Lyme borreliosis is a tick-borne disease caused by the Borrelia burgdorferi sensu lato complex. Bio-Rad Laboratories has developed a fully automated multiplex bead-based assay for the detection of IgM and IgG antibodies to B. burgdorferi. The BioPlex 2200 Lyme Total assay exhibits an improved rate of seropositivity in patients with early Lyme infection. Asymptomatic subjects from endemic and nonendemic origins demonstrated a seroreactivity rate of approximately 4% that was similar to other commercial assays evaluated in this study.
KEYWORDS: Lyme disease, borreliosis, serodiagnostics, immunodiagnostics, immunoassay, multiplex, Lyme Total, BioPlex 2200
ABSTRACT
Lyme borreliosis is a tick-borne disease caused by the Borrelia burgdorferi sensu lato complex. Bio-Rad Laboratories has developed a fully automated multiplex bead-based assay for the detection of IgM and IgG antibodies to B. burgdorferi. The BioPlex 2200 Lyme Total assay exhibits an improved rate of seropositivity in patients with early Lyme infection. Asymptomatic subjects from endemic and nonendemic origins demonstrated a seroreactivity rate of approximately 4% that was similar to other commercial assays evaluated in this study. Coupled to this result was the observation that the Lyme Total assay retained a high first-tier specificity of 96% while demonstrating a relatively high sensitivity of 91% among a well-characterized CDC Premarketing Lyme serum panel. The Lyme Total assay also performs well under a modified two-tier algorithm (sensitivity, 84.4 to 88.9%; specificity, 98.4 to 99.5%). Furthermore, the new assay is able to readily detect early Lyme infection in patient samples from outside North America.
INTRODUCTION
Lyme disease or borreliosis (LB) is a tick-transmitted disease caused by pathogenic spirochetes of the Borrelia burgdorferi sensu lato complex and is widely distributed throughout the northern hemisphere (1–3). In the United States, the geographical range of LB has expanded in recent decades, with the number of reported cases approaching 40,000 per year and a Centers for Disease Control and Prevention (CDC)-estimated actual incidence of approximately 300,000 yearly infections (4–6). Most of these reported cases are from areas of endemicity in the northeastern, upper midwestern, and mid-Atlantic states. B. burgdorferi sensu stricto is the Borreliella genospecies predominantly responsible for LB in North America—Borrelia mayonii is another pathogenic genospecies recently identified in the upper Midwest (3, 7, 8). Outside North America, in areas of endemicity of Europe and parts of Asia, there is usually a wider array of pathogenic genospecies such as B. afzelii, B. garinii, B. bavariensis, and others, in addition to B. burgdorferi sensu stricto (2, 3, 9).
The serodiagnosis of LB, when clinically needed, relies on the standardized two-tier (STT) algorithm as recommended by the CDC, wherein typically a first-tier enzyme immunoassay (EIA) with a positive or equivocal result is subsequently followed by a second-tier immunoblot. The two-tier format was recommended to improve the overall specificity of the serodiagnosis (10–12). However, the STT algorithm introduced, among other issues, a lower sensitivity for early-stage infections (7). Early sensitivity is clinically important since the diagnosis and treatment of LB in its initial stages is associated with better clinical outcomes (1, 3, 13). In recent years, modified two-tier (MTT) algorithms have been investigated, with two sequential EIA designs in particular offering better sensitivity in addition to other advantages over the STT algorithm (14–20). More recently, the U.S. Food and Drug Administration (FDA) has cleared for the first time a two EIA alternative from Zeus Scientific (21).
The laboratory diagnosis of LB is primarily dependent on the demonstration of antibodies to B. burgdorferi components (22). The major antigenic proteins expressed by B. burgdorferi are prominently cell surface proteins, and the timing of their expression is important to the development of the immune response (23–26). The outer surface protein C (OspC) is a small immunodominant lipoprotein that can generate an early immune response in patients with active Lyme infection (24). During tick feeding, its expression is upregulated in the host-adapted bacteria and required for bacterial transmission to the mammalian host, making it a highly suitable marker for early infection (27–29). Vmp-like sequence-expressed (VlsE) protein is a surface lipoprotein that is expressed only after infection is established in the mammalian host (30). Comprised of alternating variable and invariable domains, VlsE contains antigenic regions (with notably the sixth invariable region) capable of eliciting a strong immune response (31, 32). Flagellin B (FlaB), an extracellular structural protein of flagella, is expressed in the feeding tick and makes for an immunodominant antigen that can induce an early immune response (33, 34). Oligopeptide permease A2 (OppA2) is the peptide-binding component of a transporter system found in B. burgdorferi and a known early antigenic marker (26, 35).
The BioPlex 2200 Lyme Total assay is a new first-tier immunoassay designed to detect antibodies (IgG and IgM) reactive to the following B. burgdorferi proteins: OppA2 (p58), OspC serotype B (OspCB), and a synthetic fusion peptide (FVlsE) containing sequences from both FlaB and a modified VlsE. These target antigens were previously identified for potential use in a multiantigen assay: OspCB and FVlsE (36) and p58 antigen (26).
The aim of this study was to compare the performance of the BioPlex 2200 Lyme Total assay to commonly used commercial Lyme assays. When evaluated under various testing algorithms using different sample cohorts of various geographic origins, the BioPlex 2200 Lyme Total assay displayed a relatively high level of specificity and sensitivity that was not observed among other Lyme commercial kits tested here. Importantly, the relative sensitivity improvements achieved by the new Lyme Total assay were most apparent among the early stage Lyme samples.
MATERIALS AND METHODS
BioPlex 2200 Lyme Total.
The BioPlex 2200 Lyme Total assay is a fully automated bead-based multiplex flow immunoassay that comprises four Lyme-specific antigens: recombinant OspC type B (OspCB), recombinant p58 (OppA2), and the synthetic fusion peptide FVlsE containing sequences from FlaB (amino acids [aa] 211 to 223) and a modified VlsE (aa 275 to 291). A detailed description of the assay principle and a similar procedure has been previously published (37). Briefly, Lyme-specific antibodies present in serum are captured onto dyed paramagnetic beads using B. burgdorferi-derived antigens that are chemically precoupled to the beads. After washing, the beads are incubated with anti-human IgM and IgG antibody-phycoerythrin conjugates and, after further washes, the beads are passed through a detector to measure the fluorescence signal generated by the bound conjugates. While the resulting multiplexed bead signals are independently calibrated for each antigen bead and processed into individual antibody index (AI) values, only the highest AI value is reported as the final test result. Based on this value, the test reports “nonreactive” (0.2 to 0.8 AI), “equivocal” (0.9 to 1.0 AI), or “reactive” (1.1 to 8.0 AI) for Lyme antibodies. It is important to note that the Lyme Total assay does not distinguish between IgG and IgM signals due to the use of common fluorescently tagged anti-IgG and anti-IgM conjugates in a combined mixture.
