Klebsiella species are problematic pathogens in neonatal units and may cause outbreaks, for which the sources of transmission may be challenging to elucidate. We describe the use of whole-genome sequencing (WGS) to investigate environmental sources of transmission during an outbreak of extended-spectrum-β-lactamase (ESBL)-producing Klebsiella michiganensis colonizing neonates. Ceftriaxone-resistant Klebsiella spp. isolated from neonates (or their mothers) and the hospital environment were included.
KEYWORDS: Klebsiella oxytoca, Klebsiella michiganensis, whole-genome sequencing, extended-spectrum β-lactamase, outbreak
ABSTRACT
Klebsiella species are problematic pathogens in neonatal units and may cause outbreaks, for which the sources of transmission may be challenging to elucidate. We describe the use of whole-genome sequencing (WGS) to investigate environmental sources of transmission during an outbreak of extended-spectrum-β-lactamase (ESBL)-producing Klebsiella michiganensis colonizing neonates. Ceftriaxone-resistant Klebsiella spp. isolated from neonates (or their mothers) and the hospital environment were included. Short-read sequencing (Illumina) and long-read sequencing (MinION; Oxford Nanopore Technologies) were used to confirm species taxonomy, to identify antimicrobial resistance genes, and to determine phylogenetic relationships using single-nucleotide polymorphism profiling. A total of 21 organisms (10 patient-derived isolates and 11 environmental isolates) were sequenced. Standard laboratory methods identified the outbreak strain as an ESBL-producing Klebsiella oxytoca, but taxonomic assignment from WGS data suggested closer identity to Klebsiella michiganensis. Strains isolated from multiple detergent-dispensing bottles were either identical or closely related by single-nucleotide polymorphism comparison. Detergent bottles contaminated by K. michiganensis had been used for washing milk expression equipment. No new cases were identified once the detergent bottles were removed. Environmental reservoirs may be an important source in outbreaks of multidrug-resistant organisms. WGS, in conjunction with traditional epidemiological investigation, can be instrumental in revealing routes of transmission and guiding infection control responses.
INTRODUCTION
Outbreaks of extended-spectrum-β-lactamase (ESBL)-producing Enterobacterales strains within neonatal intensive care units are most commonly caused by Klebsiella species and may be associated with significant morbidity and mortality rates (1). Klebsiella michiganensis was first identified from a toothbrush holder in a home in Michigan and was initially identified as Klebsiella oxytoca, to which it is closely related (99% nucleotide sequence identity in the 16S rRNA gene sequence) (2). Since its characterization, K. michiganensis has been reported in clinical settings, including as a cause of diarrhea in a hematopoietic stem cell transplant recipient (3), from a sample of fluid from an abdominal fistula (4), in an investigation of hospital-acquired colonization by a New Delhi metallo-β-lactamase-producing organism (5), and as an invasive, Klebsiella pneumoniae carbapenemase (KPC)-producing pathogen causing bloodstream infection (6). While the role of K. oxytoca in nosocomial outbreaks is established (7–11), there have been no reported outbreaks involving K. michiganensis to date.
Similar to K. oxytoca, K. michiganensis carries a chromosomally encoded OXY-type (Ambler class A) β-lactamase (12, 13) (also labeled K1 in K. oxytoca [14]), which mediates resistance to amino- and carboxy-penicillins (e.g., ampicillin, ticarcillin, and temocillin). Overexpression of OXY enzymes arising from mutations in regulatory genes (15–17) can lead to phenotypic resistance to multiple β-lactams. Constitutive hyperproducers may also be selected during antibiotic therapy (18). OXY-type enzymes may give false-positive results in ESBL confirmation testing with the clavulanate combination disk method (19) but are not “classic” ESBLs (i.e., Bush-Jacoby group 2be enzymes [20]).
Outbreaks of multidrug-resistant (MDR) organisms in neonatal units are of significant concern because empirical antibiotic strategies defined in most guidelines may have limited efficacy against MDR Gram-negative bacilli. Hospitalized newborns, especially when premature, are at risk for infections caused by Klebsiella spp. (21), which account for significant proportions of nosocomially acquired neonatal infections, particularly in developing countries (22).
We describe an outbreak of ESBL-producing K. michiganensis (initially misidentified as K. oxytoca) that occurred within a neonatal unit and was declared after three apparently unrelated colonized cases were identified. The admissions did not overlap in time, and preliminary examinations failed to reveal any likely cross-contamination events. We explored the utility of whole-genome sequencing (WGS) in conjunction with a standard outbreak investigation to help resolve the source of the outbreak and to guide interventions to successfully halt transmission.
MATERIALS AND METHODS
Setting.
