Take Home Message
Non–muscle-invasive bladder cancer patients with COVID-19 are more likely to develop acute respiratory distress syndrome. Thus, several adjustments to the use of intravesical instillations of bacillus Calmette-Guérin should be made during the current pandemic to limit the risk of contamination.
1. Introduction
The current COVID-19 pandemic is forcing caregivers to adapt their clinical practice, especially for the management of life-threatening conditions such as urological malignancies. During this health care crisis it is important to weigh the risk of contamination related to breaking lockdown for treatment delivery against the risk of jeopardizing cancer prognosis by delaying any form of treatment. This consideration is of utmost relevance for patients with high-risk non–muscle-invasive bladder cancer (NMIBC) who require repeat visits to hospital for intravesical instillations of bacillus Calmette-Guérin (BCG) to decrease the risk of recurrence and progression after transurethral resection of the bladder (TURB). The median age at initial diagnosis in this patient population is >70 yr [1] and it has recently been reported that almost 30% of patients older than 65 yr may develop acute respiratory distress syndrome after contracting COVID-19 [2]. Therefore, most NMIBC patients should be considered at high risk of presenting with severe forms of COVID-19 that might require admission to an intensive care unit for invasive ventilation.
In this particular context, it is every urologist’s responsibility to evaluate the potential benefits and risks of delivering intravesical BCG instillations. Several factors can be taken into account in the decision-making process, which is highly limited by the absence of available data. Therefore, all the following points should not be interpreted as evidence-based guidelines but as pragmatic expert opinion for post-TURB use of BCG to treat NMIBC during the COVID-19 pandemic.
The treatment course is likely to depend on COVID-19 status, which should be evaluated via meticulous clinical examination to look for common and uncommon symptoms and a detailed history to account for recent contact with confirmed COVID-19 cases [2].
2. Patients without COVID-19 suspicion
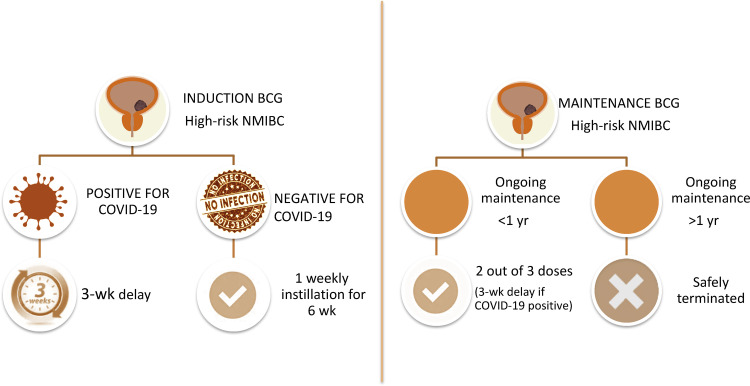
2.1. Induction BCG
In the absence of COVID-19, we believe that the six weekly doses of BCG induction should be completed for high-risk NMIBC cases, given that several randomized controlled trials and meta-analyses have shown that such treatment is associated with decreases of up to 60–70% [3], [4] and 26% [4] in the risk of recurrence and progression, respectively (Fig. 1 ).
Figure 1.
Adjustments in the use of intravesical instillations of bacillus Calmette-Guérin (BCG) for high-risk non–muscle-invasive bladder cancer (NMIBC) during the COVID-19 pandemic.
It is noteworthy that minimizing the risk of health care–acquired COVID-19 should be a priority during the instillation process. Specifically, dedicated care pathways should be set up at each institution to prevent any contact between NMIBC patients and those with COVID-19. In addition, time spent in hospital should be reduced to a minimum and appropriate asepsis protocols using adequate personal protective equipment should be strictly followed to limit the risk of contamination.
2.2. Maintenance BCG
To minimize the number of hospital visits, receipt of at least two out of the three doses of a BCG maintenance course should be considered acceptable. Intravesical BCG therapy courses that have been ongoing for longer than 1 yr can be safely terminated for high-risk NMIBC patients. Although the European Association of Urology (EAU) guidelines [5] recommend 3-weekly instillations at 3, 6, 12, 18, 24, 30, and 36 mo on the basis of European Organization for Research and Treatment of Cancer data [6], the optimal schedule for maintenance BCG remains unclear. Among high-risk NMIBC patients, maintenance BCG reduces the rates of recurrence and progression by approximately 15% and 4%, respectively [4], [7].
