Accurate detection of influenza A virus (IAV) is crucial for patient management, infection control, and epidemiological surveillance. The World Health Organization and the Centers for Disease Control and Prevention have recommended using the M gene as the diagnostic gene target for reverse-transcription-PCR (RT-PCR). However, M gene RT-PCR has reduced sensitivity for recent IAV due to novel gene mutations. Here, we sought to identify novel diagnostic targets for the molecular detection of IAV using long-read third-generation sequencing.
KEYWORDS: influenza, next-generation sequencing, diagnostic assay
ABSTRACT
Accurate detection of influenza A virus (IAV) is crucial for patient management, infection control, and epidemiological surveillance. The World Health Organization and the Centers for Disease Control and Prevention have recommended using the M gene as the diagnostic gene target for reverse-transcription-PCR (RT-PCR). However, M gene RT-PCR has reduced sensitivity for recent IAV due to novel gene mutations. Here, we sought to identify novel diagnostic targets for the molecular detection of IAV using long-read third-generation sequencing. Direct nanopore sequencing from 18 nasopharyngeal specimens and one saliva specimen showed that the 5′ and 3′ ends of the PB2 gene and the entire NS gene were highly abundant. Primers selected for PB2 and NS genes were well matched with seasonal or avian IAV gene sequences. Our novel PB2 and NS gene real-time RT-PCR assays showed limits of detection similar to or lower than that of M gene RT-PCR and achieved 100% sensitivity and specificity in the detection of A(H1N1), A(H3N2), and A(H7N9) in nasopharyngeal and saliva specimens. For 10 patients with IAV detected by M gene RT-PCR conversion in sequentially collected specimens, NS and/or PB2 gene RT-PCR was positive in 2 (20%) of the initial specimens that were missed by M gene RT-PCR. In conclusion, we have shown that PB2 or NS gene RT-PCRs are suitable alternatives to the recommended M gene RT-PCR for diagnosis of IAV. Long-read nanopore sequencing facilitates the identification of novel diagnostic targets.
INTRODUCTION
Seasonal and pandemic influenza viruses cause a rapid increase in the number of patients, overwhelming the health care system (1, 2). Animal influenza viruses occasionally jump the species barrier and cause human disease (3, 4). Though rare, human infections with avian influenza viruses are particularly severe, especially for influenza A(H5N1), A(H7N9), and A(H5N6) (5).
Accurate detection of influenza viruses is important for patient management, public health policies, and research. A highly sensitive assay would reduce the number of false-negative results, facilitating appropriate antiviral treatment and infection control measures. Asymptomatic infections are now increasingly recognized due to highly sensitive molecular assays (6). Vaccine effectiveness is determined using test-negative design, and therefore an accurate test is essential for the determination of vaccine effectiveness (7). A more sensitive assay will also allow the use of specimen types with lower viral load, such as saliva, nasal swab, and throat swab (8, 9).
The World Health Organization (WHO) has emphasized that better tools are required to detect influenza viruses (1). Currently, reverse transcription PCR (RT-PCR) is the mainstay in the laboratory detection of influenza A viruses (10). The matrix (M) gene has been chosen as the target for influenza A virus RT-PCR by the WHO and the Centers for Disease Control and Prevention (CDC) because it is highly conserved (11–13), but in recent years, M gene mutations have affected the sensitivity of influenza virus RT-PCR for both influenza A(H1N1) and A(H3N2) (12, 14–16). As a result, the WHO updated their M gene real-time RT-PCR primers in 2017 (11).
Few studies have evaluated the use of other internal gene diagnostic targets. A real-time RT-PCR targeting a nonstructural (NS) gene has been shown to be highly sensitive for the detection of A(H1N1)pdm09 virus in clinical specimens (17). In the current study, we identified novel gene targets for influenza A virus RT-PCR using nanopore sequencing (18). Since the gene region targeted is located in the coding region, our assay can detect both viral RNA (vRNA) and mRNA.
(This work was presented in part at the 35th Clinical Virology Symposium, Savannah, GA, 5 to 8 May 2019.)
MATERIALS AND METHODS
Clinical specimens.
