Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, mainly targets the respiratory system. However, cardiac arrhythmias have been reported in 16% of hospitalized patients and in 44% of patients in intensive care units [1]. Structural and/or inflammatory cardiac damage has also been described [2], [3], with up to 23% of heart failure episodes in in-hospital patient series [4].
At the time of writing, several potential treatments are under investigation. Some candidate drugs may cause PR prolongation (e.g. lopinavir/ritonavir) and/or QT prolongation (chloroquine, hydroxychloroquine, azithromycin, lopinavir/ritonavir, and others). The expectedly short treatment duration for COVID-19 (5 − 10 days) mitigates the drugs’ cardiac risks to an extent. However, an individualized approach that weighs the risks and benefits of these medications in affected patients is important. An in-depth discussion of the multiple candidate treatments being tested, and their specific cardiac risks, is beyond the scope of this editorial and is likely to quickly become outdated as new data emerge. Rather, our aim is to propose a pragmatic approach to mitigate the potential risk of lethal arrhythmias when using any drugs with potential cardiac effects in the setting of COVID-19.
Background
In the two main adverse event reporting databases, Vigibase (the World Health Organization's database of individual case safety reports from 130 countries) and FAERS (US Food and Drug Administration Adverse Event Reporting System), the number of sudden cardiac deaths reported with potential COVID-19 treatments are, respectively, 81 and 105 for hydroxychloroquine, 26 and 48 for lopinovir/ritonavir, 54 and 151 for imatinib and 27 and 251 for azithromycin. Although a degree of underreporting of adverse events in these databases is likely, it is worthwhile noting that hydroxychloroquine or azithromycin, for instance, have been prescribed for decades to hundreds of millions of patients worldwide. In a recent study by Raoult et al. on the potential benefits of combined hydroxychloroquine and azithromycin [5], an ECG was performed before initiating treatment and at day 2. All other QT-prolonging drugs were stopped, and the treatment was not started in patients with a QTc > 500 ms. Our cardiology colleagues in Marseille have collected electrocardiogram (ECG) data from 502 inpatients and outpatients who have received this drug combination (Baptiste Maille et al. manuscript under review). In their study, hydroxychloroquine/azithromycin was withheld for cardiac reasons (either clinical or electrocardiographic) in 2.3%. Among the 413 patients in whom both baseline and day 2 ECGs were available, the mean QTc prolongation was modest. Nine patients had QT prolongations of > 60 ms, none of whom had symptomatic cardiac events. In another series of 84 inpatients with more severe symptoms of COVID-19, including some in critical care, hydroxychloroquine/azithromycin prolonged the QTc to > 500 ms in 11% [6]. The development of acute renal failure was a strong predictor of extreme QTc prolongation. No lethal arrhythmic event was reported in these preliminary series. The French agency for medications safety (ANSM) reported some cases with events possibly related to hydroxychloroquine use, and recommends close follow-up on the possible adverse events of the different drugs with potential cardiac effects used off-label for COVID-19 [7].
Cardiac work-up before initiating QT-prolonging drugs in patients with COVID-19
It is worth noting that medications with the potential to prolong the QT interval do not require an ECG before being prescribed according to their labelling, at the nationwide or international level. The occurrence of QT prolongation and particularly of torsades de pointes are often multifactorial. Risk factors can be divided in three groups. First, there are modifiable risk factors including use of a drug that prolongs the QT interval (see crediblemeds.org), serum potassium < 3.5 mmol/L, bradycardia < 50 bpm, hypocalcaemia < 90 mg/L (2.2 mmol/L), hypomagnesemia < 15 mg/L (< 0.6 mmol/L). Second there are non-modifiable risk factors including inherited long QT syndrome, female sex, age > 65 years, intrinsic baseline QTc > 460 ms, cardiac disease (acute coronary syndrome or heart failure episode), history of kidney or liver disease and sepsis. Third, one could anticipate risk factors attributed to COVID-19 itself, such as myocarditis and arrhythmias, hypokalaemia and renal failure (with an associated risk of drug toxicity). As alluded to above, the short duration of treatment for COVID-19 (5 − 10 days) should be associated with a lower risk of drug accumulation and of arrhythmia than would be expected with longer-term treatments.
