Abstract
A Cu-catalyzed method for the efficient enantio- and diastereoselective synthesis of chiral homoallylic amines bearing a quaternary carbon and an alkenylboron is disclosed. Transformations are promoted by a readily prepared (phosphoramidite)–Cu complex, and involve bench-stable γ-disubstituted allyldiborons and benzyl imines; products are obtained in up to 82% yield, >20:1 dr, and >99:1 er. Reactions proceed via stereodefined boron-stabilized allylic Cu species formed by an enantioselective transmetalation. Utility of the 1-amino-3-alkenylboronate products is highlighted by a variety of synthetic transformations.
Graphical Abstract

Development of catalytic enantioselective methods for the synthesis of chiral amines and all-carbon quaternary stereocenters are both critical objectives in organic synthesis.1,2 In this regard, the enantioselective allyl addition to imines with appropriately substituted C-based nucleophiles represents a direct route for the concomitant generation of both of these important chemical motifs.3 While a number of catalytic methods have been reported for the enantio- and diastereoselective additions of γ-substituted allyl reagents (e.g., crotyl) to imines that afford secondary homoallylic amines bearing a vicinal tertiary stereocenter,4,5 the corresponding quaternary stereocenter variants remain lacking.6 One example involves the diastereoselective and enantiospecific addition of chiral allylborons to TMS-aldimines via the borinic ester recently reported by Aggarwal (Scheme 1A).7 In contrast, only two catalytic protocols have been introduced for the enantio- and diastereoselective synthesis of homoallylamines bearing quaternary carbon stereocenters. First, chiral amino alcohols have been shown to catalyze an efficient enantio- and diastereoselective addition of a chiral allyl-B(pin) to aldimines that proceeds by stereospecific allyl transfer to the catalyst (Scheme 1B).8 Secondly, chiral binaphthyl diols have been shown to catalyze the enantio- and diastereoselective additions of γ,γ-disubstituted allyl boronic acids to cyclic imines in high stereoselectivity (Scheme 1C).9 Nevertheless, both catalytic protocols present limitations related to the requirement of either pre-formed enantioenriched γ,γ-disubstituted allylboron reagents or unstable achiral allyl boronic acids. To-date, metal-free catalytic methods have proven to be most effective for the enantioselective synthesis of quaternary carbon stereogenic centers in allylic nucleophile additions to imines. Such catalytic protocols possess mechanistic characteristics that maintain the E or Z alkene isomer of the allyl reagent.7,8 In contrast, due to their configurational instability, reactions of most π-allylmetal complexes lead to poor diastereoselectivity or are limited to the synthesis of one stereoisomer.10,11,12
Scheme 1.

Enantio- and Diastereoselective Additions to Imines with γ,γ-Disubstituted Allyl Reagents
An emerging strategy for the construction of contiguous stereogenic centers via allylic nucleophile additions to C=O and C=N electrophiles involves the use of allylic gem-diboronate esters. Murakami reported the in situ generation of these reagents via Pd- or Ru-catalyzed olefin isomerization, followed by enantio- and diastereoselective addition to aldehydes promoted by a chiral phosphoric acid.13 Subsequently, Cho reported the diastereoselective addition of isolated allyldiboron ester 5 to a variety of cyclic sulfonyl imines, although, the report is limited to the E-crotyl reagent (Eq 1).14
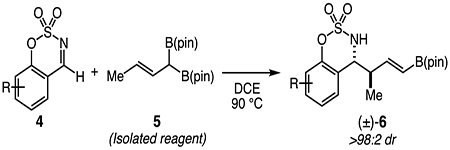 |
(1) |
At this juncture, based on our previous work with 1,1-diborylalkanes,15,16 we postulated that stereodefined γ,γ-disubstituted allyldiboron (e.g., 2) would result in increased reagent stability, and their participation in enantioselective Cu-catalyzed additions to imines would result in the enantioselective synthesis of secondary homoallylic amines bearing a quaternary carbon stereogenic center (e.g., 3) (Scheme 1D). A key aspect of this strategy requires reactions to proceed by enantioselective transmetalation through a chiral α-boryl–Cu–allyl nucleophile.
