, Supplemental Table 2). The linkage map consisted of 8 LGs ranging from 64.9 cM (LG8) to 113.5 cM (LG1), and covered 752.5 cM in total (Fig. 1). The maximum interval between adjacent markers was 14.3 cM in LG6 (Fes3265 at 82.0 cM to Fes4151 at 96.3 cM), with an average of 2.8 cM. This linkage map was integrated with a high-density linkage map (Yabe et al. 2014) using neighbor markers: P1_1 and P2_1 were designated as LG1, P1_2 and P2_2 as LG2, P1_3 and P2_3 as LG3, P1_4 and P2_4 as LG4, P1_5 and P2_5 as LG5, P1_6 and P2_6 as LG6, P1_7 and P2_7 as LG7, and P1_8.1, P1_8.2 and P2_8 as LG8.
Fig. 1.
Linkage map. LG, linkage group. ‘Fest_F’ and ‘Fest_L’ are EST-based markers. ‘Ne_FE’ markers are based on DNA micro-array markers developed by Yabe et al. (2014). ‘Fes’ markers are based on SSR-based markers developed by Konishi and Ohnishi (2006).
QTL analysis for photoperiod sensitivity
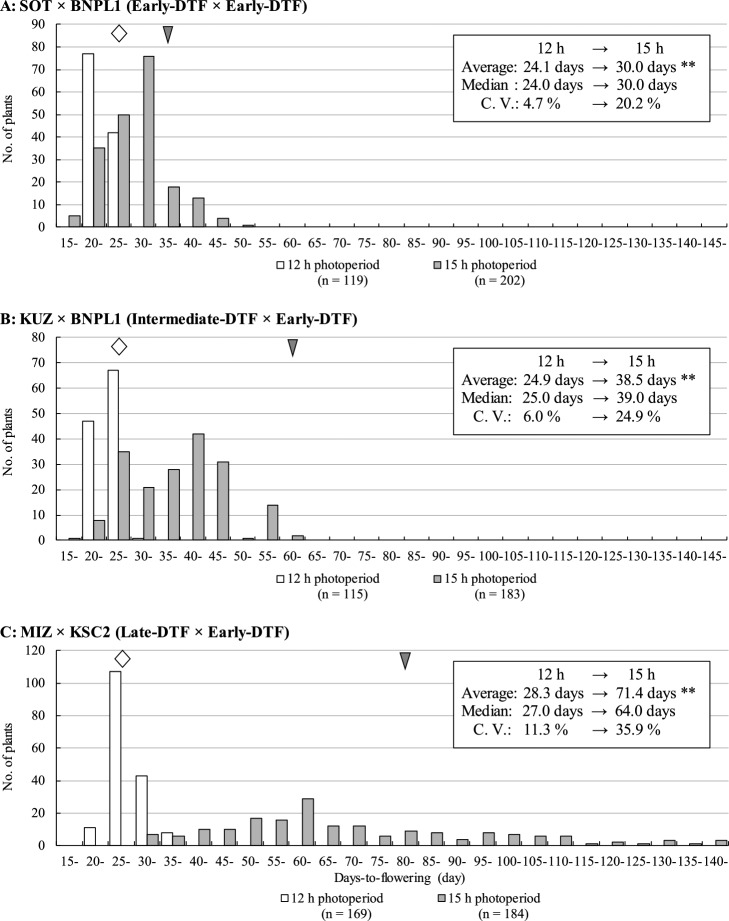
In all F2 segregating populations, cultivation under long-day conditions resulted in a significant (P < 0.01) delay in average DTF, and median DTF was also delayed (Fig. 2). The segregation of photoperiod sensitivity in all three F2 populations under long-day conditions showed a wide distribution, and C.V. was higher than under short-day conditions (Fig. 2). SOT × BNPL1 and KUZ × BNPL1 F2 segregating populations showed superdominance in early flowering; SOT × BNPL1 and especially MIZ × KSC2 showed superdominance in late flowering (Fig. 2). We performed QTL analysis using two F2 populations, KUZ × BNPL1 and MIZ × KSC2.
