Abstract
COVID-19 has become one of the worst infectious disease outbreaks of recent times, with over 2.1 million cases and 120,000 deaths so far. Our study investigated the demographic, clinical, laboratory and imaging features of 63 patients with COVID-19 in Beijing. Patients were classified into four groups, mild, moderate, severe and critically ill. The mean age of our patients was 47 years of age (range 3–85) and there was a slight male predominance (58.7%). Thirty percent of our patients had severe or critically ill disease, but only 20% of severe and 33% of critically ill patients had been to Wuhan. Fever was the most common presentation (84.1%), but cough was present in only slightly over half of the patients. We found that lymphocyte and eosinophils count were significantly decreased in patients with severe disease (p = 0.001 and p = 0.000, respectively). Eosinopenia was a feature of higher levels of severity. Peripheral CD4+, CD8+ T lymphocytes, and B lymphocytes were significantly decreased in severe and critically ill patients, but there was only a non-statistically significant downward trend in NK cell numbers with severity. Of note is that liver function tests including AST, ALT, GGT and LDH were elevated, and albumin was decreased. The inflammatory markers CRP, ESR and ferritin were elevated in patients with severe disease or worse. IL-6 levels were also higher, indicating that the presence of a hyperimmune inflammatory state portends higher morbidity and mortality. In a binary logistic regression model, C-reactive protein level (OR 1.073, [CI, 1.013–1.136]; p = 0.017), CD8 T lymphocyte counts (OR 0.989, [CI, 0.979–1.000]; p = 0.043), and D-dimer (OR 5.313, [CI, 0.325–86.816]; p = 0.241) were independent predictors of disease severity.
Keywords: 2019 novel coronavirus disease, SARS-CoV-2, Mortality, Prognosis, COVID-19, Lymphocyte subsets, Cytokine storm, Elevated liver enzymes, Interleukin-6
Highlights
-
•
China was the earliest country to experience rapid spread of the virus, with most cases in Hubei province.
-
•
We studied 63 patients with a mean age of 47 years. Thirty percent had severe or critical disease and there was a male predominance of 58.7%.
-
•
Eosinophils, CD4+, CD8+, CD19+ and total lymphocytes were significantly decreased in patients with severe disease.
-
•
Liver enzymes, C-reactive protein, erythrocyte sedimentation rate, ferritin and IL-6 levels were elevated in severe disease.
-
•
C-reactive protein, T lymphocyte count and D-dimer were independent predictors of disease severity.
1. Introduction
In December 2019, several patients with a pneumonia of unknown etiology were treated in Wuhan, China. By late December 2019, a novel coronavirus originally named 2019-nCoV was found to be the etiology of these illness. The terminology was later updated by the WHO whereby the disease is named COVID-19 and it is caused by the virus SARS-CoV-2 [1,2]. On January 21, 2020, the Department of Infectious Disease at the Fifth Medical Center of Chinese PLA General Hospital was designated to be one of the medical institutions for the diagnosis and treatment of patients with COVID-19 in Beijing, China. On February 24, 2020, the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) reported that 13.8% of those infected with COVID-19 had severe disease, with a case fatality rate of 3.8%. As of April 3, 2020, the case fatality rate of COVID-19 in China is approximately 4.0% [3]. At this time, the epidemic situation across China has been effectively controlled, and most of the new cases in China are currently imported from abroad. This study aims to evaluate the characteristics and prognostic factors of disease severity in patients with COVID-19 in Beijing.
1.1. Patients and methods
This study evaluated the characteristics and prognostic factors of disease severity in 63 confirmed non-imported COVID-19 patients in Beijing. Diagnostic criteria for COVID-19 pneumonia was based on the New Coronavirus Pneumonia Prevention and Control Program (7th edition) published by the National Health Commission of China [4]. Various specimens including respiratory samples from throat swab or sputum, urine, blood or stool samples were collected and tested for SARS-CoV-2 following WHO guidelines for quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) [5,6]. Positive results of COVID-19 infection were defined as any positive test from the above specimens. Routine serial hematologic and biochemical tests were performed after admission, and the serum samples were screened for common respiratory pathogens, including antibodies to adenovirus, chlamydia pneumoniae, mycoplasma pneumoniae, respiratory syncytial virus, coxsackie virus A16, legionella pneumophila and mycobacterium tuberculosis and all were negative. Chest X-Ray and chest CT scans were obtained for all patients and monitored serially.
Data were analyzed with SPSS V.21.0 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as the mean ± standard deviation (SD) or as the median (range). The analysis of variance or Kruskal-Wallis rank sum test was used for comparison between multiple groups. Categorical variables were expressed as a number (%). Intergroup comparison was performed using chi-square test. Spearman correlation coefficient was used to describe the association between different variables and adverse events. The logistic regression model was used to identify factors associated with adverse events. Odds ratios and their associated 95% confidence intervals (CIs) were used as measures of effect size. p value less than 0.05 (two-tailed) was considered to be statistically significant.