Successful implementation of our plan required the development of a general method for the synthesis of allylic gem-diboronate esters (e.g., 2). To accomplish this goal, we hypothesized that the direct Pd-catalyzed cross coupling of lithiated organodiboron 7, which can be prepared by deprotonation of diborylmethane with bulky lithium amide bases (LDA or LTMP) on gram-scale with readily available stereodefined vinyl halides would provide the most direct strategy for the synthesis of a variety of stereodefined bis-allylic 1,1-diborons. Building on work by Feringa,17 we found a wide variety of stereodefined alkenyl halides undergo Pd-catalyzed cross coupling with organolithium 7, to afford chromatographically stable stereodefined allyldiborons 2a–i isolated in 32–75% yield (Scheme 2); for example, n-butyl 2b is often isolated in up to 75% yield on a multigram-scale. As the synthesis of 2c and 2e illustrate, the presence of silyl ethers and aryl groups result in equally efficient coupling. The method can also be extended to alkenyl iodides containing α-branched alkyl groups (2g–h). Moreover, ethyl-substituted variants 2d, 2f, and E/Z-2i can be prepared efficiently.
Scheme 2.

Synthesis of γ,γ,-Disubstituted Allyldiboronate Esters a
aSee SI for details.
Next, we began by identifying catalytic conditions for the enantioselective reaction of phosphinoylimine 9 with 2a (Table 1). Investigation of a variety of bidentate and monodentate phosphine ligands (L1–L5, entries 1–5) revealed monodentate phosphoramidite L4 to be the most effective, delivering 11 in 77% conv. and 85:15 er (entry 4). Similar low enantio- and diastereoselectivity was observed in reactions with Me/n-Bu reagent 2b (entries 6–8), with L5 affording 12 in 94% 1H NMR yield, 1.1:1.0 dr, and 90:10 and 91:9 er (entry 8). In addition, phosphoramidite ligands L6 and L7 bearing 3,3’ mesityl and 3,5-xylyl groups, delivered 12 in low dr but with an increase in enantioselectivity. At this point we hypothesized that the high enantioselectivity of each diastereoisomer of 12 afforded with L7 is a result of a chiral (L7)-Cu-allyl formed in high er by an enantioselective transmetalation, but reacts with both prochiral faces of 9 with limited bias. Such an outcome would be consistent with the poor enantioselectivity observed in reverse prenyl additions (entries 1–5), and likely the result of non-selective binding of imine 9 to the Cu-allyl species. Changing the imine activating group to p-MeO-benzyl (PMB) 10, which results in a less electrophilic imine but more Lewis basic nitrogen, resulted in marked improvement in dr. Treatment of 10 and 2b with 5 mol % Cu-OtBu, 10 mol % L7, and 1 equivalent of MeOH at −40 °C for 18 h delivered 14 in 77% yield, >20:1 dr, and 99:1 er (entry 11). Furthermore, reaction of 2a with PMB imine 10 under identical conditions resulted in 13 in 36% yield and 99:1 er (entry 12). In reactions of PMB imine 10 with both 2a and 2b it was noted that significant quantities (15–55%) of protodeborated allyldiboron is formed through competitive reaction of the allylic Cu with methanol. To suppress protonation of the Cu-allyl species, we employed CD3OD to utilize a deuterium isotope effect.18 As illustrated in entries 13 and 14, reaction in the presence of CD3OD results in an increase in yield, furnishing 14 in 84% yield (>20:1 dr, and 99:1 er), and 13 in 65% yield (99:1 er).
Table 1.
Reaction Optimization of Enantio- and Diastereoselective Cu-Catalyzed Additions to Iminesa

|
Reactions performed under N2 atmosphere.
Yield and diastereomeric ratios (dr) determined by analysis of 400, 500, or 600 MHz 1H NMR spectra of crude reactions with hexamethyldisiloxane as internal standard.
Enantiomeric ratios (er) determined by HPLC or SFC analysis; see the SI for details.
15% proto-deboration of 2b.
55% proto-deboration of 2a.
<2% proto-deboration of 2a or 2b.