Caboolture Base Hospital is a 280-bed regional hospital in South East Queensland, Australia. The special care nursery (SCN) provides care for neonates with gestational ages of >32 weeks or birth weights of ≥1,500 g. The unit was designed to accommodate 12 neonates; however, due to demand, it is regularly forced to operate up to 14 beds. Active surveillance (rectal swabs) for multiresistant organisms, including methicillin-resistant Staphylococcus aureus, multiresistant Gram-negative bacteria, and vancomycin-resistant Enterococcus, is performed weekly and for all patients transferred into the unit. All cases of multiresistant organism colonization or infection are reviewed by the infection control (IC) team, and contact isolation is employed for all cases of methicillin-resistant S. aureus, vancomycin-resistant Enterococcus, and multiresistant Gram-negative bacteria (except ESBL-producing Escherichia coli).
Definitions, data collection, and IC responses.
After declaration of the outbreak, enhanced surveillance was introduced, with rectal swabbing of all SCN patients every 48 h. All neonates with positive screening swabs were isolated in single rooms (when available) and with contact precautions (according to routine policy). Mothers of colonized babies were also screened. Direct observation of IC procedures and hand hygiene performance was performed by independent Hand Hygiene Australia-accredited IC staff. SCN procedures and policy compliance were reviewed.
Microbial surveillance.
Rectal swabs were collected, plated onto selective chromogenic medium (chromID ESBL agar; bioMérieux), and examined after 18 to 24 h of incubation in ambient air at 35°C. Colonies that grew on selective medium were identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) (Vitek MS; bioMérieux), and antibiotic susceptibility was determined using the Vitek 2 system (bioMérieux). ESBL production was confirmed using combination disk diffusion, with an increase in the zone of inhibition of ≥5 mm with the addition of clavulanic acid (10 μg) to cefotaxime (30 μg) or ceftazidime (30 μg) disks suggesting ESBL production (23). After the incident case (case 1) was identified, any ceftriaxone-resistant K. oxytoca strain from a neonatal culture from any body site was stored; following the declaration of an outbreak (case 3), all stored and prospective isolates were sent for WGS. For comparison, additional isolates, including ESBL-producing E. coli and ceftriaxone-susceptible K. oxytoca strains from neonates, as well as any K. oxytoca strains identified from maternal samples, were sequenced. An outbreak case was defined as any individual with K. oxytoca, cultured from any site, that was determined to be resistant to ceftriaxone by Vitek 2 (irrespective of ESBL phenotypic confirmatory testing).
Environmental screening.
Swabs were taken from faucet aerators, soap dispensers, damp surfaces, high-use equipment, and the humidification apparatus of humidicribs. Swabs were inserted through the plugholes of sinks in order to sample the internal surfaces of sink drains. Consumables such as shampoo, paraffin, and ultrasound gel were collected, plated on selective chromogenic medium, and processed in the same way as the patient screening samples (see above).
Whole-genome sequencing.
All K. oxytoca isolates from neonates, their mothers, or the environment were submitted for WGS at the Queensland Health Forensic Scientific Services laboratory in five batches, between 29 January 2018 and 23 March 2018. Genomic DNA was extracted using QIAamp DNA minikits (Qiagen, Australia) and quantified by spectrophotometry (NanoDrop; Thermo Fisher) and fluorometry (Quant-iT; Thermo Fisher). Paired-end DNA libraries were prepared using Nextera XT kits (Illumina, Australia), and WGS was performed using an Illumina NextSeq system (150-bp paired-end reads).
Using Nanopore sequencing (MinION; Oxford Nanopore Technologies), we assembled the complete genome of strain M82255 for reference. In brief, 1.5 μg of DNA was used as input for the 1D sequencing by ligation kit (product number SQK-LSK108), according to the manufacturer’s instructions. The final library was loaded onto a FLO-MIN106 R9.4.1 flow cell and run for approximately 40 h on a MinION system.
In silico multilocus sequencing typing, SNP typing, and resistance gene detection.
Sample analysis was undertaken using the custom Queensland Genomics Health Alliance infectious diseases genomic analysis pipeline (version dev-0.4.0). In brief, raw Illumina sequencing data were quality trimmed using Trimmomatic (version 0.36) (24). In silico sequence typing was preformed using SRST2 and the K. oxytoca typing scheme available from PubMLST (https://pubmlst.org/koxytoca/). Single-nucleotide polymorphism (SNP) profiling and determination of core genome SNPs were undertaken by aligning the trimmed Illumina sequencing reads for each sample to the complete genome of strain M82255 using Snippy (version 4.4.0) (https://github.com/tseemann/snippy), with a minimum read coverage of 10× and a minimum base quality score of 20 being required for a site to be considered. Resistance gene profiling was performed by screening the trimmed sequence reads for each isolate against the ARG-ANNOT resistance gene database using SRST2 (25, 26). Only genes with a minimum of 90% nucleotide sequence identity and 90% sequence coverage were reported.
Phylogenetic analysis.
A list of polymorphic positions conserved in all strains was established using Snippy-core (https://github.com/tseemann/snippy). Polymorphic substitution sites were concatenated to produce an alignment that was used to reconstruct the phylogeny. A recombination-filtered, maximum likelihood phylogenetic tree was estimated using Gubbins (version 2.2.0) (http://sanger-pathogens.github.io/gubbins) (27) for the SNP alignments, under the generalized time reversible nucleotide substitution model with gamma correction for among-site rate variation. The resulting tree was rooted using the genome of K. michiganensis CAV1374 (GenBank accession number CP011636) as an outgroup.