1.2. Clinical characteristics
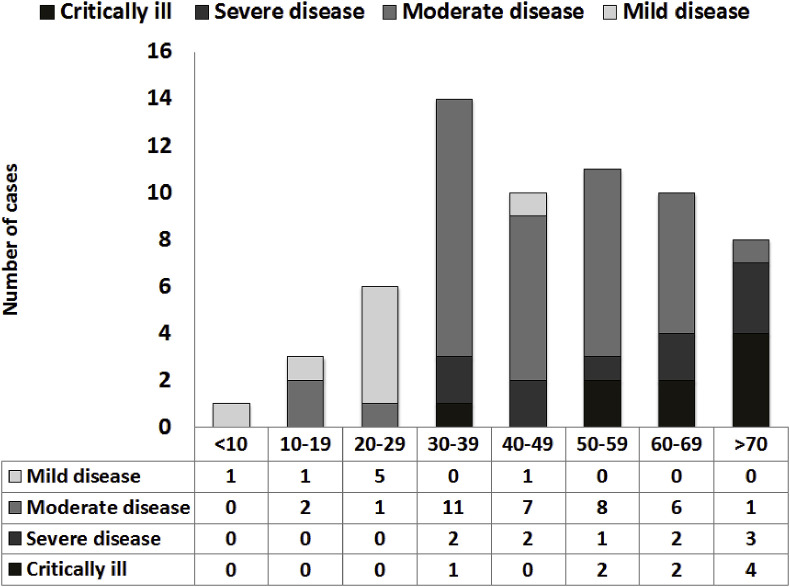
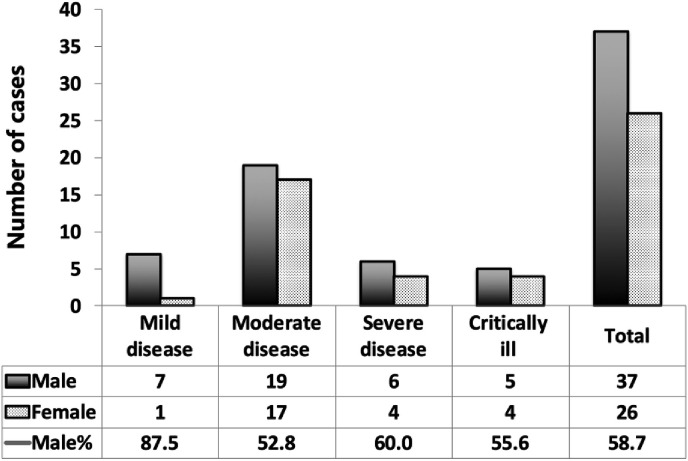
Patients were categorized into mild, moderate, severe and critically ill levels of severity based on the criteria shown in Table 1 . Eight patients were identified to have mild disease (12.7%), 36 had moderate disease (57.1%), 10 had severe diseases (15.9%) and nine were critically ill (14.3%). Patients with severe or critical disease accounted for 30.2%. Table 2 shows the demographic, epidemiologic, and clinical features of patients at admission. The median patient age was 47 years, with a range of 3–85 years of age. Fig. 1 shows the age and demographic characteristics of patients with COVID-19. COVID-19 preferentially affects males (37 of 63, 58.7%). The sex distribution for the various levels of severity showed that among the mild cases, 7 (87.5%) were male, among moderate cases, 19 (52.8%) were male, among severe cases, 6 (60.0%) were male and among critically ill patients, 5 (55.6%) were male (Fig. 2 ). There was a statistically significant difference among the four groups (p < 0.001).
Table 1.
Defining level of severity in COVID-19.
| Clinical category | Definition |
|---|---|
| Mild disease | Mild symptoms and normal or non-pneumonia findings on radiographic examination |
| Moderate disease | Respiratory symptoms and fever, with evidence of pneumonia on radiographic examination |
| Severe disease | Dyspnea, respiratory frequency ≥30/minute, blood oxygen saturation ≤93%, PaO2/FiO2 ratio <300, and/or lung infiltrates >50% of the lung field within 24–48 h |
| Critically ill | Respiratory failure and mechanical ventilation required, septic shock, and/or multiple organ dysfunction/failure and requires ICU monitoring and treatment |
Table 2.
Demographic and epidemiologic characteristics of patients with COVID-19 in beijing.