To investigate substrate scope of the allylic nucleophile addition reaction, we first focused on enantioselective reverse prenyl addition to aryl imines with allyldiboron 2a (Scheme 4). The catalytic method can be used to prepare a wide array of secondary amines bearing an alkenylboron in good yield (>55%) and high selectivity (>98:2 er). Parent phenyl imine (13a), as well as aryl imines bearing electron-donating (13c), electron-withdrawing substituents (13b, d–f), and halogens (13b, h–i) in various positions are suitable substrates. Naphthyl-derived imines also undergo efficient and selective reverse prenyl addition (13g). As the synthesis of 13j–l illustrates, use of heteroaryl imines, including pyridyl groups, results in equally efficient and enantioselective reactions.19
Scheme 4.

Enantio- and Diastereoselective Cu-Catalyzed Synthesis of Quaternary Carbon Stereogenic Centersa–d
aReactions performed under N2 atmosphere. bConversion and diastereomeric ratios (dr) determined by analysis of 400, 500, or 600 MHz 1H NMR spectra of crude reactions with hexamethyldisiloxane as internal standard. cEnantiomeric ratios (er) determined by HPLC or SFC analysis; see the SI for details. dYields of isolated product after SiO2 chromatography and represent an average of at least two runs.
The catalytic method is equally effective for reactions of non-symmetric allyldiboron reagents 2b–j with aryl imines (Scheme 4), providing stereoselective access to a range of complex homoallylic amines bearing a vicinal quaternary carbon stereogenic center, and a pendant alkenylboron group. Reaction of E-allyldiboron reagents that contain n-alkyl groups (14a-b), a pendant silyl ether (14c-d) or a homobenzyl unit (14e-f) react smoothly to furnish secondary amines bearing quaternary carbon stereogenic centers in good yield, dr, and er. Notably, reaction of smaller 2-furyl derived-imine with 2e, affords 14e with excellent stereocontrol (>20:1 dr, and 97:3 er). Stereoselective synthesis of ethyl-substituted quaternary stereocenters can be achieved with high selectivity through the application of ethyl-derived E-allyldiborons 2d-f; for example, secondary amines 14d and 14f are both isolated as single enantio- and diastereomers. Allyldiboron reagents containing more sterically congested α-branched alkyl groups participate under the reaction conditions, although, with mixed results. Cyclohexyl-substituted organodiboron reagent 2g affords 14g in 64% yield and >20:1 dr but 93:7 er. In comparison, a smaller cyclopropyl group (e.g., 2h) results in a restoration in selectivity (14h: 74% yield, >20:1 dr, and 98:2 er).
The efficient transfer of E and Z alkene stereochemistry from the allyldiboron to the product, indicated that the catalytic method could provide an effective strategy for the stereoselective synthesis of Me/Et quaternary carbon stereogenic centers. Potential diminution in selectivity could arise from poor enantioselective transmetalation and/or stereochemical isomerization of the transient allylic Cu species prior to C–C bond formation. As shown in Scheme 5, reactions of both E- and Z-2i were found to proceed stereospecifically with naphthyl imine 10m to access both diastereoisomers in high dr and er. For example, Cu-catalyzed reaction of E-2i delivers R,S-14i in 63% yield, >20:1 dr, and 97.5:2.5 er, whereas, transformation of Z-2i results in R,R-14j in 59% yield, >20:1 dr, and 97.5:2.5 er. These results indicate that the reaction proceeds by an enantioselective transmetalation, followed by Cu-allyl addition to the imine, which is either faster than isomerization or nucleophilic addition via a diastereomeric Cu-allyl isomer. The same mechanism must also be operative for the reverse prenyl addition (Scheme 3).
Scheme 5.

Cu-Catalyzed Stereospecific Additions with E- and Z-Methyl/Ethyl AllylDiboronsa
aSee SI for details.
Scheme 3.

Enantioselective Cu-Catalyzed Reverse Prenyl Addition to Aldiminesa–d
aReactions performed under N2 atmosphere. bConversion determined by analysis of 400, 500, or 600 MHz 1H NMR spectra of crude reactions with hexamethyldisiloxane as internal standard. cEnantiomeric ratios (er) determined by HPLC or SFC analysis; see the SI for details. dYields of isolated product after SiO2 chromatography and represent an average of at least two runs.