Clinical specimens used in this study were either specimens archived at the clinical microbiology laboratory of Queen Mary Hospital or were collected from our previous study (9). For nanopore sequencing, we randomly selected the nasopharyngeal specimens of 18 patients with laboratory-confirmed influenza A virus infection (see Table S1 in the supplemental material). For one patient (patient 18), we also performed nanopore sequencing on her saliva specimen. For the evaluation of the novel PB2 and NS gene RT-PCR on the detection of seasonal influenza virus, a total of 160 nasopharyngeal and 160 saliva specimens were retrieved that were previously tested using the Xpert Xpress flu/RSV assay (GeneXpert System, Cepheid, Sunnyvale, CA, USA) or by the Public Health Laboratory Services Branch in Hong Kong. Among them, 115 clinical specimens were collected during our previous study (Table S2) (9). To further determine the clinical utility of our novel assays, we identified 10 hospitalized patients with sequential nasopharyngeal specimens sent for influenza A virus detection for whom the first specimen tested negative but the subsequent specimen tested positive for influenza A virus using the M gene real-time RT-PCR. A total of 10 specimens collected in 2019 were retrieved. For the evaluation of the detection of A(H7N9), a total of 10 A(H7N9)-positive specimens were included. These were collected from patients in Hong Kong from 2014 and 2017, and six were included in our previous study (19).
This study has been approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (HKU/HA HKW IRB) (UW 19-094 and UW 13-372). This study is reported according to the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guideline (20).
Nanopore sequencing library preparation and nanopore sequencing.
RNA was extracted from nasopharyngeal specimens using a QIAamp viral RNA minikit (Qiagen, Hilden, Germany) as we described previously (21). DNase I was added to the extracted RNA to remove residual host DNA. All gene segments of influenza A were amplified simultaneously with the SuperScript III one-step RT-PCR system (Thermo Fisher Scientific, Waltham, MA, USA) using the primers ONT-uni-12 (5′-TTTCTGTTGGTGCTGATATTGCAGCRAAAGCAGG-3′) and ONT-uni-13 (5′-ACTTGCCTGTCGCTCTATCTTCAGTAGAAACAAGG-3′). The underlined bases represent 5′ universal tails that are complementary to the barcode primers of the PCR barcoding kit (SQK-PBK004) manufactured by Oxford Nanopore Technologies, whereas the remaining bases are the 12-bp and 13-bp sequences targeting the 3′ UTR and 5′ UTR, respectively, of all gene segments of influenza A virus (22). The RT-PCR conditions were 42°C for 15 min, 55°C for 15 min, 60°C for 5 min, and 94°C for 2 min and then 5 cycles of 94°C for 30 s, 45°C for 30 s, and 68°C for 3 min, followed by 30 cycles of 94°C for 30 s, 57°C for 30 s, and 68°C for 3 min.
Subsequent library preparation was performed according to the manufacturer’s instructions. Briefly, amplified PCR products were purified using 0.8× AMPure XP beads (Beckman Coulter, California, USA) and were then subjected to PCR barcoding (SQK-PBK004). A batch of 12 barcoded PCR products was purified using 0.8× AMPure XP beads before being pooled at equal concentrations for end repair and sequencing adaptor ligation (SQK-LSK109). Finally, sequencing was performed on an Oxford Nanopore MinION platform using an R9.4.1 flow cell for 6 to 12 h.
After sequencing, the raw signal data were converted into FASTQ data using Guppy v3.2.2 with the fast base-calling mode. The FASTQ files were quality-checked using MinIONQC to ensure data integrity and sequencing quality (23). The FASTQ reads were then demultiplexed and trimmed using the Guppy barcoder. Next, the DNA sequences were mapped onto a reference sequence to generate an alignment file using the Burrows-Wheeler Aligner MEM algorithm (BWA-MEM) with the “-x ont2d” setting (24). The reference influenza virus sequences chosen for A(H1N1) and A(H3N2) read mapping were the 2018–2019 WHO-recommended vaccine strains (A/Michigan/45/2015 and A/Singapore/INFIMH-16-0019/2016). Finally, the coverage data were obtained using the SAMtools depth command (25).
Selection of primers and probes.
Primers and probes targeting the polymerase basic 2 (PB2) and NS genes were designed by multiple alignment of human or zoonotic influenza A virus subtypes. Sequences used for alignment were obtained from the Global Initiative on Sharing All Influenza Data (GISAID) EpiFlu Database (http://www.gisaid.org) and NCBI GenBank (see supplemental files 4 to 7).
Analytical sensitivity and specificity.
Serially diluted virus culture isolates were used for the determination of the limit of detection (LOD). Virus culture isolates included A/HK/402978/2019 (H1N1), A/HK/797/2019 (H3N2), A/HK/459094/2010 (H5N1), and A/HK/470129/2013 (H7N9). Triplicates were performed for each dilution in two independent experiments.