Fig. 2.
Distribution of days-to-flowering (DTF) in F2 segregating populations. (A) SOT × BNPL1 (Early-DTF, 38 DTF × Early-DTF, 27 DTF), (B) KUZ × BNPL1 (Intermediate-DTF, 64 DTF × Early-DTF, 27 DTF), and (C) MIZ × KSC2 (Late-DTF, 83 DTF × Early-DTF, 25 DTF). White bars, short day (12 h photoperiod); gray bars, long day (15 h photoperiod). White rhombi and gray arrowheads indicate the DTF of parent plants (Table 2). **Significant at the 1% level compared with short-day conditions.
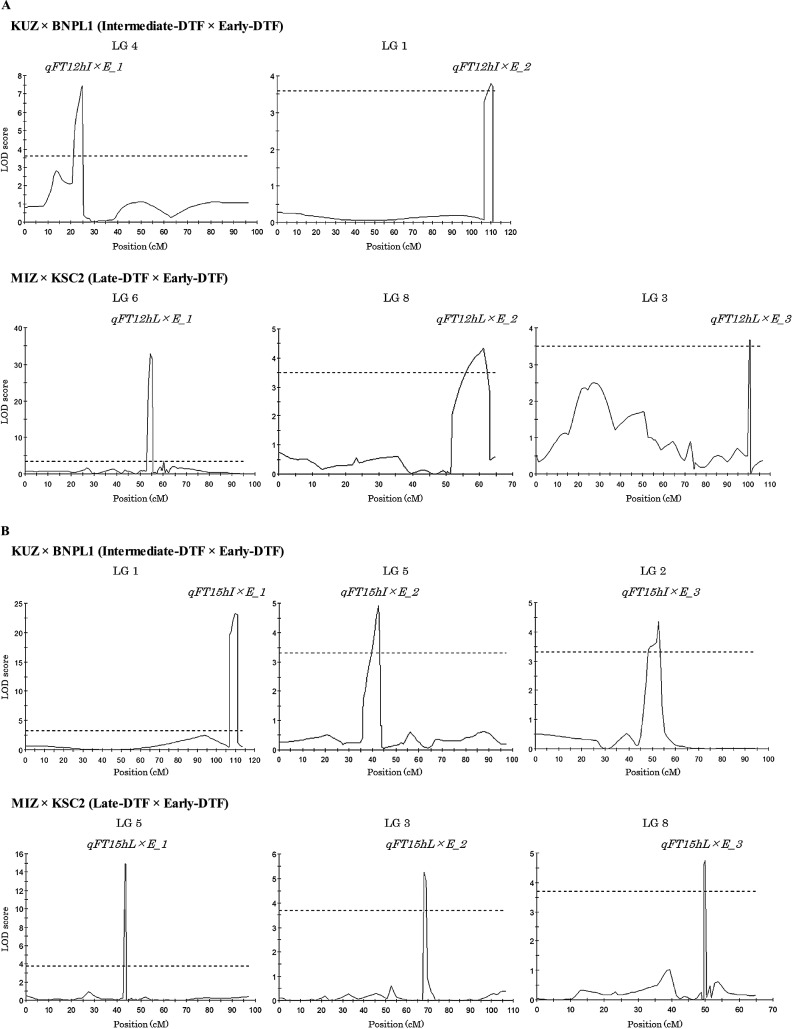
Of the 282 markers (excluding 180 SSR markers), 79 were polymorphic among the parents of the KUZ × BNPL1 F2 segregating population. We performed QTL analysis with the genotypes of these markers and with DTF at 12 and 15 h as trait values. In interval mapping, two regions in LG1 (108.6–111.2 cM) and LG4 (13.5 cM) at 12 h and one region in LG1 (62.8–113.5 cM) at 15 h were associated with photoperiod sensitivity (at LOD score thresholds of >3.5 at 12 h and >3.3 at 15 h; P < 0.05). In cofactor analysis, a significant difference (P < 0.02) was detected at two markers (FeELF3_1 in LG1 and Fest_L0021_3 in LG4) at 12 h and at three markers (FeELF3_1 in LG1, FeTOC1_7 in LG2 and Fest_L0337_10 in LG5) at 15 h. In MQM analysis, we detected QTL for flowering time under 12 h photoperiod in Intermediate-DTF × Early-DTF population 1 (qFT12hI×E_1) and qFT12hI×E_2 (for qFT12hI×E_1, nearest DNA marker was Fest_L0724_2 in LG4; for qFT12hI×E_2, the nearest maker was FeELF3_1 in LG1), which showed high LOD scores at 12 h (>3.6, P < 0.05; Table 4, Fig. 3A), and QTL for flowering time under 15 h photoperiod in Intermediate-DTF × Early-DTF population 1 (qFT15hI×E_1) to qFT15hI×E_3 (qFT15hI×E_1, nearest maker FeELF3_1 in LG1; qFT15hI×E_2, Fest_L0337_10 in LG5; qFT15hI×E_3, Ne_FE225518 in LG2), which showed high LOD scores at 15 h (>3.3, P < 0.05; Table 4, Fig. 3B). The candidate genes for photoperiod sensitivity—Fagopyrum esculentum EARLY FLOWERING 3 (FeELF3; interval, 0.0 cM), Fagopyrum esculentum GIGANTEA (FeGI; 1.5 cM) and Fagopyrum esculentum TIMING OF CAB EXPRESSION 