Ethics.
WGS activities for pathogen surveillance and IC are covered by the Queensland Genomics Health Alliance clinical demonstration project (approval no. HREC/17/QFSS/6), with approval for waiver of consent requirements.
Accession number(s).
Genome data have been deposited at NCBI under BioProject accession number PRJNA512395. Raw Nanopore and Illumina sequence read data have been deposited in the Sequence Read Archive (accessions numbers SRR8420303 to SRR8420330). The complete genome of strain M82255 has been deposited in GenBank (accessions numbers CP035214 to CP035216).
RESULTS
Species identification.
Initial species characterization, using MALDI-TOF MS, identified strain M82255 as Klebsiella oxytoca. Taxonomic assignment was confirmed in silico using Kraken (28), by comparison of sequence read data for M82255 against the NCBI RefSeq database, which contains ∼25,000 complete bacterial, archaeal, and viral genomes. However, after submission of the complete genome of strain M82255 to GenBank, a quality control test of the taxonomic assignment using average nucleotide identity (ANI) (29) revealed the genome of M82255 to be 98.903% identical to K. michiganensis (91.1% genome coverage) but only 92.455% similar to K. oxytoca. Further biochemical tests were performed with M82255 to identify its position within the genus Klebsiella (see Table S1 in the supplemental material). Similar to the expected biochemical profile of K. michiganensis, M82255 was unable to produce urease (2), which confirmed the results of the ANI approach. Consequently, M82255 was reclassified as K. michiganensis.
Incident cases.
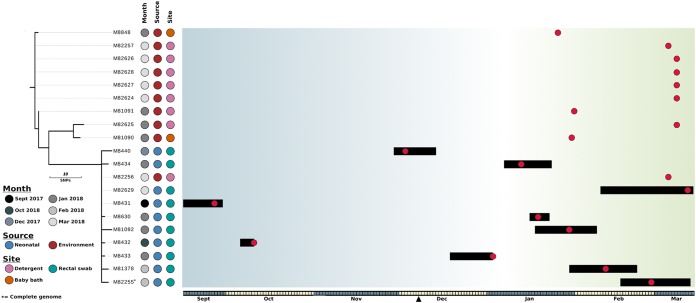
From the incident case to identification of the final case 188 days later, 10 neonates were colonized with K. michiganensis (Fig. 1). One neonate was simultaneously cocolonized with ESBL-producing E. coli. During this period, there were approximately 880 births and 163 admissions to the SCN. There were no cases of clinical infection caused by K. michiganensis. The average length of stay in the SCN was 15.7 days (range, 5 to 35 days), and there was a mean of 7.9 days (range, 2 to 15 days) from admission to the SCN to detection of ESBL-producing K. michiganensis. One neonatal patient sample was obtained from another hospital facility following discharge.
FIG 1.
Evolutionary relationship of K. michiganensis isolates. The maximum likelihood phylogenetic tree was built using 38 core genome SNPs, relative to K. michiganensis strain M82255, and was rooted using K. michiganensis strain CAV1374 (not shown). The month of isolation, source (neonatal or environmental), and specific site of isolation are indicated. Branches (black lines) represent the genetic distances between isolates in terms of SNPs. The scale is indicated. The outbreak timeline is presented adjacent to the tree. The timescale is represented at the bottom, with alternate months colored gray and yellow. The black triangle indicates the date on which the outbreak was declared and screening was switched from weekly to every 48 h. Each box in the timeline represents a period of 1 day, starting on 15 September 2017. Black bars represent the admission periods for colonized patients, and red dots represent dates on which samples were isolated (including environmental isolates). Blue shading represents periods in which there was no overlap in patient stays, and green shading represents periods in which multiple colonized patients were present in the SCN at the same time.
IC observation.
Direct observation of the SCN IC procedures revealed that all staff hand hygiene performance was above the national benchmark (80%). However, significant opportunities for cross-contamination (breakdowns in hand hygiene, leaning on equipment, and failure to observe contact isolation procedures) were noted in relation to non-staff members (family members, friends, and SCN occupants). Additionally, incomplete compliance with microbial surveillance cultures at admission was noted at the start of the outbreak.
Environmental screening.
Exhaustive screening of the SCN on multiple occasions failed to detect a source of environmental contamination. Environmental sampling was then extended to the maternity ward and birth suite. ESBL-producing K. michiganensis was identified from the drain of a baby bath in a multipurpose utility room containing laundry equipment (washing machine and laundry dryer) and from a pair of baby baths (no longer used for bathing). However, an epidemiological link between the multipurpose room in the maternity ward and the colonized cases in the SCN was not evident. No other drains were found to culture K. michiganensis.