Analytical specificity (cross-reactivity) was determined by testing genomic DNA or RNA extracted from the culture isolates of influenza B virus, influenza C virus, human adenovirus, rhinovirus, respiratory syncytial virus, human metapneumovirus, human parainfluenza virus types 1 to 4, and the human coronaviruses 229E, NL63, OC43, Middle East respiratory syndrome (MERS), and severe acute respiratory syndrome (SARS). For human coronavirus HKU1, total nucleic acid (TNA) was extracted from a patient specimen. Five microliters of TNA was used for the real-time RT-PCR assays.
PB2, NS, and M gene real-time RT-PCR using clinical specimens.
TNA was extracted using NucliSENS easyMAG (bioMérieux, Marcy l'Etoile, France) as we described previously (6, 9). Briefly, 250 μl of each specimen was mixed with lysis buffer. After extraction, TNA was recovered using 55 μl of elution buffer. Monoplex real-time RT-PCR assays was performed using a QuantiNova Probe RT-PCR kit (Qiagen, Hilden, Germany). The reagent mixture (20 μl) contained 1× QuantiNova Probe RT-PCR master mix, 1× QN Probe RT-mix, 0.8 μM each forward and reverse primer, 0.2 μM probe, and 5 μl TNA as the template. The thermal cycling conditions were 10 min at 45°C for reverse transcription, 5 min at 95°C for PCR initial activation, and 50 cycles of 5 s at 95°C and 30 s at 55°C. All reactions were performed using the LightCycler 480 real-time PCR system (Roche, Basel, Switzerland). The primers and probes are shown in Table 1. For patients with discrepant results between the WHO M gene assay and our in-house PB2 or NS gene assay, we retested with the M gene assay using the same PCR reagents and real-time RT-PCR thermocycling conditions as in the WHO protocol (11).
TABLE 1.
Primers and probes used in the present study for influenza A virus detection
| Target (source) | Primer or probe | Primer or probe sequence (5′–3′) |
|---|---|---|
| PB2 (this study) | Forward primer | GACGTRGTGTTGGTAATGAAAC |
| Reverse primer | GAATYCTTTTGGTCGCTGTCTG | |
| Probe | FAM-AAACGGGACTCTAGYATACTTACTGACAG-IABkFQ | |
| NS (this study) | Forward primer | YGAGGATGTCAAAAATGCARTTG |
| Reverse primer | GCTTCTCCAAGCGAATCTCTGTA | |
| Probe | FAM-CCTCATCGGAGGAYTTGARTGGAATG-IABkFQ | |
| M (CDC) | Forward primer | GACCRATCCTGTCACCTCTGAC |
| Reverse primer | AGGGCATTYTGGACAAAKCGTCTA | |
| Probe | FAM-TGCAGTCCTCGCTCACTGGGCACG-BHQ1 | |
| M (WHO) | Forward primer | CTTCTAACCGAGGTCGAAACGTA |
| Reverse primer | GGTGACAGGATTGGTCTTGTCTTTA | |
| Probe | FAM-TCAGGCCCCCTCAAAGCCGAG-BHQ1 |
Statistical analysis.
Statistical analysis was performed using PRISM 6.0 for Windows. The median threshold cycle (CT) values between PB2 and NS gene RT-PCRs were compared using Student’s t test.
Data availability.
The raw reads, after excluding human reads, have been deposited in the NCBI BioProject database under the BioProject number PRJNA605947. The investigators will share data used in developing the results presented in the manuscript on request. Anonymized record-level data will be made available on proposal for analysis by those who have received ethical clearance from their host institution.
RESULTS
Identification of highly abundant viral gene regions using whole-genome sequencing.
Nanopore sequencing was performed on 18 nasopharyngeal specimens from 13 patients with A(H1N1) and 5 patients with A(H3N2) virus infection and on 1 saliva specimen (from patient 18) (Table S1). All were hospitalized adult patients. The specimens from patients 13 and 18 were collected in 2017 during our previously published study (9). The other 16 specimens were collected in January 2019. The median age was 63.5 years old, ranging from 26 to 91 years old. Among these 18 patients, 8 (44.4%) were female, 8 (44.4%) required oxygen supplement, 5 (27.8%) were admitted to an intensive care or coronary care unit, and 1 (5.6%) died.