1 (FeTOC1; 2.7 cM)—were present near the QTLs qFT12hI×E_2/qFT15hI×E_1, qFT15hI×E_2 and qFT15hI×E_3, respectively (Table 4, Fig. 1). The BNPL1 alleles of all QTLs except qFT12hI×E_2 and qFT15hI×E_1 had a negative additive effect on photoperiod sensitivity (Table 4). Both QTLs detected at 12 h had a complete dominant effect on early flowering (qFT12hI×E_1 –4.4 days, qFT12hI×E_2 –0.9 days; Table 4). Two QTLs detected at 15 h had a complete dominant effect on late flowering (qFT15hI×E_1 6.9 days) and early flowering (qFT15hI×E_2 –9.1 days; Table 4).
Table 4.
Quantitative trait loci (QTLs) for photoperiod sensitivity detected in each F2 segregating population by multiple-QTL analysis
| Photoperiod | F2 segregating population | LGa | Position (cM) | Detected QTL | Nearest DNA marker | Neighborhood candidate gene (distance to QTL) | LOD score | Additiveb effect | Dominantb effect | PVEc (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 12 h | KUZ × BNPL1 (Intermediate-DTF × Early-DTF) | 4 | 24.7 | qFT12hI×E_1 | Fest_L0724_2 | None identified | 7.5 | –4.2 | –4.4 | 28.6 |
| 1 | 110.3 | qFT12hI×E_2 | FeELF3_1 | FeELF3 (0.0 cM) | 3.8 | 0.6 | –0.9 | 15.6 | ||
| MIZ × KSC2 (Late-DTF × Early-DTF) | 6 | 54.5 | qFT12hL×E_1 | Fest_L0596_1 | FeCRY1 (5.1 cM) | 32.8 | –2.6 | –3.1 | 56.6 | |
| 8 | 61.2 | qFT12hL×E_2 | Ne_FE234430 | None identified | 4.3 | –0.9 | –0.8 | 4.7 | ||
| 3 | 100.5 | qFT12hL×E_3 | Fest_L0083_3 | None identified | 3.7 | –0.8 | –0.6 | 3.8 | ||
| 15 h | KUZ × BNPL1 (Intermediate-DTF × Early-DTF) | 1 | 110 | qFT15hI×E_1 | FeELF3_1 | FeELF3 (0.0 cM) | 23.2 | 7.5 | 6.9 | 44.1 |
| 5 | 42.6 | qFT15hI×E_2 | Fest_L0337_10 | FeGI (1.5 cM) | 4.9 | –8.9 | –9.1 | 19.2 | ||
| 2 | 52.6 | qFT15hI×E_3 | Ne_FE225518 | FeTOC1 (2.7 cM) | 4.4 | –3.2 | 0.5 | 15.4 | ||
| MIZ × KSC2 (Late-DTF × Early-DTF) | 5 | 43.6 | qFT15hL×E_1 | Fes1303 | FeGI (0.5 cM) | 15.0 | –17.6 | 0.8 | 24.5 | |
| 3 | 68.0 | qFT15hL×E_2 | Fest_L0230_3 | None identified | 5.3 | –8.4 | –6.9 | 7.6 | ||
| 8 | 49.9 | qFT15hL×E_3 | Fest_L0064_2 | FePHY3 (0.5 cM) | 4.8 | –10.7 | –5.5 | 6.9 |
a Linkage group.