A proposed catalytic cycle and stereochemical model for the enantio- and diastereoselective synthesis of complex homoallylic amines is depicted in Scheme 6. In situ generated (L)-Cu–OMe (I) undergoes enantioselective transmetalation with allyldiboron II via III to generate enantioenriched allylic Cu species IV. The orientation of the B(pin) units will be positioned to minimize A(1,3)-strain. Isomerization to less congested and boron-stabilized allylic Cu E-V is followed by reaction with the E-PMB imine via 6-membered cyclic transition state VI. Coordination of the E-imine to (L)-Cu places both the aryl substituent and the PMB groups axial.20 Furthermore, to minimize additional 1,3-diaxial interactions, the B(pin) unit is placed in the equatorial position. Following allyl transfer in a highly diastereoselective manner, CD3OD (or MeOH) then releases the homoallylic amine VIII and regenerates (L)-Cu-OCD3.
Scheme 6.

Proposed Catalytic Cycle and Stereochemical Model
The secondary amines prepared through the Cu-catalyzed protocol are amenable to a range of subsequent chemical transformations (Scheme 7). For example, homoallylic amine 14e undergoes efficient Suzuki-Miyaura cross coupling with p-CF3-C6H4Br to afford alkenylarene 15 in 66% isolated yield. Allylboronic ester 16 can be efficiently prepared in 72% yield via the one carbon homologation of 14f. Additionally, proto-deboration of alkenylboronate 13a proceeds smoothly in the presence of AgF to furnish terminal olefin 17 in 67% yield.21 Of note, this two-step process represents an example of a formal enantioselective reverse prenylation of an acyclic imine.22 Finally, the secondary amines can be transformed into pyrrolidines by a two-step sequence. Treatment of alkenylboronates 13b, 14b-c with NaBO3•4H2O, results in conversion to the corresponding cyclic hemiaminal, which can be reduced with NaBH3CN to afford functionalized pyrrolidines 18, 19 and 20 in 49%, 57%, and 47% yield, respectively over two steps (Scheme 7D). Absolute stereochemistry of the products was determined through X-Ray crystallographic analysis of dimethyl-substituted pyrrolidine 18, which revealed the stereochemical orientation of the secondary amine to be R (Scheme 7D). Furthermore, examination of pyrrolidine 19 by 1D-NOESY confirmed a cis-relationship between the benzylic methine and adjacent methyl group.
Scheme 7.

Synthetic Utility of Products
aSee SI for details. bContains ~10% by-product that cannot be removed.
In conclusion, we have developed the first catalytic enantio- and diastereoselective process for the synthesis of complex homoallylic amines via the reaction of allyldiboronates with aldimines. A (phosphoramidite)-Cu catalyst promotes enantioselective transmetalation, generating a highly reactive α-boryl-Cu-allyl species. This nucleophile reacts efficiently with a variety of aryl aldimines to generate enantioenriched homoallylic amines in excellent dr and er, bearing either gem-dimethyl substitution or a quaternary carbon stereogenic center. Significantly, both product diastereoisomers are rendered accessible by choice of E- and Z-allyldiboron stereochemistry. Furthermore, utility of the products is highlighted through representative transformations that included cross coupling, homologation, and conversion to functionalized pyrrolidines. Further catalytic stereoselective reactions of allyldiborons are ongoing.
Supplementary Material
ACKNOWLEDGMENT
Financial support was provided by the United States National Institutes of Health, Institute of General Medical Sciences (R01GM116987) and the University of North Carolina at Chapel Hill. Mass spectrometry facilities in the Department of Chemistry at University of North Carolina are supported by the National Science Foundation (CHE1726291). We thank Blane Zavesky of UNC for X-ray structure elucidation of 18. AllyChem is acknowledged for donations of B2(pin)2.
Footnotes
Supporting Information. Experimental procedures and spectral and analytical data for all products. This material is available free of charge via the Internet at http://pubs.acs.org.
Authors declare no competing financial interests.