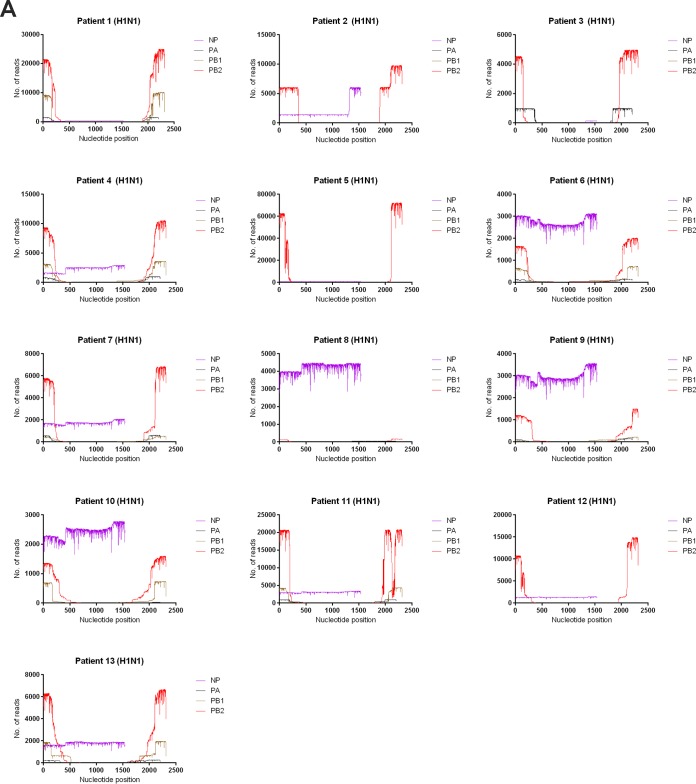
The number of reads is shown in coverage maps (Fig. 1 and 2). We focused on the internal genes because we were searching for conserved gene targets. The NS gene was highly abundant for all patients. For M and nucleoprotein (NP) genes, the number of reads was constant throughout the genome for most patients. For 9 of 18 patients (50%), the number of reads mapped to the NS gene was higher than that for the M gene (Fig. 1A and B). Therefore, the NS gene was chosen as an RT-PCR target.
FIG 1.
Coverage map for all internal gene segments in the nanopore sequencing. The x axis shows the nucleotide position, and the y axis shows the number of reads.
FIG 2.
Coverage map for NP and polymerase genes. The x axis shows the nucleotide position, and the y axis shows the number of reads.
For the polymerase genes, most reads were located in the 5′ UTR and 3′ UTR. Among these polymerase genes, the PB2 gene has the highest number of reads for all 13 patients with A(H1N1) and for 1 patient with A(H3N2), while the polymerase acid (PA) gene has the highest number of reads for 3 patients with A(H3N2) (Fig. 2A and B). For 9 patients with A(H1N1) and 3 patients with A(H3N2), the number of reads at the 5′ or 3′ end was higher for PB2 than for the NP gene. Hence, the PB2 gene was chosen as an RT-PCR target.
For patient 18, NP, NS, and the 5′/3′ ends of PB2 genes are highly expressed in both NPA and saliva. The NP gene segment was relatively more abundant for the NPA specimen, while the 5′/3′ end of the PA gene segment was relatively more abundant for the saliva specimen.
In silico analysis of our primers and probes for human and avian influenza viruses.
To assess whether the novel NS and PB2 RT-PCR can detect seasonal and zoonotic influenza viruses, we aligned the primers and probes with viral sequences that are available at GISAID and NCBI GenBank. Our NS and PB2 primers and probes were well matched with seasonal A(H1N1) and A(H3N2) influenza viruses and with H5, H6, H7, H9, and H10 influenza A viruses that were isolated from humans, especially at the 3′ ends of the primers (see Fig. S1 to S6 and Table S3 in the supplemental material). These primers and probes do not have homology with noninfluenza viruses.
Analytical sensitivity and specificity.
The LOD was determined for seasonal influenza A (H1N1 and H3N2) and the two most common avian influenza viruses affecting humans (H5N1 and H7N9). PB2 and NS RT-PCR had a lower LOD than WHO or CDC M gene RT-PCR for A(H3N2) virus, while PB2 RT-PCR had a lower LOD than all other RT-PCRs for A(H7N9) virus. The LODs for A(H1N1) and A(H5N1) were the same for all RT-PCR assays (Table 2). PB2 and NS RT-PCR assays showed no cross-reaction with other respiratory viruses.
TABLE 2.