b Values for the ‘KSC2’ or ‘BNPL1’ genotype.
c Percentage of total phenotypic variance explained by the QTL.
Fig. 3.
QTLs detected in multiple-QTL analysis. (A) QTLs detected under short-day (12 h photoperiod) conditions. (B) QTLs detected under long-day (15 h photoperiod) conditions. The QTL likelihood map for each linkage group (LG) was obtained by using the MQM procedure of MapQTL. Linkage group number is indicated at the top of each graph. Horizontal dashed lines indicate the significant (P < 0.05) LOD score threshold.
Of the 462 markers, 275 were polymorphic among the parents of the MIZ × KSC2 F2 segregating population. We performed QTL analysis with the genotypes of these markers, and with DTF at 12 and 15 h as trait values. In interval mapping, one region in LG6 (10.6–96.3 cM) at 12 h and three regions in LG3 (66.5–81.8 cM), LG5 (20.0–87.1 cM) and LG8 (44.0–45.0 cM) at 15 h were associated with photoperiod sensitivity (LOD score >3.6; P < 0.05). In cofactor analysis, a significant difference (P < 0.02) was detected at three markers (Fest_L0183 in LG3, Fest_L0596_1 in LG6 and Ne_FE234430 in LG8) at 12 h, and at three other markers (Fest_L0230_3 in LG3, Fes1303 in LG5 and Fest_L0064_2 in LG8) at 15 h. In MQM analysis, we detected QTL for flowering time under 12 h photoperiod in Late-DTF × Early-DTF population 1 (qFT12hL×E_1) to qFT12hL×E_3 (qFT12hL×E_1, nearest marker Fest_L0596_1 in LG6; qFT12hL×E_2, Ne_FE234430 in LG8; qFT12hL×E_3, Fest_L0083_3 in LG3), which showed high LOD scores at 12 h (>3.5, P < 0.05; Table 4, Fig. 3A), and QTL for flowering time under 15 h photoperiod in Late-DTF × Early-DTF population 1 (qFT15hL×E_1) to qFT15hL×E_3 (qFT15hL×E_1, Fes1303 in LG5; qFT15hL×E_2, Fest_L0230_3 in LG3; qFT15hL×E_3, Fest_L0064_2 in LG8), which showed high LOD scores at 15 h (>3.7, P < 0.05; Table 4, Fig. 3B). Three candidate genes for photoperiod sensitivity—Fagopyrum esculentum CRYPTOCHROME 1 (FeCRY1; interval, 5.1 cM), FeGI (0.5 cM) and Fagopyrum esculentum PHYTOCHROME 3 (FePHY3; 0.5 cM)—were present near the QTLs qFT12hL×E_1, qFT15hL×E_1 and qFT15hL×E_3, respectively (Table 4, Fig. 1). The KSC2 alleles of all QTLs had a negative additive effect on photoperiod sensitivity (Table 4). All QTLs detected at 12 h had a complete dominant effect on early flowering (qFT12hL×E_1 –3.1 days, qFT12hL×E_2 –0.8 days, qFT12hL×E_3 –0.6 days; Table 4). Two QTLs detected at 15 h had an incomplete dominant effect on early flowering (qFT15hL×E_2 –6.9 days, qFT15hL×E_2 –5.5 days; Table 4).