During IC unit observation, it was noted that the hospital’s volunteer flower service used the multipurpose room for cleaning of flower vases. The flower service’s equipment was then sampled, and a second K. michiganensis environmental isolate was retrieved from a sample of the flower service’s dishwashing detergent. The hospital’s detergent (Cleantec Emmy; Ecolab, Australia) was bought as bulk concentrate and then decanted into reusable detergent bottles for use around the hospital. Each drum of concentrate lasted approximately 1 month. K. michiganensis was not identified in the bulk concentrate at the time of testing; however, sampling of detergent from around the hospital identified K. michiganensis in 7 of 13 bottles.
Whole-genome sequencing.
A total of 21 organisms (10 patient-derived isolates, from 10 individuals, and 11 environmental isolates) were included in the genomic analysis (Table 1). A single non-ESBL-producing K. oxytoca strain (M8843) was identified from a maternal rectal swab and was found to belong to the sequence type 50 lineage; it was excluded from further study and was not considered part of the outbreak. All K. michiganensis strains possessed chromosomal OXY-type β-lactamases, as well as SHV-2 ESBL (in addition to LEN and TEM-1B β-lactamases). Resistance genes for aminoglycosides [aac(3)-IId, aph(3′)-Ia, and strA/B] and sulfonamides (sul1) were also detected.
TABLE 1.
Sample details
| Isolate | Isolate source | Patient gender | Sampling date | Sample type or source | Organism | Hospital |
|---|---|---|---|---|---|---|
| M8431 | Neonatal | Male | September 2017 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M8432 | Neonatal | Female | October 2017 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M8440 | Neonatal | Male | December 2017 | Skin swab | Klebsiella michiganensis | External hospital |
| M8433 | Neonatal | Female | January 2018 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M8434 | Neonatal | Female | January 2018 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M8630 | Neonatal | Female | January 2018 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M81092 | Neonatal | Female | January 2018 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M81378 | Neonatal | Male | February 2018 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M82255 | Neonatal | Female | February 2018 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M82629 | Neonatal | Female | March 2018 | Rectal swab | Klebsiella michiganensis | Caboolture Hospital |
| M8848 | Environmental | January 2018 | Baby bath drain in maternity multipurpose room | Klebsiella michiganensis | Caboolture Hospital | |
| M81090 | Environmental | January 2018 | Baby bath drain in maternity multipurpose room | Klebsiella michiganensis | Caboolture Hospital | |
| M81091 | Environmental | January 2018 | Detergent for flower service | Klebsiella michiganensis | Caboolture Hospital | |
| M82256 | Environmental | March 2018 | Detergent in SCN milk room | Klebsiella michiganensis | Caboolture Hospital | |
| M82257 | Environmental | March 2018 | Detergent at nurses station | Klebsiella michiganensis | Caboolture Hospital | |
| M82624 | Environmental | March 2018 | Detergent brush in ICUa | Klebsiella michiganensis | Caboolture Hospital | |
| M82625 | Environmental | March 2018 | Detergent in ward 2a multipurpose room | Klebsiella michiganensis | Caboolture Hospital | |
| M82626 | Environmental | March 2018 | Milk room in pediatric ward | Klebsiella michiganensis | Caboolture Hospital | |
| M82627 | Environmental | March 2018 | Detergent in ICU | Klebsiella michiganensis | Caboolture Hospital | |
| M82628 | Environmental | March 2018 | Detergent in pediatric ward | Klebsiella michiganensis | Caboolture Hospital |
ICU, intensive care unit.
To investigate the relationship between outbreak isolates at the single-nucleotide level, reads from each isolate were mapped to the complete genome of K. michiganensis strain M82255. Phylogenetically, the K. michiganensis isolates formed two distinct clusters separated by 15 core genome SNPs (Fig. 2; also see Table S2). SCN cluster 1 (SCN-C1) was composed exclusively of environmental isolates (n = 10). SCN cluster 2 (SCN-C2) was composed of the 10 patient isolates and a single environmental isolate, M82256. Isolate M82256 was collected from the milk room detergent used to clean breast milk expression equipment, thus allowing contamination of expressed milk and milk bottles and subsequent onward transmission to neonates in the SCN.
FIG 2.