Analytical sensitivity of different RT-PCR assays
| Target gene | Limit of detection (TCID50/ml)a
|
|||
|---|---|---|---|---|
| H1N1 | H3N2 | H5N1 | H7N9 | |
| PB2 | 4.22 | 0.56 | 0.0005 | 0.003 |
| NS | 4.22 | 0.56 | 0.0005 | 0.03 |
| M (WHO) | 4.22 | 5.62 | 0.0005 | 0.03 |
| M (CDC) | 4.22 | 5.62 | 0.0005 | 0.03 |
TCID50, 50% tissue culture infective dose.
Validation of PB2 and NS RT-PCR using clinical specimens.
To evaluate the use of NS and PB2 RT-PCR in clinical settings, we compared the performance of NS and PB2-RT-PCR assays with two M gene RT-PCR assays (WHO and CDC). The clinical specimens included 80 nasopharyngeal and 80 saliva specimens that had previously tested positive for seasonal influenza A virus and 80 nasopharyngeal and 80 saliva specimens that had previously tested negative for seasonal influenza A virus. PB2, NS, and CDC M gene RT-PCR yielded concordant results for all 320 specimens. There was one saliva specimen in which the WHO M gene RT-PCR was negative but that had tested positive with all other RT-PCR assays. The median CT value was significantly less for PB2 gene RT-PCR than that for NS gene RT-PCR (Table 3). To further determine the utility of the PB2 or NS RT-PCR assays, we identified 10 hospitalized patients with sequential nasopharyngeal specimens sent for influenza A virus detection for whom the first specimen tested negative but the subsequent specimen tested positive for influenza A virus using the M gene real-time RT-PCR. We retested the first nasopharyngeal specimens with PB2, NS, and M gene RT-PCR. The interval between the first and subsequent positive specimens was 1 to 8 days (Table 4). Out of these 10 patients, 2 (20%) were NS and/or PB2 gene RT-PCR positive, while the M gene RT-PCRs (CDC or WHO) were all negative. For the 3 specimens that were negative in the WHO M gene RT-PCR but positive in our PB2 RT-PCR, we repeated the WHO RT-PCR with the exact reagent and cycling conditions as described in the original protocol, and all tested negative.
TABLE 3.
CT values of the PB2 gene and NS genea
| RT-PCR | H1N1 or H3N2 (n = 160) | H7N9 (n = 10) |
|---|---|---|
| Median CT value (interquartile range) | ||
| PB2 gene | 25.79 (21.3–30.42) | 28.85 (24.78–31.29) |
| NS gene | 26.21 (21.58–31.67) | 29.94 (27.15–32.97) |
| Comparison of CT values (P values) | ||
| PB2 versus NS | <0.0001 | 0.001 |
For the purpose of statistical calculation, a CT value of 43 was assigned if one was not detected.
TABLE 4.
Retesting of specimens negative for influenza A virus RT-PCR in patients with confirmed influenza A virus infectiona
| Patient no. | Time interval between 1st specimen and subsequent positive specimen (days) |
CT value for: |
|||
|---|---|---|---|---|---|
| PB2 | NS | M (CDC) | M (WHO) | ||
| 201901 | 3 | ND | ND | ND | ND |
| 201902 | 8 | ND | 39.96 | ND | ND |
| 201903 | 3 | ND | ND | ND | ND |
| 201904 | 1 | ND | ND | ND | ND |
| 201905 | 1 | ND | ND | ND | ND |
| 201906 | 1 | ND | ND | ND | ND |
| 201907 | 1 | ND | ND | ND | ND |
| 201908 | 8 | ND | ND | ND | ND |
| 201909 | 5 | ND | ND | ND | ND |
| 201910 | 5 | 38.19 | 37.46 | ND | ND |
ND, not detected.
We also retrieved 10 archived respiratory tract specimens that were positive for A(H7N9) virus. For all 10 specimens, both PB2 and NS RT-PCR results were positive. There was no significant difference in the median CT values between the PB2 and NS RT-PCRs (Table 2).
DISCUSSION
We successfully designed novel RT-PCR assays which were able to detect influenza A virus in clinical specimens that were missed by M gene RT-PCR. First, we chose the NS and PB2 genes as the gene targets for RT-PCR assays because a large number of reads were mapped to these genes in the nanopore sequencing of clinical specimens. Second, in silico analysis showed that our newly designed NS and PB2 primers and probes can target different subtypes of seasonal and zoonotic influenza A viruses. Third, our new NS and PB2 RT-PCRs have lower LODs for contemporary seasonal and avian influenza A viruses that have caused human infections. Fourth, we validated our NS or PB2 RT-PCR assays on nasopharyngeal or saliva specimens from patients with seasonal or avian influenza A virus infection. Finally, our PB2 or NS RT-PCR successfully detected influenza A virus specimens that were missed previously by standard M gene RT-PCR. Hence, NS and PB2 genes are suitable alternative targets for influenza A virus RT-PCR.