Other commercial Lyme test kits.
The following Lyme test kits were tested according to the manufacturer’s protocol: Borrelia VlsE1/pepC10 (Zeus Scientific, Branchburg, NJ), Immunetics Lyme C6 (Oxford Immunotec, Marlborough, MA), VIDAS Lyme IgM/IgG (bioMérieux, Durham, NC), VIDAS Lyme IgM II and VIDAS Lyme IgG II (bioMérieux, Durham, NC), LIAISON Borrelia burgdorferi (DiaSorin, Saluggia, Italy), LIAISON Borrelia IgM II and LIAISON Borrelia IgG (DiaSorin, Saluggia, Italy), and MarDx B. burgdorferi Marblot IgM and MarDx B. burgdorferi Marblot IgG (Trinity Biotech, Bray, Ireland).
Sample cohorts.
A total of 200 serum samples from an asymptomatic U.S. population of apparently healthy subjects were randomly selected based on their endemic (Northeast) or nonendemic (Gulf Coast) origins. These remnant samples were acquired from commercial vendors. A 280-member premarketing panel consisting of the following sample categories were obtained from the Centers for Disease Control and Prevention (Ft. Collins, CO): 39 acute-phase samples (30 early erythema migrans [EM], 3 cardiac Lyme, and 6 neurologic Lyme), 31 convalescent-phase samples (30 early EM and 1 neurologic follow-ups), 20 late-phase samples (all Lyme arthritis/neurologic), 90 “look-alike” disease samples (15 each of fibromyalgia, mononucleosis, multiple sclerosis, periodontitis, rheumatoid arthritis, and syphilis), 50 healthy nonendemic negative controls, and 50 healthy endemic negative controls. The sample categories of acute, convalescent, and late phases were previously described by the CDC (38). For samples obtained outside the United States, 100 normal blood donor sera from an area of endemicity within Russia and 103 Ukrainian samples of early Lyme infection (all with clinically identified EM rash) were acquired from commercial vendors. All Ukrainian samples were collected within the first 3 months of the tick bite with a majority within the first 30 days.
Sample handling, storage, and confidentiality.
Serum samples were kept at –20°C for the duration of the study. After thawing at room temperature, samples were briefly vortexed and pulse centrifuged before testing in singlicate. All samples were deidentified by the vendors to ensure subject confidentiality.
Statistical analysis.
Positive and negative agreement ratios were calculated with corresponding Wilson score intervals (95% confidence interval [CI], two tailed). Sample proportions were compared by using the McNemar-Mosteller exact test (two tailed) to derive corresponding P values. Receiver operating characteristic (ROC) curves were evaluated by comparing the area under curve (AUC) proportions using the z-test (two tailed). All statistical computations and ROC curves were generated using the statistical software Analyze-It (v5.11.3).
RESULTS
Seroreactivity in U.S. asymptomatic populations.
In order to assess reactivity rates among normal U.S. populations, 100 nonendemic (Gulf Coast) and 100 endemic (Northeast) asymptomatic blood donor sera were acquired from commercial vendors and tested against various commercially available Lyme test kits (Table 1). Although the BioPlex 2200 Lyme Total (henceforth, “BioPlex Total”’) assay’s nonendemic rate of 3% was the lowest among the commercial kits tested, it was statistically comparable to the nonendemic rates of 4% observed for the Immunetics Lyme C6 (henceforth, “Immunetics”) and DiaSorin LIAISON B. burgdorferi (henceforth, “DiaSorin”) test kits (Table 1). In contrast, the Zeus Borrelia VlsE/pepC10 (henceforth, “Zeus”) kit and the combined results of the dissociated bioMérieux VIDAS Lyme II IgM and IgG tests (henceforth, “VIDAS II”) produced measurably higher nonendemic rates of 7 and 10%, respectively, of which only the VIDAS II was statistically higher than the BioPlex Total (P = 0.016). In the endemic sample cohort, the BioPlex Total reactivity was 5%, which was within the corresponding range of 3 to 8% observed with the other assays (Table 1). The combined reactivity of 4% for the BioPlex Total was statistically within the observed range of most commercial assays tested here. Only the combined VIDAS II tests yielded a statistically higher combined reactivity of 7.5% (P = 0.039).
TABLE 1.
Seroreactivity in asymptomatic U.S. populationsa
| Test kit | No. of samples |
Combined |
||||||
|---|---|---|---|---|---|---|---|---|
| Nonendemic area(s) |
Endemic area(s) |
|||||||
| Pos | Equ | Neg | Pos | Equ | Neg | % reactivity | P | |
| BioPlex 2200 Lyme Total | 2 | 1 | 97 | 5 | 0 | 95 | 4.0 | Ref |
| DiaSorin LIAISON B. burgdorferi | 2 | 2 | 96 | 3 | 0 | 97 | 3.5 | 1.000 |
| Immunetics Lyme C6 | 4 | 0 | 96 | 5 | 3 | 92 | 6.0 | 0.481 |
| Zeus Borrelia VlsE1/pepC10 | 6 | 1 | 93 | 1 | 5 | 94 | 6.5 | 0.267 |
| bioMérieux VIDAS Lyme II (lgM+IgG) | 4 | 6 | 90 | 4 | 1 | 95 | 7.5 | 0.039 |
| bioMérieux VIDAS Lyme II lgM | 3 | 6 | 91 | 2 | 1 | 97 | 6.0 | 0.289 |
| bioMérieux VIDAS Lyme II lgG | 1 | NA | 99 | 2 | NA | 98 | 1.5 | 0.180 |
A total of 200 normal blood donor samples collected from areas of nonendemicity (Gulf Coast, n = 100) and endemicity (Northeast, n = 100) in the United States were assessed for reactivity to the listed commercially available Lyme test kits. Pos, positive; Equ, equivocal; Neg, negative; Ref, reference; NA, not applicable.
CDC premarketing panel: determination of clinical sensitivity and specificity.