| Characteristic | Mild disease (n = 8) | Moderate disease (n = 36) | Severe disease (n = 10) | Critically ill (n = 9) | Total (n = 63) | p value |
|---|---|---|---|---|---|---|
| Median age (range) | 23 (3, 48) | 47 (13, 78) | 59 (33, 85) | 63 (34, 79) | 47 (3, 85) | 0.000 |
| BMI | 23.40 ± 4.98 | 24.09 ± 3.14 | 25.95 ± 3.17 | 25.13 ± 3.20 | 24.45 ± 3.43 | 0.338 |
| Epidemiologic | ||||||
| Travel history to Wuhan in 14 days before the onset | 8 (100.0%) | 22 (61.1%) | 2 (20.0%) | 3 (33.3%) | 35 (55.6%) | 0.017 |
| History of contact with a person from Wuhan or around Wuhan in the 14 days before onset | 2 (25.0%) | 17 (47.2%) | 4 (40.0%) | 3 (33.3%) | 26 (41.3%) | 0.602 |
| Contact with a confirmed case of COVID-19 infection | 3 (37.5%) | 24 (66.7%) | 3 (30.0%) | 4 (44.4%) | 34 (54.0%) | 0.549 |
| Case clustering | 2 (25.0%) | 25 (69.4%) | 5 (50.0%) | 4 (44.4%) | 36 (57.1%) | 0.363 |
| Symptoms, n (%) | ||||||
| Fever | 4 (50.0%) | 35 (97.2%) | 7 (70.0%) | 7 (77.8%) | 53 (84.1%) | 0.004 |
| Chills | 0 | 1 (2.8%) | 0 | 0 | 1 (1.6%) | 0.182 |
| Myalgia | 1 (12.5%) | 9 (25.0%) | 2 (20.0%) | 4 (44.4%) | 16 (25.4%) | 0.461 |
| Cough | 2 (25.0%) | 21 (58.3%) | 5 (50%) | 6 (66.7%) | 34 (54.0%) | 0.256 |
| Headache | 0 | 7 (19.4%) | 1 (10.0%) | 0 | 8 (12.7%) | 0.260 |
| Anorexia | 1 (12.5%) | 11 (30.6%) | 2 (20%) | 3 (33.3%) | 17 (27.0%) | 0.678 |
| Sputum production | 0 | 9 (25.0%) | 6 (60.0%) | 4 (44.4%) | 19 (30.2%) | 0.029 |
| Shortness of breath | 0 | 1 (2.8%) | 4 (40.0%) | 6 (66.67%) | 11 (17.5%) | 0.000 |
| Dizziness | 1 (12.5%) | 5 (13.9%) | 0 | 1 (11.1%) | 7 (11.1%) | 0.671 |
| Diarrhea | 0 | 4 (11.1%) | 1 (10.0%) | 0 | 5 (7.9%) | 0.549 |
| Sore throat | 2 (25%) | 9 (25.0%) | 1 (10.0%) | 0 | 5 (7.9%) | 0.301 |
| Runny nose | 0 | 1 (2.8%) | 0 | 0 | 1 (1.6%) | 0.068 |
Fig. 1.
Age and clinical type distribution of COVID-19.
Fig. 2.
Sex distribution of COVID-19 with different clinical classification.
The mean BMI of the 63 patients was 24.45 ± 3.43, and there was no statistically significant difference among the four groups. Thirty-five patients (55.6%) had a travel history to Wuhan in the 14 days before the onset of symptoms, and twenty-six (41.3%) patients without a travel history to Wuhan had a history of contact with a person from or near Wuhan in the 14 days before the onset of illness. Thirty-four (54.0%) of 63 patients had close contact with patients with COVID-19 and 36 (57.1%) had a history of family clustering.
Patients with coexisting conditions were more susceptible to severe disease and 29 of our 63 patients had 1 or more coexisting diseases, including hypertension in 12 cases, diabetes mellitus in 5, cerebral infarction in 2, cardiac arrhythmia in 2, prostate cancer in 1, bronchial asthma in 2, pulmonary tuberculosis in 1, claustrophobia in 1, thyroid disease in 3, chronic hepatitis in 2, and chicken pox in 1. This is shown in Table 3 . The most common symptoms were fever (84.1%) and cough (54.0%), but diarrhea was not common.
Table 3.
Comorbidities of patients with COVID-19.
| Comorbidities | Mild disease | Moderate disease | Severe disease | Critically ill |
|---|---|---|---|---|
| Hypertension | – | 8 | 1 | 3 |
| Diabetes mellitus | – | 2 | 2 | 1 |
| Cerebral infarction | – | – | – | 2 |
| Cardiac arrhythmia | – | – | 1 | 1 |
| Prostate cancer | – | – | 1 | – |
| Bronchial asthma | – | 1 | 1 | – |
| Pulmonary tuberculosis | 1 | – | – | – |
| Claustrophobia | – | – | – | 1 |
| Thyroid disease | 1 | 2 | – | – |
| Liver disease | – | – | 1 (Hepatitis B) | 1 (NAFLD) |
| Chicken pox | – | 1 | – | – |
NAFLD = Non-alcoholic fatty liver disease.
1.3. Laboratory features
Laboratory tests on admission showed leukopenia in 11 patients (17.5%), lymphopenia in 11 patients (17.5%), thrombocytopenia in 3 patients (4.8%), anemia in 10 patients (15.9%), and eosinopenia in 30 patients (47.6%) (Table 4 ). The leukocyte, neutrophil, lymphocyte, eosinophil counts and hemoglobin level differed significantly among the four severity groups. Eosinopenia was not found in patients with mild disease but was found in 19 patients (52.8%) with moderate disease, 7 patients (70.0%) with severe disease and 4 critically ill patients (44.4%).
Table 4.
