Serological testing for nasopharyngeal carcinoma (NPC) has recently been reinvigorated by the implementation of novel Epstein-Barr virus (EBV)-specific IgA and IgG antibodies from a proteome array. Although proteome arrays are well suited for comprehensive antigen selection, they are not applicable for large-scale studies. We adapted a 13-marker EBV antigen signature for NPC risk identified by proteome arrays to multiplex serology to establish an assay for large-scale studies. Taiwanese NPC cases (n = 175) and matched controls (n = 175) were used for assay validation.
KEYWORDS: Epstein-Barr virus, multiplex serology, nasopharyngeal carcinoma, risk stratification signature, validation
ABSTRACT
Serological testing for nasopharyngeal carcinoma (NPC) has recently been reinvigorated by the implementation of novel Epstein-Barr virus (EBV)-specific IgA and IgG antibodies from a proteome array. Although proteome arrays are well suited for comprehensive antigen selection, they are not applicable for large-scale studies. We adapted a 13-marker EBV antigen signature for NPC risk identified by proteome arrays to multiplex serology to establish an assay for large-scale studies. Taiwanese NPC cases (n = 175) and matched controls (n = 175) were used for assay validation. Spearman’s correlation was calculated, and the diagnostic value of all multiplex markers was assessed independently using the area under the receiver operating characteristic curve (AUC). Two refined signatures were identified using stepwise logistic regression and internally validated with 10-fold cross validation. Array and multiplex serology showed strong correlation for each individual EBV marker, as well as for a 13-marker combined model on continuous data. Two refined signatures with either four (LF2 and BGLF2 IgG, LF2 and BMRF1 IgA) or two (LF2 and BGLF2 IgG) antibodies on dichotomous data were identified as the most parsimonious set of serological markers able to distinguish NPC cases from controls with AUCs of 0.992 (95% confidence interval [CI], 0.983 to 1.000) and 0.984 (95% CI, 0.971 to 0.997), respectively. Neither differed significantly from the 13-marker model (AUC, 0.992; 95% CI, 0.982 to 1.000). All models were internally validated. Multiplex serology successfully validated the original EBV proteome microarray data. Two refined signatures of four and two antibodies were capable of detecting NPC with 99.2% and 98.4% accuracy.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a rare cancer globally, with an age-standardized incidence rate of less than one per 100,000 person-years (1). However, incidence rates in regions of endemicity, including Southeast Asia and southern China, are up to 30 times higher (2). Within regions of endemicity, men aged 45 to 60 years and subjects with multiple first- and second-degree relatives with NPC harbor the highest risk (2). NPC is disproportionately diagnosed at more advanced stages, when survival rates are significantly lower than in early-stage NPCs (3). The presenting stage has been shown to be the most important prognostic factor for NPC patients, with overall survival decreasing from 90% when presenting in stage I to 58% in stage IV (4).
Effective screening for NPC could facilitate early detection and thereby subsequently improve survival rates in high-risk populations (5). Because 95% of NPCs in regions of endemicity are undifferentiated carcinoma associated with Epstein-Barr virus (EBV) infection (6), antibodies against EBV represent a viable, noninvasive screening option in NPC-endemic regions.
Several studies have investigated EBV antibodies in human serum as potential NPC biomarkers in case/control (7–10) and prospective studies (11–13), including IgA antibodies against the EBV viral capsid antigen (VCA), early antigen (EA), and EBV nuclear antigen 1 (EBNA1) measured via immunofluorescence assay (IFA) or enzyme-linked immunosorbent assay (ELISA). Other EBV antigens from the roughly 100 protein-coding open reading frames and other antibody isotypes (especially IgG) have been examined less frequently. Recently, we identified a novel EBV-specific antibody risk stratification signature for early detection of NPCs in Taiwan using an EBV whole-proteome microarray (14). This array was novel in that it measured antibodies against nearly the entire EBV proteome. The identified risk signature consisted of 13 antibodies, both IgA and IgG, against 11 EBV proteins and significantly improved NPC risk prediction compared with currently used simplex VCAp18/EBNA1 IgA biomarkers (93% accuracy versus 82% for current biomarkers alone; P ≤ 0.01) included on the same array. Although the EBV whole-proteome microarray allowed simultaneous evaluation of antibodies against nearly all EBV open reading frames, protein microarrays are not suitable for large-scale studies. To facilitate large-scale testing, we adapted the improved risk stratification signature identified from proteome-wide screening for multiplex serology, a low-density high-throughput methodology (15).
Here, we report the validation of the proteome-based EBV antibody risk stratification signature with multiplex serology against the EBV proteome array. We further reduced the number of markers for risk stratification and proposed two refined antigen signatures for future use in prospective studies and high-throughput screening.
MATERIALS AND METHODS
Study material.
Sera for validation of the multiplex serology assay included 175 incident Taiwanese NPC cases (67 stage I/IIa, 16 stage IIb, 88 stage III/IV, and 4 unknown) and 175 community controls (frequency matched on age, sex, and region) that were previously assayed using the EBV whole-proteome array (14).