3. Patients with suspected or confirmed COVID-19
Any patient with a suspicion of COVID-19 should be tested. In the presence of confirmed COVID-19, a cautionary approach would be to delay instillation of BCG given that there are no data on tolerance of intravesical BCG among COVID-19 patients. The median age (70 yr) and high comorbidity status of patients diagnosed with NMIBC increase their risk of developing a very severe form of COVID-19 [2]. This could be associated with both more adverse side effects of BCG and/or more severe forms of COVID-19. On the basis of the previously described natural history of COVID-19 [2], it is advisable to delay BCG instillation for at least 3 wk after initial symptoms to allow for complete recovery.
4. BCG vaccination and COVID-19
Recent theories suggest that use of BCG as a vaccination could prevent COVID-19. This is based on the epidemiological observation that older patients are at higher risk of COVID-19, especially severe forms, while younger patients could be protected by BCG vaccination providing childhood immunity that may last for approximately 20 yr [8]. In addition, preclinical studies in mice have shown that BCG vaccination could offer protection against various DNA and RNA viruses via induction of innate immune memory and heterologous lymphocyte activation [9]. Two randomized controlled trials are currently testing BCG vaccination for COVID-19 prevention in Australia (NCT04327206) and the Netherlands (NCT04328441). Arguably, intravesical instillations of BCG for induction of a local immune response with activation of macrophages, neutrophils, and natural killer T lymphocytes [10] could help in preventing and/or controlling COVID-19, but this is only a hypothesis that cannot currently be used for clinical decision-making. Only post-crisis retrospective analyses of COVID-19 incidence among NMIBC patients treated with intravesical instillations of BCG during the pandemic will provide interesting insights into this view.
5. Management of BCG side effects
In managing side effects related to intravesical BCG instillations, careful attention should be paid to persistent fever by isolating and testing patients for COVID-19. Furthermore, although specific treatments for side effects should be delivered according to current EAU guidelines [5], nonsteroidal anti-inflammatory drugs should only be used in COVID-19–negative patients, given that these medications may worsen the course of COVID-19, potentially leading to a higher risk of hospital and intensive care unit admission [11].
To conclude, the current COVID-19 pandemic is likely to impact management of NMIBC, but patients should be reassured that management of urological malignancies remains a top priority even during the health care crisis. Reasonable adjustments are required to limit the risk of COVID-19 contamination while maintaining adequate oncological outcomes. Specifically, induction BCG should be maintained for all high-risk patients with the exception of patients infected with COVID-19, for whom BCG installations should be delayed for 3 wk. Intravesical BCG therapies that have been ongoing for >1 yr can be safely terminated for high-risk NMIBC patients.
Conflicts of interest: The authors have nothing to disclose.
References
- 1.American Cancer Society . American Cancer Society; Atlanta, GA: 2020. Cancer Facts & Figures 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. In press. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed]
- 3.Shelley M.D., Court J.B., Kynaston H. Intravesical bacillus Calmette-Guerin in Ta and T1 bladder cancer. Cochrane Database Syst Rev. 2000;2000 doi: 10.1002/14651858.CD001986. CD001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sylvester R.J., van der M.A., Lamm D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M., Burger M., Comperat E.M. European Association of Urology guidelines on non–muscle-invasive bladder cancer (TaT1 and carcinoma in situ) – 2019 update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Oddens J., Brausi M., Sylvester R. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guerin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63:462–472. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Bohle A., Jocham D., Bock P.R. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 8.Miller A., Reandelar M.J., Fasciglione K., et al. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv preprint. www.medrxiv.org/content/10.1101/2020.03.24.20042937v1.
- 9.Mathurin K.S., Martens G.W., Kornfeld H. CD4 T-cell-mediated heterologous immunity between mycobacteria and poxviruses. J Virol. 2009;83:3528–3539. doi: 10.1128/JVI.02393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettenati C., Ingersoll M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol. 2018;15:615–625. doi: 10.1038/s41585-018-0055-4. [DOI] [PubMed] [Google Scholar]
- 11.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368:m1185. doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]