Abstract
BACKGROUND:
Repurposed memantine, mefloquine, and metformin have putative anticancer activity. The objective of this phase 1 study was to determine the maximum tolerated doses (MTDs) of combinations of these agents with temozolomide (TMZ).
METHODS:
Adults with newly diagnosed glioblastoma who completed chemoradiation were eligible. The patients were assgined to receive doublet, triplet, or quadruplet therapy with TMZ combined with mefloquine, memantine, and/or metformin. Dose-limiting toxicities (DLTs) were determined, using a 3 + 3 study design.
RESULTS:
Of 85 enrolled patients, 4 did not complete cycle 1 (the DLT observation period) for nontoxicity reasons, and 81 were evaluable for DLT. The MTDs for doublet therapy were memantine 20 mg twice daily, mefloquine 250 mg 3 times weekly, and metformin 850 mg twice daily. For triplet therapy, the MTDs were memantie 10 mg twice daily, mefloquine 250 mg 3 times weekly, and metformin 850 mg twice daily. For triplet therapy, the MTDs were memantine 10 mg twice daily, mefloquine 250 mg 3 times weekly, and metformin 850 mg twice daily. For quadruplet therapy, the MTDs were memantine 10 mg twice daily, mefloquine 250 mg 3 times weekly, and metformin 500 mg twice daily. DLTs included dizziness (memantine) and gastrointestinal effects (metformin). Lymphopenia was the most common adverse event (66%). From study entry, the median survival was 21 months, and the 2-year survival rate was 43%.
CONCLUSIONS:
Memantine, mefloquine, and metformin can be combined safely with TMZ in patients with newly diagnosed glioblastoma.
Keywords: glioblastoma, mefloquine, memantine, metformin, temozolomide
INTRODUCTION
Despite improvements in therapy, the median survival of patients with glioblastoma (GBM) remains less than 2 years from diagnosis in trial-eligible individuals and less than 1 year in population-based studies.1 The addition of temozolomide (TMZ) during radiation therapy followed by 6 months of adjuvant therapy improved the 5-year survival rate from 1.9% to 9.8%.2 A tumor-treating fields (TTFields) device also was recently approved for use as adjuvant treatment with TMZ after the completion of chemoradiation and reportedly produced a 5-year survival rate of 13%.3 However, other than radiation, TMZ, and the TTFields device, there are no other effective standard therapies for patients with newly diagnosed GBM.
Oncologic drug repurposing is the use of existing, nononcology drugs that have potential anticancer activity as treatment for various tumors.4 Various repurposed drugs that cross the blood-brain barrier, such as psychotropic, antiepileptic, and antihypertensive drugs, have been investigated as potential treatments for GBM.5 Because of its favorable safety profile, TMZ has been used in combination therapeutic regimens with repurposed drugs such as interferon, thalidomide, isotretinoin, celecoxib, and marimastat for gliomas in clinical trials, with promising preliminary results.6–11
In preclinical studies, N-methyl-D-aspartic acid antagonists, including memantine, inhibit proliferation in GBM, medulloblastoma cell lines,12‘13 and high-grade gliomas in vivo12,14 by reducing tumor expansion, possibly through the neuroprotection of peritumoral tissue.14 Glutamate, which accumulates in the peritumoral fluid in glioma, leads to ionotropic glutamate receptor activation, local and tumor excitotoxicity, and probably necrosis, which is a pathologic characteristic of GBM.15,16 Recent studies suggest that the glutaminergic system enhances gliomagenesis by activation of the Akt pathway.17 A phase 2 study with talampanel, an amino-3-hydroxy-5 methyl-4-isoxazolepropionic acid receptor blocker, combined with radiation and TMZ for newly diagnosed GBM, produced promising results, with a median overall survival duration of 20.3 months for patients aged <70 years.18