Antigen selection and expression.
Antigens from the array-based antibody risk stratification signature (14), totaling 6 IgG and 7 IgA antibodies against 11 antigens, were adapted for multiplex serology. This signature included antigens VCAp18, EBNA1, and BMRF1 (EA-D), which were previously expressed and validated specifically for multiplex serology (16) (Table 1). The nucleotide sequences of the other eight novel proteins in the NPC signature (BPLF1, BXLF1, LF2, BZLF1, BORF1, BFRF1, BGLF2, and BRLF1) were optimized for expression in Escherichia coli, synthesized commercially, and cloned into pGEX4T3tag vector (17) for expression as bacterial glutathione S-transferase (GST) fusion proteins. The NPC risk stratification signature from the protein array contained two BMRF1 markers (originally 14 markers overall), which differed in the nucleotide but not in the amino acid sequence. Those two BMRF1 markers were subsequently only expressed once for use in multiplex serology.
TABLE 1.
Summary of anti-EBV antibodies targeted in multiplex serology (n = 13)
| Protein | Protein accession/DNA template | Antibody type | Spearman’s rho (array/multiplex)a | Median MFI (interquartile range) |
|||
|---|---|---|---|---|---|---|---|
| Controls | All cases | Early-stage cases | Late/unknown-stage cases | ||||
| VCAp18b | Cosmid DNA (16) | IgA | 0.74 | 1,876 (956–4,012) | 4,491 (2,588–7,111) | 3,752 (2,095–6,798) | 4,504 (2,705–7,149) |
| EBNA1c | Cosmid DNA (16) | IgA | 0.72 | 374 (197–712) | 1,636 (1,028–2,611) | 1,889 (977–3,180) | 1,626 (1,037–2,470) |
| BXLF1 | YP_001129497.1-133399-131576 | IgA | 0.82 | 107 (50–225) | 5,257 (2,857–7,787) | 6,126 (3,036–8,322) | 5,131 (2,841–7,662) |
| BRLF1 | YP_001129468.1-93725-91908 | IgA | 0.81 | 88 (43–197) | 1,829 (559–4,644) | 1,905 (681–3,707) | 1,812 (491–4,694) |
| LF2 | YP_001129504.1-151808-150519 | IgA | 0.79 | 1 (1–10) | 2,131 (549–4,526) | 2,019 (390–4,546) | 2,141 (652–4,366) |
| BMRF1 | AFY97929.1-67486-68700 | IgA | 0.86 | 325 (132–877) | 5,655 (3,692–7,960) | 5,655 (3,338–7,960) | 5,723 (4,173–7,976) |
| BPLF1 | CAA24839.1-71527-62078-2 | IgA | 0.79 | 181 (70–439) | 2,437 (887–4,204) | 2,123 (981–3,664) | 2,528 (863–4,429) |
| BZLF1 | YP_001129467.1-90855-90724 | IgG | 0.81 | 1 (1–3) | 153 (19–465) | 70 (7–319) | 196 (32–574) |
| BORF1 | YP_001129451.1-63084-64178 | IgG | 0.75 | 1 (1–5) | 150 (6–579) | 79 (2–548) | 186 (15–586) |
| BFRF1 | YP_001129446.1-46719-47729 | IgG | 0.72 | 1 (1–6) | 904 (275–2,777) | 525 (180–1,140) | 1,354 (426–3,478) |
| BGLF2 | YP_001129486.1-115415-114405 | IgG | 0.83 | 120 (56–228) | 8,099 (4,211–10,420) | 6,832 (3,889–8,911) | 8,795 (5,452–11,032) |
| BXLF1 | Identical to BXLF1 IgA | IgG | 0.75 | 61 (5–181) | 3,176 (1,500–5,691) | 2,472 (935–4,381) | 3,953 (1,996–6,556) |
| LF2 | Identical to LF2 IgA | IgG | 0.79 | 1 (1–1) | 1,327 (604–2,946) | 1,133 (309–2,156) | 1,686 (723–3,602) |
Interpretation of Spearman’s rho: 0.00–0.19, very weak; 0.20–0.39, weak; 0.40–0.59, moderate; 0.60–0.79, strong; 0.80–1.00, very strong.
Overall amino acid identity, 38% (67/176 amino acids); identity in 67 overlapping amino acids, 94%.
Overall amino acid identity, 21% (67/317 amino acids); identity in 67 overlapping amino acids, 100%; amino acid identity for all other proteins, 100%.
Multiplex serology.