In order to determine the clinical sensitivity and specificity of the BioPlex Lyme Total assay, a panel of clinically diagnosed patient samples from the CDC was acquired and blind tested (38). The CDC premarketing panel includes 90 Lyme positive samples stratified by the CDC’s assignment of Lyme disease phase: acute (nearly all EM-positive patients), convalescent (follow-up draws of the acute patients), or late (Lyme arthritis/neurologic). In addition, the panel offers 190 Lyme disease-negative samples consisting of healthy control samples and potentially cross-reactive “look-alike” disease sera (see Materials and Methods for further CDC panel details).
(i) First-tier clinical sensitivity.
Testing of the Lyme disease samples revealed a statistically higher overall sensitivity for the BioPlex Total assay than for the individual VIDAS II IgM and IgG assays (P < 0.002; Table 2). In addition, the BioPlex Total assay had a numerically higher overall sensitivity (91.1%) than the Zeus (88.9%), Immunetics (86.7%), and the combined VIDAS II (85.6%) assays, but these differences did not reach statistical significance (P > 0.05). The BioPlex Total assay also demonstrated an overall sensitivity equivalent to the bioMérieux polyvalent VIDAS results provided by the CDC, but with considerably fewer equivocal results (82 positive, 0 equivocal) than the VIDAS polyvalent (75 positive, 7 equivocal). Notably, the overall higher sensitivity of the BioPlex Total assay draws primarily from the improved detection of the earlier stages of Lyme infection—mostly acute- and, to a lesser extent, convalescent-phase samples (Table 2). There were no observed sensitivity differences among the late-stage samples.
TABLE 2.
CDC premarketing panel: first-tier sensitivity comparisonsa
| Test kit | No. of samples or PPA |
Combined |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute phase |
Convalescent phase |
Late phase |
||||||||||||
| Pos | Equ | Neg | PPA | Pos | Equ | Neg | PPA | Pos | Equ | Neg | PPA | PPA | P | |
| BioPlex 2200 Lyme Total | 33 | 0 | 6 | 84.6 | 29 | 0 | 2 | 93.5 | 20 | 0 | 0 | 100 | 91.1 | Ref |
| Immunetics Lyme C6 | 29 | 0 | 10 | 74.4 | 28 | 1 | 2 | 93.5 | 20 | 0 | 0 | 100 | 86.7 | 0.125 |
| Zeus Borrelia VlsE1/pepC10 | 31 | 1 | 7 | 82.1 | 28 | 0 | 3 | 90.3 | 20 | 0 | 0 | 100 | 88.9 | 0.500 |
| bioMérieux VIDAS Lyme polyvalent | 28 | 5 | 6 | 84.6 | 27 | 2 | 2 | 93.5 | 20 | 0 | 0 | 100 | 91.1 | 1.000 |
| bioMérieux VIDAS Lyme II (lgM+IgG) | 30 | 1 | 8 | 79.5 | 26 | 0 | 5 | 83.9 | 20 | 0 | 0 | 100 | 85.6 | 0.063 |
| bioMérieux VIDAS Lyme II lgM | 23 | 5 | 11 | 71.8 | 23 | 3 | 5 | 83.9 | 6 | 8 | 6 | 70.0 | 75.6 | <0.001 |
| bioMérieux VIDAS Lyme II lgG | 26 | NA | 13 | 66.7 | 26 | NA | 5 | 83.9 | 20 | NA | 0 | 100 | 80.0 | 0.002 |
| MarDx B. burgdorferi Marblot (lgM+IgG) | 24 | NA | 15 | 61.5 | 25 | NA | 6 | 80.6 | 20 | NA | 0 | 100 | 76.7 | <0.001 |
| MarDx B. burgdorferi Marblot lgM | 21 | NA | 18 | 53.8 | 19 | NA | 12 | 61.3 | 8 | NA | 12 | 40.0 | 53.3 | <0.001 |
| MarDx B. burgdorferi Marblot lgG | 11 | NA | 28 | 28.2 | 14 | NA | 17 | 45.2 | 20 | NA | 0 | 100 | 50.0 | <0.001 |
A CDC panel of clinically diagnosed Lyme disease-positive patient samples was assessed for reactivity to the listed commercial Lyme test kits. Disease-stage information was provided by the CDC as follows: 39 acute-phase samples (30 early EM, 3 cardiac Lyme, and 6 neurologic Lyme); 31 convalescent-phase samples (30 early EM and 1 neurologic Lyme follow-ups); and 20 late-phase samples (all Lyme arthritis/neurologic) for a combined 90 Lyme-positive samples. PPA, positive percent agreement with CDC results [PPA = (Pos + Equ)/n]. Results for the MarDx Marblot, Immunetics Lyme C6, and bioMérieux VIDAS Lyme polyvalent tests were provided by the CDC.
(ii) Two-tier clinical sensitivity.
All patient samples that tested reactive (positive/equivocal) with any first-tier immunoassay were subsequently analyzed under different two-tier algorithms. Under the STT algorithm (Table 3, “MarDx Marblot”), the sensitivity differences between the various first-tier assays were noticeably reduced, with no statistical differences. However, the BioPlex Total assay retained a slight numerical advantage in the overall STT sensitivity compared to other first-tier immunoassays, due to residual higher sensitivity for the early-stage infections (Table S1). The two-tier analysis was then extended to the MTT format wherein two first-tier immunoassays are used in lieu of the immunoblot (Table 3). These alternative two-tier algorithms consistently yielded higher overall sensitivity (80.0 to 88.9%) than the corresponding immunoblot confirmed values (73.3 to 76.7%). The highest overall two-tier sensitivity of 88.9% was obtained when the BioPlex Total was paired with the Zeus VlsE/pepC10 assay; for comparison, the highest overall two-tier sensitivity observed in the absence of the BioPlex Total was 85.6%. This difference was not statistically significant (P = 0.250); however, it is notable that the highest MTT sensitivity for each first-tier assay was observed when paired with the BioPlex Total assay.
TABLE 3.