In vitro studies of the antimalarial drugs chloroquine,19,20 quinacrine,20 and mefloquine20 have identified these agents as potential treatments for GBM. Antimalarial drugs intercalate into the DNA double helix and are lysomotropic; both of these actions can modify multiple cell functions, such as mutagenesis and resistance to chemotherapy. Chloroquine demonstrated a survival benefit in GBM when combined with standard radiotherapy and adjuvant carmustine in a retrospective study21 and in a randomized, double-blind, placebo-controlled trial.21 In vitro assays have demonstrated that mefloquine has similar activity in glioma cell lines while exhibiting higher potency, making it a more suitable choice for brain tumor treatment.20
Metformin is another drug with possible antitumor effects in various solid tumors. Metformin is used primarily in the treatment of diabetes, which is a known independent risk factor for cancer.22 A recent retrospective study indicated that diabetic patients with breast cancer who received metformin incidentally with neoadjuvant chemotherapy had a higher pathologic complete response rate.23 Preclinical studies have reported that metformin has a dual antiglioma effect, blocking cell cycle progression through decreasing cyclin D1 expression and inducing apoptosis.24 Other potential anticancer mechanisms include decreased angiogenesis through down-regulation of the vascular endothelial growth factor (VEGF) pathway, activation of 5′ adenosine monophosphate-activated protein kinase (AMPK), and inhibition of the mammalian target of rapamycin (mTOR) signaling pathway25.
There is increased interest in the use of repurposed or “repositioned” drugs selected based on molecular analysis of brain tumors26. The cytostatic effects of memantine, mefloquine, and metformin, through the aforementioned inhibition of different molecular pathways in combination with the cytotoxic effect of TMZ, potentially could prolong the survival of patients with GBM. We designed the current phase 1 clinical trial to evaluate the safety, maximum tolerated dose (MTD), and recommended phase 2 dose of the repurposed drugs memantine, mefloquine, and/or metformin in combinations with TMZ for patients with newly diagnosed GBM.
MATERIALS AND METHODS
Patients
Eligible patients were aged ≥18 years and had histologically confirmed supratentorial GBM or gliosarcoma; a Karnofsky performance status ≥60; and adequate bone marrow, liver, and renal function. Patients were required to have completed standard radiation with concurrent TMZ and to be enrolled within 5 weeks after completion of chemoradiation. All patients underwent a baseline post-treatment gadolinium-diethylenetriamine-pentacetate–enhanced magnetic resonance imaging (GD-DPTA MRI) scan within 14 days before registration on a stable or decreasing dose of steroids. Patients were required to have no evidence of progressive disease according to Response Assessment in Neuro-Oncology (RANO) criteria,27 although pseudoprogression defined according to RANO criteria was allowed. Patients who were enrolled on the mefloquine treatment arms were required to have no prolonged QTc interval >450 msec or no evidence of clinically significant arrhythmia within 14 days before registration; no concurrent cardiac disease requiring β-blocker treatment (unable to change medication to another class); no concurrent treatment with an antimalarial drug, quinine, or quinidine; no history of psychosis/schizophrenia; and were not allowed to take an enzyme-inducing anticonvulsant (which required a 2-week wash-out period before starting treatment with mefloquine). All patients were required to provide informed consent indicating that they were aware of the investigational nature of this study, in keeping with the policies of The University of Texas MD Anderson Cancer Center Institutional Review Board, which approved the protocol as consistent with the principles set forth in the Declaration of Helsinki and other ethics standards. This trial is registered on clinicaltrials.gov ( NCT01430351).