Multiplex serology is a bead-based suspension array technology for high-throughput testing of serum samples for antibodies against multiple antigens. The antigens are immobilized on beads, which are filled with different ratios of two fluorescent dyes, enabling the measurement of antibodies against multiple antigens at the same time. IgA and IgG antibodies are accessed in separate assays, using two different secondary antibodies. The basic principle of the multiplex serology assay format has been described previously (15). All sera were tested in three dilutions as follows: 1:100 for IgA and 1:1,000 and 1:10,000 for IgG testing. Preincubation of all sera at 1:50 (for a final 1:100 dilution), 1:500 (for a final 1:1,000 dilution), and 1:5,000 (for a final 1:10,000 dilution) dilutions was done in phosphate-buffered saline (PBS) containing 2 mg/ml casein, 2 g/liter GST-tag lysate, 5 g/liter polyvinyl alcohol, and 8 g/liter polyvinyl-pyrrolidone (18). To detect bound IgA and IgG serum antibodies, goat anti-human IgA-Biotin (1:1,000) (catalog number 109-065-011; Jackson ImmunoResearch) and goat anti-human IgG-Biotin (1:1,000) (catalog number 109-065-098; Jackson ImmunoResearch) were used before staining with streptavidin-R-phycoerythrin (1:750) (Moss, Inc.).
IgG testing resulted in higher sensitivities at the same specificity for the 1:10,000 dilution. The 1:1,000 dilution was hence not included in the analysis.
For quality control, 35 sera (18 cases and 17 controls) were tested blinded in duplicate to calculate intraclass correlation coefficients (ICCs) and coefficients of variation (CVs). One pair was removed due to a label mismatching.
Statistical analysis.
All statistical analyses were performed with SAS Enterprise Guide 7.1 (SAS Institute), GraphPad Prism 6 (GraphPad Software, Inc.), and RStudio 1.2.1335 (RStudio, Inc.). A P value of 0.05 was considered statistically significant.
Spearman’s rank correlation coefficients were used to compare signal intensity outputs from the proteome array and multiplex serology assays, measured on the same individuals. Spearman’s rho values were evaluated as follows: 0.00 to 0.19, very weak; 0.20 to 0.39, weak; 0.40 to 0.59, moderate; 0.60 to 0.79, strong; and 0.80 to 1.00, very strong.
Antigen-specific cutoffs for all multiplex serology antigens were determined using receiver operating characteristic (ROC) analysis on continuous data with a specificity of 95% to correctly classify control status used to dichotomize the median fluorescence intensity (MFI) values (Table 2). The resulting sensitivities for correctly classifying NPC status were calculated for all NPC cases and restricted to early-stage and late/unknown-stage cases.
TABLE 2.
Diagnostic value for multiplex serology assay, including sensitivities and antigen-specific cutoffs
| Protein | Antibody type | AUC (95% CI) continuous variables | Cutoffs for 95% specificity (MFI) | Sensitivity (%) |
||
|---|---|---|---|---|---|---|
| All cases | Early-stage cases | Late/unknown-stage cases | ||||
| 13-multiplex marker | 0.99 (0.98–1.00) | NAa | 98.9 | 100.0 | 98.1 | |
| VCAp18 | IgA | 0.74 (0.69–0.79) | 8,414 | 15.4 | 11.9 | 17.6 |
| EBNA1 | IgA | 0.89 (0.86–0.93) | 1,809 | 45.7 | 50.8 | 42.6 |
| BXLF1 | IgA | 0.97 (0.96–0.99) | 1,155 | 89.7 | 89.6 | 89.8 |
| BRLF1 | IgA | 0.92 (0.89–0.95) | 1,088 | 61.1 | 61.2 | 61.1 |
| LF2 | IgA | 0.97 (0.96–0.99) | 92 | 93.1 | 94.0 | 92.6 |
| BMRF1 | IgA | 0.94 (0.92–0.97) | 3,484 | 77.1 | 74.6 | 78.7 |
| BPLF1 | IgA | 0.90 (0.87–0.93) | 1,591 | 64.6 | 62.7 | 65.7 |
| BZLF1 | IgG | 0.88 (0.84–0.92) | 39 | 67.4 | 59.7 | 75.0 |
| BORF1 | IgG | 0.80 (0.76–0.85) | 202 | 44.0 | 37.1 | 48.2 |
| BFRF1 | IgG | 0.95 (0.93–0.97) | 347 | 74.3 | 68.7 | 79.6 |
| BGLF2 | IgG | 0.99 (0.98–1.00) | 626 | 97.1 | 98.5 | 96.3 |
| BXLF1 | IgG | 0.97 (0.95–0.98) | 645 | 88.0 | 82.1 | 91.7 |
| LF2 | IgG | 0.98 (0.97–1.00) | 36 | 96.0 | 98.5 | 95.4 |
NA, not applicable.
Stepwise logistic regression on dichotomized (positive/negative) data in SAS was used to narrow the number of antibodies from the 13-marker risk stratification signature to a more parsimonious group in an attempt to facilitate an easily implemented NPC screening test for mass application. P < 0.15 was set as the model entry criterion, and P < 0.05 was the stay criterion for an antibody to remain in the model, as previously used (14). Based on the results of this logistic regression, the set of refined markers was used to compute the diagnostic accuracy based on the area under the curve (AUC). All models in Table 3 and Fig. S2 are based on dichotomized data. Models were internally validated using 10-fold cross-validation, meaning that the data were split into 10 equally sized data sets. The AUC was then calculated using each of the 10 data sets in turn for validation and the remaining 9 data sets for training. The average of the 10 results (i.e., average of the AUC calculated from the 10 validation subsets) is reported as a value for the 10-fold cross-validation.
