Short abstract
Objective
Multimodal non-pharmacological interventions have been argued to have the potential to complement current pharmacological approaches to improving quality of life for people living with dementia. The aim of this review was to identify, synthesise and appraise the evidence for the effectiveness of multimodal non-pharmacological interventions for improving cognitive function specifically.
Method
After a comprehensive search strategy including grey literature, 26 studies were reviewed. The inclusion criteria concerned adults with a primary diagnosis of dementia. Studies used two or more different modes of intervention, and measured a cognitive outcome. Due to differences in the conceptualisations of the term ‘multimodal’, a typology of modes and methods was developed to facilitate classification of candidate studies.
Results
Twenty-one group studies and five case studies were found. Group studies used two or three modes of intervention and multiple methods to implement them. Interventions utilised were cognitive, physical, psychological and psychosocial, nutrition, fasting, gut health, sleep hygiene, stress reduction, detoxification, hormonal health and oxygen therapy. Five individual case studies were found in two separate papers. Each personalised patient treatment utilised in-depth assessments and prescribed up to nine different modes. In 19 (90%) of the 21 group comparisons, participants were reported to have cognitive improvements, stability with their dementia or a delay in their decline. The extent of these improvements in terms of meaningful clinical change was variable.
Conclusion
Multimodal non-pharmacological interventions have the potential to complement singular therapeutic approaches by addressing multiple modifiable risk factors currently understood to contribute towards cognitive decline.
Keywords: dementia, cognition, non-pharmacological, multimodal, interventions, treatment, Alzheimer’s disease
Introduction
The latest estimate of people affected by dementia worldwide is 50 million (Pickett et al., 2018), with a further nine million people developing dementia yearly (WHO, 2017). Current narratives suggest that the progression of dementia is inevitable, supported by the results of pharmacological trials which have been less than encouraging (Cummings, Morstorf, & Zhong, 2014). However, regardless of whether improvements in drug efficacy are possible, people with dementia often have other long-term conditions and so additional medication can cause unpleasant interactions with existing regimes. On the other hand, non-pharmacological interventions can provide complementary therapy, offering useful, versatile approaches to improve outcomes for people with dementia (Olazaran et al., 2010).
Whilst a number of abilities are affected in people with dementia, its primary manifestation is through reduced cognitive ability. Global cognition is a broad term covering various cognitive functions including memory, executive functioning (time management, judgement, planning), attention (ability to direct energy to perform the task at hand, organise tasks into a coherent logical pattern), language and communication. Cognitive interventions (CIs) in people with dementia have been classified by Clare and Woods (2004) into three basic types – cognitive stimulation (CS), training (CT) and rehabilitation (CR). According to Buschert et al. (2011), training for specific cognitive functions is less useful for more impaired participants, but stimulation and activation of everyday functions tend to be more meaningful and successful. Overall, studies show evidence of small but consistent effects of CI in improving cognition (Alves et al., 2013). Moreover, evidence suggests that utilising more than one method of CI can incrementally improve cognition of people with dementia (Barban et al., 2016; Cotelli et al., 2014; Lee, Choi, Oh, Sohn, & Lee, 2016; Panerai, 2016).
Another intervention with potential is routine physical exercise (Olazaran et al., 2010). Exercise increases the size of the hippocampus and improves memory in older adults (Erickson et al., 2011). For example, better cognitive scores, after 6 to 12 months of exercise, were found by Ahlskog, Geda, Graff-Radford, and Petersen (2011) who recommended exercise as a disease-modifying treatment. In particular, there is evidence of a beneficial cognitive effect of high intensity exercise interventions (Livingston et al., 2017). In a meta-analysis of 802 patients in 18 randomised controlled trials (RCTs) the combination of aerobic and non-aerobic exercise interventions (offered at both high and low frequency) positively influenced cognition in patients with dementia (Groot et al., 2016).
Another mode worthy of investigation is nutritional modification, with micronutrients, vitamins and antioxidants showing some evidence of attenuating disease progression (Aliev et al., 2013) and improving cognition through mitochondrial energy production and protein synthesis (Troesch, Weber, & Mohajeri, 2016). Proper nutrition is also related to AD through epigenetic pathways, suggesting a potential role in the prevention of late-onset AD and attenuation of cognitive deficits (Athanasopoulos, Karagiannis, & Tsolaki, 2016).
As well as CIs, physical exercise and nutrition, numerous other modes can reduce the risk for dementia or address the symptoms. Whilst several of these are trialled in the included studies, more are emerging from new evidence that nearly 600 factors can potentiate the development of AD (Kostoff, Zhang, Ma, Porter, & Buchtel, 2017). This understanding that dementia is multifactorial and determined by mechanisms that interact and intervene throughout life (Van der Linden & Juillerat Van der Linden, 2016) has given rise to the emergence of multimodal approaches to prevention.
Dementia prevention trials affirm that risk factors can be reduced when using a multimodal approach, as multiple mechanisms may be necessary for clinically significant effects on global cognition (Ngandu et al., 2015). Indeed, activities containing more than one component seem to be more beneficial in reducing risk (Karp et al., 2006). These may exert a synergistic effect, for instance, whereby physical training guided by CT may facilitate the neuroplastic potential to induce beneficial cognitive effects (Bamidis et al., 2015), controlling cognitive decline and improving quality of life (QoL) (Aliev et al., 2013).
Evidence for multimodal approaches also includes combined training for brain plasticity, neurogenesis in the hippocampus and a neuroprotective effect on the cerebral cortex (García-Mesa et al., 2011). For example, Curlik and Shors (2013) found that a combination of physical training followed by successful mental learning was more beneficial for neuronal recruitment and overall mental health than either activity alone. Furthermore, exercise in combination with dietary factors can affect molecular events related to the management of energy metabolism and the synaptic plasticity of cognition according to Gomez-Pinilla (2011). Köbe et al. (2016) found that omega-3 fatty acid intake combined with aerobic exercise and CS prevented atrophy in AD-related brain regions in mild cognitive impairment (MCI) patients.
Given the emergence of multimodal approaches, some reviews have already been conducted. Rodakowski, Saghafi, Butters, and Skidmore (2015) found small improvements in selected cognitive abilities in early stage dementia from a combination of cognitive and physical exercise. Law, Barnett, Yau, and Gray (2014) found significant cognitive function improvement in four out of five studies combining cognitive and exercise training in older adults with cognitive impairment. However, Rodakowski et al.’s (2015) scoping review looked at adults with a range of cognitive impairment. Law et al. (2014) looked only at exercise and CS in adults with and without cognitive impairment. Therefore, it remains to understand the extant evidence more comprehensively.
Three gaps in the literature have been identified which make this review both timely and relevant. Firstly, whilst there is growing interest in non-pharmacological interventions for treating dementia, little is known about the effect of complex interventions in this population. Secondly, evidence exists for people with subjective cognitive impairment (SCI) and MCI, but evidence is lacking for measured cognitive outcomes in studies specifically for people living with dementia. Thirdly, whilst some reviews, such as the two above have been conducted which look at certain combinations of modalities, a thorough systematic review of all possible modality combinations has not been conducted. Consequently, this review provides a synthesis of the evidence for multimodal non-pharmacological interventions (MNPIs) for improving cognitive function for people living with dementia.
Method
Search strategy and screening
A systematic search of peer-reviewed literature was performed on PubMed, PsycINFO, Medline, Scopus, EMBASE, Cochrane Database of Systematic Reviews, CINAHL and LILACS. Search terms included dementia, Alzheimer’s or cognitive impairment and variations of multimodal, treatment, intervention, activity or programme tailored to each database (Online Appendix 1). Grey literature was also searched for theses, dissertations, policy documents and conference proceedings.
Typology development
Multiple terms in the evidence have been used to describe ‘multimodal’ interventions. For this review, a classification system was required to determine how many modes a study utilised and hence, whether it was included. Drawing upon the categories of Choi and Twamley (2013) and Clare and Woods (2004), three initial modes were derived:
Cognitive enhancement therapies (CS, CT and CR; non-invasive brain stimulation);
Physical interventions (physical exercise, physical and occupational rehabilitation); and
Psychological and psychosocial therapies (art, music, dance, cognitive-behavioural therapy (CBT), horticultural therapy (HT), psychotherapy, recreational activities, volunteering, etc.).
Seven further modes emerged from the title and abstract screening (see Table 1).
Table 1.
Typology of modes and methods for dementia interventions – Brief.
| Modes | Methods |
|---|---|
| 1. Cognitive enhancement therapies (Choi & Twamley, 2013; Clare & Woods, 2004) | |
| a. Cognitive therapies, stimulation, rehabilitation and training (CST, ROT, RT, CR, CSPR, CT, BT, DT, GRT, MT) | |
| b. Non-invasive brain stimulation | |
| 2. Physical | |
| a. Physical exercise (HIIT, AE, ST, DT) | |
| b. Physical and occupational rehabilitation (OT, PT, KT, IPP, PE) | |
| 3. Psychological and psychosocial therapies | |
| a. Art, Music, Drama, Dance & Movement, CBT, HT, STH, PMT, Arts & Crafts, Tailored activities, Recreational activities, Spiritual elements, Community activities, volunteering | |
| 4. Nutrition and diet | |
| 5. Sleep hygiene | |
| 6. Stress reduction – Meditation, etc. | |
| 7. Detoxification – Sauna, etc. | |
| 8. Hormonal health | |
| 9. Oxygen therapy – Hyperbaric, Intermittent Hypoxic Training (IHT), Oxygen inhalation | |
| 10. Traditional Chinese Medicine – Acupuncture, Herbs | |
Inclusion criteria
All included studies met the following inclusion criteria:
Involved older people with a primary diagnosis of dementia;
At least two modes of non-pharmacological intervention;
Changes in cognitive function was an outcome measure;
Any study design or setting;
Studies with mixed participant groups (dementia, MCI, etc.) if subgroup analysis (e.g., individuals with dementia) was available;
English language of any date.
Exclusion criteria
Specifically addressed delirium, pain, incontinence or behavioural and psychological symptoms of dementia (BPSD);
If only two modes addressed and one of these delivered training, knowledge or support to care staff or family carers;
One mode was pharmacological treatment. (If participants were taking stable doses of dementia medication prior to the study it was not excluded.)
(A list of acronyms and abbreviations can be found in Online Appendix 4)
Study selection
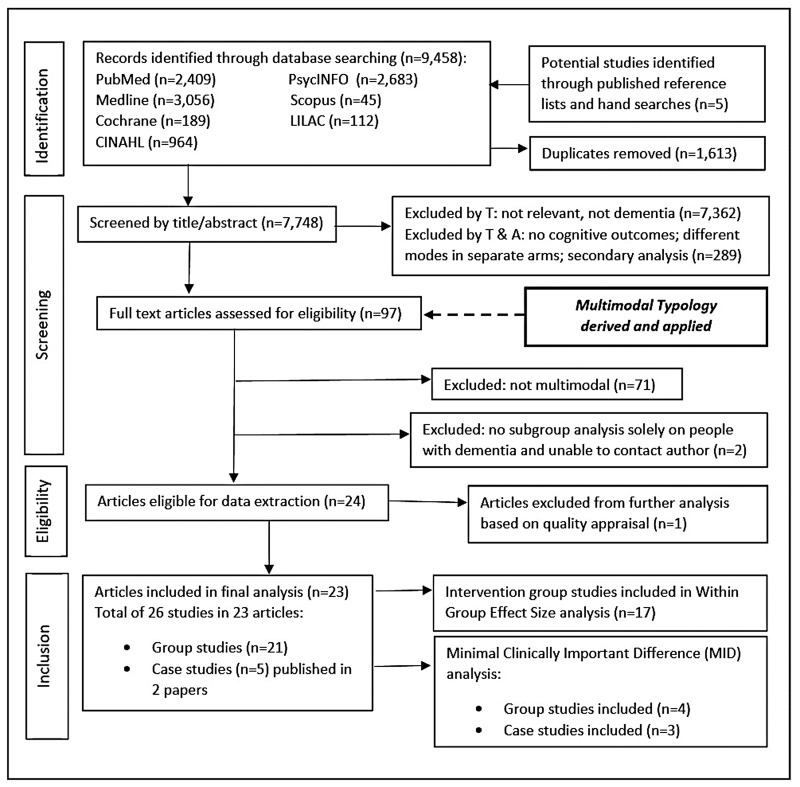
Ninety-seven candidate studies were selected at the title/abstract stage by the first author (GC). The other authors (CM and JS) checked 10% of the studies, discussed and resolved any disagreements. Figure 1 shows the searching, screening and selection process. The team consulted on the modes and methods of intervention. Twenty-four papers were initially included (27 group or case studies) finalising the Typology at 10 modes (Table 1). See Online Appendix 2 for detailed descriptions of modes and methods.
Figure 1.
Flowchart of the searching, screening and selection process.
Included studies are shown in Table 2 with their modes of intervention.
Table 2.
Included studies and their modes.
| Author, Year | Country | Cognitive therapies, Stimulation, Training & Rehabilitation | Physical Exercise, Physical & Occupational Rehabilitation | Psychological & Psychosocial | Fasting | Nutrition – Diet Supplements, Antioxidants | G I Health | Sleep hygiene | Stress reduction | Detoxification | Hormonal health | Oxygen therapy | Total modes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arkin (2007) | USA | • | • | • | 3 | ||||||||
| Baglio et al. (2015) | Italy | • | • | • | 3 | ||||||||
| Bredesen et al. (2016) * | USA | • | • | • | • | • | • | • | • | • | 9 | ||
| Burgener et al. (2008) | USA | • | • | • | 3 | ||||||||
| Christofoletti et al. (2008) | Brazil | • | • | • | 3 | ||||||||
| Coelho et al. (2013) | Brazil | • | • | 2 | |||||||||
| Graessel et al. (2011) | Germany | • | • | • | 3 | ||||||||
| Han et al. (2017) | Korea | • | • | • | 3 | ||||||||
| Ibarria et al. (2016) | Spain | • | • | • | 3 | ||||||||
| Kang et al. (2010) | Korea | • | • | • | 3 | ||||||||
| Kim et al. (2016) | Korea | • | • | • | 3 | ||||||||
| La Rue et al. (2015) | USA | • | • | • | 3 | ||||||||
| Li and Li (2017) | China | • | • | 2 | |||||||||
| Maci et al. (2012) | Italy | • | • | • | 3 | ||||||||
| Onor et al. (2007) | Italy | • | • | • | 3 | ||||||||
| Oswald et al. (2007) | Germany | • | • | • | 3 | ||||||||
| Prokopov (2010)** | Spain | • | • | • | 2 | ||||||||
| Raggi et al. (2007) | Italy | • | • | • | 3 | ||||||||
| Serda i Ferrer and del Valle (2014) | Spain | • | • | • | 3 | ||||||||
| Tay et al. (2016) | Singapore | • | • | • | 3 | ||||||||
| Vicente de Sousa et al. (2017) | Portugal | • | • | 2 | |||||||||
| Viola et al. (2011) | Brazil | • | • | • | 3 | ||||||||
| Yoon et al. (2013) | Korea | • | • | 2 |
*Contains 4 case studies; **Contains 1 case study.
Searching the grey literature revealed increasing public and practitioner interest in the area of non-pharmacological treatments for dementia, including multimodal approaches. However, no findings from the grey literature met the inclusion criteria.