Laboratory findings in Patients with COVID-19 at admission.
| Laboratory Finding | Mild disease (n = 8) | Moderate disease (n = 36) | Severe disease (n = 10) | Critically ill (n = 9) | Total (n = 63) | p value |
|---|---|---|---|---|---|---|
| Leukocyte count, 109 cells/L | 5.92 ± 1.29 | 4.56 ± 1.21 d# | 5.01 ± 1.76 | 6.84 ± 3.57 | 5.13 ± 1.95 | 0.007 |
| Neutrophil count, 109 cells/L | 3.25 ± 0.82 c# | 2.70 ± 1.01 d# | 3.76 ± 1.85 f* | 5.54 ± 3.70 | 3.32 ± 1.96 | 0.001 |
| Lymphocyte count, 109 cells/L | 2.00 ± 0.64 a.b.c# | 1.42 ± 0.56 d.e# | 0.90 ± 0.47 | 0.83 ± 0.50 | 1.32 ± 0.65 | 0.001 |
| Hemoglobin level, g/L | 144.88 ± 12.63 c# | 137.67 ± 14.07 e# | 134.5 ± 16.79 | 119.33 ± 30.85 | 135.46 ± 18.59 | 0.021 |
| Platelet count, 109 cells/L | 204.38 ± 49.16 | 180.31 ± 56.84 | 183.40 ± 71.87 | 193.56 ± 75.86 | 185.75 ± 60.50 | 0.759 |
| Eosinophils count, 109 cells/L | 0.14 ± 0.06 a.b.# | 0.03 ± 0.045* | 0.01 ± 0.00 f* | 0.09 ± 0.14 | 0.05 ± 0.07 | 0.000 |
| Monocytes count, 109 cells/L | 0.46 ± 0.16 | 0.55 ± 0.99 | 0.33 ± 0.19 | 0.37 ± 0.19 | 0.48 ± 0.75 | 0.835 |
| PT, s | 12.01 ± 1.13 c* | 12.14 ± 0.81 e* | 12.57 ± 2.19 f* | 21.30 ± 8.29 | 13.52 ± 9.63 | 0.072 |
| PTA (%) | 88.97 ± 23.33 | 84.04 ± 15.13 e* | 84.18 ± 23.10 | 66.97 ± 28.35 | 82.14 ± 20.28 | 0.097 |
| Fibrinogen, g/L | 2.04 ± 0.39 a.b.c* | 3.14 ± 1.00 | 3.69 ± 2.04 | 3.62 ± 1.52 | 3.18 ± 1.32 | 0.045 |
| D-dimer, mg/L | 0.16 ± 0.06 b.c# | 0.35 ± 0.25 d.e# | 3.15 ± 3.31 | 1.95 ± 2.38 | 1.97 ± 1.83 | 0.000 |
| Sodium level, mmol/L | 137.14 ± 3.08 | 137.66 ± 3.30 | 136.40 ± 3.10 | 135.22 ± 4.82 | 137.03 ± 3.52 | 0.288 |
| Potassium level, mmol/L | 4.64 ± 0.31 a.b.# | 4.14 ± 0.39 | 3.97 ± 0.60 | 4.26 ± 0.52 | 4.18 ± 0.47 | 0.020 |
| Albumin level, g/L | 43.62 ± 3.20 a*.b#.c# | 39.47 ± 4.09 e# | 36.80 ± 6.29 | 32.77 ± 5.17 | 38.61 ± 5.38 | 0.000 |
| Globulin level, g/L | 28.63 ± 2.39 | 29.03 ± 4.81 | 29.30 ± 4.69 | 28.00 ± 4.30 | 28.87 ± 4.41 | 0.919 |
| Total bilirubin level, mmol/L | 12.14 ± 9.98 | 12.09 ± 6.88 | 12.14 ± 4.29 | 10.83 ± 5.53 | 11.92 ± 6.69 | 0.965 |
| ALT level, U/L | 29.88 ± 20.73 | 29.39 ± 19.40 e* | 41.50 ± 47.58 | 83.11 ± 140.49 | 39.05 ± 59.03 | 0.100 |
| AST level, U/L | 31.00 ± 17.00 c* | 29.61 ± 15.24 e# | 40.10 ± 27.60 f* | 95.67 ± 153.65 | 40.89 ± 61.99 | 0.034 |
| ALP level, U/L | 97.50 ± 53.3 | 77.00 ± 43.10 | 66.30 ± 20.53 | 73.00 ± 35.93 | 77.33 ± 40.89 | 0.436 |
| GGT level, U/L | 23.25 ± 13.83 b.c* | 34.28 ± 27.65 | 53.30 ± 45.89 | 54.89 ± 29.30 | 38.84 ± 31.37 | 0.061 |
| LDH level, U/L | 231.75 ± 122.92 c# | 217.47 ± 51.12 e# | 279.70 ± 84.36 f* | 376.89 ± 161.55 | 251.94 ± 103.51 | 0.000 |
| Creatine phosphokinase level, U/L | 80.75 ± 28.93 | 101.55 ± 124.69 | 132.57 ± 111.91 | 137.00 ± 166.51 | 109.68 ± 122.79 | 0.826 |
| Urea level, mmol/L | 4.33 ± 0.67 c# | 3.99 ± 1.21 e# | 4.49 ± 1.05 f# | 9.41 ± 8.69 | 4.88 ± 3.78 | 0.001 |
| Creatinine level, μmol/L | 77.13 ± 21.33 c# | 77.3 ± 12.92 e# | 74.80 ± 10.99 e# | 103.11 ± 48.31 | 80.57 ± 23.47 | 0.017 |
| C-reactive protein level, mg/L | 5.08 ± 8.58 b# | 11.83 ± 17.074# | 48.92 ± 72.71 | 30.96 ± 29.46 | 19.53 ± 35.94 | 0.012 |
| Erythrocyte sedimentation rate, mm/60min | 5.14 ± 4.11*b#.c# | 25.16 ± 21.28 d*e# | 45.86 ± 19.07 | 52.13 ± 37.46 | 29.24 ± 26.37 | 0.001 |
| Serum ferritin (Times the upper limit of normal) | 0.55 ± 0.502.c# | 2.00 ± 2.20 e# | 3.20 ± 1.47 | 5.08 ± 3.29 | 2.45 ± 2.48 | 0.000 |
| IL-6 level, pg/ml | 5.26 ± 1.252.c# | 14.17 ± 11.37 d#e* | 33.22 ± 31.90 | 34.09 ± 26.47 | 18.50 ± 20.03 | 0.001 |
*p < 0.05.