TABLE 3.
Multiplex serology ROC models on dichotomized data
| Model | AUC | 95% CI | 10-fold cross-validation for: |
|
|---|---|---|---|---|
| AUC | 95% CI | |||
| All 13 markers | 0.992 | 0.982–1.000 | 0.984 | 0.967–1.000 |
| LF2 IgG, BGLF2 IgG, BMRF1 IgA, LF2 IgA | 0.992 | 0.983–1.000 | 0.987 | 0.972–1.000 |
| LF2 IgG, BGLF2 IgG | 0.984 | 0.971–0.997 | 0.974 | 0.952–0.996 |
| BMRF1 IgA, LF2 IgA | 0.978 | 0.964–0.992 | 0.969 | 0.949–0.989 |
RESULTS
Quality control.
Intraclass correlation coefficients (ICCs) and coefficients of variation (CVs) were calculated for quality control. ICCs from log-transformed data of all markers ranged from 0.96 to 1.00, and CVs ranged from 1 to 18%.
Correlation of multiplex serology and proteome array data.
Median fluorescence intensity (MFI) values generated from the multiplex serology assay for 13 of the anti-EBV IgA and IgG antibodies from the previously reported NPC risk stratification signature were compared to standardized signal intensities from the same EBV markers previously ascertained using the proteome microarray. Spearman’s rho correlation coefficients for each pair are shown in Table 1. Both cases and controls were used to assess Spearman’s rho correlation coefficients.
The correlation between the array and multiplex serology output for all biomarkers was either strong or very strong, with Spearman’s rho values ranging from 0.72 to 0.86. Even VCAp18 IgA and EBNA1 IgA, which displayed a fairly low amino acid coverage (overall identity of 38% for VCAp18 and 21% for EBNA1, but high identities for each with 67 overlapping amino acids (94% for VCAp18 and 100% for EBNA1), resulted in strong Spearman’s rho correlation coefficients. The amino acid identity for all other antigens was 100%.
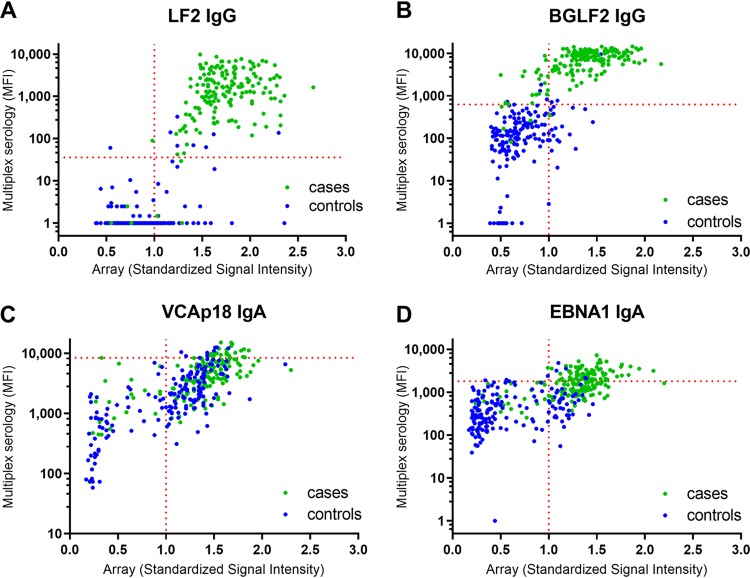
Figure 1 illustrates the correlation of the proteome array and multiplex serology data for four selected antibodies, categorized according to case/control status. Displayed are IgA antibodies against VCAp18 and EBNA1, which have previously been shown to be an effective combination for discriminating between NPC cases and controls using ELISA-based methods (19), as well as LF2 and BGLF2 IgG, which performed particularly well in separating cases and controls in our study. For all four displayed antibody markers (VCAp18 IgA, EBNA1 IgA, LF2 IgG, and BGLF2 IgG), the high correlation of proteome array and serology data is striking. However, the separation of cases and controls was primarily observed for LF2 IgG and BGLF2 IgG, compared to case/control overlap observed in the VCAp18 and EBNA1 IgA reactivities (Fig. 1). Scatterplots for the remaining antibodies are included in Fig. S1 in the supplemental material.
FIG 1.
Scatterplots for the correlation of array and multiplex serology data for four selected antibodies categorized to case/control status. (A) LF2 (IgG); (B) BGLF2 (IgG); (C) VCAp18 (IgA); (D) EBNA1 (IgA). The horizontal dotted line represents the multiplex serology cutoff, which is defined using receiver operating characteristic (ROC) analysis on continuous data with a specificity of 95% to correctly classify control status, and the vertical dotted line represents the threshold of seropositivity for the array data, which is defined as standardized signal intensity > 1.