Quality appraisal
Given that both qualitative and quantitative studies were eligible for inclusion in the review, quality appraisal of the peer-reviewed literature utilised the Mixed Methods Appraisal Tool (MMAT), allowing for assessment of qualitative, quantitative and mixed method studies within one measure (Pluye et al., 2011; Souto et al., 2015). A recommended cut-off score of 25% or less excluded lower quality papers from further analysis. At this stage, one paper was excluded (Jian, 1999) out of 24 reducing the total included papers to 23 (See Online Appendix 3: Quality Appraisal).
Data synthesis and analysis
The included studies were found to have one predominant characteristic under which they could be compiled and synthesised. Ten studies in which the intervention mode was predominantly cognitive (therapies, stimulation, rehabilitation or training) are presented in Table 3. Ten further studies in which the intervention was predominantly physical (exercise, occupation, rehabilitation or nutrition) are presented in Table 4. Finally, two case study papers (with a total of 5 individual case studies) in which the intervention mode was personalised treatment in a clinical or research setting are shown in Table 5. Study characteristics included author & year; sample, study design, intervention modes and methods, setting, study length, frequency and time involved.
Table 3.
Study characteristics – Cognitive.
|
Study |
Sample |
Intervention Mode
– CognitiveTherapies, Stimulation, Rehabilitation
& Training |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author & Year | N (IG/CG) | Age (IG/CG) | Sex – %F(IG/CG) | Diagnosis | Study design | Methods | Other Modes and Methods | Setting & Location | Length & Frequency | Weeks &Sessions |
| Burgener et al. (2008) | IG = 24 CG = 19 | 77.9 (7.9)76.0 (8.1) | 46%47% | Early to early-middle stage dementia | Repeated measures experimental, randomised, controlled | Cognitive behavioral therapies (CBT) | Physical: Taiji exercisesSupport group | Living at home: Out-patient clinic | CBT 90 min 2x/wk;Taiji 60 min 3x/wk;SG 90 min 2x/zwk540 min | 40 wks; CBT 80 sessionsTaiji 120 sessionsSG 80 sessions |
| Graessel et al. (2011) | IG = 50CG = 46Follow-up N = 61 31/30 | 84.5 ± 4.585.7 ± 5.7 | IG 88%CG 78.3% | Primary degenerative dementia (not VD); mild-mod | Randomized, controlled, single-blind longitudinal studyPP analysis | MAKS – Cognitive stimulation therapy | –Short spiritual element–ADLs practice –Motor stimulation exercises, creative tasks (work with wood, paper, etc.), gardening | 5 nursing homes | 120 min6x/wk | 48 wks288 sessions |
| Han et al. (2017) | IG = 32 MCICG = 32 DementiaIG N = 55 at follow-up | 77.13 ± 6.58Dementia subgroup | 46.9%Dementia subgroup | 28 AZD3 VaD1 FTD | Multi-center, double-blind, randomized,placebo-controlled (mock therapy MT), two 8 wk periods(4 wk washout) crossover trial | Multimodal Cognitive Enhancement Therapy (MCET)Cognitive trainingCognitive stimulationReality orientationReminiscence therapy | Physical exerciseMusic therapy | Living at home: 4 university hospital research centres | 180 min (30 min PE; 30 min RO; 30 min CT; 30 min break & 60 min of RT, CS & MT in turn)3x/wk | 8 wks24 sessionsCrossover and repeat8 wks |
| Ibarria et al. (2016) | IG = 206 | 75.88(±8.97) (54–93) | 150 women 56 men | Mild (54.2%) tomoderate AD (45.8%) | Descriptive non-random, no-control; Integrated | Psychostimulation Programme (IPP) integrating Cognitive, Motor and Mood-related rehabilitation and stimulation for cognitive functions, such as memory, praxis, language, reasoning, etc. | Exercise – Active and passive gymnastics, personal & spatial orientation, motor coordination, body languageMusic therapyRelaxationOccupational activities to maintain ADLsExpression & creativityBoard gamesCaregivers involvement | Fundació ACE Alzheimer Research Center and Memory Clinic | 8 h/day, 5x/wk, for 1 yr; M-F, 10 to 6. Some attended only 3x/wk; some only half day. Estimated 3 full days, 24 h/wk= 1440 min/wk | The mean of time spent in the IPP programme was 10.2 mos (±3.43) Estimated 24 h/wk x 40.8 wks = 979 h120 sessions |
| Kang et al. (2010) | IG = 20CG = 18 | IG 60%CG 72.2% were 65–79 yrs old | IG 80%CG 100% | Mild dementia; ≤23 on Korean MMSE-K | Quasi-experimental, non-random control group, pre–post-test design | Cognitive stimulation: session consisting of activity involving training aides; concept memory training | Exercise Music therapy Art therapy Horticultural therapy | Living at home: Senior welfare centre | 180 min2x/wk | 9 wks18 sessions |
| Li and Li (2017) | IG = 24CG = 24Final analysis: IG = 19CG = 21 | IG 83.1(±4.1 SD) CG 81.8(±6.7 SD) | IG 63.2%CG 76.2% | IG26.3% Mild47.4 Moderate26.3 Severe dementiaCG 9.5% Mild 61.9% Moderate28.6% Severe | Quasi-experimental, randomised, controlled | Folk recreational programme comprised of: Folk art activities including crafts, drawing, decorating and colouring which were mainly about Chinese tales or traditional festivals. | Games – upper body physical activities like fishing, throwing balls, ring toss, number finding, bowlingMusic activities – favourite folk songsPersonalized training on daily life activity (ADLs) based on their functional levelIndividual activity programme according to their interest and preference, like singing practice of favourite folk songs | Long-term care facility | 40–50 min3x/wk; Individual sessions 30 min 2x/wkArt – TuesdaysGames – FridaysMusic – Sundays | 16 wks48 group sessions32 individual sessions |
| Onor et al. (2007) | IG = 168 patients and carersCG = 168 patients and carers | 60–80 yrsIG 68 ± 6.5CG 72 ± 5.2 | 37.5% | Mild-to-moderate AD | Randomised, controlledpilot study | Integrated rehabilitation programme: Reality Orientation Therapy | – Occupational Therapy:Activities stimulating implicit memory– Reminiscence Therapy: Activities stimulating the memory of events – Caregiver Psychoeducation | Living at home, attending a university rehabilitation programme | 60 min group sessions3x week4 months | Phase 1 – ROT8 wks24 sessionsPhase 2 – 8 wksOT – 12 sessionsRT – 12 sessions |
| Oswald et al. (2007) | Analysis sample after 53% dropouts: IG = 64CG = 73 | IG 83.06 (6.90) CG 82.7 (7.15) | IG 87.5%CG 76.7% | Dementia MMSE scores range from 16.2 to 27.4 SISCO | 12-site controlled trial | Cognitive activation –attention, concentration, speed of processing, storage, memory retrieval, maze tasks, digit/letter cancellation, memory exercises | Physical activation to train psychomotor skills – 20 minutes physical exercise for balance, strength, stretching, coordination, warm-up; psychomotor, tactile experienceSocial interactionRelaxation | 12 Nursing homes | 60 min/ 2x a week (Cognitive 20–30 min; Physical, psychomotor & relaxation 30 min) | 52 wks104 sessions |
| Raggi et al. (2007) | 50 | 76 SD 6.33>50% aged 71–81 | 28% | Admitted with probable AD; diagnosed with mild (30%) mod (40%) severe (30%) | Pre–post-test studyTreatment course depended on MMSE score. | Comprehensive rehabilitation programme MMSE <10: informal and formal ROT.MMSE >10 ROT integrated with daily computerised cognitive training | Some patients and carers underwent support PsychotherapySome met one-on-one with an Activity therapist Mobility deficits were treated with Physical Therapy | Specialised hospital unit; and returned home | MMSE <10: 45 min 2x/day, 7 days/wkMMSE >10: ROT 7x/wkOT 5x/wk | 17 mos overall but duration of the stay varied depending on compliance and clinical requirements. Mean stay in hospital was 26 days (SD 5.52) |
| Yoon et al. (2013) | Two IGs: CA = 9CAE = 11 | 77.9 ± 7.5 70.1 ± 12.2 | Not given | Dementiawith MMSE scores ranging from 16 to 23 | Randomized, two-group, pre–post-test | Cognitive activity (CA) Memory training included sequential memory recall tasks; Three-back verbal working memory | Cycling w exercise (CAE) received the same intervention as the CA group, with the addition of a cycling exercise during their cognitive activity session. Plus conventional Physical Therapy (PT) | Long-term care facility | CA = 20 min 3x/wk + PT 30 min 5x/wk CAE = cycling 20 min 3x/wk+ CA 20 min 3x/wk+ PT 30 min 5x/wk | 12 wksCA: 96 sessionsCAE: 132 sessions |
Table 4.
Study characteristics – Physical.
|
Study |
Sample |
Intervention Mode – PhysicalExercise, Occupation, Rehabilitation & Nutrition |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author & Year | N (IG/CG) | Age (IG/CG) | Sex – %FIG, CG | Diagnosis | Study design | Methods | Other Modes | Other methods | Setting & location | Duration, Frequency | Weeks/Sessions |
| Arkin (2007) | IG N = 24CG CERAD N = 2454th year completers N = 4 | Mean (78.8/75.5) SD (8.0/7.7) | IG = 67%CERAD =60% | Mild to modProbable AD (CERAD) | Controlled, non-random, longitudinal, 4 cohorts of programme completers; students run interventions; database for matched controls | Elders Rehab Exercise – Aerobics (treadmill, bike), stretching, balance & resistance exercises, enriched with memory & language stimulation | Cognitive Psychological Psychosocial | Language-enriched physical fitness w memory & language stimulation; socialising; supervised volunteer work; 1 exercise session per week supervised by caregivers | University Medical Ctr (Wellness Centre); at home and out in community | Exercise minimum time of 1 h, 2x/wk; memory training 1x/wk; Volunteering 1x/wk; total of 300 min | 4 sessions/wk, 10 wks x 2 semesters (Spr & Fall) = 20 wks = 80 sessions |
| Christofoletti et al. (2008) | IG:1 = 17IG:2 = 17CG = 20 | 70.0 ± 1.872.9 ± 2.379.4 ± 2.0 | 65%70%70% | Mixed dementia, moderate stage | Longitudinal randomised controlledIG:2 – only physiotherapy interventions CG: no motor intervention | Physiotherapy – Individual sessions concentrated on kinesiotherapeutic exercises to stimulate strength, balance and … Cognition such as concentrated attention, recognition, immediate memory, working memory & praxis using bars, Bobath balls, elastic ribbons and proprioceptive stimulation plates | Occupational therapyPhysical education ExpressionCreativity | Arts & crafts (picture, paint, draw, embroider) connect motor coordination with cognition. Walking, upper & lower limb exercises stimulate strength, balance, motor coordination, agility, flexibility and aerobic endurance. | Long-term psychiatric institution | 120 min5x/wk | 24 wks120 sessions |
| Coelho et al. (2013) | IG = 14CG = 13 | IG 78.0 ± 7.3CG 77.1 ± 7.4 | Not given | Mild and moderate AD | Non-random controlled | Motor activities and cognitive tasks simultaneously; strength/resistance training, aerobic capacity, flexibility, balance, agility, and concomitantly cognitive activities requiring focused attention, planned organization of the answers, abstraction, motor sequencing, judgment, self-control behaviour and mental flexibility. Two sets of conditions: (i) free gait (single task); and (ii) gait w frontal cognitive task (walking and counting down: dual task). | Motor task (bouncing ball, walking or exercise with weights) combined with a Cognitive task such as finding words according to semantic criteria (animals, fruits, people, flowers…) or reacting to sensory stimuli and verbal commands. | University Department of Physical Education, Biosciences Institute | 60 min 3x/wk; | 16 wks48 sessions | |
| Kim et al. (2016) | IG = 19CG = 14 | 81.9 ± 7.080.9 ± 6.1 | IG 68.4%CG 85.7% | Moderateto severe AD | Single-blind 6-mo RCTMCP group orKEP + MCP group | Physical exercise (KEP) Supervised exercise sessions: warm up, stretching, lower limb aerobics using TERASU-ERUGO, cool-down, relaxation, stretching | Multicomponent Cognitive Program (MCP) | Music therapy, art therapy, handicraft, horticulture therapy, recreational therapy, laughing therapy and activity therapy. | Nursing home | MCP: 60 min, 2x/day, 5x/wk = 10x/wkKEP: 60 min 5x/wk | 24 wks (6 months) MCP: 240 sessionsKEP: 120 sessions |
| La Rue et al. (2015) | IG = 64N = 29 at 1st follow-up | 92% ≥ 70 | 56% | AD = 42, 66% Non AD Dem = 17, 26%; MCI/pending = 5, 8% | 1-arm trial of a quasi-experimental design, no control; 1st follow-up N = 28 42 wks/11 mos. N = 7 in 2nd follow-up avg 82 wks/20 mos | Language-Enriched Exercise Plus Socialization (LEEPS) Programme: combines physical exercise with cognitive-linguistic stimulation | Cognitive Psychological Psychosocial | Participant & volunteer meet for exercise plus language stimulation and for a social outing or volunteer work | University Wellness Center, at the person’s own home and in the community | Exercise + Language session, 90 min, 1x/wk; outing or volunteer work, 1x/wk | 44 wks/11 mos. Exercise + lang. sessions 27.5 (9.7) Social/volunteer Sessions 6.86 (9.14) |
| Maci et al. (2012) | IG = 7CG = 7 | IG 75.0 ± 12.3CG 70.3 ± 5.8 | 57%57% | Mild to mod AD, MMSE 16–24 | Pilot study, random, controlled, pre-test post-test | GAIA: Physical activity, mental stimulation and socialisation. Physical exercises included mild intensity aerobics, exercises for balance and gait, eye-hand coordination, segmental coordination, respiration and muscle trophism. All exercises were performed every day. | Cognitive Social | Cognitive stimulation activities related to enhancement of spatio-temporal orientation, memory, executive skills and language. Socialisation was encouraged during transport/intervals and at the end of the morning during the group discussion. | University gymnasium and whilst travelling as a group enroute from home to gymnasium and back again | 240 min, 5x/wk: 60 min physical activity60 min cognitive stimulation; 30 min group discussion; 60 min transport to and from home | 12 wks60 sessions |
| Serdà i Ferrer and del Valle (2014) | IG = 64 | Mean 75.53 SD 6.28 (64–87 years) | 54.69% | ADMild 29.69%Mod 31.25%Sev 39.06% | Quasi-experimental, non-controlled, random (selected at random based on their clinical records); 7 groups of 8–10 participants grouped by dementia severity | Rehabilitation Programme – multicomponent/ modular therapy to rehabilitate the motor, cognitive, affective, and social dimensions. Three categories of tasks: physical exercise, cognitive re-education and psychomotor stimulation, with the social dimension throughout. Physical exercise or meaningful recreational activity is linked or combined with cognitive exercises so physical activity indirectly activates cognitive functions. Social cognitive training exercises aimed to bridge cognitive and procedural motor activation. | Physical: Aerobics, resistance, balance, strength; Cognitive: Memory, attention, orientation, language, symbolism, decision-making, calculation and comprehension. Psychomotor: Basic, perceptual and neuromotor functions. Relaxation & feedback | Day hospitals in the Alt Empordà and Baix Empordà districts ofCatalonia (Spain) | 60 min2x/wk | 12 mos80 sessions | |
| Tay et al. (2016) | IG = 39 | 79 ± 6.2 years | 43.6% | Mild dementiaMixed types | Prospective cohort study | Combined cognitive stimulation and physical exercise programme (MINDVital) on gait performance under single- and dual-task conditions; (1) Multicomponent physical exercise programme (45 min) (2) Cognitive stimulation and rehabilitation (60 min) (3) Art therapy as part of the cognitive intervention to stimulate cognitive, emotional and interpersonal skills (4) Tailored individualized activities delivering person centred care (30 min) | (1) Light aerobics, resistance, range of motion, balance training (2) Social and mental activities for spatial and temporal orientation, language and memory (3) Expressive techniques, art therapy, non-verbal expression (4) Engage in an enjoyable activity such as iPAD games, calligraphy. | Outpatient Geriatric Clinic | 180 min1x/wk | 8 wks cycles 2 cycles each 16 weeks16 sessions | |
| Vicente de Sousa et al. (2017) | IG,1 = 25CG = 43IG,2 = 11 This 2nd IG (NSPRG) in the day centre was our focus | NSG 77.8 (7.2) NSPRG 80.0 (6.4) | NSG60%NSPRG36%CG65% | Mild to moderate AD | Prospective, randomized controlled trial with IG & CG; A further IG performed a nutritional supplementation psychomotor rehabilitation programme (NSPRG). This IG was a convenience sample with no control. Follow-up lasted 180 days. | Oral Nutritional Supplementation (ONS) Small volume high-protein energy-dense liquid ONS (125 mL) containing 300 kcal/d 12 g protein, 37.1 g carbohydrates and 11.6 g fat. Available in 4 flavours. Caregivers recorded the amount consumed. | Psychomotor Rehabilitation Programme including a multicomponent modular therapy programme | Targeted objectives consisted of: attentional tasks, strength, tonicity, static and dynamic balance exercises, body awareness, spatial and temporal restructuration, immediate and working memories and praxis, fine motor skills, and gross motor skills. | Day care centre | 60 min2x wk | 3 wks12 sessions |
| Viola et al. (2011) | IG = 25CG = 16 and their carers | Patients: Avg age 75Caregivers: Mean age 51.6 | IG 64% CG 62% | Mild ADCDR = 0.5IG = 9 CG = 7 CDR = 1.0IG = 16CG = 9 | Single-blind, controlled; Four intervention groups were formed, N = 12 plus their caregivers | Multidisciplinary cognitive rehabilitation programme; Group sessions included memory training, computer-assisted cognitive stimulation, rehabilitation and cognitive training to improve attention, memory, spatial and temporal orientation, and self-adaptations to cognitive impairment. | Art therapy Occupational therapyPhysiotherapy Physical trainingSpeech therapyCognitive stimulationReading Logic gamesCaregiver interventions | Expressive activities such as writing and art, painting to stimulate cognitive, emotional, and interpersonal skills through expressive and artistic techniques; develop resources and strategies to complete functional goals, train ADLs; improve balance, prevent falls; enhance communication; improve concentration, rapid thinking, decision-making, etc. | University-based day-hospital memory facility | 300 min (5 h) 2x/wk | 12 wks24 sessions |
Table 5.