CDC premarketing panel: two-tier test kit sensitivity comparisonsa
| Test kit | No. of samples or PPA |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One tier | STT |
MTT |
|||||||||||
| MarDx Marblot (IgM+IgG) |
BioPlex 2200 Lyme Total |
Immunetics Lyme C6 |
Zeus borrelia VlsE1/pepC10 |
bioMérieux polyvalent |
bioMérieux VIDAS II (M+G) |
||||||||
| Pos/Equ | Pos | PPA | Pos/Equ | PPA | Pos/Equ | PPA | Pos/Equ | PPA | Pos/Equ | PPA | Pos/Equ | PPA | |
| BioPlex 2200 Lyme Total | 82 | 69 | 76.7 | 78 | 86.7 | 80 | 88.9 | 76 | 84.4 | 77 | 85.6 | ||
| Immunetics Lyme C6 | 78 | 67 | 74.4 | 78 | 86.7 | - | 77 | 85.6 | 74 | 82.2 | 75 | 83.3 | |
| Zeus Borrelia VlsE1/pepC10 | 80 | 67 | 74.4 | 80 | 88.9 | 77 | 85.6 | 75 | 83.3 | 77 | 85.6 | ||
| bioMérieux VIDAS Lyme polyvalent | 82 | 66 | 73.3 | 76 | 84.4 | 74 | 82.2 | 75 | 83.3 | 72 | 80.0 | ||
| bioMérieux VIDAS Lyme II (lgM+IgG) | 77 | 66 | 73.3 | 77 | 85.6 | 75 | 83.3 | 77 | 85.6 | 72 | 80.0 | ||
| bioMérieux VIDAS Lyme II IgM | 68 | 59 | 65.6 | 68 | 75.6 | 66 | 73.3 | 68 | 75.6 | 63 | 70.0 | ||
| bioMérieux VIDAS Lyme II lgG | 72 | 62 | 68.9 | 72 | 80.0 | 72 | 80.0 | 72 | 80.0 | 70 | 77.8 | ||
All CDC clinically diagnosed Lyme disease-positive samples (n = 90) that tested first-tier positive/equivocal (Pos/Equ) from Table 2 were analyzed under various two-tier algorithms. STT, standardized two tier; MTT, modified two tier; PPA, positive percent agreement with CDC results [PPA = (Pos + Equ)/n]. Results for the MarDx Marblot, Immunetics Lyme C6, and bioMérieux VIDAS Lyme polyvalent results were provided by the CDC.
(iii) First-tier clinical specificity.
Analysis of the CDC’s premarketing panel of Lyme negative-control samples revealed further critical performance differences among the commercial assays. Within the overall 190 Lyme-negative samples (Table 4), the test results revealed a statistically higher specificity for the BioPlex Total than for the Zeus VlsE/pepC10 assay (P = 0.004) and the combined VIDAS II tests (P < 0.001), as well as the VIDAS polyvalent assay (P < 0.001). The BioPlex Total assay demonstrated an overall specificity (96.8%), which was equivalent to the Immunetics C6 assay but fell statistically short of the two-tier MarDx Marblot’s specificity of 100% (P = 0.031). The BioPlex Total and Immunetics assays had the lowest cross-reactivity among the “look-alike” diseases and healthy control samples with specificities ranging between 96.0 to 98.0% and 96.7 to 100%, respectively. In comparison, the Zeus and combined VIDAS II tests incurred noticeably more cross-reactivity among the “look-alike” diseases with specificities ranging between 87.8 to 94.0% and 82.2 to 88.0%, respectively. Notably, many of these false reactivities were the result of a relatively high number of equivocal results observed with the Zeus VlsE/pepC10 and the VIDAS II IgM assays. The VIDAS polyvalent assay displayed the lowest specificity of 74.4%, with all false-positive results coming from the “look-alike” disease category.
TABLE 4.
CDC premarketing panel: first-tier specificity comparisonsa
| Test kit | No. of samples or NPA |
Combined |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy, nonendemic |
Healthy, endemic |
Look-alike diseases |
||||||||||||
| Pos | Equ | Neg | NPA | Pos | Equ | Neg | NPA | Pos | Equ | Neg | NPA | NPA | P | |
| BioPlex 2200 Lyme Total | 1 | 0 | 49 | 98.0 | 0 | 2 | 48 | 96.0 | 1 | 2 | 87 | 96.7 | 96.8 | Ref |
| Immunetics Lyme C6 | 1 | 0 | 49 | 98.0 | 2 | 0 | 48 | 96.0 | 2 | 1 | 87 | 96.7 | 96.8 | 1.000 |
| Zeus Borrelia VlsE1/pepC10 | 3 | 3 | 44 | 88.0 | 0 | 3 | 47 | 94.0 | 6 | 5 | 79 | 87.8 | 89.5 | 0.004 |
| bioMérieux VIDAS Lyme polyvalent | 0 | 0 | 50 | 100 | 0 | 0 | 50 | 100 | 22 | 1 | 67 | 74.4 | 87.9 | <0.001 |
| bioMérieux VIDAS Lyme II (lgM+IgG) | 2 | 5 | 43 | 86.0 | 1 | 5 | 44 | 88.0 | 6 | 10 | 74 | 82.2 | 84.7 | <0.001 |
| bioMérieux VIDAS Lyme II lgM | 2 | 5 | 43 | 86.0 | 1 | 5 | 44 | 88.0 | 4 | 10 | 76 | 84.4 | 85.8 | <0.001 |
| bioMérieux VIDAS Lyme II lgG | 0 | NA | 50 | 100 | 0 | NA | 50 | 100 | 2 | NA | 88 | 97.8 | 98.9 | 0.289 |
| MarDx B. burgdorferi Marblot (lgM+IgG) | 0 | NA | 50 | 100 | 0 | NA | 50 | 100 | 0 | NA | 90 | 100 | 100 | 0.031 |
| MarDx B. burgdorferi Marblot lgM | 0 | NA | 50 | 100 | 0 | NA | 50 | 100 | 0 | NA | 90 | 100 | 100 | 0.031 |
| MarDx B. burgdorferi Marblot lgG | 0 | NA | 50 | 100 | 0 | NA | 50 | 100 | 0 | NA | 90 | 100 | 100 | 0.031 |
A CDC panel of clinically diagnosed Lyme negative controls (n = 190) were assessed for reactivity to the listed commercial Lyme test kits. Sample descriptions were provided by the CDC as follows: 50 healthy nonendemic, 50 healthy endemic, and 90 “look-alike” disease controls for a combined 190 Lyme disease-negative samples. NPA, negative percent agreement with CDC results [NPA = (Neg)/n]. The results for the MarDx Marblot, Immunetics Lyme C6, and bioMérieux VIDAS Lyme polyvalent tests were provided by the CDC.
(iv) Two-tier clinical specificity.