Study Design
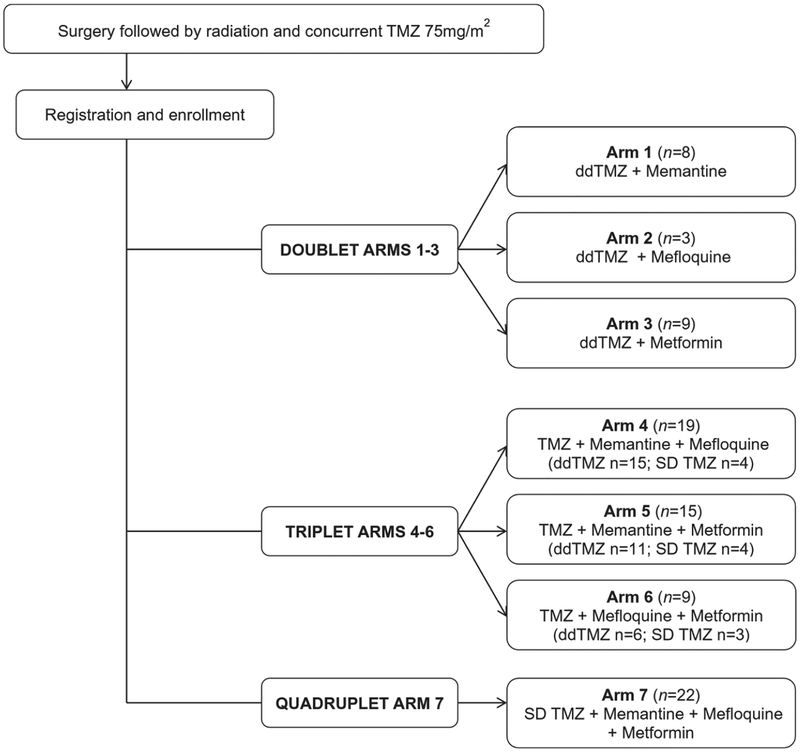
This phase 1 study with a factorial design had 7 arms (Fig. 1): arms 1, 2, and 3 consisted of doublet therapy with TMZ and 1 other drug; arms 4, 5, and 6 consisted of triplet therapy with TMZ and 2 other drugs; and arm 7 consisted of quadruplet therapy with TMZ and all 3 drugs. TMZ monotherapy was not studied in this phase 1 trial, because the safety and MTD of TMZ are well documented.28,29 Dose-limiting toxicity (DLT) was assessed at the end of the first 28-day cycle and was graded according to the National Cancer Institute’s Common Toxicity Criteria, version 4.03. A DLT included the following events: grade 3 thrombocytopenia, grade 4 anemia, grade 4 neutropenia (except afebrile neutropenia that resolved/regressed to grade 1 or less within 7 days), any grade 3 or greater nonhematologic toxic effect (excluding alopecia) lasting for ≥14 days that did not resolve/regress to grade 1 or less within 14 days of onset after optimal supportive measures; and any intolerable grade 2 toxic effect, such as fatigue or nausea (optimal medical therapy must have failed for the event to be considered a DLT).
Figure 1.
The current study design is illustrated. ddTMZ indicates dose-dense temozolomide; SD TMZ, standard-dose temozolomide; TMZ, temozolomide.
Once the MTDs were determined for the 3 doublet therapies (arms 1–3), the triplet therapies (arms 4–6) were tested; and, once the MTDs were determined for the 3 triplet therapy arms, the quadruplet therapy (arm 7) was tested.
The primary endpoint was the occurrence of DLT. Secondary endpoints were the median progression-free survival (PFS) rate, the PFS rate at 6 months, the median overall survival (OS) rate, and the 2-year survival rate.
Treatments
After undergoing maximal safe surgical resection or biopsy, patients received radiation therapy to a total dose of 60 grays (Gy) (in 2-Gy fractions) with concurrent, daily TMZ (75 mg/m2 daily) over a period of 6 weeks. Patients who had no evidence of tumor progression on a GD-DPTA MRI scan of the brain within 4 weeks after completing chemoradiation, as defined below (see Assessments), were assigned sequentially to 1 of the adjuvant chemotherapy arms, as described below (see Statistical Analysis) (Fig. 1).