Study characteristics – Personalised treatment – N of 1.
|
Study |
Sample |
Intervention Mode – Personalised Treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author & Year | N (IG/CG) | Age (IG/CG) | Sex – %F IG, CG | Diagnosis | Study design | Primary method | Other Modes | Methods | Setting & location | Frequency | Weeks/ Sessions |
| Bredesen et al. (2016) | N = 4 Case studies: Patient 2 Patient 6Patient 7Patient 9 | P2 – 69P6 – 74P7 – 62P9 – 54 | MMMF | AD type 2 (atrophic) AD type 1 (glycotoxic) AD type 1 AD type 3 | Programmatic, personalised approach | MEND Protocol – Nutrition, diet, vitamins, supplements, herbs, fasting, antioxidants Responsive to suboptimal metabolic parameters; continued optimization, iterative treatment and metabolic characterization | SleepStressExerciseBrain stimulationHormonesGI healthToxins | Sleep hygiene, stress reduction, aerobics, strength training, brain training, hormone therapy, intranasal VIP (vasoactive intestinal peptide), address heavy metal toxicity | Laboratory/clinic assessment; Individual sessions; Lifestyle changes at home:Use of life coaches | Variable, repetitive, ongoing; periodic consultations | P2 – 24 monthsP6 – 9 monthsP7 – 10 monthsP9 – 3 months |
| Prokopov (2010) | N = 1 | 78 yrs | 100% | MRI, degenerative changes; progressive mental deterioration; MRI hippocampal, cortical atrophy, enlarged volume of ventricles; typical of AZD-type dementia | Rejuvenative Personalised treatment | Repeated sessions of intermittent hypoxic training (IHT). Patients comfortably relax in a recliner, their cells and mitochondria go through multiple oscillations of pO2. Intermittent oxygen restriction (IOR) is a universal stimulus rapidly triggering multiple compensatory strategies that support genome integrity. IHT is the most ‘engineered’ mitochondria-targeting intervention among IOR protocols. | Individualized vitamins, amino acids, microelements and supplementation consisted of two formulas of Dr Rath’s programme: VitacorAnd Epican ForteNutritional adjustmentFasting | Advised to eat a low-glycaemic-index, low carbohydrate, ketogenic diet, enriched with animal proteins & omega-3 fatty acids. Advised on fasting protocol: limit food intake to within 6–7 h window to extend physiological night fasting time to 18 h | Laboratory/clinicassessment; Individual sessions | IHT 3-4x/wkAfter each cycle of 15 IHT sessions, one month pause, then repeated the whole cycle. | 4 cycles of IHT; 8 mos ofsupplementation programme |
Participants and settings. Studies were from Korea (4), USA (4), Italy (3), Spain (3), Brazil (3), Germany (2), China (1), Portugal (1) and Singapore (1). Participant numbers in the group studies ranged from 14 (Maci et al., 2012) to 206 (Ibarria et al., 2016), with a mean of 58, and an age range of 54–93. Two case studies reported on five patients with dementia, aged 54–78 (Bredesen et al., 2016a; Prokopov, 2010). Participants totalled 1,178, of which 388 in six studies lived in long-term care (nursing home or a psychiatric hospital) and 790 in 16 studies lived at home, attended a day service or were hospitalised for a period of weeks. Two studies (Onor et al., 2007; Viola et al., 2011) also enlisted the caregivers as participants during the intervention.
Pharmacology. Some participant groups were on stable doses of memantine, cholinesterase inhibitors and/or antidepressants prior to, and during, the studies although no study declared participants to be drug-naïve. Individualised treatment programmes that purported to address the root cause of the dementia symptoms (Bredesen et al., 2016; Prokopov, 2010) prescribed pharmaceutical adjuncts such as bio-identical hormones to address specific imbalances.
Study designs. Whilst the methodology was designed to be inclusive of qualitative and mixed methods studies, all studies meeting the inclusion and quality criteria were quantitative in design, but quite heterogeneous. Whilst all carried out pre–post-tests, only 11 (48%) were RCTs (Quality Appraisal 84%). A further five were non-randomised (QA 100%) and seven were quantitative descriptive (QA 100%) (see Quality Appraisal in Online Appendix 3). Han et al. (2017) was the only double-blind or cross-over trial. Three were conducted across multiple centres: Graessel et al. (2011) and Oswald, Gunzelmann, and Ackermann (2007) in German nursing homes, and Serdà i Ferrer and del Valle (2014) in Spanish day hospitals. For characteristics of all studies, see Tables 3 to 5.
Intervention modes and methods. Li and Li (2017) had no cognitive mode, and Prokopov (2010) and Vicente de Sousa et al. (2017) had neither cognitive nor physical mode, but all others had both. Only four lacked a psychological, psychosocial, psychomotor, spiritual, caregiver or support mode. One used oxygen therapy (Prokopov, 2010) and three addressed diet and nutrition and/or fasting (Bredesen, 2016; Prokopov, 2010; Vicente de Sousa et al., 2017). Four studies termed ‘multicomponent’ or ‘dual-task’ combined modes simultaneously and are shown in Table 4 spanning two columns. Methods utilised within the studies illustrated the creative variability of the main modes. Cognitive mode (Table 3) included methods such as therapies, stimulation, rehabilitation and training. Physical mode (Table 4) included methods such as exercise, occupation, rehabilitation and nutrition. Table 5 elucidates multiple methods found in personalised treatment interventions.
Study duration. The length of group intervention ranged from 20 minutes (Yoon et al., 2013) to 8 hours (Ibarria et al., 2016). The shortest timeframe was three weeks (Vicente de Sousa et al., 2017), whereas Arkin (2007) continued a facilitated intervention for up to four years. Personalised N-of-1 treatments were followed-up through clinic visits for 24 months (Bredesen et al., 2016).
Stage and type of dementia. Seven studies included participants with mild dementia, 11 with mild to moderate, three with mild, moderate or severe and one with moderate to severe. Twelve studies included participants with AD whilst 10 included unspecified or mixed types of dementia. A study by La Rue, Felten, and Turkstra (2015) included 8% (N = 5) of participants with MCI. The decision was taken to include this study as >90% of participants fit the inclusion criteria.
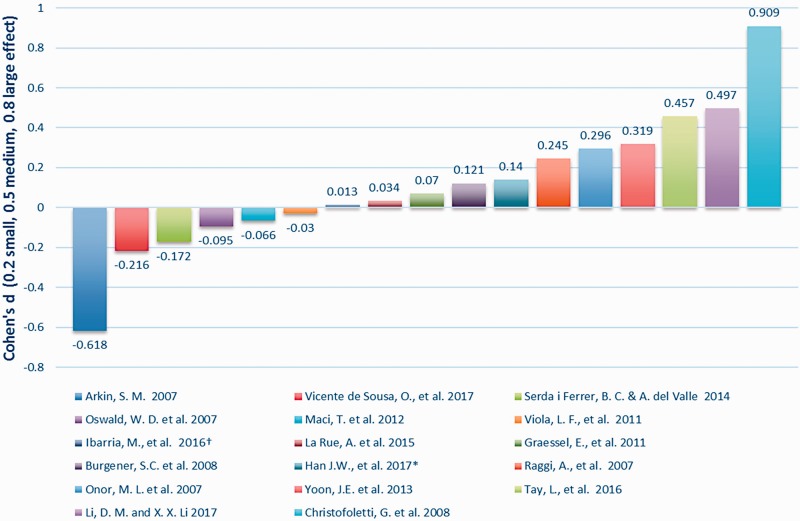
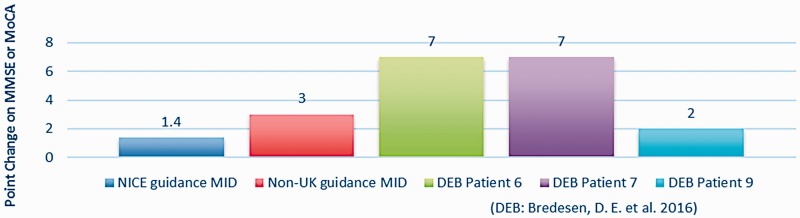
Outcome measures. For cognitive assessment, multiple tools were utilised. Predominantly, 21 studies used the MMSE (English, Korean and Chinese versions), nine used the Clinical Dementia Rating (CDR) and fouir used the ADAS-Cog (Cognitive subscale of the Alzheimer’s Disease Assessment Scale) or ADAS-K (Korean version). Fourteen other scales were used once or twice (see Table 6 for details). Of the 21 group studies, 17 (81%) reported pre–post-test scores using the Mini Mental State Examination (MMSE), the Chinese MMSE or the 7MST (7 Minute Screening Test), and could therefore be included in an effect size (ES) analysis (Figure 2). Oswald et al. (2007) uniquely included a staff survey on residents’ cognitive change. In the five case studies only three provided pre–post-test MMSE or similar which hindered comparison of clinical importance (Figure 4). The case studies in Bredesen et al. (2016) reported over 10 instruments including magnetic resonance imaging (MRI) and fluorodeoxyglucose (FDG)-positron emission tomography (PET) scans, metabolic testing, quantitative neuropsychological testing, genetic testing and a battery of cognitive tests. Whilst three of these case studies reported pre–post-test MMSE, one (Patient 9) reported the Montreal Cognitive Assessment (MoCA) instead, which substituted for the MMSE in the ES analysis. Interestingly, all four group studies that showed clinically important differences used MMSE exclusively (Figure 3).
Table 6.
Cognitive outcomes – Efficacy.