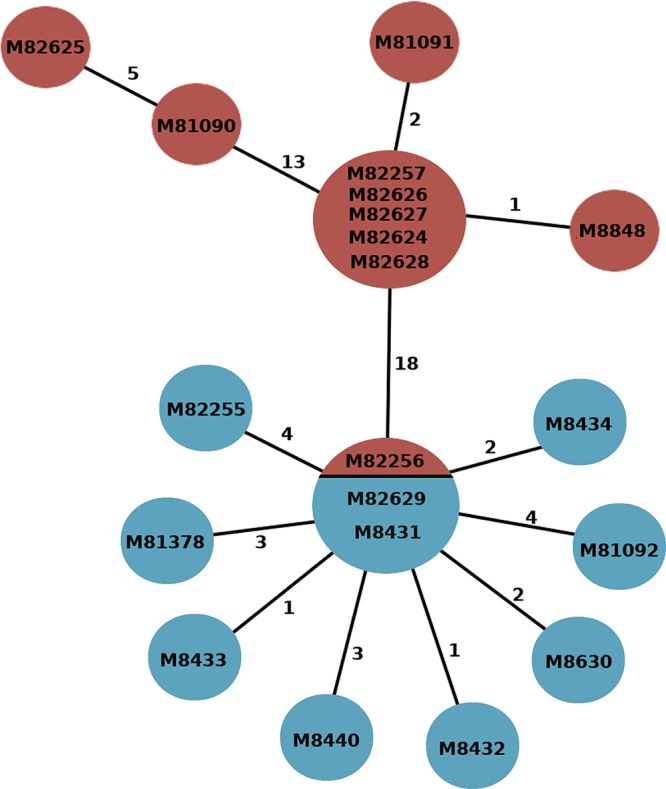
Evolutionary relationship of K. michiganensis strains. The minimal spanning tree shows relationships between environmental (red) and patient (blue) samples. Numbers attached to branches represent the numbers of SNPs between genomes. Isolate codes are represented within each node; if >1 code is listed, then the strains were identical at the core genome level.
WGS and an in-depth epidemiological investigation were used to determine whether K. michiganensis transmission resulted from direct patient-to-patient transfer or through contact with a common environmental reservoir. Between 6 January and 14 March (when the outbreak resolved), 6 colonized patients had overlapping stays in the SCN (Fig. 1), 4 of whom were resident at the same time, providing opportunities for isolate transmission between neonates. However, when WGS was used to establish relationships between these isolates, there was no genetic evidence to support patient-to-patient transmission.
SCN-C2 was composed of a tight cluster of closely related isolates (median pairwise SNP distance of 4 SNPs). Central to this cluster was a group of 3 isolates that were indistinguishable at the core genome SNP level. The cluster was composed of isolates M8431 and M82629, which were isolated from case 2 and case 10, respectively (hospital admissions separated by 147 days), and a single environmental isolate (M82256) collected from a detergent located in the SCN milk room. The remaining 8 patient isolates diverged directly from this central group but possessed no common discriminatory SNPs, suggesting that each colonized patient independently acquired K. michiganensis from a common source, probably the SCN milk room detergent.
Within SCN-C1, environmental isolates collected from a baby bath drain (isolate M8848) and detergent samples from four of the eight tested detergent-dispensing bottles (isolates M82257, M82624, M82627, and M82628) were indistinguishable at the core genome level. The relatedness of isolates collected from the detergent suggested that contamination from a central source was likely responsible for introducing K. michiganensis into the hospital, possibly during dispensing of the bulk detergent or from the drum itself. Testing of the bulk detergent drum did not identify any biological contaminants. However, the large number of SNPs (14 core genome SNPs) separating environmental and patient isolates suggests that the outbreak strain might have been present in the local environment for some time.
Outbreak management.
All identified cases were isolated under contact precautions. After isolation of K. michiganensis in the multipurpose room, the room was immediately decommissioned and refurbished as a single-use facility. Redundant equipment (baby baths and laundry equipment) was removed, and the flower service was temporarily discontinued. Reusable detergent bottles from across the hospital were destroyed, and the detergent supply was switched to prefilled single-use detergent bottles.
Because the contamination of the detergent bottles might have originated from contaminated concentrate, a search for similar cases of ESBL-producing K. michiganensis (or K. oxytoca) at other hospitals served by Pathology Queensland was performed, using the statewide laboratory information system; however, there was no indication of unrecognized outbreaks at other facilities.
DISCUSSION
Klebsiella species are frequently implicated in outbreaks in the neonatal intensive care setting, although sources are not always evident despite extensive investigation and screening (30). For example, outbreaks involving MDR K. oxytoca strains have been previously linked to environmental reservoirs, with described sources as varied as handwashing sinks (7), wastewater drainage systems (9), water humidifiers for neonatal incubators (16), transducers used for blood pressure monitoring (31), and washing machines (11). Contaminated medical solutions, such as heparin (32), sodium chloride (33), and insulin (8), have also been described as sources.
Nosocomial outbreaks of K. michiganensis have not been reported previously. However, based on the ANI approach (34), numerous strains previously identified as K. oxytoca have now been reclassified as K. michiganensis, including a carbapenem-resistant strain, E718 (GenBank accession number CP003683), that was isolated from the abdomen of a renal transplant patient (35). In fact, of the seven complete K. michiganensis genomes in GenBank, including strain M82255 from the outbreak described here and the representative K. michiganensis genome at NCBI (GenBank accession number CP003683), all were originally classified as K. oxytoca before correction by taxonomic curators at NCBI. Consequently, it is possible that hospital outbreaks of K. michiganensis may go undetected because they are prone to misclassification in the absence of genomic data.