Performance of antibodies using the multiplex serology assay.
The diagnostic value of the multiplex serology assay was determined by calculating the AUC for the combined 13-marker model on continuous data. An obtained accuracy of 99.3% (95% CI, 98 to 100%) confirmed the ability of the multiplex serology assay to reproduce the effective risk stratification derived from this signature when applied in the EBV proteome array format (Table 2). This AUC is composed of a combination of sensitivity (case identification) and specificity (control identification). At 95% specificity, sensitivity for all cases and for stage I/IIa cases was 98.8% and 100%, respectively. To further evaluate the performance of the multiplex serology assay, the individual AUC of each of the 13 markers on continuous data was calculated (Table 2). A marker-specific cutoff was determined at a specificity of 95% according to case/control status for evaluating the diagnostic value of each antibody separately. Sensitivities for all cases (n = 175) and stage I/IIa cases (n = 67) were determined (Table 2) and reached from 11.4% (VCAp18 IgA stage I/IIa cases) to 98.5% (LF2 IgG and BGLF2 IgG stage I/IIa cases). Of the 13 individual antibodies, 10 showed a sensitivity higher than 60% at 95% specificity; VCAp18, EBNA1, and BORF1 did not reach this threshold (Table 2).
Refined risk signature model.
In order to refine the number of antibodies in the NPC risk stratification signature necessary to identify NPC to a parsimonious set, a stepwise selection procedure was applied using dichotomized data. The resulting model contained the following four antibodies: LF2 IgG, BGLF2 IgG, LF2 IgA, and BMRF1 IgA (logit P = –5.0015 + 3.4328 × BMRF1 IgA + 2.6795 × LF2 IgA + 3.5153 × BGLF2 IgG + 2.4180 × LF2 IgG). The AUC of this model was 0.992 (95% CI, 0.983 to 1.000) and was 0.987 (95% CI, 0.972 to 1.000) after 10-fold cross-validation. The accuracy of the refined 4-marker signature did not significantly differ from the model including all 13 markers (logit P = –5.5382 + 1.6361 × VCAp18 IgA + 1.5281 × EBNA1 IgA + 1.1913 × BXLF1 IgA – 1.3314 × BRLF1 IgA + 2.6507 × LF2 IgA + 2.7506 × BMRF1 IgA + 0.5645 × BPLF1 IgA – 1.5870 × BZLF1 IgG + 1.3984 × BORF1 IgG + 0.1354 × BFRF1 IgG + 3.9171 × BGLF2 IgG – 0.3760 × BXLF1 IgG + 2.7950 × LF2 IgG; AUC, 0.992 [95% CI, 0.982 to 1.000], P = 0.77) (Table 3, Fig. S2).
Finally, the performance of the IgG and IgA antibodies of this refined model was assessed separately. A model including LF2 IgG and BGLF2 IgG (logit P = –4.4302 + 4.4580 × BGLF2 IgG + 3.9562 × LF2 IgG) resulted in an AUC of 0.984 (95% CI, 0.971 to 0.997; 10-fold cross-validation AUC, 0.974 [95% CI, 0.952 to 0.996]) and did not significantly differ from the 4-marker model (P = 0.07) or from the 13-marker model (P = 0.10) reported above. The IgA model, however, comprising BMRF1 IgA and LF2 IgA (logit P = –3.5991 + 5.3166 × LF2 IgA + 3.6278 × BMRF1 IgA), received an AUC of 0.978 (95% CI, 0.964 to 0.992; 10-fold cross-validation AUC, 0.969 [95% CI, 0.949 to 0.989]) and differed significantly from the 4-marker model (P = 0.01) and the 13-marker model (P = 0.01).
DISCUSSION
Nasopharyngeal carcinoma is typically diagnosed at more advanced stages, where survival rates are dramatically lower than in early-stage NPCs (3). Because the majority of endemic, undifferentiated NPC is associated with EBV, anti-EBV antibodies have been proposed as tools to improve early diagnosis rates in screening for NPC. We previously utilized a peptide-based proteome array to identify a 13-marker set to improve serological testing for NPC risk prediction and validated this signature in two independent cohorts with prospectively collected samples (14). However, although peptide-based proteome arrays are well-suited for high-dimensional antigen screening, their technical requirements make a wide-scale, high-throughput application in a population screening setting impractical. In this study, we adapted a previously reported EBV antibody risk stratification signature identified from the EBV proteome array for application in a bead-based multiplex serology platform, which will enable standardized use of this panel in future large-scale studies. We reported strong correlations between the output data for the proteome array and multiplex serology platform for all EBV markers tested. Furthermore, we refined the set of 13 markers to a reduced panel of two or four antibodies to facilitate large-scale translation in an attempt to facilitate an easily implemented NPC screening test for mass application.