| Study | Country | Cognition tools to diagnose for study inclusion, assessment measures to prescribe treatment | Cognitive efficacy measures, global cognitive function, executive function & attention, memory | Pre-test/Baseline IG mean (SD) CG mean (SD) | Post-test(s) IG mean (SD) CG mean (SD) | Control group activity | Outcomes |
|---|---|---|---|---|---|---|---|
| Arkin (2007) | USA | MMSE; CERAD (7 tests); structured clinical interview; dementia stage determined via CDR; WAIS-R (Picture Completion, Comprehension, and Similarities). Confirmatory diagnostic neurological exam by head of the University of Arizona, Department of Neurology. | CERAD (60 Second Verbal Fluency –category animals; 15-Item Boston Naming, MMSE, Sum of Boxes, Word List Memory, Word List Recall, Constructional Praxis, Word List Recognition); WAIS-R (Picture Completion, Comprehension, and Similarities) | 1 Year Completers – AD Rehab: MMSE (N = 4) IG 23.4 (4.0) CERAD CG MMSE values not given | 1 Year Completers: AD Rehab: MMSE (N = 4) IG 20.5 (5.3) CERAD CG MMSE values not given | Comparison group was a matched group from the CERAD database of untreated AD patients from 1986–1994. | Significant annual decline on MMSE occurred for all cohorts except 4-yr completers; Mean annual decline: 2.9 points for 1-yr completers (n = 24), 2.5 for 2-yr, 2.0 for 3-yr, and 1.0 for 4-yr. Only 42% of the CERAD group (n = 245) had an average annual rate of decline of less than 3 points on the MMSE. This 8% difference was statistically significant (p = .02). There was no significant between-yr decline on 5 or 6 tests of global and cognitive functioning after 2 or more semesters of participation. |
| Bredesen et al. (2016) | USA | MMSE, MoCA, MRI, FDG PET scan, ApoE genotype, online quantitative neuropsychological testing (Brain HQ); extensive metabolic testing such as fasting insulin, haemoglobin A1c, HLADR/DQ, C4a and TGF-β1, anti-thyroglobulin antibodies, anti-thyroid peroxidase antibodies, homocysteine. | MMSE, MRI, MoCA, FDG PET scan, quantitative neuropsychological testing w Neuroquant & Neuroreader, California Verbal Learning Test, Stroop colour test, immediate and delayed recall, semantic knowledge, executive function, processing speed, MFI (phagocytosis index) | P2 FDG PET: Early AD; CVLT-IIB 3rd percentileP6 MMSE 23 MFI = 230P7 MMSE 22P9 MoCA 19 | P2 FDG PET: Early AD; CVLT-IIB 84th percentileP6 MMSE 30MFI >1000P7 MMSE 29P9 MoCA 21 | No control group | P2 – Marked subjective and quantitative neuropsychological testing improvement, decline halted; business reinvigorated, a new business site was added (follow-up 24 mos) P6 – Subjective improvement, MMSE 23->30; MFI >1000 (12 mos) P7 – Subjective improvement, MMSE 22->29 (10 mos) P9 – Clear subjective improvement, modest objective improvement MoCA 19->21 (3 mos) |
| Burgener et al. (2008) | USA | Confirmed diagnosis of irreversible dementia (AZD, Lewy body, vascular, frontal lobe, or mixed dementia); a score <2.0 on the CDR indicating an early to early-middle disease stage. | MMSE; Baseline, 20 and 40 wks. | BaselineIG 24.8 (3.5) CG 22.9 (5.2) | 20 wksIG 25.2 (3.1) CG 22.4 (7.6) 40 wksIG 25.2 (2.4) | Attention-controleducational programmes; delayed 20 wks treatment | Treatment group showed improved cognitive functioning following the 20 wks intervention; Significant differences in MMSE scores was evident for treatment group subjects (+0.4), whereas for control group subjects the scores declined over the first 20 wks of the intervention (−0.5). |
| Christofoletti et al. (2008) | Brazil | MMSE, Brief Cognitive Screening Battery; primary diagnosis of dementia based on ICD-1011 Classification of Mental and Behavioral Disorders; Katz Activities Daily Living Scale | Baseline and 6 mos – MMSE, Brief Cognitive Screening Battery including the Semantic Verbal Fluency Test and the Clock Drawing Test. | IG:1 MMSE18.7 ± 1.7IG:2 MMSE12.7 ± 2.1CG MMSE14.6 ± 1.2 | IG:1 MMSE20.2 ± 1.6IG:2 MMSE14.9 ± 2.2CG MMSE14.8 ± 1.3 | IG:2 – only physiotherapy CG: No motor intervention | MANOVA did not indicate benefits on the cognitive functions between IG:1 and CG (F = 1.1, p > 0.05) and groups IG:2 and CG (F = 1.6, p > 0.05). Univariate analysis indicated some benefits of IG:1 on two specific domains measured by the BCSB (F = 26.5, p < 0.05; F = 4.4, p < 0.05). Global cognition did not improve through treatment, but an attenuation in the decline was observed on two specific cognitive domains. |
| Coelho et al. (2013) | Brazil | Diagnosis of AD according to international criteria Diagnostic and Statistical Manual of Mental Disorders 4th edition APA; a clinical and neuropsychological evaluation carried out by a trained team; CDR was used for the classification of dementia severity; MMSE. | MMSE (IG 19.5 ± 4.1; CG 19.0 ± 2.9) Frontal Assessment Battery, Clock Drawing Test, Symbol Search Subtest | IG FAB total8.6 ± 3.6CG FAB total9.9 ± 3.8 | IG FAB total13.3 ± 3.5CG FAB total8.6 ± 4.4 | Kept to their samedaily routine and did not participate in any regular orstructured exercise programs | Favourable effects on frontal cognitive function in AD patients after the 16-wks period. Frontal Assessment Battery (p < .001) and Symbol Search Subtest (p < .001); significant improvements in abstraction, organization, motor sequencing and attention. The control group worsened significantly in frontal cognitive functions, particularly in planning, organization and motor sequencing. The control group decreased the scores in the Clock Drawing Test (p = .001) and increased the number of counting errors during the dual task (p = .008) after the same period. |
| Graessel et al. (2011) | Germany | Primary degenerative dementia according to ICD-10; <24 on MMSE; confirmed by physician. | Cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog); Baseline and 12 mos | ADAS-Cog subscale IG 32.6 ± 11.5CG 35.6 ± 14.8 | 12 mos IG 32.5 ± 15.3CG 40.8 ± 17.0 | Treatment as usual | Cognitive function and the ability to carry out activities of daily living had remained stable in the intervention group but had decreased in the control patients (ADAS-Cog: adjusted mean difference: −7.7, 95% CI −14.0 to −1.4, p = .018, Cohen’s d = 0.45; E-ADL test: adjusted meandifference: 3.6, 95% CI 0.7 to 6.4, p = .015, Cohen’s d = 0.50). The effect sizes for the intervention were greater in the subgroup of patients (n = 50) with mild to moderate disease (ADAS-Cog: Cohen’s d = 0.67; E-ADL test: Cohen’s d = 0.69). |
| Han et al. (2017) | Korea | Diagnosed with DSM-IV criteria; all patients had a Clinical Dementia Rating (CDR) of 0.5 or 1. | MMSE and ADAS-Cog assessed treatment effects on cognitive function; all outcome measures administered at weeks 0, 9 and 21. | MMSE20.18 ± 4.75ADAS-Cog21.85 ± 9.51 | MMSE20.89 ± 5.36ADAS-Cog20.41 ± 9.66 Change within-group: MMSE0.71 ± 2.27ADAS-Cog−1.44 ± 3.73 | MT – Mock Therapy: health videos, gymnastics exercises, conversing, recreation | In the MCET group, 58.3% and 70.0% of subjects showed improvement in MMSE (effect size = 0.47, p = .013) and ADAS-Cog scores (effect size = 0.35, p = .045), respectively, whereas, in the Mock Therapy group, significantly fewer subjects showed improvement. For the dementia subgroup MCET was more beneficial than MT in global cognitive function measures: the effect between MCET versus MT was 0.71 ± 2.27 versus −0.03 ± 2.78 for the MMSE and −1.44 ± 3.73 vs. −0.21 ± 3.92 for the ADAS-Cog. |
| Ibarria et al. (2016) | Spain | Diagnosis of Probable or Possible AD according to the (NINCDS-ADRDA) criteria; mild to moderate severity of dementia, with a Clinical Dementia Rating (CDR) staging of 1 to 2 and a Global Deterioration Scale (GDS) staging of 4 to 5 | MMSE and ADAS-Cog Baseline, 3, 6, 9 and 12 mos follow-ups | MMSE19.60 (.33 SE) ADAS-Cog25.63 (.59 SE) | MMSE3 mos 19.66 (.30 SE) 6 mos 19.19 (.31 SE) 9 mos 18.63 (.34 SE) 12 mos 17.54 (.35 SE) ADAS-Cog3 mos 25.48 (.58 SE) 6 mos 26.03 (.62 SE) 9 mos 27.18 (.60 SE) p < .0512 mos 29.19 (.67 SE) p < .05 | No control group | Patients remained cognitively stable (MMSE/ADAS-Cog) for at least 6 mos and significantly worsened at 9 and 12 mos follow-ups. The mean annual changes were MMSE (2.06) and ADAS-Cog (3.56) points. 42.7% of patients maintained or improved global cognitive scores between baseline and 12-mo follow-up. The patients who maintained cognitive functions were older than those who did not (77.5 vs. 74.7 yrs). |
| Kang et al. (2010) | Korea | Researchers and RAs conducted cognitive function tests on participants and chose those with MMSE-K ≤23. | Korean MMSE | IG MMSE-K17.78 (14–23) CG MMSE-K21.42 (15–26) | IG MMSE-K22.03 (17–29) CG MMSE-K16.69 (16–25) Change within group: MMSE-KIG 3.00 (0–7) CG −1.00 (−5–7) | Dementia prevention education & consultations | Median cognitive function score in the IG increased from 17 at pre-test to 23 post-test. Cognitive function is greater in the control group and drops over time (21–16), experimental group is lower at the start and increases (17–23) |
| Kim et al. (2016) | Korea | Diagnosis of AD by a neurologist; moderate to severe AD as determined by a baseline MMSE score of ≤20 | ADAS-K; MMSE; CDT; Baseline and 6 mos | MMSEIG 13.4 (±4.2) CG 16.6 (±4.0) (Post-test MMSE scores not given) | Graph Figure 2. P 228. Change scores – MMSE did not show significant improvementbetween groups (F = 0.00, p = .98) | No placebo control, this comparator group received only the MCP (essentially 2 IGs) | There were significant within-group differences for the ADAS-Cog score but not for the MMSE. No cognitive measures improved significantly after 6 mos in the KEP + MCP group compared to MCP group however the ADAS-Cog score was significantly lower between the two groups in secondary analysis adjusted for baseline value, age, sex, and education yr. ADAS-Cog score was significantly lower after 6 mos in the KEP+MCP group than in the MCP group (F = 5.20, p = .03). |
| La Rue et al. (2015) | USA | Physician’s diagnosis of ADRD or Dementia Questionnaire results consistent with probable ADRD and GDS ratings of 3 (mild cognitive impairment to very mild dementia) or 4 (mild dementia). | MMSE, CERAD, WAIS-R, ABCD at baseline and 1st follow-up (11 mos) & 2nd (20 mos) | MMSE N = 28 with 1 follow-up (11 mos): 22.46 (5.07) N = 7 with 2 follow-ups: 23.57 (4.08) | N = 28 1st follow-up: 22.64 (5.48); (71% Same or improved) N = 7 1st follow-up: 25.43 (2.51) N = 7 2nd follow-up: 22.57 (4.79) | No control group | Participants generally remained stable in cognitive function through 1st follow-up (11 mos.) The modal change in the MMSE was a 1-point improvement, and there was no significant change in mean MMSEscores (t = 0.35, df = 27, p = .731, 95% CI = −0.99 to 1.23). N = 7 completed 2nd follow-up (avg 20 mos) performing near baseline levels for cognition – relative stability at nearly 2 yrs. No statistically significant differences between baseline scores and 2nd follow-up on measures of cognition (physical fitness, or well-being) (p ≥ .15). |
| Li and Li (2017) | China | Screened by researchers using CDR >0.5 and MMSE (cut-off score for cognitive impairment was corrected for education: ≤19 for illiterate, ≤22 for primary education, ≤26 secondary education or higher); Physician-diagnosed dementia | MMSE | MMSE IG 14.58 (±5.59) CG 14.48 (±4.40) | MMSEIG 17.00 (±4.03) CG 13.05 (±5.48) | Routine care without any special intervention | For the experimental group, the scores of MMSE and BI had a statistically significant increase after 16 wks (p < .01). Control group, the mean score of MMSE decreased significantly (p < .01) |
| Maci et al. (2012) | Italy | Diagnosis of AD made according to the diagnostic criteria proposed by NINCDS-ADRDA for probable or possible AD; Inclusion criteria: MMSE score 16–24 Mild to Moderate AD | MMSEFAB – Frontal Assessment Battery assessing executive functions (values corrected for age and education); CDR assessing severity of dementia | MMSEIG 17.5 ± 2.7CG 18.2 ± 2.9FAB IG 8.9 ± 2.8FAB CG 7.9 ± 1.9 | 3 mos: MMSEIG 17.3 ± 3.3CG 17.0 ± 2.7FAB IG 9.9 ± 3.1FAB CG 6.9 ± 1.6 p < .05 | Usual activities at home | No significant changes in cognitive performances were observed; participants submitted for 3 mos to the stimulation protocol exhibited a good stability of their cognitive condition; FAB scores rose 1 point in the IG and dropped 1 point in the CG. The MMSE also lowered 1.2 points in controls who displayed a worsening of cognitive abilities. |
| Onor et al. (2007) | Italy | Diagnoses according to the criteria of the DSM-IV and the NINCDS-ADRDA | Milan Overall Dementia Assessment (MODA) was administered at baseline (T0) and after 4 months (T2) of rehabilitation to assess cognitive function. | MMSE T0 – IG 23.12 ± 4.15T0 – CG 20.00 ± 2.20MODA ΔT0–T2IG –0.28 ± 14.17 CG –2.08 ± 10.72 | MMSE 2 mos/4 mosT1 – IG 23.62 ± 4.92 CG 21.25 ± 3.01T2 – IG 24.37 ± 4.30 CG 21.25 ± 2.76 | 8 patients and 8 caregivers in thecontrol group received no form of intervention | No within group difference in MMSE between T0 and T1 and between T0 and T2. A significant difference was found between T1 and T2 (t = −2.393; p = .048). Comparison between CG & IGs: no differences in MMSE scores. MODA results: Comparison between groups and Δ – cognitive performance remained stationary. Multimodal programme had only limited efficacy, maybe due to short duration of the rehabilitation programme. |
| Oswald et al. (2007) | Germany | No cognitive inclusion criteria for participation in the study. MMSE score of >10 or <10 determined assignment to a specific cognitive activation programme | SISCO SIDAM, MMSE, Subtests of the NAI: Number Connection Test ZVT-G + Memory Span ZN-G; picture Test BT. External rating questionnaire administered to nursing staff on residents cognitive performance; Baseline, 6, 12 mos | IG t0 mean s21.80 (5.60) CG21.53 (5.36) | IG t12mean s 21.18 (7.35) CG 17.77 (9.01) | Treatment as usual | Both the MMSE and the SISCO score, a global measure of cognitive performance/impairment, indicate that the general cognitive status of IG participants remained stable, whereas that of CG members deteriorated significantly (p = .001). There was a significant improvement in memory skills that involve dynamic encoding (picture test) in the IG. Nursing staff perceived residents to be more independent in everyday life and to show higher levels of psychological well-being and mental alertness. |
| Prokopov (2010) | Spain | MRI, detailed biomedical history and lifestyle investigation; suffered mental decline for about 1 yr; declining memory, low energy, low-quality sleep; loss of interests/motivations; could no longer conduct her usual activities and home chores; could not hear without a hearing aid; past medical history of moderate hypertension. | MRI, ongoing biomedical monitoring, lifestyle monitoring | Brain magnetic resonance imaging (MRI) in February 2008 showed hippocampal and cortical atrophy, enlarged volume of ventricles. | MRI April 2009showed no degenerative changes. | No control group | Improvement in mood and vitality was noticeable after the first 5 IHT sessions. Gradually, the mental and cognitive state recovered. Patient reported increased energy and activity, better memory and cognition, a slight weight loss, improved sleep, and better mood. The patient gradually recovered her healthy mental state; resumed shopping and cooking and began playing piano again, which she was not capable of doing the previous year. Only needs the hearing aid for a few hours a day, compared to the whole-day use several months before. |
| Raggi et al. (2007) | Italy | Patients with probable AD (mild to severe) diagnosed by a senior neurologist (DSM-IV criteria), CDR, cognitive status MMSE, a structured medical history collected from the patient and the primary caregiver, a neurological examination, routine laboratory analyses, a neuropsychological assessment and neuroimaging studies consistent with an AD diagnosis. Basal assessment by a staff nurse, psychologist and education specialist. | MMSE | MMSE at admission16.06 (SD 5.60) | MMSE at discharge17.54 (SD 6.45) | No control group | The mean MMSE scores at admission and discharge were respectively 16.06 and 17.54 (Wilcoxon Ranks Test: p = .005). Authors contribute the beneficial effect to continuous stimulation, positive group feeling secondary to the programme (ROT) development, and socialization. |