Contaminated disinfectant has been recognized previously as a potential source of K. oxytoca sepsis among hospitalized infants (36). Organism factors such as a mucoid phenotype based on enhanced capsule formation may enable the bacteria to survive in this environment (36). Additionally, it has been suggested that, in Klebsiella, outer membrane proteins, such as peptidoglycan-associated lipoprotein (Pal) and murein lipoprotein (LppA), contribute to resistance against detergents by modulating the integrity and selective impermeability of the cell membrane independent of lipopolysaccharide and capsule (37). Strains of K. oxytoca that are able to withstand active ingredients of commonly used detergents, such as sodium dodecyl sulfate (SDS), have been described (38).
This report clearly demonstrates the effectiveness of high-throughput WGS as a tool to enhance and to support established IC outbreak responses. The high resolution available with WGS to compare outbreak strains was sufficient to rule out patient-to-patient transmission and suggested that an environmental reservoir might be the source of colonization. The ensuing IC investigation successfully identified several environmental sites contaminated with K. michiganensis. WGS of the environmental isolates linked them unequivocally to patient isolates and identified a liquid detergent bottle in the milk dispensing room as the most likely source for onward transmission in the SCN. Washing detergent in the milk room was used by mothers to clean milk expression equipment, yielding cross-contamination between this equipment and expressed milk and thus resulting in neonate colonization. Although the initial source of the contamination could not be confirmed, K. michiganensis isolates related to the outbreak were identified in liquid detergent dispensers located at different sites throughout the hospital. Liquid detergent dispensers were refilled from a central bulk drum of detergent concentrate. At the time of the outbreak, however, the bulk concentrate was negative for K. michiganensis. It is possible either that a contaminated drum was introduced into the hospital, its contents were dispensed, and the drum was disposed of prior to the outbreak or that contamination occurred at some point during the dispensing process. It should be noted that hospital employees and volunteers were not sampled during the outbreak. However, the lack of any additional patient or environmental cases after removal of the detergent bottles indicates that current staff members are unlikely to have been the source of contamination. It is also possible that environmental sampling, without broth enrichment, may limit sensitivity.
The hospital procedures and practices for preparation of cleaning materials were reviewed. It became apparent that detergent bottles would often be “topped up” when approaching empty, rather than being emptied, washed, and left to dry before refilling. This practice would have allowed propagation of the organism in the detergent bottles after contamination. In response to this observation, reusable detergent bottles around the hospital were collected and destroyed and single-use prediluted detergent bottles were sourced. Although the topping up of detergent bottles was noncompliant with existing procedures, from the point of view of the maintenance staff it was intuitive and convenient. This gap between “practice imagined” and “practice performed” illustrates the importance of direct observation of staff practices and procedures during investigation of an outbreak, the importance of all hospital staff members understanding the IC policies relevant to them, and the need for a well-resourced, active, hospital IC team.
Conclusions.
Environmental reservoirs are increasingly being recognized as a source of outbreaks involving MDR pathogens. We demonstrate how WGS, in conjunction with standard epidemiological investigation, was instrumental in confirming the source of ESBL-producing K. michiganensis in a neonatal unit and directing effective measures to interrupt transmission. Liquid detergent can become contaminated with pathogenic bacteria and should be considered a potential source in such outbreaks.
Supplementary Material
ACKNOWLEDGMENTS
We thank the scientists at Pathology Queensland for their assistance in the laboratory work.
This work was supported by funding from the Queensland Genomics Health Alliance (now Queensland Genomics), Queensland Health, the Queensland Government. L.W.R. was supported by an Australian Government Research Training Program Scholarship. P.N.A.H. was supported by a National Health and Medical Research Council Early Career Fellowship (grant GNT1157530).
P.N.A.H. has received research grants from Merck Sharp and Dohme and Shionogi, outside the submitted work, and speaker’s fees from Pfizer, paid to the University of Queensland. D.L.P. reports receiving grants and personal fees from Shionogi and Merck Sharp and Dohme and personal fees from Pfizer, Achaogen, AstraZeneca, Leo Pharmaceuticals, Bayer, GlaxoSmithKline, Cubist, Venatorx, and Accelerate. The other authors have no conflicts of interest to declare.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Stapleton PJ, Murphy M, McCallion N, Brennan M, Cunney R, Drew RJ. 2016. Outbreaks of extended spectrum beta-lactamase-producing Enterobacteriaceae in neonatal intensive care units: a systematic review. Arch Dis Child Fetal Neonatal Ed 101:F72–F78. doi: 10.1136/archdischild-2015-308707. [DOI] [PubMed] [Google Scholar]
- 2.Saha R, Farrance CE, Verghese B, Hong S, Donofrio RS. 2013. Klebsiella michiganensis sp. nov., a new bacterium isolated from a tooth brush holder. Curr Microbiol 66:72–78. doi: 10.1007/s00284-012-0245-x. [DOI] [PubMed] [Google Scholar]