Discussion
Relationship between photoperiod sensitivity and difference in ecotype in common buckwheat
Using landraces collected across Japan, we found a negative correlation (τ = –0.4519, P < 0.01) between latitude and photoperiod sensitivity (Table 3). This result is similar to that of Ujihara and Matano (1974). However, it was difficult to strictly classify the landraces into the four ecotypes because the distribution of DTF in each landrace and among the landraces was continuous (Supplemental Fig. 1). Moreover, no correlation (τ = –0.1635, P < 0.05) was found between latitude and the within-landrace diversity of photoperiod sensitivity (C.V.) (Table 3), and although the low-latitude landraces were more diverse than the high-latitude landraces, the middle-latitude landraces had the highest diversity (Table 3). In Japan, the summer ecotype may have differentiated from the autumn ecotype through an intermediate ecotype (Hara and Ohsawa 2013, Matano and Ujihara 1979, Minami and Namai 1986, Morishita et al. 2020, Ujihara and Matano 1978). In line with this hypothesis, the genetic and phenotypic diversities of summer-ecotype (high-latitude) landraces tend to be lower than those of autumn-ecotype (low-latitude) landraces, and the genetic structure differs between summer and autumn ecotypes (Hara and Ohsawa 2013, Iwata et al. 2005, Michiyama and Hayashi 1998, Onda and Takeuchi 1942). However, the present study, which accurately evaluated the photoperiod sensitivity of landraces collected across Japan, showed that the middle-latitude landraces are the most diverse. To clarify what kinds of ecological changes have occurred in Japan, it will be necessary to conduct further research using landraces from all over the world.
Genetic analysis of photoperiod sensitivity associated with differences in common buckwheat ecotypes
We constructed a linkage map that included 15 candidate gene markers likely related to photoperiod sensitivity in 8 LGs containing 269 markers and covering 752.5 cM in total; the average interval between adjacent markers was 2.8 cM (Fig. 1). Some linkage maps with 8 LGs have been previously developed for common buckwheat. Yasui et al. (2004) constructed a linkage map that coverd a total of 508.3 cM and contained 223 amplified fragment-length polymorphism markers. Yabe et al. (2014) constructed a high-density linkage map that coverd a total of 800.4 cM and contained 1631 contigs and 4657 DNA micro-array markers. The linkage map constructed here contained markers located at both ends of each LG in the high-density linkage map (Yabe et al. 2014) as neighbor markers, and the markers covered almost the entire common buckwheat genome. Therefore, it will be suitable for comprehensive and efficient genome-wide searching for QTLs for photoperiod sensitivity.
In the MQM analysis with the 12 h photoperiod, we detected two QTLs in the KUZ × BNPL1 F2 segregating population and three in the MIZ × KSC2 F2 segregating population (Table 4, Fig. 3). With the 15 h photoperiod, we detected three QTLs in each F2 segregating population (Table 4, Fig. 3). Hara et al. (2011) and Minami (1985) suggested that photoperiod sensitivity of common buckwheat is controlled by multiple genes, and our study supports this suggestion. The distribution of DTF in the MIZ × KSC2 F2 segregating population at 15 h was broad (30 to 140 days), and the values of the percentage of total phenotypic variance explained by the detected QTLs were lower than those in the KUZ × BNPL1 F2 segregating population (Fig. 2, Table 4). These results suggest the existence of unidentified minor QTLs, and DTF in the MIZ × KSC2 F2 segregating population at 15 h may be controlled by the accumulation of the effects of such QTLs.