| Serdà i Ferrer and del Valle (2014) | Spain | Neuropsychological diagnosis of AD at any stage of the pathology confirmed by a medical practitioner based on the results of the MMSE and in accordance with standard international diagnostic criteria. | MMSE | MMSE14.65 (SD 5.68) | MMSE13.59 (SD 6.60) | No control group | Results show a significant reduction in cognitive capacity (p < .001). |
| Tay et al. (2016) | Singapore | Diagnosis of early dementia in accordance with the DSM-III-R. | Locally validated Chinese Mini-Mental State Examination (CMMSE) for evaluation of cognitive performance at T0 and at the end of each 8-wk cycle. | CMMSE17.2 ± 4.8 | CMMSE After MINDVital – 119.2 ± 3.9After MINDVital – 219.0 ± 4.1 | No control group | Significant improvements in dual-task walking in early dementia, which may be contributed by improvement in cognitive performance, as single-task gait performance remained stable. Improvement in cognitive performance on CMMSE was evident following the first cycle of MINDVital and sustained through the second cycle, with an estimated 0.9 point improvement in the CMMSE score following each MINDVital cycle (random effects coefficient (SE) of MINDVital cycle on CMMSE = +0.90 (0.31), p = .003)). |
| Vicente de Sousa et al. (2017) | Portugal | Outpatients with AD from the geriatric department of a psychiatric hospital were recruited to the IG and CG. A second IG recruited on a convenience basis from an AD day care center is of interest: NSPRG. | Baseline psychomotor performance scores, clock drawing test (CDT) and MMSE; Follow up at 21, 90 and 180 days (6 mos) | MMSENSPRG 19.3 (5.4) CG 20.0 (4.9) | MMSE 21 days: NSPRG 18.0 (6.6) CG 20.0 (4.9) 90 days: NSPRG 18.0 (6.6) CG 20.0 (4.9) 180 days: NSPRG 17.6 (8.4) CG 20.0 (4.9) | Standard dietetic advice | Cognitive status baseline to 21 days a slight decrease in the MMSE score was observed in the NSPRG −1.2 (3.1) and also on the 180th day of follow-up –1.7 (5.1) p < .05. This compares to the control group showing no declines from baseline 20.0 (4.9). The compliance with the ONS and to the psychomotor rehabilitation programme was excellent, without any refusals or dropouts in both intervention groups, the NSG and the NSPRG. |
| Viola et al. (2011) | Brazil | Diagnoses according to NINCDS-ADRDA; a score of 0.5 or 1.0 in the CDR, a score of 16 or more in the MMSE and concomitant standard pharmacological treatment for AD (cholinesterase inhibitors and/or memantine in stable therapeutic doses for at least 3 mos). | MMSE, Short Cognitive Test (SKT) pre- and post-treatment | MMSEIG 22.6 (2.9) CG 23.3 (3.9) SKT total scoreIG 14.5 (5.4) CG 12.6 (5.4) | MMSEIG 22.5 (3.8) p = .9CG 22.4 (2.8) p = .1SKT total scoreIG 14.6 (6.1) p = .9CG 13.8 (5.5) p = .05 | Standard outpatient care with monthlyfollow-up visits to the memory clinic. Wait list for future intervention group | Paired-sample t tests addressing within-group differences (baseline vs. endpoint) in test scores showed that patients in the control group had a tendency for cognitive decline, which was indicated by a slight, but significant, increase in total SKT scores and in the attention SKT subscore (i.e., higher scores in the SKT mean worse performance). Conversely, patients in the experimental group remained stable with respect to these cognitive measures of attention and global performance. |
| Yoon et al. (2013) | Korea | MMSE-K | DSF; DSB; 7MS; MMSE-K | MMSECAE 18.0 ± 1.5CA 18.7 ± 1.2 (MMSE post-test not given) | Within group post-preDSF CAE −2.1 ± 1.1 CA −1.2 ± 1.2DSB CAE −0.9 ± 0.5 CA −0.2 ± 0.47MST CAE 10.8 ± 8.4 CA 4.3 ± 5.0 | 2 group design (CA & CAE) no control | Working memory performance (DSF, DSB 7MST scores) improved significantly in the CAE group (p < .05). There were significant beneficial effects of the therapeutic programme on memory performance in the CAE group compared to CA group, and between pre-test and post-test. After the 12-wks intervention, the CAE group showed significant improvement compared to the CA group in all the measures studied. |
ABCD: The Arizona Battery for Communication Disorders of Dementia; ADAS-Cog: Cognitive subscale of the Alzheimer’s Disease Assessment Scale (scoring range 0 to 70, higher scores indicate greater cognitive impairment); ADAS-K: Korean version of Alzheimer’s Disease Assessment Scale; BCSB: Brief Cognitive Screening Battery; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; CDR: Clinical Dementia Rating; CDT: Clock Drawing Test; CMMSE: Chinese Mini-Mental State Examination; CVLT: California Verbal Learning Test; DSB: Digit Span Backward; DSF: Digit Span Forward; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; GDS: Global Deterioration Scale; KADLS: Katz Activities Daily Living Scale; MDRS: Mattis Dementia Rating Scale; MFI: phagocytosis index; MMSE: Mini Mental State Examination; MMSE-K: Korean version of the Mini Mental State Examination; MoCA: Montreal Cognitive Assessment; MODA: Milan Overall Dementia Assessment; NAI: Neuropsychological Aging Inventory; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association; SISCO SIDAM: a global measure of cognitive performance/impairment; SKT: Short Cognitive Test; TMT: Trail-Making Test; WAIS-R: Logical Memory subtest of the Wechsler Memory Scale – Revised; 7MST: 7-Minute Screening Test; SD: standard deviation (given except where ‘SE’ is specified); SE: standard error.
Figure 2.
Within group effect size – intervention groups.
Figure 4.
Evaluating case study MMSE (or MoCA) change according to NICE and non-UK guidance on MID.
Figure 3.
Evaluating group study MMSE change according to NICE guidance on MID.
Findings
Eighteen group studies or case studies out of a total of 26 (69%) reported either statistically significant or meaningful within-group improvements in cognition (Baglio et al., 2015; Bredesen et al., 2016; Burgener, Yang, Gilbert, & Marsh-Yant, 2008; Christofoletti et al., 2008; Coelho et al., 2013; Han et al., 2017; Kang et al., 2010; Kim et al., 2016; Li & Li, 2017; Onor et al., 2007; Oswald et al., 2007; Prokopov, 2010; Raggi et al., 2007; Tay, Lim, Chan, Ali, & Chong, 2016; Yoon et al., 2013). Stability of scores or an attenuation of decline was reported in six group studies (Arkin, 2007; Graessel et al., 2011; Ibarria et al., 2016; La Rue et al., 2015; Maci et al., 2012; Viola et al., 2011). Two group studies, Serdà i Ferrer and del Valle (2014) and Vicente de Sousa et al. (2017) reported no cognitive improvement and continual decline. Cognitive outcomes are summarised in Table 6.
(1) What is the evidence for what works and does not work (the predictors of efficacy)?
The ES of each intervention group was evaluated. Cohen’s ES, d, was used to calculate pre–post comparisons (Cohen, 1988). In dementia studies the tendency is for cognition in a control group to decline in the absence of an intervention. Studies that report an ES between groups at the end of the assessment period may therefore show inflation, in part from the declining controls, and consequently give a misleading over-estimate of the effect of the specific treatment or intervention. Therefore, we first chose to examine the within-group ES (Figure 2) to discern which of the interventions worked, in a calculated score uninflated by the tendency to deteriorate in the control group. Using accepted criteria (0.2 – small, ≤0.5 – medium and ≤0.8 – large) (Cohen, 1988), this analysis indicated that of the 17 studies suitable for ES, two studies had a large ES (Christofoletti et al., 2008; Li and Li, 2017), four had a medium ES (Onor et al., 2007; Raggi et al., 2007; Tay et al., 2016; Yoon et al., 2013) and five had a small ES (Burgener et al., 2008; Graessel et al., 2011; Han et al., 2017; Ibarria et al., 2016; La Rue et al., 2015). A further six studies showed a negative ES reflecting the disease course. Interestingly, one study which reported that, based on conventional inferential testing, ‘global cognition did not improve through treatment’ had the largest ES (d = 0.909) (Christofoletti et al., 2008).
The following four group studies were excluded from this analysis for the following reasons. Coelho et al. (2013) used the Frontal Assessment Battery (FAB) which was designed to assess frontal lobe function and to distinguish frontal lobe dementia from AD (Slachevsky et al., 2004). Kim et al. (2016) provided a graph showing positive mean score change. Kang et al. (2010) provided median change scores and Baglio et al. (2015) provided no measures for within-group analysis.
Of equal importance in interpreting change is the minimal clinically important difference (MCID) (Burback, Molnar, St John, & Man-Son-Hing, 1999), defined as the smallest change in an outcome that a clinician would identify as important. The ADAS-Cog is a 70-point scale on which lowered scores indicate improvement. A change of 4 points or more on the ADAS-Cog scale would define a MCID for mild to moderate dementia (Huntley, Gould, Liu, Smith, & Howard, 2015). Again, looking specifically at the intervention group data, two studies reported improvement on the ADAS-Cog (Graessel et al., 2011; Han et al., 2017) but not clinically important.
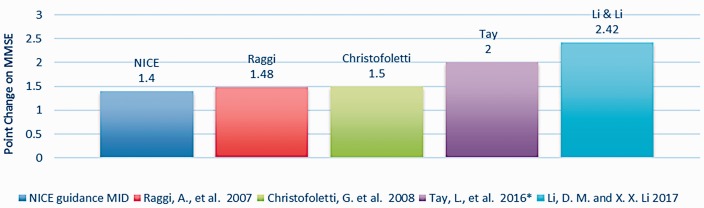
The MMSE is a 30-point scale on which a higher score indicates improvement and a change of at least 3 points is considered clinically important in North America (Burback et al., 1999; Qaseem et al., 2008). To assess studies with the National Institute for Health and Care Excellence (NICE) guidance in the UK (NICE, 2018) a Minimal Important Difference (MID) of 1.4 is also evaluated. Accordingly, four group studies (Christofoletti et al., 2008; Li & Li, 2017; Raggi et al., 2007; Tay et al., 2016) (Figure 3) reported change in mean MMSE above the NICE threshold of minimal important difference (Figure 3). However, no intervention group outcomes from the group studies reached an MID of 3 points on the MMSE.
These four, plus two more group studies were identified in the Effect Size analysis (Figure 2) as having Good or Medium efficacy. Table 7 shows these Top Six studies and the modes and methods they used.
Table 7.
Top Six most effective group studies.
| Study | Modes and Methods | Other Modes | Other Methods |
|---|---|---|---|
| Christofoletti et al. (2008) Brazil | Physiotherapy – Individual sessions concentrated on kinesiotherapeutic exercises to stimulate strength and balance. Cognition such as concentrated attention, recognition, immediate memory, working memory & praxis using bars, Bobath balls, elastic ribbons and proprioceptive stimulation plates | Occupational therapyPhysical education ExpressionCreativity | Arts & crafts (picture, paint, draw, embroider) connect motor coordination with cognition. Walking, upper & lower limb exercises stimulate strength, balance, motor coordination, agility, flexibility and aerobic endurance. |
| Li and Li (2017) China | Folk recreational programme comprised of: Folk art activities including crafts, drawing, decorating and colouring which were mainly about Chinese tales or traditional festivals. | Games – upper body physical activities like fishing, throwing balls, ring toss, number finding, bowling; Music activities – favourite folk songs; Personalized training on daily life activity (ADLs) based on their functional level; Individual activity programme according to their interest and preference, like singing practice of favourite folk songs | |
| Tay et al. (2016) Singapore | Combined cognitive stimulation and physical exercise programme (MINDVital) on gait performance under single- and dual-task conditions; (1) Multicomponent physical exercise programme (45 min) (2) Cognitive stimulation and rehabilitation (60 min) (3) Art therapy as part of the cognitive intervention to stimulate cognitive, emotional and interpersonal skills(4) Tailored individualized activities delivering person centred care (30 min) | (1) Light aerobics, resistance, range of motion, balance training(2) Social and mental activities for spatial and temporal orientation, language and memory(3) Expressive techniques, art therapy, non-verbal expression(4) Engage in an enjoyable activity such as iPAD games, calligraphy. | |
| Yoon et al. (2013) Korea | Cognitive activity (CA) Memory training included sequential memory recall tasks; Three-back verbal working memory | Cycling w exercise (CAE) received the same intervention as the CA group, with the addition of a cycling exercise during their cognitive activity session. Plus conventional Physical Therapy (PT) | |
| Onor et al. (2007) Italy | Integrated rehabilitation programme: Reality Orientation Therapy | – Occupational Therapy – Activities stimulating implicit memory– Reminiscence Therapy – Activities stimulating the memory of events – Caregiver Psychoeducation | |
| Raggi et al. (2007) Italy | Comprehensive rehabilitation programme MMSE <10: informal and formal ROT. MMSE >10 ROT integrated with daily computerised cognitive training | Some patients and carers underwent support PsychotherapySome met one-on-one with an Activity therapist Mobility deficits were treated with Physical Therapy | |
Two case studies (Bredesen et al., 2016) (P6 and P7) reported change in mean MMSE above the NICE guidance on MID. Both case studies also exceeded a non-UK MID of 3 points. A further case study (Bredesen et al., 2016), Patient 9, reported an MID on the MoCA which is significantly positively correlated (Stewart, O'Riley, Edelstein, & Gould, 2012) and translatable to the MMSE for comparison (Trzepacz, Hochstetler, Wang, Walker, & Saykin, 2015). Patient 9 showed clinically important improvement according to NICE guidance (Figure 4).
Besides the three case studies that used MMSE or MoCA in Figure 4 below, two more were reported in the included papers (Bredesen et al., 2016; Prokopov, 2010). When providing personalised N-of-1 treatment, the efficacy of the multimodal interventions was determined by the clinician through a range of assessment indicators, including MRI, FDG PET scans, CVLT-IIB and numerous metabolic and neuropsychological tests. These reported clinically important pre–post-test results for these two patients (Table 8).
Table 8.
Case studies – Cognitive assessments showing clinically important differences.
| Study | Cognitive efficacy measures | Pre-tests | Post-tests | Outcomes |
|---|---|---|---|---|
| Bredesen et al. (2016) USA | MMSE, MRI, MoCA, FDG PET scan, quantitative neuropsychological testing w Neuroquant & Neuroreader, California Verbal Learning Test, Stroop colour test, immediate and delayed recall, semantic knowledge, executive function, processing speed, MFI (phagocytosis index) | P2: FDG PET: Early AD; CVLT-IIB 3rd %ileP6: MMSE 23 MFI = 230P7: MMSE 22P9: MoCA 19 | P2: FDG PET: Early AD; CVLT-IIB 84th %ileP6: MMSE 30MFI > 1000P7: MMSE 29P9: MoCA 21 | P2: Marked subjective and quantitative neuropsychological testing improvement, decline halted; business reinvigorated, a new business site was added (follow-up 24 mos) P6: Subjective improvement, MMSE 23->30; MFI >1000 (12 mos) P7: Subjective improvement, MMSE 22->29 (10 mos) P9: Clear subjective improvement, modest objective improvement MoCA 19->21 (3 mos) |
| Prokopov, (2010) Spain | MRI, detailed biomedical history, lifestyle investigation; suffered mental decline for about 1 yr; declining memory, low energy, low-quality sleep; loss of interests/motivations; could no longer conduct her usual activities and home chores; could not hear without a hearing aid; past medical history of moderate hypertension. | Mrs KG Brain magnetic resonance imaging (MRI) February 2008: hippocampal and cortical atrophy, enlarged ventricular volume | MRI April 2009showed no degenerative changes. | Improvement in mood and vitality was noticeable after the first 5 IHT sessions. Gradually, the mental and cognitive state recovered. Patient reported increased energy and activity, better memory and cognition, a slight weight loss, improved sleep, and better mood. The patient gradually recovered her healthy mental state; resumed shopping and cooking and began playing piano again, which she was not capable of doing the previous year. Only needs the hearing aid for a few hours a day, compared to the whole-day use several months before. |
These case studies utilised the following modes and methods (Table 9).