#p < 0.01.
Compared between mild and moderate disease.
Compared between mild and severe disease.
Compared between mild disease and critically ill.
Compared between moderate and severe disease.
Compared between moderate disease and critically ill.
Compared between severe disease and critically ill.
Lymphocyte subset analysis (Table 5 ) showed that CD4+, CD8+ T lymphocytes and B lymphocytes were significantly decreased in severe and critically ill patients. Natural killer (NK) cells, a key component of innate immunity against infection [7], trended lower with increasing severity, but there was no statistically significant difference among the four groups. Prothrombin time (PT) and prothrombin activity (PTA) did not significantly differ among the four groups, but fibrinogen (FIB) and D-dimer levels showed a statistically significant difference, in that the levels were much lower in the mild and moderate disease group compared with the severe disease groups.
Table 5.
Lymphocyte Subsets levels in patients with COVID-19.
| Laboratory finding | Mild disease | Moderate disease | Severe disease | Critically ill | Total | p value |
|---|---|---|---|---|---|---|
| Lymphocyte count, cells/μl | 1767.57 ± 587.82 a.b.c# | 1202.35 ± 483.66 e* | 861.50 ± 464.96 | 746.88 ± 503.44 | 1172.49 ± 560.25 | 0.001 |
| T lymphocyte count, cells/μl | 1210.75 ± 408.81 a.b.c# | 808.97 ± 371.22 d*e# | 522.57 ± 318.73 | 464.67 ± 339.68 | 778.98 ± 417.65 | 0.000 |
| CD4 T lymphocyte count, cells/μl | 689.38 ± 251.29 a.b.c# | 436.8 ± 225.08 d*e# | 257.86 ± 129.48 | 270.11 ± 162.75 | 425.36 ± 242.55 | 0.000 |
| CD8 T lymphocyte count, cells/μl | 462.88 ± 154.43 b.c# | 355.33 ± 166.86 d*e# | 205.14 ± 153.09 | 202.22 ± 199.10 | 330.43 ± 184.26 | 0.004 |
| B lymphocyte count, cells/μl | 330.71 ± 177.65 a.b.c# | 148.92 ± 89.33 | 128.83 ± 42.44 | 119.38 ± 59.07 | 164.71 ± 113.66 | 0.000 |
| NK cell count, cells/μl | 288 ± 175.93 | 203.63 ± 209.433 | 185.00 ± 180.11 | 102.88 ± 72.28 | 196.14 ± 189.03 | 0.310 |
| CD4/CD8 ratio | 1.53 ± 0.41 | 1.62 ± 1.86 | 1.28 ± 0.76 | 2.42 ± 1.56 | 1.63 ± 1.57 | 0.671 |
*p < 0.05.
#p < 0.01.
Compared between mild and moderate disease.
Compared between mild and severe disease.
Compared between mild disease and critically ill.
Compared between moderate and severe disease.
Compared between moderate disease and critically ill.
Alanine aminotransferase (ALT) levels were elevated in 16 patients (25.4%), aspartate transaminase (AST) levels were elevated in 14 patients (22.2%), alkaline phosphatase levels (ALP) were elevated in 3 patients (4.8%), and gamma-glutamyl transferase (GGT) was elevated in 21 patients (33.3%), but only AST level had statistically significant difference among the four groups (p = 0.034). Albumin level was decreased in 11 patients (17.5%), lactate dehydrogenase (LDH) levels were increased in 26 patients (41.3%), and both of albumin and LDH levels showed a statistically significant difference among the four groups. Both creatinine and urea levels were increased in patients with severe diseases, and there were significant differences between the four groups.
Creatine phosphokinase, which is mainly found in cardiac myocytes, was elevated in 25 patients (39.7%). Though there was no statistically significant difference in these four different clinical classifications, the levels of creatine phosphokinase in the severe and critically ill groups were much higher than in the mild and moderate patients.
C-reactive protein level (CRP), erythrocyte sedimentation rate (ESR), serum ferritin and interleukin-6 (IL-6) levels all had a significant elevation in the severe and critically ill groups.
1.4. Radiographic features
On admission, all patients in the mild group had normal imaging. Ground-glass opacities were found in the remaining 55 cases. Seven moderately ill patients had single lobe focal ground-glass opacities and 4 moderately ill patients had multiple unilateral ground-glass opacities. Twenty-four moderately ill patients, 10 severely ill patients and 9 critically ill patients had diffuse multiple bilateral ground-glass opacities. Pleural effusions were found in 1 case with moderate illness, 3 with severe illness and 4 patients who were critically ill (Table 6 ).