Our QTL analysis under long-day conditions identified qFT15hI×E_2 (KUZ × BNPL1 population) and qFT15hL×E_1 (MIZ × KSC2 population) as located extremely close to each other (Table 4, Fig. 3B). Hara et al. (2011) detected three QTLs for photoperiod sensitivity (FeELF3, Fest_L0606_4 and Fest_L0337_6) in an Early-DTF × Early-DTF segregating population. Fest_L0337_6 was derived from the same cDNA clone as Fest_L0337_10 used here (DNA marker near qFT15hI×E_2 and qFT15hL×E_1; Table 4). Therefore, we presume that qFT15hI×E_2 and qFT15hL×E_1 are important QTLs for the difference in ecotype in common buckwheat.
Although not confirmed in the MIZ × KSC2 population, qFT12hI×E_2 and qFT15hI×E_1 identified in the KUZ × BNPL1 population are the same QTL common to both long- and short-day conditions, and explained a large percentage of total phenotypic variance under long-day conditions (Table 4, Fig. 3). A QTL near FeELF3_1 (DNA marker nearest to qFT12hI×E_2 and qFT15hI×E_1) has also been identified by Hara et al. (2011); in both studies, it had an additive effect in the direction of late flowering, unlike other QTLs (in MIZ × KSC2, the LOD value was not significant at 2.4, but the additive effect was in the direction of early flowering). These facts suggest that the QTL corresponding to qFT12hI×E_2 and qFT15hI×E_1 is related to the difference in photoperiod sensitivity among ecotypes.
Candidate genes of the photoperiod pathway (FeELF3, FeCRY1, FeGI, FeTOC1 and FePHY3) were present near 7 of the 11 QTLs identified here (Table 4). In Arabidopsis, ELF3 and TOC1 connect the circadian clock to the photoperiod pathway (Covington et al. 2001, Liu et al. 2001b, Putterill et al. 2004, Wang et al. 1997, Wang and Tobin 1998), and CRY (CRYPTOCHROME) and PHY (PHYTOCHROME) connect light perception to it (Putterill et al. 2004). GI connects the long-day pathway to it (Fowler et al. 1999, Park et al. 1999, Putterill et al. 2004). Our results suggest that genes orthologous to photoperiod pathway genes regulate photoperiod sensitivity in common buckwheat, as proposed by Hara et al. (2011). These candidate genes may be useful for the future detailed elucidation of the photoperiod sensitivity mechanism in common buckwheat. In particular, we consider FeGI and FeELF3, located in the immediate vicinity of four QTLs (qFT15hI×E_2, qFT15hL×E_1, qFT12hI×E_2 and qFT15hI×E_1; Table 4), as remarkable candidate genes important for the differences among ecotypes.
In summary, we have demonstrated the relationship between photoperiod sensitivity and difference in ecotype using landraces collected across Japan and cultivated under long-day conditions, and identified some QTLs associated with photoperiod sensitivity. Although unresolved points remain, our results confirm the previous hypotheses that differences among ecotypes are due to differences in photoperiod sensitivity. From the results of this study, we expect that genomics-assisted ecotype breeding for local adaptation in common buckwheat will be made possible by development of DNA markers related to photoperiod sensitivity.
Author Contribution Statement
All authors conceived, designed and performed the experiments, and analyzed and interpreted the data. TH and TS conducted field experiments. TH, TS and HN genotyped the markers. TH conducted QTL analysis. TH and RO wrote the paper.
Supplementary Material
Acknowledgments
This work was supported by a grant from JSPS KAKENHI (grant number JP16K18642) and as a commissioned project study on “Development of Soybean and Buckwheat Cultivars with Processability and Wide Area Adaptability According to the Users’ Needs”, Ministry of Agriculture, Forestry and Fisheries, Japan.