Table 9.
Case studies – Modes and methods.
| Study | Personalised treatment intervention | Other Modes | Methods |
|---|---|---|---|
| Bredesen et al. (2016) USA | MEND Protocol – Nutrition (diet, vitamins, supplementation, herbs, antioxidants) FastingResponsive to suboptimal metabolic parameters; continued optimization, iterative treatment and metabolic characterization | Sleep Stress Exercise Brain stimulation Hormones GI health Detoxification | Sleep hygiene, stress reduction, aerobics, strength training, brain training, hormone therapy, intranasal vasoactive intestinal peptide (VIP), address heavy metal toxicity |
| Prokopov (2010) Spain | Repeated sessions of Intermittent Hypoxic Training (IHT) Patients comfortably relax in a recliner, their cells and mitochondria go through multiple oscillations of pO2. Intermittent oxygen restriction (IOR) is a universal stimulus rapidly triggering multiple compensatory strategies that support genome integrity. | Nutritional adjustmentIndividualized vitamins, amino acids, microelements and supplementation Fasting | Advised to eat a low-glycaemic-index, low carbohydrate, ketogenic diet, enriched with animal proteins & omega-3 fatty acids. Advised on fasting protocol: limit food intake to within 6–7 h window to extend physiological night fasting time to 18 h |
(2) Does multimodal matter?
Nine of the 11 studies showing positive cognitive ES (Figure 2) used three modes rather than two, indicating that more may be better. However, as well as the number of modes, the number of methods used to carry out the modes is multiple and diverse. For instance, Ibarria et al. (2016) initiated an Integral Psychostimulation Program (IPP) integrating cognitive, motor and mood-related rehabilitation and stimulation for cognitive functions. They also used exercise (active and passive gymnastics, personal & spatial orientation, motor coordination and body language), music therapy, relaxation, occupational activities to maintain ADLs, expression, creativity, board games and caregivers involvement. Also, the focus or general character of the intervention overall (was it largely cognitive or physical?) helped determine the effectiveness of the cognitive mode. For example, five out of six studies which showed a negative ES were predominantly physical with other modes in addition. Conversely, 8 of the 11 studies which showed a positive ES were predominantly cognitive with other modes in addition.
(3) What is the evidence for different groups of people with dementia (early, mid or late stage)?
Analysis of the cognitive impairment levels of participant groups did not indicate that interventions necessarily work any better for the least or the most impaired. In the six papers with the highest ES there was a range of dementia from mild to severe. In fact, the second best ES was achieved by Li and Li (2017) with a participant group that was 82% moderate to severe dementia in a nursing home. Arkin (2007) found that most early stage participants tended to improve or maintain on test scores, but occasionally a person in the moderate stage did also. Graessel et al. (2011) showed that in nursing homes the ESs for the intervention were greater in the subgroup of patients (n = 50) with mild to moderate disease. Raggi et al.’s study (2007) of 30% mild, 40% moderate and 30% severe patients concluded that subjects in all stages of dementia showed some improvement from attending the treatment programme. Kim et al.’s (2016) study of nursing home residents reported improvement in moderate to severe AD taking physical exercise (Kohzuki Exercise Program – KEP) along with a multi-component cognitive programme (MCP), (KEP + MCP).
Predictably, Serdà i Ferrer and de Valle’s (2014) study reported that the magnitude of the effects of the programme diminished progressively in relation to the stage of the disease. Indeed, the most significant and continuous reduction occurred in the dimension of patient cognitive capacity among day hospital patients with mild (29.69%), moderate (31.25%) or severe (39.06%) dementia. Hence, the staging of dementia among the participants seemed to have no direct bearing on the ES of the intervention group, but perhaps had more to do with the level of optimisation of the intervention for participants in different stages of dementia. Given the available study data, the potential for different types of dementia was inconclusive.
(4) What are the strengths and limitations of different study designs used in testing these outcomes?
The most clinically important cognitive differences were achieved in case study designs. Meanwhile, 7 out of 11 group studies with positive ES had a randomised design, whereby the mode was not chosen specifically for the individual but for the cohort generally. However, this did not prove detrimental to the outcomes, as a personalised approach was also evident in the most effective group interventions. For example, Christofoletti et al. (2008), Li and Li (2017), Raggi et al. (2007) and Tay et al. (2016) tailored interventions or individual sessions to the person with dementia, whilst Burgener et al. (2008), Ibarria et al. (2016), La Rue et al. (2015) and Onor et al. (2007) added components of caregiver involvement.
In the design of the sessions (minutes per week, # of sessions), a roughly inverse pattern could be found between time and effectiveness. The time commitment required of participants in the six most effective studies was 24 weeks or less. Some studies requiring a greater time commitment were less effective. For example, Graessel et al. (2011) had the most sessions (288), Oswald et al. (2007) had the most weeks (52) and Ibarria et al. (2016) had the greatest total time commitment of 979 hours (approximated, see Table 3). Because of the nature of a cross-over trial, the length of wash-out period and the relatively short intervention time of eight weeks in Han et al. (2017) they acknowledged that these may have limited the effect of the Multimodal Cognitive Enhancement Therapy (MCET). Since they achieved a small ES for the dementia subgroup, perhaps the overall ES was only hindered by the study duration, and that a longer study might have been more efficacious.
Analysis of the settings found that three of the top four most effective studies were carried out in nursing homes or a long-term care hospital, whereas 83% of the studies with a negative ES were carried out in a day care, day hospital, university facility or research centre with people who attended for sessions but lived at home. One further point about study design, Serdà i Ferrer and de Valle’s (2014) study suggested a mixed method design may be more appropriate for older participants lacking high levels of literacy.
(5) What are the strengths and limitations of outcome measures?
Choice of outcome measures varied across studies (see Table 1) which possibly facilitated statistically significant findings. Also, some screening tests, such as the MMSE have well-reported weaknesses (Stewart et al., 2012; Verma et al., 2015). However, there was a value to using well-recognised instruments as it facilitated at least some quantitative analyses (Figures 2 to 4). The studies were largely heterogeneous and the requirements for individual studies varied depending on the intervention tested. Therefore, conclusions on the relative merits of different measures were not possible and further work is needed specific to multifactorial studies.
(6) What is the evidence for theory on the likely process of change for each mode of intervention?
Of the 10 modes utilised in the studies, other than those modes highlighted in the Introduction (i.e. exercise, especially high-intensity exercise, CS and nutrition), evidence is lacking for primary studies correlating efficacy of these various modes to improved cognition for people with dementia. However, Alzheimer’s disease has been shown to involve multiple pathophysiological factors for which physical, mental activities and exercises normalise and regulate cerebral blood flow (CBF) (Aliev et al., 2013) and promote the production of brain derived neurotrophic factor (BDNF) which correlates with neuroplasticity in the hippocampus (Colcombe et al., 2006; Erickson et al., 2011). Aerobic exercise in particular is associated with increased neurogenesis and angiogenesis, as well as the production of BDNF and other growth factors involved in neuroprotection such as the promotion of cell survival, neurite outgrowth and synaptic plasticity (Cramer et al., 2011) and protecting deoxyribonucleic acid (DNA) from oxidative damage and rejuvenating the mitochondria (Garatachea et al., 2015). Cognitive activity has increased neuronal plasticity and cognitive reserve, a lack of which hastens cognitive decline in dementia (Panerai, 2016). ‘The changes we saw in fMRI support the notion that even the AD brain still has plasticity resources and can react to positive environmental stimuli,’ said Baglio et al. (2015).
Stress relief, meditation and relaxation lowered cortisol, decreased inflammation and increased BDNF. The effect of meditation caused significant changes in CBF (Khalsa, Amen, Hanks, Money, & Newberg, 2009). Neurotoxins such as heavy metals and mould have been associated with cognitive decline and subsequently removed through detoxification with for instance sauna or chelation (Bredesen, 2016; Shoemaker & House, 2006). Hormone balancing optimises thyroid function and regulates sex hormones. Sleep disturbances and disorders may disrupt neuronal pathways, impair working memory, lead to cognitive impairment and are a significant risk factor for dementia (Miller, 2015).
Because DNA methylation, histone modifications, and microRNAs are the principal epigenetic mechanisms involved in AD pathophysiology, some argue that nutrition can prevent the onset of dementia and attenuate cognitive decline, especially if combined with brain exercise and physical training (Athanasopoulos et al., 2016). Dietary factors can complement the action of exercise at the cellular level of energy metabolism and synaptic plasticity (Gomez-Pinilla, 2011). Given the growing evidence base on the process of change for the above interventions, multimodal approaches that target several dysfunctions simultaneously, and that emphasize nutritional, botanical and stimulatory therapies may offer the most benefit (Wollen, 2010).
(7) What are the strengths and limitations of different evaluation tools used to assess the effectiveness of MNPIs on improving cognitive functioning?
In Kim et al. (2016), nursing home participants with moderate-severe AD did not improve significantly after six months in the KEP + MCP group compared to the MCP group on the MMSE and CDT. However, the ADAS-Cog score showed significant improvement at the same time point, which may relate to its greater sensitivity to detect change. The MMSE is widely criticised for this relative lack of ability. Indeed, Ibarria et al. (2016) also reported cognition as stable on the MMSE but slightly declined on the ADAS-Cog. Two studies used the FAB (possible score 18, higher indicates improvement). With so much heterogeneity among the studies the strengths and limitations of different tools are unclear.
(8) What is the effectiveness of different modes of delivery on the effectiveness of MNPIs for improving cognitive functioning for people with a diagnosis of dementia?
Different modes may take more time to register an effect than others. For example, Vicente de Sousa et al. (2017) investigated a nutritional supplementation psychomotor rehabilitation programme (NSPRG) lasting 21 days. But cognitive benefit might require a longer intervention, as evidenced by the improved test scores found in 91% (N = ?) of analysed studies in this review, all of which exceeded 21 days. Some of the most effective group studies included individualised aspects to their interventions.
Christofoletti et al. (2008) (d = 0.909) provided tailored physiotherapeutic sessions concentrating on specific kinesiotherapeutic exercises that stimulated strength, balance and cognition. This study observed an attenuation of cognitive decline, in particular verbal fluency and executive function. Li and Li (2017) (d = 0.497) provided 30-minute individual sessions twice a week according to their likes and preference, such as singing practice of favourite folk songs. Tay et al. (2016) (d = 0.457) gave tailored individualised 30-minute activities. A further personalised approach was found in Raggi et al. (2007) (d = 0.245) which illustrated a comprehensive rehabilitation programme in a specialised hospital unit. Although the study lasted 17 months overall, the mean stay in the hospital was only 26 days, beginning with a thorough investigation including neurological examination and laboratory analyses to inform each person’s integrated, supportive and individualised treatment.
In summary, this review found multimodal intervention research predominantly in Spain, Portugal, USA, Italy and Asia. Group studies commonly utilised 2–3 modes and occurred in long-term care, day care, clinic, hospital, university facilities or in the community. A very small number of case studies were found, utilising up to nine modes. Study duration varied from three weeks to four years and sessions lasted from 20 minutes to 8 hours. Time commitment required of participants in the six most effective studies was 24 weeks or less. People with all stages of dementia participated and studies occasionally involved caregivers.
Discussion and implications
The results of this review have important implications for dementia treatments, as 92% (24/26) of included studies demonstrated statistical improvement, stability or attenuation of decline. Studies that personalised the multimodal approach by identifying individual needs of participants or patients and tailoring interventions accordingly, resulted in the greatest ESs. These findings should encourage extensive research of complex multimodal interventions for dementia, including cognitive, physical or psychological therapies alongside novel ones such as brain stimulation (Raggi, Tasca, & Ferri, 2017), oxygen therapy, detoxification, stress reduction, sleep hygiene, hormonal health, fasting and nutrition. Some considerations have emerged:
Length of study. A multimodal or ‘complex’ intervention contains interacting components requiring specific design guidance (Craig et al., 2013). One difficulty in designing multimodal interventions is accounting for the varying timeframes within which different effects may impact individuals. Because the shortest study (Vicente) had one of the smallest ESs and four of the longest studies showed maintenance of cognition, we investigated whether study length was an indicator of ES. We found that the length of intervention was not an indicator of effectiveness as evidenced by the results of Raggi, Han, Tay, Li and Li and Kang, all of which lasted four months of less. As Vicente was predominantly a nutritional intervention, which also assessed the effect on cognition, it is not surprising that a short study length would have proved unproductive cognitively. Similarly, in the case of Onor et al. (2007), there was a ‘lack of improvement of cognitive function … probably due to the short duration of the rehabilitation program’ (p. 268).
Effect size versus benefit. Reviewing multimodal studies by looking at a measurable gain in only one mode such as cognition, can lead to erroneous assumptions about the success of the research and indeed the benefits of the intervention for the participants with dementia. An example of this is Arkin’s study (2007), which scored lowest on ES of the intervention groups. In spite of this, maintenance of function, or improvement on several discourse measures, was achieved by the programme’s 11 first-year participants. This study was also successful in reducing the annual rate of decline between 3rd and 4th year to just 1 point, which was significantly less than the CERAD comparator group. Therefore, a small amount of decline can be a benefit when compared to rapid decline in the untreated population.
Holistic approach. Since dementia is multifactorial in terms of the numerous risk factors involved, then a holistic approach to treatment would perhaps stand the best chance of addressing a range of symptoms. This is the premise for multimodal interventions, at least as they are currently designed, which cannot be unpacked to determine which element helped to delay or reverse cognitive decline. Positive emotions, enjoyment, creativity, belief, even spirituality can contribute to cognition as much as exercise and diet for example. Onor et al. (2007) reported that socialization itself can lead to improvements, and ‘programs that take a more holistic approach to the individual are more effective than those focusing on cognitive rehabilitation alone’ (p. 270). Maci et al. (2012) echoed this with their integrated approach of CS, physical activity and socialisation to slow down affective decline and reduce carer burden.
Assessment tools. Judging from the multiple tools that have been reported one might jump to the conclusion that many tests are administered in hopes of finding positive outcomes to report. However, multimodal studies require numerous tests – a complete battery for each mode. This enables more insight into the various ways the intervention might be helping to improve cognition. But testing can also contribute to participant burden and might even encourage drop-outs. There is thus a pressing need in multimodal research to analyse the strengths and limitations of different evaluation tools.
Recently, Webster et al. (2017) found that cognitive measures such as the MMSE can be distressing and demoralising for people with dementia and their carers who preferred that cognition should be taken in context of previous ability. It was felt that ‘a larger package of specific measures would give a holistic view of an individual and include more detail’ (p. 13) and that timed measures which estimate cognitive processing speed should be considered, along with the recommended validated measures of MMSE and ADAS-Cog. In keeping with this, studies reported a range of testing including CSF biomarkers, blood work, metabolic analysis as well as in-depth narrative assessment. One paper (Oswald et al., 2007) included data from another informant, a questionnaire for nursing staff on the changes in cognitive and functional ability of residents.