At the time this trial was designed, dose-dense TMZ (ddTMZ) was under investigation to determine whether there was a survival advantage compared with standard-dose TMZ (SD TMZ) in patients with GBM, possibly by the depletion of O6-methylgiuanine-DNA methyltransferase (MGMT)30 For this reason, the initial design included ddTMZ 150 mg/m2 daily on a week-on/week-off schedule (28-day cycle). However, because the final results from a phase 2 trial of ddTMZ 150 mg/m2 daily on a week-on/week-off schedule for recurrent GBM and a phase 3 trial of ddTMZ on a 3-weeks-on/l-week-off schedule for patients with newly diagnosed GBM produced no improvement in median OS compared with SD TMZ,30,31 the protocol for the current trial was modified, and ddTMZ was changed to SD TMZ at 150 to 200 mg/m2 daily on days 1 through 5 of a 28-day cycle for a total of 12 cycles. Patients on the doublet therapy arms (arms 1–3) received ddTMZ. Figure 1 summarizes the TMZ dose schedule for each treatment arm. Pneumocystis pneumonia prophylaxis was not mandated according to the protocol.
The starting doses of the repurposed drugs were memantine 30 mg twice daily, mefloquine 250 mg 3 times weekly, and metformin 1000 mg twice daily and were selected based on previously published studies.32–35 Patients were enrolled into the first cohort of arms 1, 2 and 3 at these predetermined target doses, with a plan for dose de-escalation in subsequent cohorts if DLTs were observed. At minimum, 3 patients were enrolled at each dose level. If the starting dose was not associated with DLTs, then that dose was used for the triplet therapy arms. If 1 of the initial 3 patients at a dose level developed a DLT, then the cohort was expanded by an additional 3 patients. If 2 of the initial 3 patients developed a DLT, then the dose was de-escalated to the next lower dose level. The dose at which less than 2 of 6 patients experienced a DLT was considered the MTD for each arm.
After the MTD of each single drug in combination with TMZ (doublet therapy arms 1, 2, and 3) was determined, enrollment was started to the triplet therapy arms (arms 4, 5, and 6). The first cohort of patients was started at a dose 1 level below the MTD for each repurposed drug determined from the doublet therapy arms. If there were no DLTs, then the doses of each drug were escalated to the target levels in subsequent cohorts. Triplet therapy arms followed the same protocol as doublet therapy arms if there was a DLT.
Once the MTDs of the combinations of 2 drugs plus TMZ (triplet therapy) were determined, enrollment was started to the quadruplet therapy arm (arm 7; TMZ plus all 3 repurposed drugs). A dose-escalation protocol similar to that used for the triplet therapy arms was followed.
Assessments
Complete blood counts, renal and hepatic function tests, and pregnancy test for women of childbearing potential were obtained before randomization. Blood counts were assessed weekly during cycle 1 and every 2 weeks during subsequent cycles, and blood chemistries were assessed every 4 weeks. For patients enrolled in arms containing mefloquine, an electrocardiogram (ECG) was obtained before the initiation of treatment and before cycles 3 and 7.
DLT was assessed at the end of the first 28-day cycle and was graded according to the National Cancer Institute’s Common Toxicity Criteria, version 4.03. Treatment response was evaluated after every two 28-day cycles with a GD-DPTA MRI using RANO criteria.27
Statistical Analysis
Participants were sequentially assigned to a treatment arm at the time they completed chemoradiation. Toxicity analysis included all enrolled patients who received treatment. DLT and survival analyses included all patients who received treatment on protocol for at least 4 weeks. Participants who were lost to follow-up were censored at their last clinic visit. Patients who discontinued treatment for reasons other than progression or death were censored for PFS. The median PFS and OS were estimated using the Kaplan-Meier method from time of registration to the time of progression, death, or last follow-up. The data were analyzed using TIBCO S+ software for Windows (version 8.2; TIBCO Software Inc, Palo Alto, CA).