Since our primary goal was to develop RT-PCR assays for clinical use, we specifically selected clinical specimens instead of virus culture isolates for nanopore sequencing. Moreover, the panel of clinical specimens encompassed patients with different influenza subtypes, demographics, and clinical severity. Indeed, there were notable differences in the gene abundance among different patients. For example, the NP gene was highly abundant for some patients but almost undetectable for others.
The polymerase genes are conserved among influenza A viruses but are rarely used for diagnosis because of the low level of polymerase gene RNA detected using traditional methods (13). Polymerase genes with internal deletions, also known as defective interfering genes (DIG), are frequently found during influenza A virus infection (26). These DIGs have been shown to inhibit viral replication and modulate the immune response (26, 27). Due to the presence DIGs, the 5′ and 3′ ends of the polymerase genes are much more abundant than the internal region. For patients with A(H1N1)pdm09 virus infection, Saira et al. showed that only the 5′ or 3′ end of the polymerase genes were abundant, with few reads in the internal region (28). Using long-read sequencing, we previously showed that >90% of the reads mapping to PB2, PB1, or PA were defective interfering (DI) sequences for patients with A(H7N9) virus infection (19). In this study, we designed primers and probes to target the 5′ end of the PB2 gene because the number of reads at the 5′ end of PB2 was much higher than at those of PA and PB1 for most patients. Since DIGs are also found in other viruses, our strategy in choosing primers and probes can be generalized to other viruses that also carry DIGs with large deletions.
In addition to PB2, we also found that the NS gene is highly expressed, and the NS gene RT-PCR assay was positive for all specimens that were positive with M gene RT-PCR. However, the median CT value was slightly greater for NS gene than for PB2 gene RT-PCR. The NS gene codes for NS1 and NS2 proteins. NS1 is an antagonist of interferon, while NS2 is involved in exporting the viral ribonucleoprotein from the nucleus to the cytoplasm. Live attenuated virus with deletion of NS1 has been used successfully as a vaccine platform (29). In a previous study, the mutation rate for the influenza virus NS gene is similar to that of the M gene (30). We designed our primers and probes to detect both human and zoonotic influenza A virus subtypes. Hence, we chose gene regions that are conserved among different subtypes. Furthermore, we avoided any mismatches at the 3′ end of the primers because such mismatches can severely affect the sensitivity of detection (31).
There are several limitations in this study. First, we did not perform nanopore sequencing for influenza A viruses other than A(H1N1) and A(H3N2). Second, we did not test any influenza viruses that were isolated from nonhuman samples. Our PB2 and NS RT-PCR should be further evaluated in a veterinary setting. Third, the quantitation using next-generation sequencing can be affected by several factors, such as bias during RT-PCR, nanopore sequencing, or bioinformatics analysis. However, a previous study showed that that quantitative analysis of cDNA using nanopore sequencing has similar performance as that of PacBio RS II and Illumina HiSeq 2500 (32).
In this study, we demonstrated that PB2 and NS RT-PCR assays are suitable alternatives to the recommended M gene RT-PCR. With improving technology in microfluidic and multiplex PCR, simultaneous detection of multiple gene targets can now be performed quickly and easily and is currently used in some commercial assays. The combination of different gene targets may enhance the accuracy for surveillance of influenza A viruses in both humans and animals.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge Joy-Yan Lam for assistance in processing clinical specimens. We gratefully acknowledge the originating and submitting laboratories that contributed sequences used in the phylogenetic analysis to GISAID (supplemental files 4 to 7).
C.C.-Y.Y., W.-M.C., J.D.I., R.W.-S.P., K.-H.C., K.-H.K., and K.K.-W.T. designed the study. C.C.-Y.Y., W.-M.C., J.D.I., C.W.-M.S., and K.-H.L. acquired the data. K.K.-W.T. carried out the statistical analysis. All authors interpreted the data, revised the manuscript critically for important intellectual content, and approved the final report.
We declare no conflict of interest.
This study was supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for the Department of Health of the HKSAR Government and by donations from the Shaw Foundation Hong Kong, Richard Yu and Carol Yu, Michael Seak-Kan Tong, the Respiratory Viral Research Foundation Limited, and The Hui Ming, Hui Hoy, and Chow Sin Lan Charity Fund Limited.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2019. Global influenza strategy 2019-2030. https://apps.who.int/iris/handle/10665/311184. Accessed 25 April 2019.