Table 6.
Radiographic features in patients with COVID-19.
| Radiographic features | Mild disease (n = 8) | Moderate disease (n = 36) | Severe disease (n = 10) | Critically ill (n = 9) |
|---|---|---|---|---|
| No Ground-glass opacities (GGO) | 8 | 0 | 0 | 0 |
| Single lobe Focal GGO | 0 | 7 | 0 | 3 |
| Multiple unilateral GGO | 0 | 4 | 0 | 0 |
| Multiple bilateral GGO | 0 | 24 | 10 | 9 |
| Consolidation | 0 | 8 | 3 | 2 |
| Pleural effusion | 0 | 1 | 3 | 4 |
| Peripheral lung distribution | 0 | 13 | 4 | 2 |
1.5. Clinical outcomes
Patients were categorized into two groups according to clinical outcome, defined by those with or without an adverse event. Adverse events were defined as any of the following: respiratory rates ≥30 breaths per minute, PaO2/FiO2 ≤300 mmHg, peripheral oxygen saturation (SpO2) ≤93%, or admission to the intensive care unit. The group with adverse events includes patients with severe disease or who are critically ill.
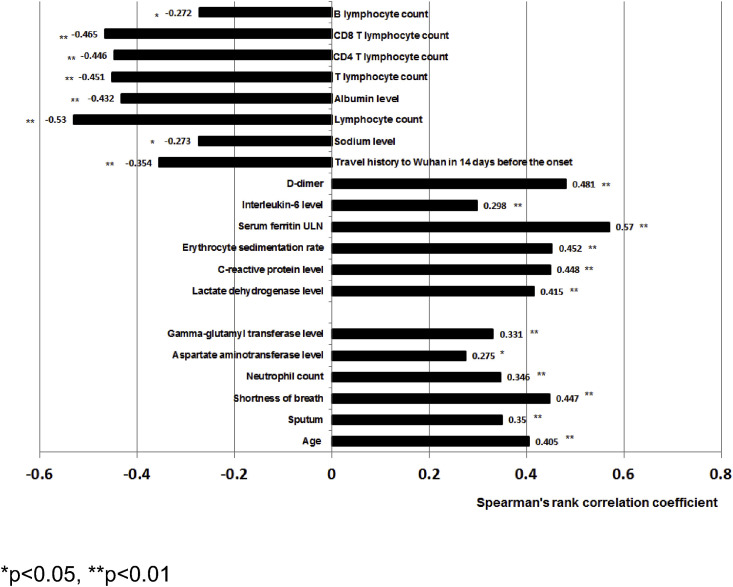
Fig. 3 shows the relationships between clinical characteristics and disease severity, using the Spearman correlation coefficient. The following variables showed significant positive correlation to the disease severity (p < 0.01): advanced age, sputum production, shortness of breath, higher neutrophil count, AST level (p < 0.05), LDH level, GGT level, CRP level, ESR level, serum ferritin level and interleukin-6. Of these, D-dimer, and serum ferritin level had a particularly strong correlation. There was also a positive correlation between ferritin levels and liver function tests (Table 7 ). Patients with higher total lymphocyte count (p < 0.01), T lymphocyte count (p < 0.01), CD8+ T lymphocyte count (p < 0.01), CD4+ T lymphocyte count (p < 0.01), B lymphocyte count (p < 0.05), albumin level (p < 0.01), and sodium level (p < 0.05), were negatively correlated with disease severity, and a travel history to Wuhan in the 14 days prior to the onset of disease was also negatively correlated with disease severity (p < 0.01).
Fig. 3.
Correlation coefficient and p value between clinical characteristics and disease severity.
Table 7.
Correlation coefficient and p value between liver enzymes and serum ferritin.
| Parameter | Spearman correlation coefficient | p values |
|---|---|---|
| ALT | 0.385 | 0.002 |
| AST | 0.437 | 0.000 |
| ALP | −0.054 | 0.673 |
| GGT | 0.204 | 0.109 |
| LDH | 0.394 | 0.001 |
The variables which showed a significant correlation with disease severity were included in a binary logistic regression model to identify that C-reactive protein level, CD8+ T lymphocyte count, and D-dimer were independent predictors of disease severity (Table 8 ).
Table 8.
Independent predictors of disease severity by multivariate Cox regression.
| Parameter | Odds Ratio | 95% CI | p value |
|---|---|---|---|
| C-reactive protein level | 1.073 | 1.013–1.136 | 0.017 |
| CD8 T lymphocyte count | 0.989 | 0.979–1.000 | 0.043 |
| D-dimer | 5.313 | 0.325–86.816 | 0.241 |
2. Discussion
The identification of predictive variables may assist physicians in the evidence-based treatment of COVID-19. We analyzed the demographic, clinical, laboratory and imaging features of 63 patients with COVID-19 in Beijing to determine potential biomarkers that may affect the prognosis and management of these patients.