RESULTS
Patients
This study was open for enrollment from September 2011 to November 2015. In total, 100 patients were assessed for eligibility. Fifteen patients were excluded because of insurance denial (n = 10), noneligibility (n = 4; infratentorial tumor, elevated creatinine, prolonged QTc interval, inability to discontinue β-blocker drugs), and withdrawal of consent (n = 1). The remaining 85 patients were enrolled sequentially to 1 of the 7 treatment arms (Fig. 1). Of these 85 patients, 4 did not complete cycle 1 of treatment for reasons other than toxicity and were replaced in arm 4 (n = 1; hospitalization because of intercurrent illness not related to treatment), arm 5 (n = 2; patients’ decision to discontinue treatment), and arm 7 (n = 1; withdrawal of consent). The 4 replaced patients were not evaluable for DLT and treatment response but were evaluable for toxicity. One patient on arm 5 (combined TMZ, memantine, and metformin) developed an allergic reaction (grade 3 rash) to metformin during cycle 1 and continued therapy with TMZ plus memantine alone. Baseline characteristics of the patients who received treatment are provided in Table 1.
TABLE 1.
Patient Characteristics
| Characteristic | No. of Patients (%) | |
|---|---|---|
| All Patients, n = 85 | Quadruplet Arm 7: Memantine, Mefloquine, and Metformin, n = 22 | |
| Age: Median [range], y | 53 [21–77] | 53 [34–68] |
| Sex | ||
| Men | 54 (64) | 11 (50) |
| Women | 31 (36) | 11 (50) |
| Karnofsky performance status, | ||
| 100 | 37 (44) | 8 (36) |
| 90 | 30 (35) | 11 (50) |
| 80 | 12 (14) | 3 (14) |
| 70 | 6 (7) | 0 (0) |
| Extent of resection | ||
| Biopsy | 10 (12) | 3 (14) |
| Partial resection | 23 (27) | 9 (41) |
| Gross total resection | 52 (61) | 10 (45) |
| IDH status | ||
| Not available | 24 (30) | 3 (14) |
| Mutateda | 8/61 (13) | 1/19 (5) |
| Wild typeb | 53/61 (87) | 18/19 (95) |
Abbreviations: IDH, isocitrate dehydrogenase; IHC, immunohistochemistry;
In the IDH-mutated group, of all patients, 4 were tested by IHC only, and 4 were tested by IHC and polymerase chain reaction (PCR); and, in quadruplet arm 7, 1 patient was tested by IHC and PCR.
In the IDH wild-type group, of all patients, 5 were tested by IHC only (including 2 patients aged >55 years), 42 were tested by PCR for IDH1/IDH2, and 6 were tested by PCR IDH1 only; and, in quadruplet arm 7, 3 were tested by IHC only (including 2 patients aged >55 years), and 15 patients were tested by PCR for IDH1/IDH2.
Safety
Dose adjustments were required for 13 patients who experienced DLTs; among these patients, dizziness related to memantine was the most frequent DLT, and gastrointestinal adverse events related to metformin constituted the second most frequent. Table 2 summarizes the DLTs observed for memantine and metformin. There were no DLTs observed for mefloquine. The final dosing for each arm is indicated in Table 3. Ten patients discontinued treatment because of toxicity, and the adverse events are summarized in Table 4.
TABLE 2.
Dose-Limiting Toxic Effects
| Patient No. | Treatment Arm | Agenta | Dose, mg | Adverse Event | Grade |
|---|---|---|---|---|---|
| 1 | 1 | Memantine | 30 | Dizziness | 2 |
| 2 | 1 | Memantine | 30 | Dizziness | 3 |
| 3 | 1 | Memantine | 30 | Dizziness/confusion | 3 |
| 4 | 3 | Metformin | 1000 | Nausea | 2 |
| 5 | 3 | Metformin | 1000 | Dysgeusia | 2 |
| 6 | 4 | Memantine | 20 | Dizziness | 2 |
| 7 | 4 | Memantine | 20 | Dizziness | 3 |
| 8 | 4 | Memantine | 10 | Back pain | 2 |
| 9 | 5 | Memantine | 10 | Dizziness | 2 |
| 10 | 5 | Metformin | 850 | Fatigue, anorexia | 2 |
| 11 | 7 | Metformin | 500 | Anorexia, nausea | 2 |
| 12 | 7 | Memantine | 10 | Dizziness | 2 |
| 13 | 8 | Memantine | 10 | Dizziness | 2 |
Patients received memantine and metformin twice daily.