- 2.Cheng VC, To KK, Tse H, Hung IF, Yuen KY. 2012. Two years after pandemic influenza A/2009/H1N1: what have we learned? Clin Microbiol Rev 25:223–263. doi: 10.1128/CMR.05012-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D, Li Y, Yao X, Zhang Y, Wu H, Zheng S, Diao H, Xia S, Zhang Y, Chan KH, Tsoi HW, Teng JL, Song W, Wang P, Lau SY, Zheng M, Chan JF, To KK, Chen H, Li L, Yuen KY. 2013. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.To KK, Tsang AK, Chan JF, Cheng VC, Chen H, Yuen KY. 2014. Emergence in China of human disease due to avian influenza A(H10N8)–cause for concern? J Infect 68:205–215. doi: 10.1016/j.jinf.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 5.To KK, Chan JF, Chen H, Li L, Yuen KY. 2013. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: a tale of two cities. Lancet Infect Dis 13:809–821. doi: 10.1016/S1473-3099(13)70167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To KKW, Chan KH, Ho J, Pang PKP, Ho DTY, Chang ACH, Seng CW, Yip CCY, Cheng VCC, Hung IFN, Yuen KY. 2019. Respiratory virus infection among hospitalized adult patients with or without clinically apparent respiratory infection: a prospective cohort study. Clin Microbiol Infect 25:1539–1545. doi: 10.1016/j.cmi.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman LA, Kieke B, Irving S, Shay DK, Vandermause M, Lindstrom S, Belongia EA. 2011. Comparison of influenza vaccine effectiveness using different methods of case detection: clinician-ordered rapid antigen tests vs. active surveillance and testing with real-time reverse-transcriptase polymerase chain reaction (rRT-PCR). Vaccine 29:387–390. doi: 10.1016/j.vaccine.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 8.To KK, Lu L, Yip CC, Poon RW, Fung AM, Cheng A, Lui DH, Ho DT, Hung IF, Chan KH, Yuen KY. 2017. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg Microbes Infect 6:e49. doi: 10.1038/emi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To KKW, Yip CCY, Lai CYW, Wong CKH, Ho DTY, Pang PKP, Ng ACK, Leung KH, Poon RWS, Chan KH, Cheng VCC, Hung IFN, Yuen KY. 2019. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect 25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S 3rd, Theel ES, Thomson RB Jr, Weinstein MP, Yao JD. 2018. A guide to utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 67:e1–e94. doi: 10.1093/cid/ciy381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. 2017. WHO information for the molecular detection of influenza viruses. http://www.who.int/influenza/gisrs_laboratory/WHO_information_for_the_molecular_detection_of_influenza_viruses_20171023_Final.pdf. Accessed 15 August 2018.
- 12.Stellrecht KA. 2018. The drift in molecular testing for influenza: mutations affecting assay performance. J Clin Microbiol 56:e01531-17. doi: 10.1128/JCM.01531-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatada E, Hasegawa M, Mukaigawa J, Shimizu K, Fukuda R. 1989. Control of influenza virus gene expression: quantitative analysis of each viral RNA species in infected cells. J Biochem 105:537–546. doi: 10.1093/oxfordjournals.jbchem.a122702. [DOI] [PubMed] [Google Scholar]
- 14.Stellrecht KA. 2018. History of matrix genes mutations within PCR target regions among circulating influenza H3N2 clades over ten-plus-years. J Clin Virol 107:11–18. doi: 10.1016/j.jcv.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Yang JR, Kuo CY, Huang HY, Wu FT, Huang YL, Cheng CY, Su YT, Chang FY, Wu HS, Liu MT. 2014. Newly emerging mutations in the matrix genes of the human influenza A(H1N1)pdm09 and A(H3N2) viruses reduce the detection sensitivity of real-time reverse transcription-PCR. J Clin Microbiol 52:76–82. doi: 10.1128/JCM.02467-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overmeire Y, Vanlaere E, Hombrouck A, De Beenhouwer H, Simons G, Brink A, Van den Abeele AM, Verfaillie C, Van Acker J. 2016. Severe sensitivity loss in an influenza A molecular assay due to antigenic drift variants during the 2014/15 influenza season. Diagn Microbiol Infect Dis 85:42–46. doi: 10.1016/j.diagmicrobio.