Abstract
The prevalence of hypertension is high in patients affected by coronavirus disease 2019 (COVID-2019) and it appears to be related to an increased risk of mortality, as shown in many epidemiological studies. The angiotensin-converting enzyme (ACE) system is not uniformly expressed in all of the human races, and current differences could explain some of the geographical discrepancies in infection around the world. Furthermore, animal studies have shown that the ACE2 receptor is a potential pathway for host infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. As two-thirds of hypertensive patients take ACE inhibitors/angiotensin receptor blockers, several concerns have been raised about the detrimental role of current antihypertensive drugs in COVID-19. This report summarizes the recent evidence for and against the administration of ACE blockade in the COVID-19 era.
Blockage of the angiotensin-converting enzyme (ACE) system is the cornerstone of treatment for several conditions including hypertension and heart failure. The inhibition of ACE has also demonstrated favourable effects in many metabolic diseases, such as diabetes, obesity, and chronic kidney disease. In clinical practice, two main drug types able to modulate the ACE system exist: ACE inhibitors (ACEI), which block the conversion of angiotensin 1 (AT1) to angiotensin 2 (AT2), and angiotensin receptor blockers (ARBs), which exert their effect via blockage of the AT1 receptor. Both classes of drug have an important role in cardiovascular risk reduction, blood pressure control, and maintenance of cardiac function.
Recently some concerns have been raised about the role of the ACE system in the facilitation and worsening of coronavirus disease 2019 (COVID-2019). However, to date, no large cohort studies have shown such a relationship, and these concerns have been based mostly on certain biomolecular evidence (Vaduganathan et al., 2020 March 30).
The first large-scale analysis of the Chinese population affected by COVID-19 demonstrated that 15% had hypertension. However, only a small percentage of these patients were on treatment and only a quarter of these were being treated with ACEI/ARBs (Guan et al., 2020 Feb 28). Indeed, in the general Chinese population, the prevalence of hypertension ranges from about 18% to 25% and only half of these people are on treatment. In Western countries, the rate of hypertension is higher when compared to China, ranging from 20% to 35%, depending on age, ethnicity, region, and baseline cardiovascular risk (Williams et al., 2018), and hypertension is the main risk factor associated with adverse outcomes during hospitalization for COVID-19. The spread of COVID-19 in European countries has shown an increased incidence among older people (60–70 years old), who are usually affected by hypertension. Furthermore, different ACE polymorphisms have been observed in the Chinese race and could be related to different ACE activity and subsequent ACEI use and efficacy (He et al., 2013). Moreover, a lower incidence of COVID-19 disease has been observed in African countries. These discrepancies could be explained by the different ACE system expression and ACE activity among the races, suggesting a possible link with the spread of COVID-19 and with the different outcomes observed in the European Union when compared to China. Despite these epidemiological findings, a recent study involving a population-based cohort showed that black Americans with COVID-19 had a higher incidental rate of adverse events. This is probably due to poor socio-economic conditions, dietary habits, and insufficient adherence to the distance rule and wearing of face masks (Yancy, 2020 Apr 15).
The mechanisms behind the potential association between hypertension and modulation of the ACE system are different for ACEI and ARBs, but the pathophysiological basis is supported by experimental studies. The main effect of ACEI on the cardiovascular system is due to angiotensin blockade, resulting in a reduction in bradykinin degradation, with consequent escape and an increased plasma level. Bradykinin has several important cardiovascular effects on fibrinolysis and vasodilatation, but it is also involved in some inflammatory and oxidative stress processes via kinin–kallikrein activation. Bradykinin plays a potential inflammatory role at different sites and in different cells: it is responsible for the stimulation of alveolar macrophages to releases monocytic eosinophil and neutrophil activators, which in turn stimulate the release of prostaglandins and some cytokines involved in the inflammatory cascade, such as interleukin (IL)-1β and IL-6. The effects of bradykinin are mediated by B1 and B2 receptors. A recent in vitro study showed that antagonists of B1 and agonists of B2 are capable of directly inducing prostaglandin E (PGE) production. Activation of cyclooxygenase modulated by nuclear factor kappa B (NF-κB) transcription, leading to an increase in arachidonic acid levels, is an alternative inflammatory pathway (Muscella et al., 2020). Such mechanisms have been demonstrated in bronchial fibroblast and smooth muscle cells, in which IL-6 production and expression have been reported. Blockade of the B2 receptor and specific protein kinase administration both reduce the inflammatory activity mediated by bradykinin.