TABLE 3.
Final Doses
| Treatment Arm | Agenta | Dose, mg |
|---|---|---|
| 1 | Memantine | 20 |
| 2 | Mefloquine | 250 |
| 3 | Metformin | 850 |
| 4 | Memantine | 10 |
| Mefloquine | 250 | |
| 5 | Memantine | 10 |
| Metformin | 850 | |
| 6 | Mefloquine | 250 |
| Metformin | 850 | |
| 7 | Memantine | 10 |
| Mefloquine | 250 | |
| Metformin | 500 |
Patients received memantine and metformin twice daily and mefloquine 3 times weekly.
TABLE 4.
Grade 3 and 4 Adverse Events Leading to Treatment Discontinuation
| Treatment Arma | AEs Leading to Treatment Discontinuation (No. of Patients) | Grade 3 (No. of AEs) | Grade 4 (No. of AEs) |
|---|---|---|---|
| Memantine | Pancytopenia (1), poor performance status (1) | Lymphopenia (5); dizziness (3); anemia, fatigue, generalized muscle weakness, rash (2 each); thrombocytopenia, abdominal pain, pruritus, hypersomnia, hyperglycemia (1 each) | Lymphopenia, neutropenia, leukopenia, thromboembolic event (1 each) |
| Mefloquine | None | Lymphopenia, rash (1) | None |
| Metformin | Nausea with weight loss (1), intolerable nausea and vomiting (1), intolerable dysgeusia (1) | Lymphopenia (6); fatigue, generalized muscle weakness, nausea (2 each); anorexia, pruritus, dry skin, URI, urinary tract infection (1 each) | None |
| Memantine and mefloquine | Pancytopenia (1), thrombocytopenia (1), back pain and weight loss (1) | Lymphopenia (7); thrombocytopenia (3); neutropenia, leukopenia, anemia, fatigue (2 each; abdominal pain, nausea, pain in extremity, URI, back pain (1 each) | Lymphopenia (5); neutropenia (1); leukopenia, thrombocytopenia, joint infection, sepsis (1 each) |
| Memantine and metformin | Fatigue with anorexia and abdominal pain (1), fatigue with anorexia (1) | Lymphopenia (8); fatigue (3); hypersomnia (2); neutropenia, leukopenia, chest wall pain, gait disturbance (1 each) | None |
| Mefloquine and metformin | None | Lymphopenia (2); neutropenia, thrombocytopenia, anemia, weight loss (1 each) | Lymphopenia (3); URI, respiratory failure, hypoxia, headache (1 each) |
| Memantine, mefloquine, and metformin | None | Lymphopenia (6); thrombocytopenia (2); neutropenia, leukopenia, nausea, URI (1 each) | Thrombocytopenia (1) |
Abbreviations: AEs, adverse events; TMZ, temozolomide; URI, upper respiratory infection.
All arms included TMZ.
Overall, hematologic toxic effects were the most common adverse events, with lymphopenia the most frequent (n = 56; 66% of all patients) and thrombocytopenia the second most frequent (n = 44; 52% of all patients). Most lymphopenic events were grade 3 (n = 35; 41% of all patients); only 10% of patients experienced grade 4 lymphopenia (n = 9). Most of the thrombocytopenic events were grade 1. Grade 1, 2 and (less frequently) 3 fatigue was the second most common adverse event (n = 55; 65% of all patients; only 10% grade 3).