All samples that tested reactive with any first-tier assay were once again subjected to the two-tier algorithms (Table 5). The differences in overall specificity among the two-tier algorithms were mostly small. For reference, the second-tier immunoblot (“MarDx Marblot”) conferred 100% specificity with all first-tier immunoassays. However, the alternative two-tier algorithms also yielded high specificity values with an overall range of 96.8 to 99.5% that was in some cases only slightly below the specificity of the STT algorithm. The lowest of these MTT specificities (96.8%) was observed when the two bioMérieux assays, VIDAS polyvalent and combined VIDAS II tests, were paired together due to some apparent similarity in cross-reactivity with samples from mostly the “look-alike” disease category (see Table S2 in the supplemental material). The highest overall MTT specificity of 99.5% was observed when the BioPlex Total was paired with the Immunetics C6 assay (Table 5). But the pairing of the BioPlex Total with either the Zeus (VlsE/pepC10) assay or the combined VIDAS II tests also produced relatively high two-tier specificities of 98.9%. For comparison, the highest overall MTT specificity observed in the absence of the BioPlex Total assay was also 98.9%.
TABLE 5.
CDC premarketing panel: overall two-tier specificity comparisonsa
| Test kit | No. of samples or NPA |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One tier | STT |
MTT |
|||||||||||
| MarDx Marblot (IgM+IgG) |
BioPlex 2200 Lyme Total |
Immunetics Lyme C6 |
Zeus borrelia VlsE1/pepC10 |
bioMérieux polyvalent |
bioMérieux VIDAS II (M+G) |
||||||||
| Pos/Equ | Pos | NPA | Pos/Equ | NPA | Pos/Equ | NPA | Pos/Equ | NPA | Pos/Equ | NPA | Pos/Equ | NPA | |
| BioPlex 2200 Lyme Total | 6 | 0 | 100 | 1 | 99.5 | 2 | 98.9 | 2 | 98.9 | 3 | 98.4 | ||
| Immunetics Lyme C6 | 6 | 0 | 100 | 1 | 99.5 | 2 | 98.9 | 2 | 98.9 | 2 | 98.9 | ||
| Zeus Borrelia VlsE1/pepC10 | 20 | 0 | 100 | 2 | 98.9 | 2 | 98.9 | 5 | 97.4 | 5 | 97.4 | ||
| bioMérieux VIDAS Lyme polyvalent | 23 | 0 | 100 | 2 | 98.9 | 2 | 98.9 | 5 | 97.4 | 6 | 96.8 | ||
| bioMérieux VIDAS Lyme II (lgM+IgG) | 29 | 0 | 100 | 3 | 98.4 | 2 | 98.9 | 5 | 97.4 | 6 | 96.8 | ||
| bioMérieux VIDAS Lyme II IgM | 27 | 0 | 100 | 3 | 98.4 | 2 | 98.9 | 5 | 97.4 | 5 | 97.4 | ||
| bioMérieux VIDAS Lyme II lgG | 2 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 1 | 99.5 | ||
A total of 190 CDC clinically diagnosed Lyme disease-negative controls that tested first-tier positive/equivocal (Pos/Equ) from Table 4 were analyzed under various two-tier algorithms. STT, standardized two tier; MTT, modified two tier; NPA, negative percent agreement with CDC results [NPA = (Neg)/n]. The results for the MarDx Marblot, Immunetics Lyme C6, and bioMérieux VIDAS Lyme polyvalent tests were provided by the CDC.
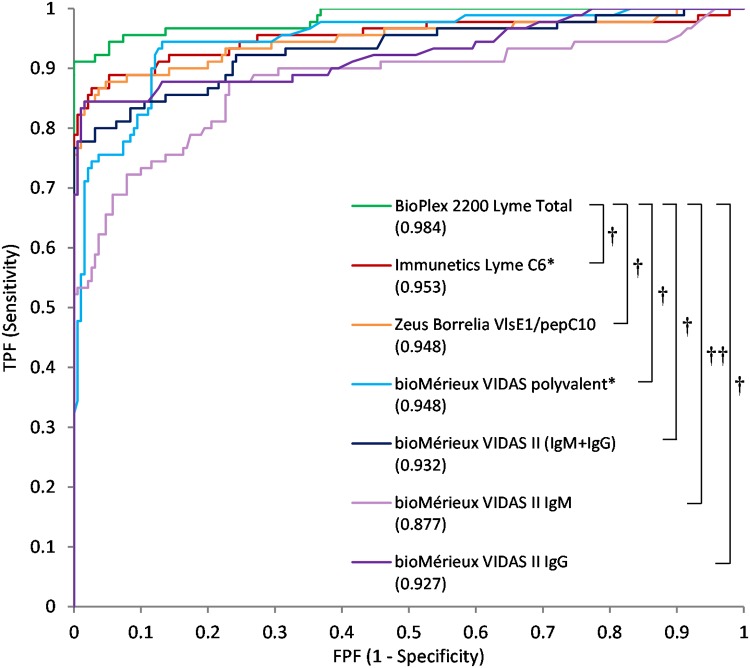
ROC analysis.
To better compare the overall performance of the first-tier assays, receiver operating characteristic (ROC) curves were generated using the CDC premarketing data sets (Fig. 1). Area under curve (AUC) calculations revealed statistically better results for the BioPlex Total assay compared to the Immunetics (P = 0.009), Zeus (VlsE/pepC10) (P = 0.004), VIDAS polyvalent (P = 0.005), and combined VIDAS II tests (P = 0.001). The ROC curves also permitted more careful comparisons of the sensitivity differences by measuring sensitivity at an equal specificity level (Table 6). In all cases wherein the specificity was fixed between 90 and 99%, the BioPlex Total assay consistently yielded a higher sensitivity matching the CDC results and was statistically more sensitive in head-to-head comparisons.
FIG 1.
CDC premarketing panel: ROC analysis. First-tier sensitivity and specificity with the CDC diagnosed Lyme-positive and -negative samples from Tables 2 and 4 were used to plot receiver operating characteristic (ROC) curves. Corresponding AUC values are indicated in parentheses. *, Test results for the Immunetics Lyme C6 and bioMérieux VIDAS Lyme polyvalent were provided by the CDC; all other test results were obtained internally. †, P < 0.01; ††, P < 0.001; TPF, true positive fraction; FPF, false positive fraction.
TABLE 6.