REFERENCE
- (1).For representative reviews that cover the stereoselective synthesis of amines, see:; (a) Enders D; Reinhold U Asymmetric Synthesis of Amines by Nucleophilic 1,2-Addition of Organometallic Reagents to the CN-Double Bond. Tetrahedron: Asymmetry 1997, 8, 1895–1946. [Google Scholar]; (b) Bloch R Additions of Organometallic Reagents to C=N Bonds: Reactivity and Selectivity. Chem. Rev 1998, 98, 1407–1438. [DOI] [PubMed] [Google Scholar]; (c) Kobayashi S; Ishitani H Catalytic Enantioselective Addition to Imines. Chem. Rev 1999, 99, 1069–1094. [DOI] [PubMed] [Google Scholar]; (d) Ding H; Friestad GK Asymmetric Addition of Allylic Nucleophiles to Imino Compounds. Synthesis 2005, 17, 2815–2829. [Google Scholar]; (e) Friestad GK; Mathies AK Recent Developments in Asymmetric Catalytic Addition to CN Bonds. Tetrahedron 2007, 63, 2541–2569. [Google Scholar]; (f) Shibasaki M; Kanai M Asymmetric Synthesis of Tertiary Alcohols and α-Tertiary Amines via Cu-Catalyzed C−C Bond Formation to Ketones and Ketimines. Chem. Rev 2008, 108 (8), 2853–2873. [DOI] [PubMed] [Google Scholar]; (g) Robak MT; Herbage MA; Ellman JA Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev 2010, 110, 3600–3740. [DOI] [PubMed] [Google Scholar]
- (2).For recent reviews on the enantioselective synthesis of quaternary carbon stereogenic centers, see:; (a) Das JP; Marek I Enantioselective Synthesis of All-Carbon Quaternary Stereogenic Centers in Acyclic Systems. Chem. Commun 2011, 47, 4593–4623. [DOI] [PubMed] [Google Scholar]; (b) Minko Y; Marek I Stereodefined Acyclic Trisubstituted Metal Enolates Towards the Asymmetric Formation of Quaternary Carbon Stereocentres. Chem. Commun 2014, 50, 12597–12611. [DOI] [PubMed] [Google Scholar]; (c) Marek I; Minko Y; Pasco M; Mejuch T; Gilboa N; Chechik H; Das JP All-Carbon Quaternary Stereogenic Centers in Acyclic Systems through the Creation of Several C−C Bonds per Chemical Step. J. Am. Chem. Soc 2014, 136, 2682–2694. [DOI] [PubMed] [Google Scholar]; (d) Quasdorf KW; Overman LE Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocentres. Nature 2014, 516, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Liu Y; Han S-J; Liu W-B; Stoltz BM Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res 2015, 48, 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zeng X-P; Cao Z-Y; Wang Y-H; Zhou F; Zhou J Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev 2016, 116, 7330–7396. [DOI] [PubMed] [Google Scholar]; (g) Feng J; Holmes M; Krische MJ Acyclic Quaternary Carbon Stereocenters via Enantioselective Transition Metal Catalysis. Chem. Rev 2017, 117, 12564–12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Marek I; Sklute G Creation of quaternary stereocenters in carbonyl allylation reactions. Chem. Commun 2007, 1683–1691. [DOI] [PubMed] [Google Scholar]; (b) Kolodney G; Sklute G; Perrone S; Knochel P; Marek I Diastereodivergent Synthesis of Enantiomerically Pure Homoallylic Amine Derivatives Containing Quaternary Carbon Stereocenters. Angew. Chem. Int. Ed 2007, 46, 9291–9294. [DOI] [PubMed] [Google Scholar]
- (4).Yus M; González-Gómez JC; Foubelo F Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem. Rev 2011, 111, 7774–7854. [DOI] [PubMed] [Google Scholar]