Grade 3 and 4 adverse events related to each treatment arm (definite, probable, and possible) are provided in Table 4. None of the grade 3 or 4 adverse events had a definite association with treatment. The 13 adverse events definitely related to treatment were grade 1 and 2 and comprised anemia and lymphopenia, which occurred in the same patient (n = 1; arm 2); hyperuricemia (n = 1; arm 1); hypoalbuminemia (n = 1; arm 4); hyperglycemia (n = 1; arm 5); nausea (n = 3; arm 7); vomiting (n = 1; arm 7); constipation (n = 1; arm 7); allergic reaction (n = 1; arm 7); dizziness (n = 1; arm 7); and fatigue (n = 1; arm 7). There were 2 deaths from pneumonia possibly related to treatment: the first patient was treated on arm 4 with ddTMZ, memantine, and mefloquine and developed pneumonia from influenza type A, and the second was treated on arm 6 with ddTMZ, metformin, and mefloquine and developed pneumonia not otherwise specified.
Abnormal ECG findings related to mefloquine occurred in 2 patients who were treated in arm 6. One patient developed QTc interval prolongation (grade 1) definitely related to mefloquine, which corrected after discontinuation of the agent; the other patient developed grade 1 sinus bradycardia possibly related to mefloquine.
Survival
Treatment was discontinued in 40 patients before completing all 12 cycles because of disease progression; these patients were treated in arm 1 (n = 4), arm 2 (n = 3), arm 3 (n = 3), arm 4 (n = 8), arm 5 (n = 9), arm 6 (n = 3), and arm 7 (n = 10).
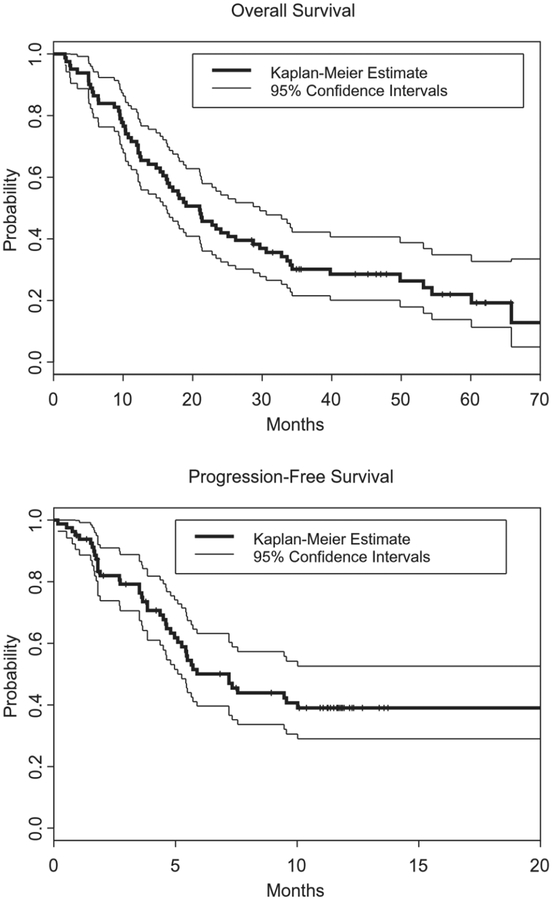
Eighty-one patients (95%) were included in the survival analysis. The Kaplan-Meier plots in Figure 2 indicates that the median PFS measured from time of registration was 7.2 months (95% confidence interval [CI], 5.3 months to not reached), with 43 events after a median follow-up of 11 months. The PFS rate at 6 months was 50% (95% CI, 40%–63%), the median OS was 21 months (95% CI, 16.2–29.7 months), and the 2-year survival rate was 43% (95% CI, 34%–56%). The overall median vital status follow-up duration at the time of the current analysis was 57 months, and there had been 62 deaths (Fig. 2).
Figure 2.