ROC curve-predicted sensitivity at fixed specificitya
| Test kit | Fixed specificity |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 99% |
98% |
97% |
96% |
95% |
90% |
|||||||
| % | P | % | P | % | P | % | P | % | P | % | P | |
| BioPlex 2200 Lyme Total | 91.1 | Ref | 91.1 | Ref | 91.1 | Ref | 92.2 | Ref | 92.2 | Ref | 95.6 | Ref |
| Immunetics Lyme C6 | 82.2 | 0.008 | 83.3 | 0.016 | 86.7 | 0.125 | 86.7 | 0.063 | 86.7 | 0.063 | 88.9 | 0.031 |
| Zeus Borrelia VlsE1/pepC10 | 76.7 | <0.001 | 84.4 | 0.031 | 84.4 | 0.031 | 86.7 | 0.063 | 87.8 | 0.125 | 88.9 | 0.031 |
| bioMérieux VIDAS Lyme polyvalent | 47.8 | <0.001 | 71.1 | <0.001 | 74.4 | <0.001 | 75.6 | <0.001 | 75.6 | <0.001 | 82.2 | 0.002 |
| bioMérieux VIDAS Lyme II (lgM+IgG) | 77.8 | <0.001 | 77.8 | <0.001 | 77.8 | <0.001 | 80.0 | 0.001 | 80.0 | 0.001 | 83.3 | 0.001 |
| bioMérieux VIDAS Lyme II lgM | 50.0 | <0.001 | 50.0 | <0.001 | 52.2 | <0.001 | 57.8 | <0.001 | 58.9 | <0.001 | 67.8 | <0.001 |
| bioMérieux VIDAS Lyme II lgG | 77.8 | <0.001 | 84.4 | 0.031 | 84.4 | 0.031 | 84.4 | 0.016 | 84.4 | 0.016 | 84.4 | 0.002 |
The ROC curves generated in Fig. 1 were used to measure the predicted sensitivity of the Lyme assays at the selected specificity levels shown. Ref, reference.
Seroreactivity in a non-U.S. asymptomatic population.
The BioPlex Total assay displayed a 10% reactivity rate among 100 Russian asymptomatic blood donors that was higher than the rate observed for the Immunetics C6 (7%) and Zeus VlsE/pepC10 (7%) assays, but this difference was not statistically significant (P > 0.05, Table 7). However, the combined DiaSorin LIAISON Borrelia IgM and IgG tests displayed a substantially higher reactivity of 37% that was statistically higher than the BioPlex Total assay (P < 0.001). The higher seroreactivity of the combined DiaSorin tests was primarily due to the relatively high apparent reactivity of its IgM test.
TABLE 7.
Seroreactivity in a non-U.S. endemic populationa
| Test kit | No. of samples |
% reactive | 95% CI | P | ||
|---|---|---|---|---|---|---|
| Pos | Equ | Neg | ||||
| BioPlex 2200 Lyme Total | 9 | 1 | 90 | 10.0 | 5.5–17.4 | Ref |
| Immunetics Lyme C6 | 7 | 0 | 93 | 7.0 | 3.4–13.8 | 0.581 |
| Zeus Borrelia VlsE1/pepC10 | 2 | 5 | 93 | 7.0 | 3.4–13.8 | 0.629 |
| DiaSorin LIAISON Borrelia (lgM+IgG) | 29 | 8 | 63 | 37.0 | 28.2–46.8 | <0.001 |
| DiaSorin LIAISON Borrelia IgM II | 26 | 2 | 72 | 28.0 | 20.1–37.5 | 0.003 |
| DiaSorin LIAISON Borrelia IgG | 3 | 6 | 91 | 9.0 | 4.8–16.2 | 1.000 |
Asymptomatic donor samples (n = 100) collected from an area of endemicity within Russia were assessed for reactivity to the following commercially available Lyme test kits. 95% CI, 95% confidence interval; Ref, reference.
Sensitivity comparison in a non-U.S. retrospective Lyme-positive cohort.
To determine the sensitivity of the assay in a non-U.S. population, a cohort of 103 early-stage Lyme samples of Ukrainian origin from patients with clinically identified EM rash were tested using commercially available Lyme assays (Table 8). Similar to the results obtained with the U.S. cohort, the BioPlex Total assay yielded the highest seropositivity with the lowest number of equivocal results. The BioPlex Total assay produced a sensitivity of 75.7% that was higher than the Immunetics C6 (67.0%), Zeus VlsE/pepC10 (61.2%), and combined DiaSorin IgG and IgM tests (68.0%); of these, the comparisons to Immunetics and Zeus were statistically significant (P values of 0.049 and <0.001, respectively).
TABLE 8.
Retrospective Lyme positive non-U.S. cohorta
| Test kit | No. of samples |
PPA | 95% CI | P | ||
|---|---|---|---|---|---|---|
| Pos | Equ | Neg | ||||
| BioPlex 2200 Lyme Total | 76 | 2 | 25 | 75.7 | 66.6–83.0 | Ref |
| Immunetics Lyme C6 | 66 | 3 | 34 | 67.0 | 57.4–75.3 | 0.049 |
| Zeus Borrelia VlsE1/pepC10 | 57 | 6 | 40 | 61.2 | 51.5–70.0 | <0.001 |
| DiaSorin LIAISON Borrelia (lgM+IgG) | 65 | 5 | 33 | 68.0 | 58.4–76.2 | 0.077 |
| DiaSorin LIAISON Borrelia IgM II | 49 | 3 | 51 | 50.5 | 41.0–60.0 | <0.001 |
| DiaSorin LIAISON Borrelia IgG | 45 | 7 | 51 | 50.5 | 41.0–60.0 | <0.001 |
Vendor-tested Lyme disease-positive patient sera (n = 103) collected from an area of endemicity within Ukraine were assessed for reactivity to commercially available Lyme test kits. All sera were characterized by the vendor as “early-stage” Lyme infections (EM rash positive). PPA, positive percent agreement; 95% CI, 95% confidence interval; Ref, reference.