For future studies the use of a range of testing relevant to the population sample is recommended, as well as a standardised, free and widely available instrument for pre–post-test assessment. For instance, the CDR global score has been recommended as a staging or impression of change outcome measure in future studies (Webster et al., 2017). The Short Cognitive Test (SKT) has also been known to be ‘more sensitive to subtle changes’ than the MMSE as it is a ‘more comprehensive cognitive assessment battery and takes account processing speed and response accuracy’ (Viola et al., 2011).
Study design – Pharmacology and the Naturalistic. Although this review searched for, selected and focused on non-pharmacological approaches, the advent of disease-modifying drug treatments is near for preclinical and prodromal stages as disease models improve their ability to predict the likely course of dementia (Ritchie et al., 2017). Drug-naïve patients with a diagnosis of dementia are practically non-existent as this review discovered. Therefore, the opportunity arises for collaborative interventions that combine and integrate pharmacology with non-pharmacological treatments as shown in Ibarria et al. (2016). So-called ‘naturalistic’ studies, which follow a group of patients in an outpatient clinic over many months are relevant and informative to clinical practice. Bragin et al. (2012) showed arrest in cognitive decline for 60 months for patients with depression, dementia, physical disability and medical illnesses. Patients were treated with a multimodal intervention specific to their needs, including anti-depressants, cholinesterase inhibitors, N-methyl-D-aspartate (NMDA) receptor antagonists, vitamins and supplements as well as physical and cognitive exercises. This example strengthens the evidence base, informs clinical practice and is relevant to people living with dementia who (1) would routinely be prescribed a dementia drug, and (2) often have comorbidities. Such a patient consequently may benefit from a combined, integrative treatment model, as Bragin et al. (2012) aptly demonstrated.
Terminology. Because innumerable descriptive terms for utilising more than one mode pervaded the intervention literature, an added benefit of this review was to develop the typology described herein. The Typology of Modes and Methods for Dementia Interventions (Online Appendix 2) organises them into a comprehensive and logical structure. This typology enables a systematic evaluation of where a study sits relevant to the extant literature. It also facilitates multidisciplinary comparison of studies as different fields have their own ways of describing modes of interventions. This typology can help to classify research involving multimodal, multicomponent, dual-task, integrative, combined or complex interventions.
Involving people with dementia. There are opportunities in research design and implementation to include participants in a meaningful way. Perhaps one of the reasons why N-of-1 interventions worked so well is the involvement of the patient, particularly by helping to formulate and then agree the treatment approach with the practitioner or researcher. This goes beyond mere consent to participate and freedom to withdraw. This is about collaborative engagement – co-ownership throughout the process. Another paper by Tay et al. (2016) went into more depth about their goal-oriented approach (Chew, Chong, Fong, & Tay, 2015). Cognition goals as well as goals to improve engagement and socialization; reduce caregiver stress; and improve physical function, behaviour and mood were set and 61.8% of participants met them.
Diversity of needs. Groups are complicated by the diversity of needs within them. Serdà i Ferrer and del Valle (2014) found, on the one hand, that advanced age, diverse symptoms, the stage of the disease and the impracticalities of working with a group led to significant and continuous reduction in patient cognitive status. On the other hand, they noted that the improvement in QoL was ‘decisive and groundbreaking’, suggesting that ‘psychological factors may have helped mediate the relationship between exercise and QoL’ (p. 197).
Personalised approach. It is a commonly held belief across the lay population and mainstream media that no disease-modifying treatments for dementia exist (Webster et al., 2017). We have now shown that optimised and targeted interventions can help to address the underlying causes of cognitive decline. The role of neurotoxins, nutritional deficiencies, inflammation and the gut microbiome in neurodegenerative disease (Bland, 2016) can lead to a personalised care plan and enhanced precision of treatment decisions (Galvin, 2017). Moreover, a person’s dementia has a unique etiology which must be understood individually to develop personalized treatment (Pomorska & Ockene, 2017). Likewise, effort must also be taken to add some component of support for individuals in research studies. Should personalised treatments for individuals become more widespread, the learning and application can benefit research as well as patients and residents in long term care (Bodai et al., 2018).
Limitations
The wide diversity of nomenclature in multimodal studies means our search strategy may not be as comprehensive or successful as intended. Interventions not described in-depth may have led to erroneous categorisation. Decisions about which assessment measures to include in order to maintain consistency but also to include as many studies as possible may have weakened our conclusions. Lack of response from some authors meant we had to omit some studies from the computational analyses.
Conclusion
Overall, there is some evidence that MNPIs can improve cognitive function in adults with a primary diagnosis of dementia by addressing multiple modifiable risk factors currently understood to contribute towards cognitive decline. In cases where cognitive outcomes were improved, the following seven research components tended to be in place:
At least three modes were utilised and the methods for implementing the modes were multiple and diverse;
The general character or focus of the intervention was on cognitive therapies, stimulation, training or rehabilitation, augmented with other modes such as physical, psychosocial, nutritional, etc.;
A personalised one-to-one interaction specific to each individual was included which involved engagement, investigation and assessment helping to focus or fine-tune their intervention;
Social, logistical or practical support involved caregivers or students, or a general widening of social networks;
The degree of a person’s cognitive impairment was not seen as an impediment to intervention but rather was optimised for those with moderate to severe impairment to maximise potential benefits;
Study design and outcome measures allowed for the generation of measurable improvement and the timely and meaningful data capture of the results;
Interventions leveraged recent advances in our understanding of the underlying causes of dementia and ways to disrupt these neurodegenerative mechanisms through nutrition, fasting, oxygen therapies, stress reduction, sleep hygiene and so on.
The aim of this review was to determine the effectiveness of MNPIs for improving cognition in people with a dementia diagnosis. As research increases in this area, healthcare practitioners will be more able to treat people with cognitive impairment in ways that help to reduce the symptoms and slow the decline by addressing the underlying causes of dementia. Health and social care providers need to be better informed about lifestyle medicine as an adjunct to existing pharmacotherapies in order to improve choices for their patients and service users. It is socially and economically imperative that practice does not fall behind emerging evidence. Current relevant findings on effective treatments may empower and encourage people with dementia and their carers towards personal health through self-care, whilst directing and stimulating research that continually improves clinical understanding and therefore patient outcomes.
Supplemental Material
Supplemental material, Supplementary material1 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia
Supplemental material, Supplementary material2 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia
Supplemental material, Supplementary material3 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia
Supplemental material, Supplementary material4 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia
Biography
Garuth Chalfont (landscape architect www.chalfontdesign.com, Geography MA, Architecture PhD) roots his research in therapeutic nature environments. He promotes a holistic approach to dementia treatment in the UK, integrating ‘root cause’ investigations with mainstream NHS practice. RAD:ISH (Research Addressing Dementia: Interventions Supporting Health) will involve neighbourhood food-growing, multimodal activities, social support and education to inspire awareness for emerging evidence; stimulate healing with lifestyle medicine; and nurture human capacity for self-care and healthy ageing.
Christine Milligan is a professor of Health Geography and Co-Director of Lancaster University Centre for Ageing Research. A social scientist, Christine has over twenty years experience of undertaking research in health, ageing and dementia in both national and international settings. In particular, her work on dementia focuses on interventions to support and maintain the wellbeing of people with dementia and their family care-givers. Christine has over a hundred publications in international refereed journals, sits on the research grant board of the Alzheimer’s Society UK and is an International Science Advisory Panel member for the New Zealand Government National Science Challenge for Ageing Well.
Jane Simpson is a clinical and academic psychologist whose main research interest is in the psychological functioning and well-being of people with neurodegenerative disease. She has been part of the Centre for Ageing Research at Lancaster University since its inception 10 years ago and holds an honorary professorship in clinical psychology from the University of Crete. She has over 100 articles published in peer-reviewed papers and holds a number of grants from international and national funders.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the AIM Foundation.
Supplemental Material
Appendices are available online.
References
- Ahlskog J. E., Geda Y. E., Graff-Radford N. R., Petersen R. C. (2011). Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic Proceedings, 86(9), 876–884. DOI: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G., Ashraf G. M., Kaminsky Y. G., Sheikh I. A., Sudakov S. K., Yakhno N. N., Bachurin S. O. (2013). Implication of the nutritional and nonnutritional factors in the context of preservation of cognitive performance in patients with dementia/depression and Alzheimer disease. American Journal of Alzheimer’s Disease & Other Dementias, 28(7), 660–670. DOI: 10.1177/1533317513504614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves J., Magalhaes R., Machado A., Goncalves O. F., Sampaio A., Petrosyan A. (2013). Non-pharmacological cognitive intervention for aging and dementia: Current perspectives. World Journal of Clinical Cases, 1(8), 233–241. DOI: 10.12998/wjcc.v1.i8.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkin S. M. (2007). Language-enriched exercise plus socialization slows cognitive decline in Alzheimer's disease. American Journal of Alzheimer’s Disease & Other Dementias, 22(1), 62–77. DOI: 10.1177/1533317506295377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasopoulos D., Karagiannis G., Tsolaki M. (2016). Recent findings in Alzheimer disease and nutrition focusing on epigenetics. Advances in Nutrition, 7, 917–927. DOI: 10.3945/an.116.012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio F., Griffanti L., Saibene F. L., Ricci C., Alberoni M., Critelli R., Farina E. (2015). Multistimulation group therapy in Alzheimer's disease promotes changes in brain functioning. Neurorehabilitation and Neural Repair, 29(1), 13–24. DOI: 10.1177/1545968314532833. [DOI] [PubMed] [Google Scholar]
- Bamidis P. D., Fissler P., Papageorgiou S. G., Zilidou V., Konstantinidis E. I., Billis A. S., Kolassa I.-T. (2015). Gains in cognition through combined cognitive and physical training: The role of training dosage and severity of neurocognitive disorder. Frontiers in Aging Neuroscience, 7, 152–152. DOI: 10.3389/fnagi.2015.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban F., Annicchiarico R., Pantelopoulos S., Federici A., Perri R., Fadda L., Caltagirone C. (2016). Protecting cognition from aging and Alzheimer's disease: A computerized cognitive training combined with reminiscence therapy. International Journal of Geriatric Psychiatry, 31(4), 340–348. DOI: 10.1002/gps.4328. [DOI] [PubMed] [Google Scholar]
- Bland J. (2016). Mild cognitive impairment, neurodegeneration, and personalized lifestyle medicine. Integrative Medicine, 15(2), 12–14. [PMC free article] [PubMed] [Google Scholar]
- Bodai B. I., Nakata T. E., Wong W. T., Clark D. R., Lawenda S., Tsou C., Campbell T. M. (2018). Lifestyle medicine: A brief review of its dramatic impact on health and survival. The Permanente Journal, 22, 17–25. DOI: 10.7812/TPP/17-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin V., Chemodanova M., Bragin I., Dzhafarova N., Mescher I., Chernyavskyy P., Aliev G. (2012). A 60-month follow-up of a naturalistic study of integrative treatment for real-life geriatric patients with depression, dementia and multiple chronic illnesses. Open Journal of Psychiatry, 02(02), 129–140. DOI: 10.4236/ojpsych.2012.22018. [Google Scholar]
- Bredesen D. E. (2016). Inhalational Alzheimer’s disease: An unrecognized and treatable epidemic. Aging (Albany NY), 8(2), 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen D. E., Amos E. C., Canick J., Ackerley M., Raji C., Fiala M., Ahdidan J. (2016). Reversal of cognitive decline in Alzheimer's disease. Aging (Albany NY), 8(6), 1250–1258. DOI: 10.18632/aging.100981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burback D., Molnar F. J., St John P., Man-Son-Hing M. (1999). Key methodological features of randomized controlled trials of Alzheimer's disease therapy. Minimal clinically important difference, sample size and trial duration. Dementia and Geriatric Cognitive Disorders, 10(6), 534–540. DOI: 10.1159/000017201. [DOI] [PubMed] [Google Scholar]
- Burgener S. C., Yang Y., Gilbert R., Marsh-Yant S. (2008). The effects of a multimodal intervention on outcomes of persons with early-stage dementia. American Journal of Alzheimer's Disease and Other Dementias, 23(4), 382–394. DOI: 10.1177/1533317508317527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschert V. C., Friese U., Teipel S. J., Schneider P., Merensky W., Rujescu D., Buerger K. (2011). Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer's disease: A pilot study. Journal of Alzheimer's Disease: JAD, 25(4), 679–694. DOI: 10.3233/JAD-2011-100999. [DOI] [PubMed] [Google Scholar]
- Chew J., Chong M.-S., Fong Y.-L., Tay L. (2015). Outcomes of a multimodal cognitive and physical rehabilitation program for persons with mild dementia and their caregivers: A goal-oriented approach. Clinical Interventions in Aging, 10, 1687–1694. DOI: 10.2147/CIA.S93914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Twamley E. W. (2013). Cognitive rehabilitation therapies for Alzheimer’s disease: A review of methods to improve treatment engagement and self-efficacy. Neuropsychology Review, 23(1), 48–62. DOI: 10.1007/s11065-013-9227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofoletti G., Oliani M. M., Gobbi S., Stella F., Bucken Gobbi L. T., Renato Canineu P. (2008). A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clinical Rehabilitation, 22(7), 618–626. DOI: 10.1177/0269215507086239. [DOI] [PubMed] [Google Scholar]
- Clare L., Woods R. T. (2004). Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer's disease: A review. Neuropsychological Rehabilitation, 14(4), 385–401. DOI: 10.1080/09602010443000074. [Google Scholar]
- Coelho F. G., Andrade L. P., Pedroso R. V., Santos-Galduroz R. F., Gobbi S., Costa J. L., Gobbi L. T. (2013). Multimodal exercise intervention improves frontal cognitive functions and gait in Alzheimer's disease: A controlled trial. Geriatrics & Gerontology International, 13(1), 198–203. DOI: 10.1111/j.1447-0594.2012.00887.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. (2nd ed). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Colcombe S. J., Erickson K. I., Scalf P. E., Kim J. S., Prakash R., McAuley E., Kramer A. F. (2006). Aerobic exercise training increases brain volume in aging humans. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 61(11), 1166–1170. [DOI] [PubMed] [Google Scholar]