Literature Cited
- Covington M.F., Panda S., Liu X.L., Strayer C.A., Wagner D.R. and Kay S.A. (2001) ELF3 modulates resetting of the circadian clock in Arabidopsis. Plant Cell 13: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler S., Lee K., Onouchi H., Samach A., Richardson K., Morris B., Coupland G. and Putterill J. (1999) GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J. 18: 4679–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M., Inoue N. and Matano T. (1998) Variability in the length of flower bud differentiation period of common buckwheat. Fagopyrum 15: 55–64. [Google Scholar]
- Hara T., Iwata H., Okuno K., Matsui K. and Ohsawa R. (2011) QTL analysis of photoperiod sensitivity in common buckwheat by using markers for expressed sequence tags and photoperiod-sensitivity candidate genes. Breed. Sci. 61: 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T. and Ohsawa R. (2013) Accurate evaluation of photoperiodic sensitivity and genetic diversity in common buckwheat under a controlled environment. Plant Prod. Sci. 16: 247–254. [Google Scholar]
- Hayama R. and Coupland G. (2004) The molecular basis of diversity in the photoperiodic flowering responses of arabidopsis and rice. Plant Physiol. 135: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata H., Imon K., Tsumura Y. and Ohsawa R. (2005) Genetic diversity of common buckwheat varieties in Japan based on microsatellite markers. Genome 48: 367–377. [DOI] [PubMed] [Google Scholar]
- Kojima S., Takahashi Y., Kobayashi Y., Monna L., Sasaki T., Araki T. and Yano M. (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol. 43: 1096–1105. [DOI] [PubMed] [Google Scholar]
- Konishi T. and Ohnishi O. (2006) A linkage map for common buckwheat based on microsatellite and AFLP markers. Fagopyrum 23: 1–6. [Google Scholar]
- Liu J., Yu J., McIntosh L., Kende H. and Zeevaart J.A.D. (2001a) Isolation of a COSTANS ortholog fro Pharbitis nil and its role in flowering. Plant Physiol. 125: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.L., Covington M.F., Fankhauser C., Chory J. and Wagner D.R. (2001b) ELF3 encodes a circadian clock–regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell 13: 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matano T. and Ujihara A. (1979) Agroecological classification and geographical distribution of the common buckwheat, Fagopyrum esculentum M. in the East Asia. Jpn. Agric. Res. Q. 13: 157–162. [Google Scholar]
- Matsui K., Tetsuka T., Hara T. and Morishita T. (2008) Breeding and characterization of a new self-compatible common buckwheat parental line, “Buckwheat Norin-PL1”. Bull. Natl. Agric. Res. Cent. Kyushu Okinawa Reg. 49: 1–17. [Google Scholar]
- Michiyama H. and Hayashi H. (1998) Differences of growth and development between summer and autumn-type cultivars in common buckwheat (Fagopyrum esculentum Moench). Jpn. J. Crop Sci. 67: 323–330. [Google Scholar]
- Michiyama H., Tsuchimoto K., Tani K., Hirano T., Hayashi H. and Campbell C. (2005) Influence of day length on stem growth, flowering, morphology of flower clusters, and seed-set in buckwheat (Fagopyrum esculentum Moench). Plant Prod. Sci. 8: 44–50. [Google Scholar]
- Minami, H. (1985) Ecological-genetic studies of difference of ecotype in common buckwheat. Doctoral thesis, University of Tsukuba, p. 62. [Google Scholar]
- Minami H. and Namai H. (1986) Populational change in flowering time caused by different harvesting date observed in a late-summer type cultivar Miyazakizairai of buckwheat (Fagopyrum esculentum Moench). Japan. J. Breed. 36: 155–162. [Google Scholar]
- Morishita T., Hara T. and Hara T. (2020) Important agronomic characteristics of yielding ability in common buckwheat; ecotype and ecological differentiation, preharvest sprouting resistance, shattering resistance, and lodging resistance. Breed. Sci. 70: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase, Y. (2001) Soba no kigen to tokusei. Rural Culture Association Japan. Tensakuzensyo 3zakkoku. Tokyo, pp. 447–467. [Google Scholar]