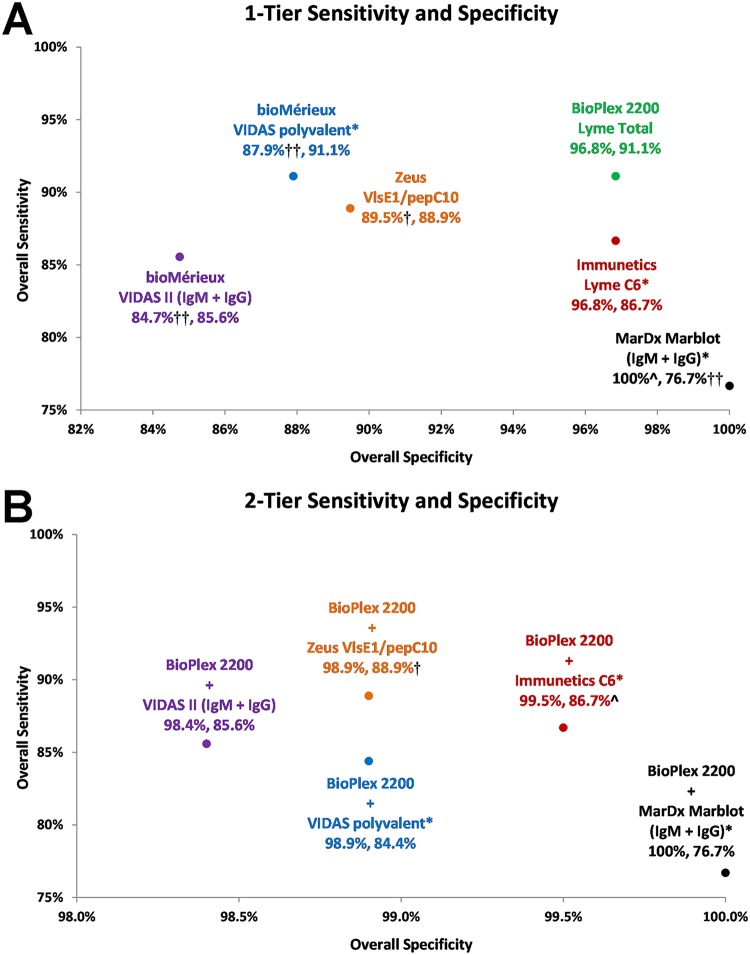
DISCUSSION
The results of the comparative study indicate that the BioPlex 2200 Lyme Total assay is more sensitive and specific than many commonly used commercial first-tier immunoassays (Fig. 2A). It is important to note that the relatively higher sensitivity of the BioPlex 2200 Lyme Total assay is primarily driven by the improved detection of early stage Lyme infections. Consistent with this improved performance, the new assay yielded noticeably fewer equivocal results, perhaps reflective of a wider equivocal range and/or lower cutoff employed by some of the other commercial assays observed in this study. The sensitivity and specificity levels measured in this study for the other commercial assays are comparable to those published by other groups using similar CDC panels (15, 19). In particular, the performance of the VIDAS polyvalent and dissociated Lyme II tests reported here as first-tier tests and as STT or MTT algorithms are within close range (1% to 6%) of the corresponding values reported by Molins et al. using a larger panel of CDC samples (39).
FIG 2.
CDC premarketing panel: overall sensitivity and specificity. (A) Graphical summary of the overall one-tier (1-Tier) sensitivity and specificity levels from Tables 2 and 4, respectively. ^, P < 0.01 (compared to BioPlex 2200 Lyme Total); †, P < 0.01; ††, P < 0.001. (B) Graphical summary of the overall two-tier (2-Tier) sensitivity and specificity levels from Tables 3 and 5, respectively. ^, P < 0.05 (compared to BioPlex 2200 Lyme Total + MarDx Marblot [IgM + IgG]); †, P < 0.01; ††, P < 0.001.
Despite these significant improvements, subjecting the BioPlex Total assay to second-tier immunoblot testing consistently limited its improved first-tier sensitivity. Lyme immunoblots are known for having lower sensitivity than most first-tier immunoassays and are generally regarded as labor-intensive and prone to subjectivity or reproducibility issues. These limitations and issues have intensified the search for modified testing methods over the years (14–20). As an alternative modality, pairing the new BioPlex assay with one of the other first-tier assays consistently preserved most of the high sensitivity of the first-tier assay while retaining a high level of specificity expected of the STT method (Fig. 2B). Therefore, consistent with other studies reporting the performance viability of a two-EIA approach, the BioPlex Total assay in conjunction with a highly complementary immunoassay provides another compelling option to address some of the deficiencies of the current STT approach.
The improved performance of the new BioPlex Total assay owes in large part to its specific antigens. In particular, the synthetic fusion peptide FVlsE that combines selected immunogenic excerpts from the outer surface proteins FlaB and VlsE has been designed to allow its inclusion without incurring the type of specificity issues typically seen with Lyme immunoassays using longer native sequences of these two immunogenic proteins. Furthermore, despite apparent genetic diversity in Borrelia strains and the differential expression of antigenic components due to geographic differences, the antigens used in the BioPlex 2200 Lyme Total assay contain immunoreactive domains that are largely conserved across genospecies endemic in other parts of the world (40). Testing the endemic cohorts of Russian and Ukrainian origins (where B. afzelii and B. garinii are more prevalent) revealed that the BioPlex Total assay also has relatively high sensitivity for early Lyme infection in samples from outside the United States, while maintaining a relatively low asymptomatic seroreactivity. Thus, there may be potential for the new assay to be applied to other areas of endemicity where the predominant genospecies is not B. burgdorferi sensu stricto. However, further studies using additional larger cohorts from other geographic regions are needed to confirm that the performance is sufficient to extend its general use beyond North America.
Supplementary Material
ACKNOWLEDGMENTS
We thank the CDC in general for providing the clinical premarketing panel, and we specifically thank Christopher Sexton for providing the accompanying test results. We also thank Biopeptides Corp. for providing the FVlsE antigen. We are also grateful for critical reviews of the manuscript from Sadie Eicher and Catalina Esteban-Christen.