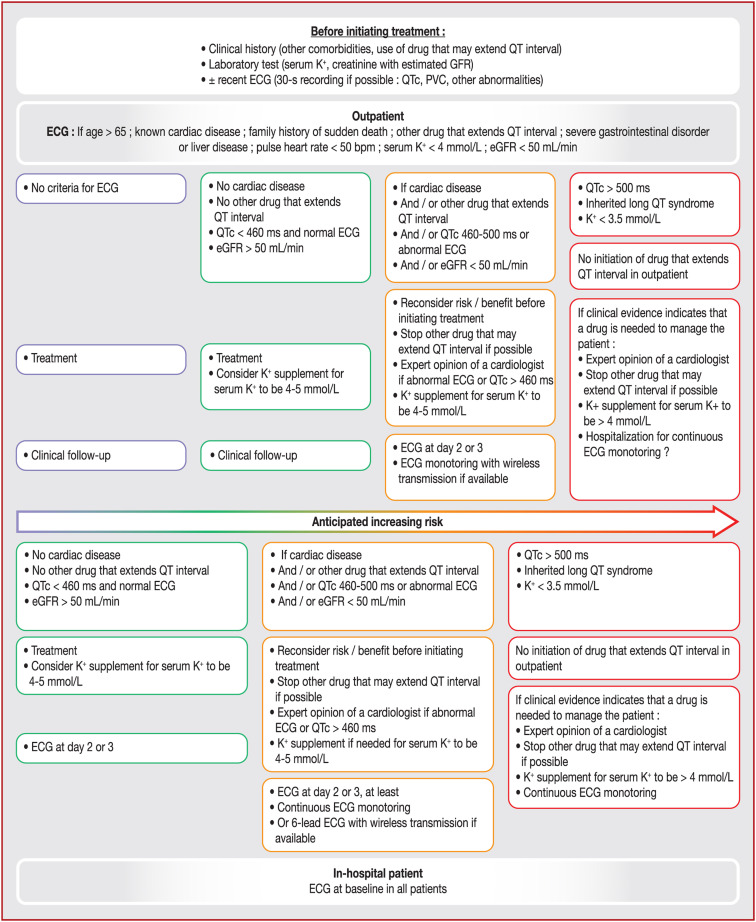
To minimize the risk of a cardiac event for COVID-19 patients treated with drugs that have a cardiac effect, we suggest different work-up and follow-up depending on their clinical status, biochemical parameters (potassium and creatinine blood level at a minimum) and disease severity (outpatients vs. inpatients) (Fig. 1 ). For outpatients under the age of 65 years without cardiac, hepatic, digestive or renal comorbidities, an ECG does not seem to be mandatory in the absence of other QT-prolonging drugs. However, if an ECG can be readily performed, it is reasonable to do so. The initiation of QT-prolonging drugs should be deferred in patients with serum potassium levels below 3.5 mmol/L until they are corrected to at least 3.5 mmol/L. QT-prolonging drugs may be initiated in patients with serum potassium levels between 3.5 and 4.0 mmol/L, but concurrent potassium supplementation should be given.
Figure 1.
Cardiac algorithm when prescribing drug that may prolong QT interval in patients with COVID-19 (top panel for outpatients, lower panel for in-hospital patients). bpm: beats per min; ECG: electrocardiogram; eGFR: estimated glomerular filtration rate; K+: potassium; PVC: premature ventricular contractions.
If two QT-prolonging drugs are being initiated, an ECG should be considered mandatory before drug administration. Follow-up will depend on the context and is summarized in the Figure. In intensive care, an ECG is systematic as well as a close and permanent rhythm monitoring, but a focus on QTc interval remains important.
The lower QTc limit under which the risk is considered low has been set at 460 ms, which represents 98% or the 99th percentile of the different normal population studied [8], [9]. The upper limit above which the risk appears to become high has been set at 500 ms. In cases of QTc intervals > 500 ms, a QT-prolonging drug should not be prescribed unless deemed necessary and if close and continuous rhythm monitoring with a strategy to maintain serum potassium > 4 mmol/L is instituted. Any other QT-prolonging drug should be discontinued in this scenario as well.