COVID-19 can occur in any age group. Our data showed that mild illness appears predominately in those under 30 years of age. Older individuals develop higher levels of severity of disease. Overall, we showed that there was a slight male predominance. Laboratory analysis at the time of admission in our cohort of patients demonstrated that lymphocyte and eosinophil counts were significantly decreased in patients with severe disease (p = 0.001 and p = 0.000, respectively). Eosinopenia was not found in mild disease, but was present in 52.8%, 70% and 44.4% of patients with moderate, severe and critical illness. Evaluation of lymphocytes subsets demonstrated that CD4+, CD8+ T lymphocytes and B lymphocytes were both reduced in severe disease and critically ill patients. Natural killer (NK) cell counts were on a downward trend with increasing severity, but there was no statistical difference among the four groups.
Adam et al. reported that in patients with acute exacerbations of chronic obstructive pulmonary disease, eosinopenia increases the risk of treatment failure and in-hospital mortality [8]. Other studies have also demonstrated eosinopenia in late stage bacterial pneumonias, as well as COVID-19 [9]. This is consistent with our results of eosinopenia in more severely affected patients.
It has been shown that both SARS-CoV-1 and SARS-CoV-2 use the same angiotensin-converting enzyme 2 (ACE2) receptor to enter host cells [10,11]. The pathophysiology following viral entry is under intense study, and our data showed the total T lymphocytes, CD4+, CD8+ T lymphocytes and B lymphocyte counts were all significant decreased in severe and critically ill patients on admission. These immunological characteristics were also seen in SARS-CoV-1 infection [12]. These results indicate that SARS-CoV-2, like its predecessor has a negative impact on T-cell mediated immunity [13].
In other viral infections, such as HIV, CD4+ lymphocytes have been found to migrate to various tissues such as lymph nodes and bone marrow, supporting the theory that homing of CD4+ or CD8+ lymphocytes may be responsible for depletion of these cells in the peripheral blood [14]. In dengue virus, T cells have been capable of skin homing [15]. So the possibility exists that the reason for the lymphopenia in COVID-19 could be the result of homing of lymphocyte subsets to the lung, although the actual clinical evidence for this has yet to be uncovered.
Liver injury in patients with SARS-CoV-2 infection is not rare [16]. We found that ALT levels were elevated in 25.4% of patients, AST elevated in 22.2%, ALP elevated in 4.8%, and GGT elevated in 33.3% of patients, although only AST level was much higher in the critically ill patients and showed significance difference among the four groups. However, albumin level was much lower and LDH levels were much higher in the severity patients, and there was a statistically significant difference among the four groups (p = 0.000 and p = 0.000). Of these 63 patients, only two had a history of liver disease with normal liver function, one with hepatitis B undergoing antivirus treatment, the other with NAFLD.
We found that the inflammatory markers CRP level, ESR, serum ferritin and interleukin-6 levels were elevated in severe and critically ill groups, and that serum ferritin level was positively associated with ALT, AST and LDH levels, but not with ALP and GGT. Serum ferritin levels can be affected by iron status and may indicate a hyperimmune state. It is a marker for hemophagocytic lymphohistiocytosis [17,18], which is a known complication of viral infections. It is often accompanied by an increase in certain cytokines such as IL-2Rα [19]. Serum ferritin has been found to correlate with the presence of or severity of disease in other diseases states, including inflammatory conditions such as macrophage activation syndrome [20].
Severe disease, characterized by massive alveolar damage and progressive respiratory failure, may be related to a cytokine storm event [21]. In cytokine storm, increases in interleukin (IL)-2, IL-7, granulocyte colony stimulating factor (G-CSF), interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α may be observed. In addition to the above cytokines, the pro-inflammatory cytokines such as IL-1β and IL-6 are often significant contributors to the host response to infections and increased morbidity and mortality [22]. IL-6 is an example of a multifunctional cytokine involved in the mediation of the immune response and inflammation [23,24]. IL-6 is produced mainly by monocytes, but also be produced by interstitial fibroblasts and alveolar macrophages in the lung [25,26]. IL-6 has also been shown to be involved in the pathogenesis of acute lung injury [27]. Histological characteristics in the lung of COVID-19 patients showed diffuse alveolar damage and interstitial lymphocytes infiltrates [11]. IL-6 may act as a functional mediator to recruit lymphocytes into the lung lesions, which can further secrete IL-6 in a vicious cycle, thus aggravating the lung injury and hastening death.
Early clinical and laboratory factors contributing to disease severity were advanced age, sputum production, shortness of breath, elevated neutrophil count, AST, LDH and GGT levels, elevated CRP and ESR, high serum ferritin level, increased interleukin-6 and D-dimer. In the binary logistic regression model C-reactive protein level (OR 1.073,[CI, 1.013–1.136]; p = 0.017), CD8+ T lymphocyte count(OR 0.989,[CI, 0.979–1.000]; p = 0.043), and D-dimer (OR 5.313,[CI, 0.325–86.816]; p = 0.241) were independent predictors of disease severity. D-dimer did not reach statistical significance possibly due to its effect on other factors.
3. Conclusions
COVID-19 primarily attacks the lungs, but other organ systems can sustain injury as well, including the heart and liver. Disease severity increased with advancing age. Our findings indicated that C-reactive protein level, CD8 T lymphocyte count, and D-dimer were independent predictors of disease severity in COVID-19 patients in Beijing.