- Cotelli M., Manenti R., Brambilla M., Petesi M., Rosini S., Ferrari C., Miniussi C. (2014). Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Frontiers in Aging Neuroscience, 6. DOI: 10.3389/fnagi.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig P., Dieppe P., Macintyre S., Michie S., Nazareth I., Petticrew M. (2013). Developing and evaluating complex interventions: The new Medical Research Council guidance. International Journal of Nursing Studies, 50, 587–592. DOI: 10.1016/j.ijnurstu.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Cramer S. C., Sur M., Dobkin B. H., O'Brien C., Sanger T. D., Trojanowski J. Q., Vinogradov S. (2011). Harnessing neuroplasticity for clinical applications. Brain, 134(Pt 6), 1591–1609. DOI: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. L., Morstorf T., Zhong K. (2014). Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Research & Therapy, 6(4), 37. DOI: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curlik D. M., 2nd, & Shors T. J. (2013). Training your brain: Do mental and physical (MAP) training enhance cognition through the process of neurogenesis in the hippocampus?. Neuropharmacology, 64, 506–514. DOI: 10.1016/j.neuropharm.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. I., Voss M. W., Prakash R. S., Basak C., Szabo A., Chaddock L., Kramer A. F. (2011). Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences, 108(7), 3017. DOI: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin J. E. (2017). Prevention of Alzheimer's disease: Lessons learned and applied. Journal of the American Geriatrics Society, 65(10): 2128–2133. DOI: 10.1111/jgs.14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garatachea N., Pareja-Galeano H., Sanchis-Gomar F., Santos-Lozano A., Fiuza-Luces C., Moran M., Lucia A. (2015). Exercise attenuates the major hallmarks of aging. Rejuvenation Research, 18(1), 57–89. DOI: 10.1089/rej.2014.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mesa Y., López-Ramos J. C., Giménez-Llort L., Revilla S., Guerra R., Gruart A., Sanfeliu C. (2011). Physical exercise protects against Alzheimer's disease in 3xTg-AD mice. Journal of Alzheimer's Disease, 24(3), 421–454. DOI: 10.3233/JAD-2011-101635. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F. (2011). The combined effects of exercise and foods in preventing neurological and cognitive disorders. Preventive Medicine: An International Journal Devoted to Practice and Theory, 52(Suppl), S75–S80. DOI: 10.1016/j.ypmed.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessel E., Stemmer R., Eichenseer B., Pickel S., Donath C., Kornhuber J., Luttenberger K. (2011). Non-pharmacological, multicomponent group therapy in patients with degenerative dementia: A 12-month randomizied, controlled trial. BMC Medicine, 9, 129–129. DOI: 10.1186/1741-7015-9-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot C., Hooghiemstra A. M., Raijmakers P. G., van Berckel B. N., Scheltens P., Scherder E. J., Ossenkoppele R. (2016). The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Research Reviews, (25), 13–23. DOI: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Han J. W., Lee H., Hong J. W., Kim K., Kim T., Byun H. J., Kim K. W. (2017). Multimodal cognitive enhancement therapy for patients with mild cognitive impairment and mild dementia: A multi- center, randomized, controlled, double-blind, crossover trial. Journal of Alzheimer’s Disease, 55(2), 787–796. DOI: 10.3233/JAD-160619. [DOI] [PubMed] [Google Scholar]
- Huntley J. D., Gould R. L., Liu K., Smith M., Howard R. J. (2015). Do cognitive interventions improve general cognition in dementia? A meta-analysis and meta-regression. BMJ Open, 5, e005247. DOI: 10.1136/bmjopen-2014-005247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarria M., Alegret M., Valero S., Morera A., Guitart M., Canabate P., Tarraga L. (2016). Beneficial effects of an integrated psychostimulation program in patients with Alzheimer's disease. Journal of Alzheimer’s Disease, 50(2), 559–566. DOI: 10.3233/jad-150455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian, G. (1999). Treatment, of 50 cases of senile dementia by acupuncture combined with inhalation of herbal drugs and oxygen. Journal of Traditional Chinese Medicine, 19(4), 287–289. [PubMed] [Google Scholar]
- Kang H. Y., Bae Y. S., Kim E. H., Lee K. S., Chae M. J., Ju R. A. (2010). An integrated dementia intervention for Korean older adults. Journal of Psychosocial Nursing and Mental Health Services, 48(12), 42–50. DOI: 10.3928/02793695-20100930-01. [DOI] [PubMed] [Google Scholar]
- Karp A., Paillard-Borg S., Wang H. X., Silverstein M., Winblad B., Fratiglioni L. (2006). Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dementia and Geriatric Cognitive Disorders, 21(2), 65–73. DOI: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- Khalsa D. S., Amen D., Hanks C., Money N., Newberg A. (2009). Cerebral blood flow changes during chanting meditation. Nuclear Medicine Communications, 30(12), 956–961. DOI: 10.1097/MNM.0b013e32832fa26c. [DOI] [PubMed] [Google Scholar]
- Kim M.-J., Han C.-W., Min K.-Y., Cho C.-Y., Lee C.-W., Ogawa Y., Kohzuki M. (2016). Physical exercise with multicomponent cognitive intervention for older adults with Alzheimer's disease: A 6-month randomized controlled trial. Dementia and Geriatric Cognitive Disorders Extra, 6(2), 222–232. DOI: 10.1159/000446508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köbe T., Witte A. V., Schnelle A., Lesemann A., Fabian S., Tesky V. A., Flöel A. (2016). Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. NeuroImage, 131, 226–238. DOI: 10.1016/j.neuroimage.2015.09.050. [DOI] [PubMed] [Google Scholar]
- Kostoff R. N., Zhang Y., Ma J., Porter A. L., Buchtel H. A. (2017). Prevention and reversal of Alzheimer's disease Retrieved from https://smartech.gatech.edu/handle/1853/56646
- La Rue A., Felten K., Turkstra L. (2015). Intervention of multi-modal activities for older adults with dementia translation to rural communities. American Journal of Alzheimer’s Disease & Other Dementias, 30(5), 468–477. DOI: 10.1177/1533317514568888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law L. L. F., Barnett F., Yau M. K., Gray M. A. (2014). Effects of combined cognitive and exercise interventions on cognition in older adults with and without cognitive impairment: A systematic review. Ageing Research Reviews, 15, 61–75. DOI: 10.1016/j.arr.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Lee J., Choi B. H., Oh E., Sohn E. H., Lee A. Y. (2016). Treatment of Alzheimer's disease with repetitive transcranial magnetic stimulation combined with cognitive training: A prospective, randomized, double-blind, placebo-controlled study. Journal of Clinical Neurology, 12(1), 57–64. DOI: 10.3988/jcn.2016.12.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. M., Li X. X. (2017). The effect of folk recreation program in improving symptoms: A study of Chinese elder dementia patients. International Journal of Geriatric Psychiatry, 32(8), 901–908. DOI: 10.1002/gps.4543. [DOI] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S. G., Huntley J., Ames D., Mukadam N. (2017). Dementia prevention, intervention, and care. Lancet, 390(10113), 2673–2734. DOI: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Maci T., Pira F. L., Quattrocchi G., Nuovo S. D., Perciavalle V., Zappia M. (2012). Physical and cognitive stimulation in Alzheimer disease. The GAIA Project. A pilot study. American Journal of Alzheimer's Disease and Other Dementias, 27(2), 107–113. DOI: 10.1177/1533317512440493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A. (2015). The role of sleep and sleep disorders in the development, diagnosis, and management of neurocognitive disorders. Frontiers in Neurology, 6: 224. DOI: 10.3389/fneur.2015.00224. [DOI] [PMC free article] [PubMed]
- Ngandu T., Lehtisalo J., Solomon A., Levälahti E., Ahtiluoto S., Antikainen R., Kivipelto M. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. The Lancet, 385(9984), 2255–2263. DOI: 10.1016/s0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- NICE. (2018). Dementia: Assessment, management and support for people living with dementia and their carers. In Clinical guideline: Methods, evidence and recommendations Retrieved from https://www.nice.org.uk/guidance/gid-cgwave0792/documents/full-guideline-updated: NICE [PubMed]
- Olazaran J., Reisberg B., Clare L., Cruz I., Pena-Casanova J., Del Ser T., Muniz R. (2010). Nonpharmacological therapies in Alzheimer's disease: A systematic review of efficacy. Dementia and Geriatric Cognitive Disorders, 30(2), 161–178. DOI: 10.1159/000316119. [DOI] [PubMed] [Google Scholar]
- Onor M. L., Trevisiol M., Negro C., Signorini A., Saina M., Aguglia E. (2007). Impact of a multimodal rehabilitative intervention on demented patients and their caregivers. American Journal of Alzheimer's Disease and Other Dementias, 22(4), 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald W. D., Gunzelmann T., Ackermann A. (2007). Effects of a multimodal activation program (SimA-P) in residents of nursing homes. European Review of Aging and Physical Activity, 4(2), 91–102. DOI: 10.1007/s11556-007-0025-y. [Google Scholar]
- Panerai S. (2016). Group intensive cognitive activation in patients with major or mild neurocognitive disorder. Frontiers in Behavioral Neuroscience, 10: 34. DOI: 10.3389/fnbeh.2016.00034. [DOI] [PMC free article] [PubMed]
- Pickett J., Bird C., Ballard C., Banerjee S., Brayne C., Cowan K., Walton C. (2018). A roadmap to advance dementia research in prevention, diagnosis, intervention, and care by 2025. International Journal of Geriatric Psychiatry, 1–7. DOI: 10.1002/gps.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluye, P., Cargo. M, Robert, E., Bartlett, G., O’Cathain, A., Griffiths, F., Boardman F., Gagnon M., Rousseau, M. (2011). A pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies reviews. In: Abstracts of the 19th Cochrane Colloquium; 2011, 19–22 Oct; Madrid, Spain. John Wiley & Sons, 2011.
- Pomorska G., Ockene J. K. (2017). A general neurologist's perspective on the urgent need to apply resilience thinking to the prevention and treatment of Alzheimer's disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 3, 498–506. DOI: 10.1016/j.trci.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopov A. F. (2010). A case of recovery from dementia following rejuvenative treatment. Rejuvenation Research, 13(2–3), 217–219. DOI: 10.1089/rej.2009.0947. [DOI] [PubMed] [Google Scholar]
- Qaseem A., Snow V., Cross J. T., Forciea M. A., Hopkins R., Shekelle P., Owens D. K. (2008). Current pharmacologic treatment of dementia: A clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Annals of Internal Medicine, 148(5), 370–378. DOI: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- Raggi A., Iannaccone S., Marcone A., Ginex V., Ortelli P., Nonis A., Cappa S. F. (2007). The effects of a comprehensive rehabilitation program of Alzheimer's disease in a hospital setting. Behavioural Neurology, 18(1), 1–6. DOI: 10.1155/2007/782959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raggi A., Tasca D., Ferri R. (2017). A brief essay on non-pharmacological treatment of Alzheimer's disease. Reviews in the Neurosciences, 28(6), 587–597. DOI: 10.1515/revneuro-2017-0002. [DOI] [PubMed]
- Ritchie C. W., Russ T. C., Banerjee S., Barber B., Boaden A., Fox N. C., Burns A. (2017). The Edinburgh Consensus: Preparing for the advent of disease-modifying therapies for Alzheimer’s disease. Alzheimer’s Research & Therapy, 9(1), 85. DOI: 10.1186/s13195-017-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodakowski J., Saghafi E., Butters M. A., Skidmore E. R. (2015). Non-pharmacological interventions for adults with mild cognitive impairment and early stage dementia: An updated scoping review. Molecular Aspects of Medicine, 43–44, 38–53. DOI: 10.1016/j.mam.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdà I Ferrer B. C., del Valle A. (2014). A rehabilitation program for Alzheimer's disease. Journal of Nursing Research, 22(3), 192–199. DOI: 10.1097/jnr.0000000000000046. [DOI] [PubMed] [Google Scholar]
- Shoemaker R. C., House D. E. (2006). Sick building syndrome (SBS) and exposure to water‐damaged buildings: Time series study, clinical trial and mechanisms. Neurotoxicology and Teratology, 28, 573–588. DOI: 10.1016/j.ntt.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Slachevsky A., Villalpando J. M., Sarazin M., Hahn-Barma V., Pillon B., Dubois B. (2004). Frontal assessment battery and differential diagnosis of frontotemporal dementia and Alzheimer disease. Archives of Neurology, 61(7), 1104–1107. DOI: 10.1001/archneur.61.7.1104. [DOI] [PubMed] [Google Scholar]
- Souto R. Q., Khanassov V., Hong Q. N., Bush P. L., Vedel I., Pluye P. (2015). Systematic mixed studies reviews: Updating results on the reliability and efficiency of the mixed methods appraisal tool. International Journal of Nursing Studies, 52(1), 500–501. DOI: 10.1016/j.ijnurstu.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Stewart S., O'Riley A., Edelstein B., Gould C. (2012). A preliminary comparison of three cognitive screening instruments in long term care: The MMSE, SLUMS, and MoCA. Clinical Gerontologist, 35(1), 57–75. DOI: 10.1080/07317115.2011.626515. [Google Scholar]
- Tay L., Lim W. S., Chan M., Ali N., Chong M. S. (2016). A combined cognitive stimulation and physical exercise programme (MINDVital) in early dementia: Differential effects on single- and dual-task gait performance. Gerontology, 62(6), 604–610. DOI: 10.1159/000444084. [DOI] [PubMed] [Google Scholar]
- Troesch B., Weber P., Mohajeri M. H. (2016). Potential links between impaired one-carbon metabolism due to polymorphisms, inadequate B-vitamin status, and the development of Alzheimer's disease. Nutrients, 8(12). DOI: 10.3390/nu8120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzepacz P. T., Hochstetler H., Wang S., Walker B., Saykin A. J. (2015). Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatrics, 15, 107. DOI: 10.1186/s12877-015-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden M., Juillerat Van der Linden A. C. (2016). A life-course and multifactorial approach to Alzheimer's disease: Implications for research, clinical assessment and intervention practices. Dementia (London), 17(7), 880–895. [DOI] [PubMed]
- Verma N., Beretvas S. N., Pascual B., Masdeu J. C., Markey M. K., Alzheimer's Disease Neuroimaging I. (2015). New scoring methodology improves the sensitivity of the Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) in clinical trials. Alzheimer’s Research & Therapy, 7(1), 64. DOI: 10.1186/s13195-015-0151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente de Sousa O., Soares Guerra R., Sousa A. S., Pais Henriques B., Pereira Monteiro A., Freitas Amaral T. (2017). Impact of nutritional supplementation and a psychomotor program on patients with Alzheimer's disease. American Journal of Alzheimer’s Disease & Other Dementias, 32(6), 329–341. DOI: 10.1177/1533317517705221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola L. F., Nunes P. V., Yassuda M. S., Aprahamian I., Santos F. S., Santos G. D., Forlenza O. V. (2011). Effects of a multidisciplinary cognitive rehabilitation program for patients with mild Alzheimer's disease. Clinics (Sao Paulo), 66(8), 1395–1400. DOI: 10.1590/S1807-59322011000800015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster L., Groskreutz D., Grinbergs-Saull A., Howard R., O'Brien J. T., Mountain G., Livingston G. (2017). Development of a core outcome set for disease modification trials in mild to moderate dementia: A systematic review, patient and public consultation and consensus recommendations. Health Technology Assessment, 21(26), 1–192. DOI: 10.3310/hta21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2017). Global action plan on the public health response to dementia 2017-2025 (Licence: CC BY-NC-SA 3.0 IGO). Retrieved from Geneva: http://www.who.int/mental_health/neurology/dementia/action_plan_2017_2025/en/
- Wollen K. A. (2010). Alzheimer's disease: The pros and cons of pharmaceutical, nutritional, botanical, and stimulatory therapies, with a discussion of treatment strategies from the perspective of patients and practitioners. Alternative Medicine Review, 15(3), 223–244. [PubMed] [Google Scholar]
- Yoon J. E., Lee S. M., Lim H. S., Kim T. H., Jeon J. K., Mun M. H. (2013). The effects of cognitive activity combined with active extremity exercise on balance, walking activity, memory level and quality of life of an older adult sample with dementia. Journal of Physical Therapy Science, 25(12), 1601–1604. DOI: 10.1589/jpts.25.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary material1 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia
Supplemental material, Supplementary material2 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia
Supplemental material, Supplementary material3 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia
Supplemental material, Supplementary material4 for A mixed methods systematic review of multimodal non-pharmacological interventions to improve cognition for people with dementia by Garuth Chalfont, Christine Milligan and Jane Simpson in Dementia