If the baseline QTc interval is > 460 ms, a repeat ECG should be performed 2 − 3 days after treatment initiation. This ECG can be performed using an ambulatory system with remote monitoring capabilities if it allows for reliable QTc measurements. If the QTc interval prolongs to > 500 ms, prolongs by > 60 ms relative to baseline or if ventricular arrhythmias occur, treatment with the QT-prolonging drug should be stopped. If it is deemed necessary to continue the QT-prolonging medication in such a scenario, the patient should be hospitalized and closely monitored. Any adverse events should be reported to the French healthcare authorities (https://signalement.social-sante.gouv.fr/psig_ihm_utilisateurs/index.html#/accueil).
Measuring the corrected QT interval
The QTc interval is difficult to measure accurately [10]. When T waves are well-defined, automated measurements provided on ECGs are generally reliable. To manually measure the QTc, the tangent method [11] is appropriate. The QT interval should be measured in lead II or V5 and corrected for heart rate using the preceding RR interval as per Bazett's or Fridericia's formulae if the heart rate is > 90 bpm (https://www.qtcalculator.org). In case of PVC on the ECG, a marked QTc lengthening on the following sinus beat may be a marker of higher risk for torsades de pointe [12], [13].
In patients with QRS durations of > 120 ms (e.g. bundle branch block or paced ventricular rhythms), a modified approach to measuring the adjusted QTc is needed. We propose that the following formula be used [14]: adjusted QTc = QTc–QRS duration + 90 ms. The same thresholds of 460 and 500 ms used for QTc can be applied for adjusted QTc.
When in doubt or for difficult cases, we advise healthcare providers to consider seeking advice from colleagues with expertise in this area, such as electrophysiologists with experience caring for patients with congenital long QT syndromes. To facilitate this, relevant centres of expertise could consider establishing methods for rapid communication on these matters (e.g. dedicated email addresses that could be centralized in a national/regional registry/website).
Funding
None.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Wang D., Hu B., Hu C., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautret P., Lagier J.C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [105949] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Chorin E., Matthew Dai M., Shulman E., et al. The QT Interval in Patients with SARS-CoV-2 Infection Treated with Hydroxychloroquine/Azithromycin. MedRxiv. 2020 doi: 10.1101/2020.04.02.20047050. [Accessed on 17 April 2020] [DOI] [Google Scholar]
- 7.Agence Nationale de Sécurité du Médicament et des produits de santé. 2020. Medicines used in patients with COVID-19: enhanced surveillance for adverse effects - Information point. https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Medicaments-utilises-chez-les-patients-atteints-du-COVID-19-une-surveillance-renforcee-des-effets-indesirables-Point-d-information [accessed 17 April 2020] [Google Scholar]
- 8.Mason J.W., Ramseth D.J., Chanter D.O., Moon T.E., Goodman D.B., Mendzelevski B. Electrocardiographic reference ranges derived from 79,743 ambulatory subjects. J Electrocardiol. 2007;40:228–234. doi: 10.1016/j.jelectrocard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Vink A.S., Neumann B., Lieve K.V.V., et al. Determination and Interpretation of the QT Interval. Circulation. 2018;138:2345–2358. doi: 10.1161/CIRCULATIONAHA.118.033943. [DOI] [PubMed] [Google Scholar]
- 10.Viskin S., Rosovski U., Sands A.J., et al. Inaccurate electrocardiographic interpretation of long QT: the majority of physicians cannot recognize a long QT when they see one. Heart Rhythm. 2005;2:569–574. doi: 10.1016/j.hrthm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Postema P.G., De Jong J.S., Van der Bilt I.A., Wilde A.A. Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart Rhythm. 2008;5:1015–1018. doi: 10.1016/j.hrthm.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Haissaguerre M., Lemetayer P., Montserrat P., Massiere J.P., Warin J.F. Post-extrasystolic long QT: evaluation and significance. Ann Cardiol Angeiol (Paris) 1991;40:15–22. [PubMed] [Google Scholar]
- 13.Viskin S., Alla S.R., Barron H.V., et al. Mode of onset of torsade de pointes in congenital long QT syndrome. J Am Coll Cardiol. 1996;28:1262–1268. doi: 10.1016/s0735-1097(96)00311-7. [DOI] [PubMed] [Google Scholar]
- 14.Yankelson L., Hochstadt A., Sadeh B., et al. New formula for defining “normal” and “prolonged” QT in patients with bundle branch block. J Electrocardiol. 2018;51:481–486. doi: 10.1016/j.jelectrocard.2017.12.039. [DOI] [PubMed] [Google Scholar]