- (5).For recent examples of enantioselective Cu-catalyzed allyl additions to imines and ketimines that do not generate quaternary carbon stereogenic centers, see:; (a) Wada R; Shibuguchi T; Makino S; Oisaki K; Kanai M; Shibasaki M Catalytic Enantioselective Allylation of Ketoimines. J. Am. Chem. Soc 2006, 128, 7687–7691. [DOI] [PubMed] [Google Scholar]; (b) Yazaki R; Nitabaru T; Kumagai N; Shibasaki M Direct Catalytic Asymmetric Addition of Allylic Cyanides to Ketoimines. J. Am. Chem. Soc 2008, 130, 14477–14479. [DOI] [PubMed] [Google Scholar]; (c) Vieira EM; Snapper ML; Hoveyda AH Enantioselective Synthesis of Homoallylic Amines through Reactions of (Pinacolato)Allylborons with Aryl-, Heteroaryl-, Alkyl-, or Alkene-Substituted Aldimines Catalyzed by Chiral C1-Symmetric NHC−Cu Complexes. J. Am. Chem. Soc 2011, 133, 3332–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yeung K; Ruscoe RE; Rae J; Pulis AP; Procter DJ Enantioselective Generation of Adjacent Stereocenters in a Copper-Catalyzed Three-Component Coupling of Imines, Allenes, and Diboranes. Angew. Chem. Int. Ed 2016, 55, 11912–11916. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Jiang L; Cao P; Wang M; Chen B; Wang B; Liao J Highly Diastereo- and Enantioselective Cu-Catalyzed Borylative Coupling of 1,3-Dienes and Aldimines. Angew. Chem. Int. Ed 2016, 55, 13854–13858. [DOI] [PubMed] [Google Scholar]; (f) Jang H; Romiti F; Torker S; Hoveyda AH Catalytic Diastereo- and Enantioselective Additions of Versatile Allyl Groups to N–H Ketimines. Nature Chemistry 2017, 9, 1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Deng H; Meng Z; Wang S; Zhang Z; Zhang Y; Shangguan Y; Yang F; Yuan D; Guo H; Zhang C Enantioselective Copper‐Catalyzed Three‐Component Carboboronation of Allenes: Access to Functionalized Dibenzo [b,f][1,4]Oxazepine Derivatives. Adv. Synth. Catal 2019, 361, 3582–3587. [Google Scholar]; (h) Bhakta U; Kattamuri PV; Siitonen JH; Alemany LB; Kürti L Enantioselective Catalytic Allylation of Acyclic Ketiminoesters: Synthesis of α-Fully-Substituted Amino Esters. Org. Lett 2019, 21, 9208–9211. [DOI] [PubMed] [Google Scholar]; (i) Li D; Park Y; Yoon W; Yun H; Yun J Asymmetric Synthesis of 1-Benzazepine Derivatives via Copper-Catalyzed Intramolecular Reductive Cyclization. Org. Lett 2019, 21, 9699–9703. [DOI] [PubMed] [Google Scholar]
- (6).For examples of enantioselective allyl additions to aldehydes that generate quaternary carbon stereogenic centers, see:; (a) Denmark SE; Fu J Catalytic, Enantioselective Addition of Substituted Allylic Trichlorosilanes Using a Rationally-Designed 2,2’-Bispyrrolidine-Based Bisphosphoramide. J. Am. Chem. Soc 2001, 123, 9488–9489. [DOI] [PubMed] [Google Scholar]; (b) Denmark SE; Fu J Asymmetric Construction of Quaternary Centers by Enantioselective Allylation: Application to the Synthesis of the Serotonin Antagonist LY426965. Org. Lett 2002, 4, 1951–1953. [DOI] [PubMed] [Google Scholar]; (c) Feng J; Garza VJ; Krische MJ Redox-Triggered C–C Coupling of Alcohols and Vinyl Epoxides: Diastereo- and Enantioselective Formation of All-Carbon Quaternary Centers via Tert -(Hydroxy)-Prenylation. J. Am. Chem. Soc 2014, 136, 8911–8914. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Nguyen KD; Herkommer D; Krische MJ Enantioselective Formation of All-Carbon Quaternary Centers via C–H Functionalization of Methanol: Iridium-Catalyzed Diene Hydrohydroxymethylation. J. Am. Chem. Soc 2016, 138, 14210–14213. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Holmes M; Nguyen KD; Schwartz LA; Luong T; Krische MJ Enantioselective Formation of CF3-Bearing All-Carbon Quaternary Stereocenters via C–H Functionalization of Methanol: Iridium Catalyzed Allene Hydrohydroxymethylation. J. Am. Chem. Soc 2017, 139, 8114–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Schwartz LA; Holmes M; Brito GA; Gonçalves TP; Richardson J; Ruble JC; Huang K-W; Krische MJ Cyclometalated Iridium–PhanePhos Complexes Are Active Catalysts in Enantioselective Allene–Fluoral Reductive Coupling and Related Alcohol-Mediated Carbonyl Additions That Form Acyclic Quaternary Carbon Stereocenters. J. Am. Chem. Soc 2019, 141, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Chen JL-Y; Aggarwal VK Highly Diastereoselective and Enantiospecific Allylation of Ketones and Imines Using Borinic Esters: Contiguous Quaternary Stereogenic Centers. Angew. Chem. Int. Ed 2014, 53, 10992–10996. [DOI] [PubMed] [Google Scholar]
- (8).Silverio DL; Torker S; Pilyugina T; Vieira EM; Snapper ML; Haeffner F; Hoveyda AH Simple Organic Molecules as Catalysts for Enantioselective Synthesis of Amines and Alcohols. Nature 2013, 494, 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).For cyclic imines, see:; (a) Alam R; Diner C; Jonker S; Eriksson L; Szabó KJ Catalytic Asymmetric Allylboration of Indoles and Dihydroisoquinolines with Allylboronic Acids: Stereodivergent Synthesis of up to Three Contiguous Stereocenters. Angew. Chem. Int. Ed 2016, 55, 14417–14421. [DOI] [PMC free article] [PubMed] [Google Scholar]; For ketones, see:; (b) Alam R; Vollgraff T; Eriksson L; Szabó KJ Synthesis of Adjacent Quaternary Stereocenters by Catalytic Asymmetric Allylboration. J. Am. Chem. Soc 2015, 137, 11262–11265. [DOI] [PubMed] [Google Scholar]
- (10).For recent examples of π-allylmetal E/Z scrambling, see:; Tan KL; Jacobsen EN Indium-Mediated Asymmetric Allylation of Acylhydrazones Using a Chiral Urea Catalyst. Angew. Chem. Int. Ed 2007, 46, 1315–1317. [DOI] [PubMed] [Google Scholar]; Ref 5c.
- (11).For examples of enantio- and diastereoselective metal-catalyzed crotyl addition to cyclic imines, where E- and Z-crotyl isomerization is slow, see:; (a) Luo Y; Hepburn HB; Chotsaeng N; Lam HW Enantioselective Rhodium-Catalyzed Nucleophilic Allylation of Cyclic Imines with Allylboron Reagents. Angew. Chemie. Int. Ed 2012, 51, 8309–8313. [DOI] [PubMed] [Google Scholar]; (b) Hepburn HB; Chotsaeng N; Luo Y; Lam HW Enantioselective Rhodium-Catalyzed Allylation of Cyclic Imines with Potassium Allyltrifluoroborates. Synthesis, 2013, 45, 2549–2661. [Google Scholar]
- (12).For examples of rapid metal-allyl interconversion and reaction through a single regiosiomeric species, see: Ref. 5c–f.
- (13).(a) Miura T; Nakahashi J; Murakami M Enantioselective Synthesis of (E)-δ-Boryl-Substituted Anti-Homoallylic Alcohols Using Palladium and a Chiral Phosphoric Acid. Angew. Chem. Int. Ed 2017, 56, 6989–6993. [DOI] [PubMed] [Google Scholar]; (b) Miura T; Nakahashi J; Zhou W; Shiratori Y; Stewart SG; Murakami M Enantioselective Synthesis of Anti −1,2-Oxaborinan-3-Enes from Aldehydes and 1,1-Di(Boryl)Alk-3-Enes Using Ruthenium and Chiral Phosphoric Acid Catalysts. J. Am. Chem. Soc 2017, 139, 10903–10908. [DOI] [PubMed] [Google Scholar]; (c) Miura T; Oku N; Murakami M Diastereo- and Enantioselective Synthesis of (E)-δ-Boryl-Substituted Anti -Homoallylic Alcohols in Two Steps from Terminal Alkynes. Angew. Chem. Int. Ed 2019, 58, 14620–14624. [DOI] [PubMed] [Google Scholar]
- (14).Park J; Choi S; Lee Y; Cho SH Chemo- and Stereoselective Crotylation of Aldehydes and Cyclic Aldimines with Allylic Gem-Diboronate Ester. Org. Lett 2017, 19, 4054–4057. [DOI] [PubMed] [Google Scholar]