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Ronkko E, Ikonen N, Kontio M, Haanpaa M, Kallio-Kokko H, Mannonen L, Lappalainen M, Julkunen I, Ziegler T. 2011. Validation and diagnostic application of NS and HA gene-specific real-time reverse transcription-PCR assays for detection of 2009 pandemic influenza A (H1N1) viruses in clinical specimens. J Clin Microbiol 49:2009–2011. doi: 10.1128/JCM.00259-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petersen LM, Martin IW, Moschetti WE, Kershaw CM, Tsongalis GJ. 2019. Third-generation sequencing in the clinical laboratory: exploring the advantages and challenges of nanopore sequencing. J Clin Microbiol 58:e01315-19. doi: 10.1128/JCM.01315-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lui WY, Yuen CK, Li C, Wong WM, Lui PY, Lin CH, Chan KH, Zhao H, Chen H, To KKW, Zhang AJX, Yuen KY, Kok KH. 2019. SMRT sequencing revealed the diversity and characteristics of defective interfering RNAs in influenza A (H7N9) virus infection. Emerg Microbes Infect 8:662–674. doi: 10.1080/22221751.2019.1611346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, Lijmer JG, Moher D, Rennie D, de Vet HC, Kressel HY, Rifai N, Golub RM, Altman DG, Hooft L, Korevaar DA, Cohen JF, Group S. 2015. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.To KKW, Chan WM, Li KSM, Lam CSF, Chen Z, Tse H, Lau SKP, Woo PCY, Yuen KY. 2017. High prevalence of four novel astrovirus genotype species identified from rodents in China. J Gen Virol 98:1004–1015. doi: 10.1099/jgv.0.000766. [DOI] [PubMed] [Google Scholar]
- 22.Zhou B, Donnelly ME, Scholes DT, St George K, Hatta M, Kawaoka Y, Wentworth DE. 2009. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza A viruses. J Virol 83:10309–10313. doi: 10.1128/JVI.01109-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanfear R, Schalamun M, Kainer D, Wang W, Schwessinger B. 2019. MinIONQC: fast and simple quality control for MinION sequencing data. Bioinformatics 35:523–525. doi: 10.1093/bioinformatics/bty654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vignuzzi M, Lopez CB. 2019. Defective viral genomes are key drivers of the virus-host interaction. Nat Microbiol 4:1075–1087. doi: 10.1038/s41564-019-0465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, To KKW, Chu H, Ding Q, Zhao X, Li C, Shuai H, Yuan S, Zhou J, Kok KH, Jiang S, Yuen KY. 2018. Dual-functional peptide with defective interfering genes effectively protects mice against avian and seasonal influenza. Nat Commun 9:2358. doi: 10.1038/s41467-018-04792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saira K, Lin X, DePasse JV, Halpin R, Twaddle A, Stockwell T, Angus B, Cozzi-Lepri A, Delfino M, Dugan V, Dwyer DE, Freiberg M, Horban A, Losso M, Lynfield R, Wentworth DN, Holmes EC, Davey R, Wentworth DE, Ghedin E, INSIGHT FLU002 Study Group, INSIGHT FLU003 Study Group. 2013. Sequence analysis of in vivo defective interfering-like RNA of influenza A H1N1 pandemic virus. J Virol 87:8064–8074. doi: 10.1128/JVI.00240-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang P, Zheng M, Lau SY, Chen P, Mok BW, Liu S, Liu H, Huang X, Cremin CJ, Song W, Chen Y, Wong YC, Huang H, To KK, Chen Z, Xia N, Yuen KY, Chen H. 2019. Generation of DelNS1 influenza viruses: a strategy for optimizing live attenuated influenza vaccines. mBio 10:e02180-02119. doi: 10.1128/mBio.02180-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. 2008. The genomic and epidemiological dynamics of human influenza A virus. Nature 453:615–619. doi: 10.1038/nature06945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stadhouders R, Pas SD, Anber J, Voermans J, Mes TH, Schutten M. 2010. The effect of primer-template mismatches on the detection and quantification of nucleic acids using the 5′ nuclease assay. J Mol Diagn 12:109–117. doi: 10.2353/jmoldx.2010.090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oikonomopoulos S, Wang YC, Djambazian H, Badescu D, Ragoussis J. 2016. Benchmarking of the Oxford Nanopore MinION sequencing for quantitative and qualitative assessment of cDNA populations. Sci Rep 6:31602. doi: 10.1038/srep31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw reads, after excluding human reads, have been deposited in the NCBI BioProject database under the BioProject number PRJNA605947. The investigators will share data used in developing the results presented in the manuscript on request. Anonymized record-level data will be made available on proposal for analysis by those who have received ethical clearance from their host institution.