Reproducible assays detecting EBV markers are important for the implementation of NPC screening tools. Whole-protein microarrays, which are highly efficient in terms of antigen selection, are not suitable for large-scale testing, due to greater cost, limitations in large-scale expression of the proteins for printing on an array, a requirement for more specialized equipment, and the array proteins being expressed in a cell-free system so protein folding may not mimic native structure. To overcome these limitations, we assessed the performance of the 13-antigen signature identified by proteome arrays in a more conventional immunoscreening assay, namely, the Luminex bead-based multiplex serology platform, which has been characterized as a highly reproducible method and widely applied in many large-scale seroepidemiological studies (20–22).
In addition to strong correlations between proteome array and multiplex serology for 13 EBV markers, we explored two refined models, consisting of four (LF2 IgG, BGLF2 IgG, LF2 IgA, and BMRF1 IgA) and two (LF2 IgG and BGLF2 IgG) antibodies, respectively, which could further streamline population NPC screening if validated in an independent study. The models for the 4-antigen and 2-antigen signatures showed an accuracy of 99.2% and 98.4%, respectively, and neither differed significantly from the 13-marker model. Intriguingly, our models are predicted to have a high ability to identify stage I/IIa NPC cases, which is essential for their potential utilization in a population screening setting since screen-detected NPC cases are more likely to be at early stages.
BMRF1, also known as EA-D (early antigen diffuse) is an early gene essential for lytic replication (23). EA-D IgG is used as a marker of acute infection (24), and IgA antibodies against EA-D have been investigated previously in NPC patients (7, 25). Antibodies against LF2 and BGLF2, however, are newly identified NPC biomarkers (14). Both proteins are less investigated and have not yet been explored for their role in NPC development. LF2 has been shown to be able to bind to BRLF1 (Rta), while BGLF2 influences BZLF1 (Zta) activity. Both BRLF1 (Rta) and BZLF1 (Zta) are essential for viral DNA replication and thus lytic viral reproduction (26). While LF2 inhibits the ability of BRLF1 (Rta) to activate early lytic promoters (27), BGLF2 can enhance protein expression of BZLF1 (Zta) (28). As a result, LF2 displays a potential inhibitor for lytic EBV replication by inhibiting BRLF1 (Rta), while BGLF2 has been shown to enhance expression of BZLF1 (Zta) and thus reactivation of a lytic infection. The balance of these contrary roles of LF2 and BGLF2, leading to inhibition and promotion of the viral lytic cycle, needs to be further explored in relation to long-term EBV control and NPC pathogenesis.
Intriguingly, our refined model consisting of only two IgG antibodies, LF2 and BGLF2, had almost the same diagnostic performance (AUC, 0.984 [95% CI, 0.971 to 0.997]) as the full 13-marker set (AUC, 0.992 [95% CI, 0.982 to 1.000]). This finding is in line with our previous report highlighting the importance of not only IgA but also a smaller set of IgG markers for NPC screening (14), markers which have not been explored extensively in other studies. The refined antibody markers could also facilitate an even more efficient experimental setup. Simultaneous testing of IgA and IgG antibodies requires doubling the amount of all reactions. IgG antibody testing alone reduces the number of interactions, and IgG can also be assayed using a higher dilution of sera than IgA testing. Since IgG antibody responses to both LF2 and BGLF2 are quite low but clearly different for cases and controls, they could easily be implemented in larger studies with the two IgG antibodies tested at a 1:1,000 or even a 1:100 dilution. For future screening approaches, measuring antibodies to two antigens is much more feasible than looking at a whole antibody panel. Even alone, LF2 IgG and BGLF2 IgG show high sensitivities of 96.0% and 97.1%, respectively, in identifying NPC cases at 95% specificity in this study.
Our results should be interpreted in light of several limitations. First, samples used in the present study were collected at NPC diagnosis. It is unclear whether our markers could have the same prediction ability for NPC cases occurring years after blood collection (i.e., prospective risk stratification). Second, all samples for this study and array-based studies were collected in Taiwan, and marker performance in other NPC-endemic regions, such as southern China and Southeast Asia, is unknown and merits further investigation. Validation of the proposed risk stratification model is only based on internal 10-fold cross-validation, rather than on a separate validation data set. Further studies are needed to validate this EBV antibody signature in other populations and evaluate the antibody pattern prospectively to estimate the use of the new antibodies for screening approaches. Finally, although our marker panel had a much better performance than previous markers (VCA IgA and EBNA1 IgA) (29), we did not include assays which have been widely used in the high-risk population, such as VCA IgA and EBNA1 IgA tested using ELISA (30).