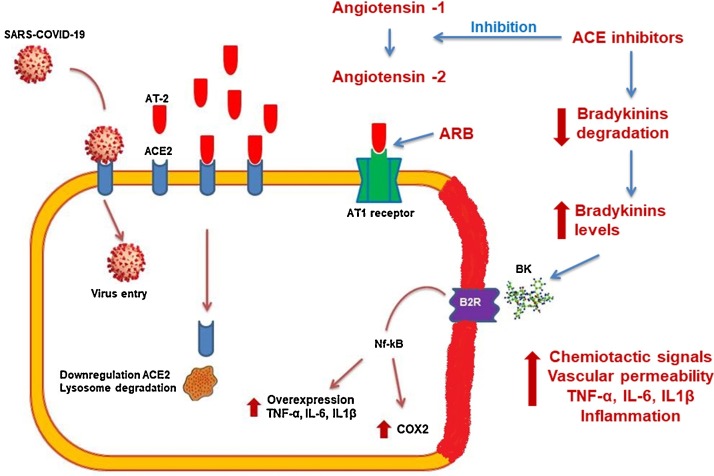
The bradykinin escape subsequent to ACEI administration could theoretically be avoided by ARB use, as these do not influence its production because they act at the AT1 receptor level antagonizing the effect of the ACE system in the cardiovascular bed. Unfortunately, in theory this is not the case in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during COVID-19 disease, because it has been revealed that the virus enters human cells via the ACE2 receptor and the cellular serine protease TMPRSS2. By a second messenger S glycoprotein, virus hosts cell receptors ACE that is the fundamental step for virus insertion inside the cell and consequent replication. The S glycoprotein includes two subunits with different actions: S1 influences virus tropism and attachment to the external membrane, and S2 is responsible for virus cell fusion and effective cell inclusion. After these processes the virus is able to enter into the sarcoplasmic reticulum and begin its RNA replication (Hoffmann et al., 2020). ACEI could increase plasma AT1, which is tissue protective, and ARBs could blunt AT2 activity, which is able to induce inflammation and acute immune reactivity in the lungs (Touyz et al., 2020) (Fig. 1 ). Given the paucity of information about (1) the molecular mechanisms existing in the host–pathogen interaction, (2) the lack of knowledge about the mechanisms by which SARS-CoV-2 causes infection, and (3) the complexity of RAS ligand and binding protein, it has become imperative to understand the effective role of the ACE system in viral infection, diffusion, and development. Indeed, despite a reasonable hypothesis demonstrated only in an animal model, no data have yet been reported from human studies. Considering that two-thirds of the entire Western population affected by hypertension or other cardiovascular diseases are taking ACEI/ARB drugs, a specific investigation to determine the potential harms/beneficial role of ACE inhibition appears mandatory.
Fig. 1.
Schematic diagram of the potential mechanisms linking the ACE system and COVID-19 infection. The virus could enter directly inside the epithelial cell of the respiratory system via the ACE2 receptor or induce an inflammatory cascade by bradykinin escape related to ACEI therapy. The subsequent increase in prostaglandins and cyclooxygenases leads to interleukin production, which causes cell membrane inflammation potentially leading to apoptosis. Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; AT, angiotensin; B2R, bradykinin 2 receptor; BK, bradykinin; COX, cyclooxygenase.
Because of these contradictory findings and the variation in epidemiological data, specific studies are warranted to elucidate the real impact of ACEI/ARB therapy on the spread of COVID-19.
Declarations
Funding source: None.
Ethical approval: Approval was not required.
Conflict of interest: None to declare.
References
- Vaduganathan M., Vardeny O., Michel T., McMurray J.J., Pfeffer M.A., Solomon S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med. 2020 March 30 doi: 10.1056/NEJMsr2005760. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 Feb 28 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B., Mancia G., Spiering W., Agabiti Rosei E., Azizi M., Burnier M., Clement D.L., Coca A., de Simone G., Dominiczak A., Kahan T., Mahfoud F., Redon J., Ruilope L., Zanchetti A., Kerins M., Kjeldsen S.E., Kreutz R., Laurent S., Lip G.Y.H., McManus R., Narkiewicz K., Ruschitzka F., Schmieder R.E., Shlyakhto E., Tsioufis C., Aboyans V., Desormais I., Authors/Task Force Members 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- He Q., Fan C., Yu M., Wallar G., Zhang Z.F., Wang L., Zhang X., Hu R. Associations of ACE gene insertion/deletion polymorphism, ACE activity, and ACE mRNA expression with hypertension in a Chinese population. PLoS One. 2013;8:e75870. doi: 10.1371/journal.pone.0075870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy C.W. COVID-19 and African Americans. JAMA. 2020 Apr 15 doi: 10.1001/jama.2020.6548. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Muscella A., Cossa L.G., Vetrugno C., Marsigliante S. Bradykinin stimulates prostaglandin E2 release in human skeletal muscular fibroblasts. Mol Cell Endocrinol. 2020;507:110771. doi: 10.1016/j.mce.2020.110771. [DOI] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C. Pöhlmann SSARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. pii: S0092-8674(20)30229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz R.M., Li H., Delles C. ACE2 the Janus-faced protein – from cardiovascular protection to severe acute respiratory syndrome-coronavirus and COVID-19. Clinical Science. 2020;134:747–750. doi: 10.1042/CS20200363. [DOI] [PubMed] [Google Scholar]