- 3.Zheng B, Xu H, Yu X, Lv T, Jiang X, Cheng H, Zhang J, Chen Y, Huang C, Xiao Y. 2018. Identification and genomic characterization of a KPC-2-, NDM-1- and NDM-5-producing Klebsiella michiganensis isolate. J Antimicrob Chemother 73:536–538. doi: 10.1093/jac/dkx415. [DOI] [PubMed] [Google Scholar]
- 4.Hazen TH, Mettus R, McElheny CL, Bowler SL, Nagaraj S, Doi Y, Rasko DA. 2018. Diversity among blaKPC-containing plasmids in Escherichia coli and other bacterial species isolated from the same patients. Sci Rep 8:10291. doi: 10.1038/s41598-018-28085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Founou RC, Founou LL, Allam M, Ismail A, Essack SY. 2018. Genomic characterisation of Klebsiella michiganensis co-producing OXA-181 and NDM-1 carbapenemases isolated from a cancer patient in uMgungundlovu District, KwaZulu-Natal Province, South Africa. S Afr Med J 109:7–8. doi: 10.7196/SAMJ.2018.v109i1.13696. [DOI] [PubMed] [Google Scholar]
- 6.Seiffert SN, Wuthrich D, Gerth Y, Egli A, Kohler P, Nolte O. 2019. First clinical case of KPC-3-producing Klebsiella michiganensis in Europe. New Microbes New Infect 29:100516. doi: 10.1016/j.nmni.2019.100516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowe C, Willey B, O'Shaughnessy A, Lee W, Lum M, Pike K, Larocque C, Dedier H, Dales L, Moore C, McGeer A. 2012. Outbreak of extended-spectrum β-lactamase-producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg Infect Dis 18:1242–1247. doi: 10.3201/eid1808.111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mete B, Aybar Bilir Y, Aygun G, Yilmaz M, Urkmez S, Dilmen Y, Ozturk R. 2013. Klebsiella oxytoca outbreak in an intensive care unit: a probable link to common insulin vial use. Anaesth Intensive Care 41:266–268. [PubMed] [Google Scholar]
- 9.Vergara-López S, Domínguez MC, Conejo MC, Pascual Á, Rodríguez-Baño J. 2013. Wastewater drainage system as an occult reservoir in a protracted clonal outbreak due to metallo-β-lactamase-producing Klebsiella oxytoca. Clin Microbiol Infect 19:E490–E498. doi: 10.1111/1469-0691.12288. [DOI] [PubMed] [Google Scholar]
- 10.Leitner E, Zarfel G, Luxner J, Herzog K, Pekard-Amenitsch S, Hoenigl M, Valentin T, Feierl G, Grisold AJ, Hogenauer C, Sill H, Krause R, Zollner-Schwetz I. 2015. Contaminated handwashing sinks as the source of a clonal outbreak of KPC-2-producing Klebsiella oxytoca on a hematology ward. Antimicrob Agents Chemother 59:714–716. doi: 10.1128/AAC.04306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmithausen RM, Sib E, Exner M, Hack S, Rosing C, Ciorba P, Bierbaum G, Savin M, Bloomfield SF, Kaase M, Jacobshagen A, Gemein S, Gebel J, Engelhart S, Exner D. 2019. The washing machine as a reservoir for transmission of extended-spectrum-beta-lactamase (CTX-M-15)-producing Klebsiella oxytoca ST201 to newborns. Appl Environ Microbiol 85:e01435-19. doi: 10.1128/AEM.01435-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arakawa Y, Ohta M, Kido N, Mori M, Ito H, Komatsu T, Fujii Y, Kato N. 1989. Chromosomal β-lactamase of Klebsiella oxytoca, a new class A enzyme that hydrolyzes broad-spectrum β-lactam antibiotics. Antimicrob Agents Chemother 33:63–70. doi: 10.1128/aac.33.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fevre C, Jbel M, Passet V, Weill FX, Grimont PA, Brisse S. 2005. Six groups of the OXY β-lactamase evolved over millions of years in Klebsiella oxytoca. Antimicrob Agents Chemother 49:3453–3462. doi: 10.1128/AAC.49.8.3453-3462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joris B, De Meester F, Galleni M, Frere JM, Van Beeumen J. 1987. The K1 β-lactamase of Klebsiella pneumoniae. Biochem J 243:561–567. doi: 10.1042/bj2430561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier B, Lagrange PH, Philippon A. 1996. β-Lactamase gene promoters of 71 clinical strains of Klebsiella oxytoca. Antimicrob Agents Chemother 40:460–463. doi: 10.1128/AAC.40.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong SH, Kim WM, Chang CL, Kim JM, Lee K, Chong Y, Hwang HY, Baek YW, Chung HK, Woo IG, Ku JY. 2001. Neonatal intensive care unit outbreak caused by a strain of Klebsiella oxytoca resistant to aztreonam due to overproduction of chromosomal β-lactamase. J Hosp Infect 48:281–288. doi: 10.1053/jhin.2001.1018. [DOI] [PubMed] [Google Scholar]
- 17.Zarate MS, Gales AC, Picao RC, Pujol GS, Lanza A, Smayevsky J. 2008. Outbreak of OXY-2-producing Klebsiella oxytoca in a renal transplant unit. J Clin Microbiol 46:2099–2101. doi: 10.1128/JCM.00194-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Then RL, Glauser MP, Angehrn P, Arisawa M. 1983. Cephalosporin resistance in strains of Klebsiella oxytoca isolated during antibiotic therapy. Zentralbl Bakteriol Mikrobiol Hyg A 254:469–479. [PubMed] [Google Scholar]
- 19.Potz NA, Colman M, Warner M, Reynolds R, Livermore DM. 2004. False-positive extended-spectrum β-lactamase tests for Klebsiella oxytoca strains hyperproducing K1 β-lactamase. J Antimicrob Chemother 53:545–547. doi: 10.1093/jac/dkh112. [DOI] [PubMed] [Google Scholar]
- 20.Bush K, Jacoby GA. 2010. Updated functional classification of β-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hart CA. 1993. Klebsiellae and neonates. J Hosp Infect 23:83–86. doi: 10.1016/0195-6701(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. 2005. Hospital-acquired neonatal infections in developing countries. Lancet 365:1175–1188. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 23.European Committee on Antimicrobial Susceptibility Testing. 2017. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, version 2.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf.