- Nagato K., Sato T. and Sugahara K. (1951) On the fruiting of buckwheat. Jpn. J. Crop Sci. 19: 299–302. [Google Scholar]
- Nagatomo T. (1961) Studies on physiology of reproduction and some cases of inheritance in buckwheat. Research Report of plant Breeding Laboratory, Faculty of Agriculture, Miyazaki University 1: 1–213. [Google Scholar]
- Nakamura M. and Nakayama H. (1950) On the enervative sterility in buckwheat. Jpn. J. Crop Sci. 19: 122–125. [Google Scholar]
- Nemoto Y., Kisaka M., Fuse T., Yano M. and Ogihara Y. (2003) Characterization and functional analysis of three wheat genes with homology to the CONSTANS flowering time gene in transgenic rice. Plant J. 36: 82–93. [DOI] [PubMed] [Google Scholar]
- Ohsawa, R., K. Ishikawa and H. Namai (2001) Assortative mating in the population of intermediate ecotype of common buckwheat with special reference to flowering time, pollen fertility and a rate of malformed pistil. Adv. Buckwheat Res. 676–680. [Google Scholar]
- Onda S. and Takeuchi T. (1942) Ecotypes of Japanese buckwheat varieties. Nogyo oyobi Engei 17: 971–974. [Google Scholar]
- Park D.H., Somers D.E., Kim Y.S., Choy Y.H., Lim H.K., Soh M.S., Kim H.J., Kay S.A. and Nam H.G. (1999) Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285: 1579–1582. [DOI] [PubMed] [Google Scholar]
- Putterill J., Laurie R. and Macknight R. (2004) It’s time to flower: the genetic control of flowering time. Bioessays 26: 363–373. [DOI] [PubMed] [Google Scholar]
- Sugawara K. and Sugiyama K. (1954) An ecological study on the flowering and seed setting of buckwheat. The annual report of the Department of Liberal Arts, the Iwate University 6: 55–68. [Google Scholar]
- Sugawara K. (1958) On the injury of buckwheat pistil: retardation of pistil growth as influenced by day-length. Jpn. J. Crop Sci. 26: 269–270. [Google Scholar]
- Ujihara A. and Matano T. (1974) Studies on the geographical variation of buckwheat (Fagopyrum esculentum M.). Journal of the Faculty of Agriculture Shinshu University 11: 221–230. [Google Scholar]
- Ujihara A. and Matano T. (1978) Tsushima’s buckwheat—on the propagation and ecotype differentiation of Japanese buckwheat. Noukounogijyutu 1: 43–59. [Google Scholar]
- Van Ooijen, J.W. (2004) MapQTL 5, Software for the mapping of quantitative trait loci in experimental populations. Wageningen, Netherlands. Kyazma B.V. [Google Scholar]
- Van Ooijen, J.W. (2006) JoinMap 4, Software for the calculation of genetic linkage maps in experimental populations. Wageningen, Netherlands. Kyazma B.V. [Google Scholar]
- Wang Z.Y., Kenigsbuch D., Sun L., Harel E., Ong M.S. and Tobin E.M. (1997) A Myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell 9: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.Y. and Tobin E.M. (1998) Constitutive expression of the CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93: 1207–1217. [DOI] [PubMed] [Google Scholar]
- Yabe S., Hara T., Ueno M., Enoki H., Kimura T., Nishimura S., Yasui Y., Ohsawa R. and Iwata H. (2014) Rapid genotyping with DNA micro-arrays for high-density linkage mapping and QTL mapping in common buckwheat (Fagopyrum esculentum Moench). Breed. Sci. 64: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M., Katayose Y., Ashikan M., Yamanouchi U., Monna L., Fuse T., Baba T., Yamamoto K., Umehara Y., Nagamura Y. et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12: 2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y., Wang Y., Ohnishi O. and Campbell C. (2004) Amplified fragment length polymorphism linkage analysis of common buckwheat (Fagopyrum esculentum) and its wild self-pollinated relative Fagopyrum homotropicum. Genome 47: 345–351. [DOI] [PubMed] [Google Scholar]
- Yasui Y., Hirakawa H., Ueno M., Matsui K., Katsube-Tanaka T., Yang S.J., Aii J., Sato S. and Mori M. (2016) Assembly of the draft genome of buckwheat and its applications in identifying agronomically useful genes. DNA Res. 23: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.