In conclusion, we validated a novel 13-marker EBV antibody risk stratification signature for the detection of NPCs identified using EBV proteome microarrays in Taiwan using multiplex serology and report high correlations with markers previously assayed using a high-dimensional proteome array. We further refined this 13-antigen signature to identify two models enabling NPC detection based on a reduced panel of either four or two anti-EBV antibodies. Future studies should focus on studying the value of the markers for predicting incident NPC and replication in other NPC-endemic populations.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. D.L.D. is supported by a principal research fellowship from the National Health and Medical Research Council of Australia. The funding organization played no role in the study design, collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2015. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chang ET, Adami H-O. 2006. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. 2017. Cancer Facts & Figures 2017. American Cancer Society, Atlanta, GA. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2017.html.
- 4.Lee AWM, Sze WM, Au JSK, Leung SF, Leung TW, Chua DTT, Zee BCY, Law SCK, Teo PML, Tung SY, Kwong DLW, Lau WH. 2005. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 5.Ji MF, Sheng W, Cheng WM, Ng MH, Wu BH, Yu X, Wei KR, Li FG, Lian SF, Wang PP, Quan W, Deng L, Li XH, Liu XD, Xie YL, Huang SJ, Ge SX, Huang SL, Liang XJ, He SM, Huang HW, Xia SL, Ng PS, Chen HL, Xie SH, Liu Q, Hong MH, Ma J, Yuan Y, Xia NS, Zhang J, Cao SM. 2019. Incidence and mortality of nasopharyngeal carcinoma: interim analysis of a cluster randomized controlled screening trial (PRO-NPC-001) in southern China. Ann Oncol 30:1630–1637. doi: 10.1093/annonc/mdz231. [DOI] [PubMed] [Google Scholar]
- 6.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. 2012. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 7.Henle G, Henle W. 1976. Epstein-Barr virus-specific IgA serum antibodies as an outstanding feature of nasopharyngeal carcinoma. Int J Cancer 17:1–7. doi: 10.1002/ijc.2910170102. [DOI] [PubMed] [Google Scholar]
- 8.Lanier AP, Bornkamm GW, Henle W, Henle G, Bender TR, Talbot ML, Dohan PH. 1981. Association of Epstein-Barr virus with nasopharyngeal carcinoma in Alaskan native patients: serum antibodies and tissue EBNA and DNA. Int J Cancer 28:301–305. doi: 10.1002/ijc.2910280308. [DOI] [PubMed] [Google Scholar]
- 9.Lin TM, Yang CS, Chiou JF, Tu SM, Chen TY, Tu YC, Lin PJ, Kawamura A Jr, Hirayama T. 1977. Antibodies to Epstein-Barr virus capsid antigen and early antigen in nasopharyngeal carcinoma and comparison groups. Am J Epidemiol 106:336–339. doi: 10.1093/oxfordjournals.aje.a112470. [DOI] [PubMed] [Google Scholar]
- 10.Hadar T, Sidi J, Rahima M, Rakowsky E, Kahan E, Sarov B, Sarov I. 1986. Significance of specific Epstein-Barr virus IgA and elevated IgG antibodies to viral capsid antigens in nasopharyngeal carcinoma patients. J Med Virol 20:329–339. doi: 10.1002/jmv.1890200405. [DOI] [PubMed] [Google Scholar]
- 11.Cao SM, Liu Z, Jia WH, Huang QH, Liu Q, Guo X, Huang TB, Ye W, Hong MH. 2011. Fluctuations of Epstein-Barr virus serological antibodies and risk for nasopharyngeal carcinoma: a prospective screening study with a 20-year follow-up. PLoS One 6:e19100. doi: 10.1371/journal.pone.0019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chien YC, Chen JY, Liu MY, Yang HI, Hsu MM, Chen CJ, Yang CS. 2001. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J Med 345:1877–1882. doi: 10.1056/NEJMoa011610. [DOI] [PubMed] [Google Scholar]
- 13.Ji MF, Wang DK, Yu YL, Guo YQ, Liang JS, Cheng WM, Zong YS, Chan KH, Ng SP, Wei WI, Chua DTT, Sham JST, Ng MH. 2007. Sustained elevation of Epstein-Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer 96:623–630. doi: 10.1038/sj.bjc.6603609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coghill AE, Pfeiffer RM, Proietti C, Hsu W-L, Chien Y-C, Lekieffre L, Krause L, Teng A, Pablo J, Yu KJ, Lou P-J, Wang C-P, Liu Z, Chen C-J, Middeldorp J, Mulvenna J, Bethony J, Hildesheim A, Doolan DL. 2018. Identification of a novel, EBV-based antibody risk stratification signature for early detection of nasopharyngeal carcinoma in Taiwan. Clin Cancer Res 24:1305–1314. doi: 10.1158/1078-0432.CCR-17-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. 2005. Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin Chem 51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 16.Brenner N, Mentzer AJ, Butt J, Michel A, Prager K, Brozy J, Weißbrich B, Aiello AE, Meier HCS, Breuer J, Almond R, Allen N, Pawlita M, Waterboer T. 2018. Validation of multiplex serology detecting human herpesviruses 1–5. PLoS One 13:e0209379. doi: 10.1371/journal.pone.0209379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sehr P, Zumbach K, Pawlita M. 2001. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods 253:153–162. doi: 10.1016/s0022-1759(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 18.Waterboer T, Sehr P, Pawlita M. 2006. Suppression of non-specific binding in serological Luminex assays. J Immunol Methods 309:200–204. doi: 10.1016/j.jim.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Coghill AE, Hsu WL, Pfeiffer RM, Juwana H, Yu KJ, Lou PJ, Wang CP, Chen JY, Chen CJ, Middeldorp JM, Hildesheim A. 2014. Epstein-Barr virus serology as a potential screening marker for nasopharyngeal carcinoma among high-risk individuals from multiplex families in Taiwan. Cancer Epidemiol Biomarkers Prev 23:1213–1219. doi: 10.1158/1055-9965.EPI-13-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreimer AR, Ferreiro-Iglesias A, Nygard M, Bender N, Schroeder L, Hildesheim A, Robbins HA, Pawlita M, Langseth H, Schlecht NF, Tinker LF, Agalliu I, Smoller SW, Ness-Jensen E, Hveem K, D’Souza G, Visvanathan K, May B, Ursin G, Weiderpass E, Giles GG, Milne RL, Cai Q, Blot WJ, Zheng W, Weinstein SJ, Albanes D, Brenner N, Hoffman-Bolton J, Kaaks R, Barricarte A, Tjønneland A, Sacerdote C, Trichopoulou A, Vermeulen RCH, Huang W-Y, Freedman ND, Brennan P, Waterboer T, Johansson M. 2019. Timing of HPV16-E6 antibody seroconversion before OPSCC: findings from the HPVC3 consortium. Ann Oncol 30:1335–1343. doi: 10.1093/annonc/mdz138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt J, Varga MG, Blot WJ, Teras L, Visvanathan K, Le Marchand L, Haiman C, Chen Y, Bao Y, Sesso HD, Wassertheil-Smoller S, Ho GYF, Tinker LE, Peek RM, Potter JD, Cover TL, Hendrix LH, Huang LC, Hyslop T, Um C, Grodstein F, Song M, Zeleniuch-Jacquotte A, Berndt S, Hildesheim A, Waterboer T, Pawlita M, Epplein M. 2019. Serologic response to Helicobacter pylori proteins associated with risk of colorectal cancer among diverse populations in the United States. Gastroenterology 156:175–186.e172. doi: 10.1053/j.gastro.2018.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gossai A, Waterboer T, Nelson HH, Michel A, Willhauck-Fleckenstein M, Farzan SF, Hoen AG, Christensen BC, Kelsey KT, Marsit CJ, Pawlita M, Karagas MR. 2016. Seroepidemiology of human polyomaviruses in a US population. Am J Epidemiol 183:61–69. doi: 10.1093/aje/kwv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhierl B, Delecluse H-J. 2006. The Epstein-Barr virus BMRF1 gene is essential for lytic virus replication. J Virol 80:5078–5081. doi: 10.1128/JVI.80.10.5078-5081.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Paschale M, Clerici P. 2012. Serological diagnosis of Epstein-Barr virus infection: problems and solutions. World J Virol 1:31–43. doi: 10.5501/wjv.v1.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tay JK, Chan SH, Lim CM, Siow CH, Goh HL, Loh KS. 2016. The role of Epstein-Barr virus DNA load and serology as screening tools for nasopharyngeal carcinoma. Otolaryngol Head Neck Surg 155:274–280. doi: 10.1177/0194599816641038. [DOI] [PubMed] [Google Scholar]
- 26.Feederle R, Kost M, Baumann M, Janz A, Drouet E, Hammerschmidt W, Delecluse HJ. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J 19:3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calderwood MA, Holthaus AM, Johannsen E. 2008. The Epstein-Barr virus LF2 protein inhibits viral replication. J Virol 82:8509–8519. doi: 10.1128/JVI.00315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Cohen JI. 2016. Epstein-Barr virus (EBV) tegument protein BGLF2 promotes EBV reactivation through activation of the p38 mitogen-activated protein kinase. J Virol 90:1129–1138. doi: 10.1128/JVI.01410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Huang Q, Liu W, Liu Q, Jia W, Chang E, Chen F, Liu Z, Guo X, Mo H, Chen J, Rao D, Ye W, Cao S, Hong M. 2012. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two-stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer 131:406–416. doi: 10.1002/ijc.26380. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Ji MF, Huang QH, Fang F, Liu Q, Jia WH, Guo X, Xie SH, Chen F, Liu Y, Mo HY, Liu WL, Yu YL, Cheng WM, Yang YY, Wu BH, Wei KR, Ling W, Lin X, Lin EH, Ye W, Hong MH, Zeng YX, Cao SM. 2013. Two Epstein-Barr virus-related serologic antibody tests in nasopharyngeal carcinoma screening: results from the initial phase of a cluster randomized controlled trial in southern China. Am J Epidemiol 177:242–250. doi: 10.1093/aje/kws404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.