- 24.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, Rolain JM. 2014. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inouye M, Dashnow H, Raven LA, Schultz MB, Pope BJ, Tomita T, Zobel J, Holt KE. 2014. SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, Parkhill J, Harris SR. 2015. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res 43:e15. doi: 10.1093/nar/gku1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood DE, Salzberg SL. 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15:R46. doi: 10.1186/gb-2014-15-3-r46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Federhen S, Rossello-Mora R, Klenk H-P, Tindall BJ, Konstantinidis KT, Whitman WB, Brown D, Labeda D, Ussery D, Garrity GM, Colwell RR, Hasan N, Graf J, Parte A, Yarza P, Goldberg B, Sichtig H, Karsch-Mizrachi I, Clark K, McVeigh R, Pruitt KD, Tatusova T, Falk R, Turner S, Madden T, Kitts P, Kimchi A, Klimke W, Agarwala R, DiCuccio M, Ostell J. 2016. Meeting report: GenBank microbial genomic taxonomy workshop (12–13 May, 2015). Stand Genomic Sci 11:15. doi: 10.1186/s40793-016-0134-1. [DOI] [Google Scholar]
- 30.Ronning TG, Aas CG, Stoen R, Bergh K, Afset JE, Holte MS, Radtke A. 2019. Investigation of an outbreak caused by antibiotic-susceptible Klebsiella oxytoca in a neonatal intensive care unit in Norway. Acta Paediatr 108:76–82. doi: 10.1111/apa.14584. [DOI] [PubMed] [Google Scholar]
- 31.Ransjo U, Good Z, Jalakas K, Kuhn I, Siggelkow I, Aberg B, Anjou E. 1992. An outbreak of Klebsiella oxytoca septicemias associated with the use of invasive blood pressure monitoring equipment. Acta Anaesthesiol Scand 36:289–291. doi: 10.1111/j.1399-6576.1992.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 32.Toldos CM, Ortiz G, Camara M, Segovia M. 1997. Application of pulsed-field gel electrophoresis in an outbreak of infection due to Klebsiella oxytoca. J Med Microbiol 46:889–890. [PubMed] [Google Scholar]
- 33.Watson JT, Jones RC, Siston AM, Fernandez JR, Martin K, Beck E, Sokalski S, Jensen BJ, Arduino MJ, Srinivasan A, Gerber SI. 2005. Outbreak of catheter-associated Klebsiella oxytoca and Enterobacter cloacae bloodstream infections in an oncology chemotherapy center. Arch Intern Med 165:2639–2643. doi: 10.1001/archinte.165.22.2639. [DOI] [PubMed] [Google Scholar]
- 34.Tindall BJ, Rosselló-Móra R, Busse H-J, Ludwig W, Kämpfer P. 2010. Notes on the characterization of prokaryote strains for taxonomic purposes. Int J Syst Evol Microbiol 60:249–266. doi: 10.1099/ijs.0.016949-0. [DOI] [PubMed] [Google Scholar]
- 35.Liao TL, Lin AC, Chen E, Huang TW, Liu YM, Chang YH, Lai JF, Lauderdale TL, Wang JT, Chang SC, Tsai SF, Chen YT. 2012. Complete genome sequence of Klebsiella oxytoca E718, a New Delhi metallo-β-lactamase-1-producing nosocomial strain. J Bacteriol 194:5454. doi: 10.1128/JB.01216-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiss I, Borkhardt A, Füssle R, Sziegoleit A, Gortner L. 2000. Disinfectant contaminated with Klebsiella oxytoca as a source of sepsis in babies. Lancet 356:310. doi: 10.1016/S0140-6736(00)02509-5. [DOI] [PubMed] [Google Scholar]
- 37.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shukor MY, Husin WS, Rahman MF, Shamaan NA, Syed MA. 2009. Isolation and characterization of an SDS-degrading Klebsiella oxytoca. J Environ Biol 30:129–134. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.