Ethical statement
There are no conflicts of interest.
Funding
No funding.
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., Sun J., Chang C. The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J. Autoimmun. 2020:102434. doi: 10.1016/j.jaut.2020.102434. 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P., Wang X., Hu C., Ping R., Hu P. Clinical features of 85 fatal cases of COVID-19 from wuhan: a retrospective observational study. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0543OC. 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.China, o N.H.C. seventh ed. 2020. New Coronavirus Pneumonia Prevention and Control Program. [Google Scholar]
- 5.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brunink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization W.H. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected. Interim guidance. 2020:28. [Google Scholar]
- 7.Hillion S., Arleevskaya M.I., Blanco P., Bordron A., Brooks W.H., Cesbron J.Y., Kaveri S., Vivier E., Renaudineau Y. The innate part of the adaptive immune system. Clin. Rev. Allergy Immunol. 2020;58:151–154. doi: 10.1007/s12016-019-08740-1. [DOI] [PubMed] [Google Scholar]
- 8.Bialas A K.K., Ciebiada M. Eosinopenia as a prognostic factor in patients with acute exacerbation of chronic obstructive pulmonary disease. Eur Respiratory Soc. 2017;50:PA2110. doi: 10.1183/1393003. [DOI] [Google Scholar]
- 9.Lavoignet C.E., Le Borgne P., Chabrier S., Bidoire J., Slimani H., Chevrolet-Lavoignet J., Lefebvre F., Jebri R., Sengler L., Bilbault P. White blood cell count and eosinopenia as valuable tools for the diagnosis of bacterial infections in the ED. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1523–1532. doi: 10.1007/s10096-019-03583-2. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30076-X. 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui W., Fan Y., Wu W., Zhang F., Wang J.Y., Ni A.P. Expression of lymphocytes and lymphocyte subsets in patients with severe acute respiratory syndrome. Clin. Infect. Dis. 2003;37:857–859. doi: 10.1086/378587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J.J., Huang J.C., Shirtliff M., Briscoe E., Ali S., Cesani F., Paar D., Cloyd M.W. CD4 lymphocytes in the blood of HIV(+) individuals migrate rapidly to lymph nodes and bone marrow: support for homing theory of CD4 cell depletion. J. Leukoc. Biol. 2002;72:271–278. [PubMed] [Google Scholar]
- 15.Rivino L. Understanding the human T cell response to dengue virus. Adv. Exp. Med. Biol. 2018;1062:241–250. doi: 10.1007/978-981-10-8727-1_17. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30057-1. 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grange S., Buchonnet G., Besnier E., Artaud-Macari E., Beduneau G., Carpentier D., Dehay J., Girault C., Marchalot A., Guerrot D. The use of ferritin to identify critically ill patients with secondary hemophagocytic lymphohistiocytosis. Crit. Care Med. 2016;44:e1045–e1053. doi: 10.1097/CCM.0000000000001878. [DOI] [PubMed] [Google Scholar]
- 18.Lin T.F., Ferlic-Stark L.L., Allen C.E., Kozinetz C.A., McClain K.L. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr. Blood Canc. 2011;56:154–155. doi: 10.1002/pbc.22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humblet-Baron S., Franckaert D., Dooley J., Ailal F., Bousfiha A., Deswarte C., Oleaga-Quintas C., Casanova J.L., Bustamante J., Liston A. IFN-gamma and CD25 drive distinct pathologic features during hemophagocytic lymphohistiocytosis. J. Allergy Clin. Immunol. 2019;143:2215–2226. doi: 10.1016/j.jaci.2018.10.068. e2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kell D.B. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genom. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahoo M., Ceballos-Olvera I., del Barrio L., Re F. Role of the inflammasome, IL-1beta, and IL-18 in bacterial infections. ScientificWorldJournal. 2011;11:2037–2050. doi: 10.1100/2011/212680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) Faseb. J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 24.Garcia-Ramirez R.A., Ramirez-Venegas A., Quintana-Carrillo R., Camarena A.E., Falfan-Valencia R., Mejia-Arangure J.M. TNF, IL6, and IL1B polymorphisms are associated with severe influenza A (H1N1) virus infection in the Mexican population. PloS One. 2015;10 doi: 10.1371/journal.pone.0144832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elias J.A., Lentz V. IL-1 and tumor necrosis factor synergistically stimulate fibroblast IL-6 production and stabilize IL-6 messenger RNA. J. Immunol. 1990;145:161–166. [PubMed] [Google Scholar]
- 26.Kotloff R.M., Little J., Elias J.A. Human alveolar macrophage and blood monocyte interleukin-6 production. Am. J. Respir. Cell Mol. Biol. 1990;3:497–505. doi: 10.1165/ajrcmb/3.5.497. [DOI] [PubMed] [Google Scholar]
- 27.Butt Y., Kurdowska A., Allen T.C. Acute lung injury: a clinical and molecular review. Arch. Pathol. Lab Med. 2016;140:345–350. doi: 10.5858/arpa.2015-0519-RA. [DOI] [PubMed] [Google Scholar]