- (15).(a) Joannou MV; Moyer BS; Meek SJ Enantio- and Diastereoselective Synthesis of 1,2-Hydroxyboronates through Cu-Catalyzed Additions of Alkylboronates to Aldehydes. J. Am. Chem. Soc 2015, 137, 6176–6179. [DOI] [PubMed] [Google Scholar]; (b) Joannou MV; Moyer BS; Goldfogel MJ; Meek SJ Silver(I)-Catalyzed Diastereoselective Synthesis of Anti −1,2-Hydroxyboronates. Angew. Chemie. Int. Ed 2015, 54, 14141–14145. [DOI] [PubMed] [Google Scholar]; (c) Murray SA; Green JC; Tailor SB; Meek SJ Enantio- and Diastereoselective 1,2-Additions to α-Ketoesters with Diborylmethane and Substituted 1,1-Diborylalkanes. Angew. Chem. Int. Ed 2016, 55, 9065–9069. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Murray SA; Liang MZ; Meek SJ Stereoselective Tandem Bis-Electrophile Couplings of Diborylmethane. J. Am. Chem. Soc 2017, 139, 14061–14064. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Murray SA; Luc ECM; Meek SJ Synthesis of Alkenyl Boronates from Epoxides with Di-[B(Pin)]-Methane via Pd-Catalyzed Dehydroboration. Org. Lett 2018, 20, 469–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For additional examples of Cu-catalyzed stereoselective 1,2-addition of diborylalkanes, see:; (a) Kim J; Ko K; Cho SH Diastereo- and Enantioselective Synthesis of β-Aminoboronate Esters by Copper(I)-Catalyzed 1,2-Addition of 1,1-Bis[(Pinacolato)Boryl]Alkanes to Imines. Angew. Chem. Int. Ed 2017, 56, 11584–11588. [DOI] [PubMed] [Google Scholar]; (b) Kim J; Shin M; Cho SH Copper-Catalyzed Diastereoselective and Enantioselective Addition of 1,1-Diborylalkanes to Cyclic Ketimines and α-Imino Esters. ACS Catal 2019, 9, 8503–8508. [Google Scholar]; (c) Kim J; Hwang C; Kim Y; Cho SH Improved Synthesis of β-Aminoboronate Esters via Copper-Catalyzed Diastereo- and Enantioselective Addition of 1,1-Diborylalkanes to Acyclic Arylaldimines. Org. Process Res. Dev 2019, 23, 1663–1668. [Google Scholar]
- (17).Giannerini M; Fañanás-Mastral M; Feringa BL Direct Catalytic Cross-Coupling of Organolithium Compounds. Nature Chem. 2013, 5, 667–672. [DOI] [PubMed] [Google Scholar]
- (18).For a related example of that employs t-BuOD, see:; Ascic E; Buchwald SL Highly Diastereo- and Enantioselective CuH-Catalyzed Synthesis of 2,3-Disubstituted Indolines. J. Am. Chem. Soc 2015, 137, 4666–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Under the current catalytic protocol, reactions of aliphatic and α,β-unsaturated imines are not efficient; for example, reaction of cinnamaldehyde-derived N-Bn imine with 2a results in 15% conversion and 63:37 er.
- (20).For an example of (L)-Cu-allyl reaction through the E-isomer of N-Bn-imine, see:; Liu RY; Yang Y; Buchwald SL Regiodivergent and Diastereoselective CuH-Catalyzed Allylation of Imines with Terminal Allenes. Angew. Chem. Int. Ed 2016, 55, 14077–14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Nave S; Sonawane RP; Elford TG; Aggarwal VK Protodeboronation of Tertiary Boronic Esters: Asymmetric Synthesis of Tertiary Alkyl Stereogenic Centers. J. Am. Chem. Soc 2010, 132, 17096–17098. [DOI] [PubMed] [Google Scholar]
- (22).For examples of stereoselective reverse prenyl additions to acyclic imines, see:; Bosque I; Foubelo F; Gonzalez-Gomez JC A General Protocol to Afford Enantioenriched Linear Homoprenylic Amines. Org. Biomol. Chem 2013, 11, 7507. [DOI] [PubMed] [Google Scholar]; Ref 5d.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