All of the authors are Bio-Rad employees.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JWR, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mead PS. 2015. Epidemiology of Lyme disease. Infect Dis Clin North Am 29:187–210. doi: 10.1016/j.idc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Borchers AT, Keen CL, Huntley AC, Gershwin ME. 2015. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun 57:82–115. doi: 10.1016/j.jaut.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. 2017. Surveillance for Lyme disease—United States, 2008–2015. MMWR Surveill Summ 66:1–12. doi: 10.15585/mmwr.ss6622a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2018. Recent surveillance data (CDC Lyme): November 7, 2019. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 7.Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM. 2016. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis 16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kingry LC, Anacker M, Pritt B, Bjork J, Respicio-Kingry L, Liu G, Sheldon S, Boxrud D, Strain A, Oatman S, Berry J, Sloan L, Mead P, Neitzel D, Kugeler KJ, Petersen JM. 2018. Surveillance for and discovery of Borrelia species in US patients suspected of tickborne illness. Clin Infect Dis 66:1864–1871. doi: 10.1093/cid/cix1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estrada-Peña A, Cutler S, Potkonjak A, Vassier-Tussaut M, Van Bortel W, Zeller H, Fernández-Ruiz N, Mihalca AD. 2018. An updated meta-analysis of the distribution and prevalence of Borrelia burgdorferi s.l. in ticks in Europe. Int J Health Geogr 17:41. doi: 10.1186/s12942-018-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the Second National Conference on the Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2018. Two-step laboratory testing process: Lyme disease. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 12.Miraglia CM. 2016. A review of the Centers for Disease Control and Prevention’s guidelines for the clinical laboratory diagnosis of Lyme disease. J Chiropr Med 15:272–280. doi: 10.1016/j.jcm.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush LM, Vazquez-Pertejo MT. 2018. Tick-borne illness: Lyme disease. Dis Mon 64:195–212. doi: 10.1016/j.disamonth.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Marques AR. 2018. Revisiting the Lyme disease serodiagnostic algorithm: the momentum gathers. J Clin Microbiol 56:e00749-18. doi: 10.1128/JCM.00749-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegalajar-Jurado A, Schriefer ME, Welch RJ, Couturier MR, MacKenzie T, Clark RJ, Ashton LV, Delorey MJ, Molins CR. 2018. Evaluation of modified two-tiered testing algorithms for Lyme disease laboratory diagnosis using well-characterized serum samples. J Clin Microbiol 56:e01943-17. doi: 10.1128/JCM.01943-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theel ES. 2016. The past, present, and (possible) future of serologic testing for Lyme disease. J Clin Microbiol 54:1191–1196. doi: 10.1128/JCM.03394-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branda JA, Strle K, Nigrovic LE, Lantos PM, Lepore TJ, Damle NS, Ferraro MJ, Steere AC. 2017. Evaluation of modified two-tiered serodiagnostic testing algorithms for early Lyme disease. Clin Infect Dis 64:1074–1080. doi: 10.1093/cid/cix043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wormser GP, Molins CR, Levin A, Lipsett SC, Nigrovic LE, Schriefer ME, Branda JA. 2018. Evaluation of a sequential enzyme immunoassay testing algorithm for Lyme disease demonstrates lack of test independence but high diagnostic specificity. Diagn Microbiol Infect Dis 91:217–219. doi: 10.1016/j.diagmicrobio.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molins CR, Delorey MJ, Sexton C, Schriefer ME. 2016. Lyme borreliosis serology: performance of several commonly used laboratory diagnostic tests and a large resource panel of well-characterized patient samples. J Clin Microbiol 54:2726–2734. doi: 10.1128/JCM.00874-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradshaw GL, Thueson RK, Uriona TJ. 2017. Improved serodiagnostic performance for Lyme disease by use of two recombinant proteins in enzyme-linked immunosorbent assay compared to standardized two-tier testing. J Clin Microbiol 55:3046–3056. doi: 10.1128/JCM.01004-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Food and Drug Administration. 2019. 510(k) substantial equivalence determination decision summary K190907. U.S. Food and Drug Administration, Bethesda, MD. [Google Scholar]
- 22.Marques AR. 2015. Laboratory diagnosis of Lyme disease: advances and challenges. Infect Dis Clin North Am 29:295–307. doi: 10.1016/j.idc.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaz A, Glickstein L, Field JA, McHugh G, Sikand VK, Damle N, Steere AC. 2001. Cellular and humoral immune responses to Borrelia burgdorferi antigens in patients with culture-positive early Lyme disease. Infect Immun 69:7437–7444. doi: 10.1128/IAI.69.12.7437-7444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun 61:2182–2191. doi: 10.1128/IAI.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Luft BJ, Schubach W, Dattwyler RJ, Gorevic PD. 1992. Mapping the major antigenic domains of the native flagellar antigen of Borrelia burgdorferi. J Clin Microbiol 30:1535–1540. doi: 10.1128/JCM.30.6.1535-1540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Signorino G, Arnaboldi PM, Petzke MM, Dattwyler RJ. 2014. Identification of OppA2 linear epitopes as serodiagnostic markers for Lyme disease. Clin Vaccine Immunol 21:704–711. doi: 10.1128/CVI.00792-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwan TG, Piesman J. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease- associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 38:382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun 74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnaboldi PM, Seedarnee R, Sambir M, Callister SM, Imparato JA, Dattwyler RJ. 2013. Outer surface protein C peptide derived from Borrelia burgdorferi sensu stricto as a target for serodiagnosis of early Lyme disease. Clin Vaccine Immunol 20:474–481. doi: 10.1128/CVI.00608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang JR, Hardham JM, Barbour AG, Norris SJ. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 31.Liang FT, Alvarez AL, Gu Y, Nowling JM, Ramamoorthy R, Philipp MT. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J Immunol 163:5566–5573. [PubMed] [Google Scholar]
- 32.Lawrenz MB, Hardham JM, Owens RT, Nowakowski J, Steere AC, Wormser GP, Norris SJ. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J Clin Microbiol 37:3997–4004. doi: 10.1128/JCM.37.12.3997-4004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craft JE, Fischer DK, Shimamoto GT, Steere AC. 1986. Antigens of Borrelia burgdorferi recognized during Lyme disease: appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest 78:934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coleman JL, Benach JL. 1987. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis 155:756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- 35.Wang X-G, Lin B, Kidder JM, Telford S, Hu LT. 2002. Effects of environmental changes on expression of the oligopeptide permease (opp) genes of Borrelia burgdorferi. J Bacteriol 184:6198–6206. doi: 10.1128/jb.184.22.6198-6206.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahey LJ, Panas MW, Mao R, Delanoy M, Flanagan JJ, Binder SR, Rebman AW, Montoya JG, Soloski MJ, Steere AC, Dattwyler RJ, Arnaboldi PM, Aucott JN, Robinson WH. 2015. Development of a multiantigen panel for improved detection of Borrelia burgdorferi infection in early Lyme disease. J Clin Microbiol 53:3834–3841. doi: 10.1128/JCM.02111-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaul R, Chen P, Binder SR. 2004. Detection of immunoglobulin M antibodies specific for Toxoplasma gondii with increased selectivity for recently acquired infections. J Clin Microbiol 42:5705–5709. doi: 10.1128/JCM.42.12.5705-5709.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molins CR, Sexton C, Young JW, Ashton LV, Pappert R, Beard CB, Schriefer ME. 2014. Collection and characterization of samples for establishment of a serum repository for Lyme disease diagnostic test development and evaluation. J Clin Microbiol 52:3755–3762. doi: 10.1128/JCM.01409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molins CR, Delorey MJ, Replogle A, Sexton C, Schriefer ME. 2017. Evaluation of bioMérieux’s dissociated Vidas Lyme IgM II and IgG II as a first-tier diagnostic assay for Lyme disease. J Clin Microbiol 55:1698–1706. doi: 10.1128/JCM.02407-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes-Solecki MJC, Meirelles L, Glass J, Dattwyler RJ. 2007. Epitope length, genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin Vaccine Immunol 14:875–879. doi: 10.1128/CVI.00122-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.