Kaplan-Meier survival estimates of (A) overall survival and (B) progression-free survival are illustrated for all evaluable patients (n = 81).
Molecular Studies
MGMT methylation status was not available for the majority of the patients (92%). Of the 7 patients for whom it was available, 4 had methylation of the MGMT gene promoter, and 3 had unmethylated MGMT. Isocitrate dehydrogenase (IDH) status results are summarized in Table 1.
DISCUSSION
The survival of patients with GBM is slowly improving but remains poor. Repurposed drugs used to treat other medical conditions have demonstrated potential anticancer activity for the treatment of GBM.5 In the current phase 1 trial in patients with newly diagnosed GBM, we observed that the combination of TMZ with 1 or more repurposed drugs (mefloquine, memantine, and metformin) was feasible and overall well tolerated.
Lymphopenia was the most common adverse event and the most common of all grade 3 and 4 adverse events. This was expected, because the backbone of each arm was TMZ, a cytotoxic drug known to induce lymphopenia. The patients with lymphopenia did not have a significantly higher rate of infections during the study period. Our results are consistent with the reported incidence of lymphopenia during a phase 1 trial combining ddTMZ with thalidomide, isotretinoin, and/or celecoxib10. The rate of grade 3 and grade 4 lymphopenia also was similar to that in a phase 2 study of ddTMZ for the treatment of recurrent GBM on a 1-week-on/l-week-off schedule31. The addition of 1 or more of the 3 repurposed drugs memantine, mefloquine, and/or metformin to TMZ did not appear to increase the risk of lymphopenia. Although fatigue was the most common nonhematologic adverse event, only 10% of patients experienced grade 3 fatigue. It is noteworthy that the adverse events, DLTs, and recommended phase 2 doses of memantine, mefloquine, and metformin in different combinations with TMZ may differ for patients who did not meet all eligibility criteria of the current study.
Dizziness was the most frequent DLT for memantine; because this agent has been associated with dizziness in patients with Alzheimer disease, this was not an unexpected adverse event in the current study36. The memantine dose had to be reduced from the original target dose of 30 mg twice daily to 20 mg twice daily in combination with TMZ and then to 10 mg twice daily in combination with TMZ and mefloquine. After dose reduction, the combination of memantine with TMZ with or without mefloquine was well tolerated. To our knowledge, there are no reported studies that evaluated the safety of combined TMZ and memantine, but our results are in accord with those from studies of memantine monotherapy in similar doses for patients with multiple sclerosis.32,37 A randomized trial for patients with brain metastasis who underwent whole-brain radiation therapy and received memantine for the prevention of cognitive dysfunction reported no difference in the rate of grade 3 and 4 adverse events compared with those who did not receive memantine. However, the maximum memantine dose in that study was 10 mg twice daily, which was the final reduced dose for the doublet therapy arm of our study.38
The most significant DLT for arms that included metformin was anorexia. The dose of metformin had to be reduced from the original target dose of 1000 mg twice daily to 850 mg twice daily in combination with TMZ because of nausea and dysgeusia, and it was reduced to 500 mg twice daily in combination with TMZ, memantine, and mefloquine. Of the patients who discontinued treatment because of toxicity, 50% were treated with metformin. Treatment was stopped because of fatigue or various gastrointestinal adverse events, such as nausea, anorexia, abdominal pain, dysgeusia, or weight loss. This indicates that metformin in combination with TMZ with or without mefloquine might increase the incidence of overlapping adverse events from these agents, such as anorexia, nausea, or weight loss. To our knowledge there are no previous studies evaluating the toxicity of the combination of TMZ and metformin. Studies evaluating the toxicity of aromatase inhibitors in combination with metformin reported no significant difference in the rate of adverse events, but the metformin dose in those studies was relatively low at 500 mg twice daily.39 In accordance with the results from our study, a phase 2 trial of metformin 1000 mg twice daily in combination with carboplatin and pemetrexed for nonsmall cell lung cancer reported 2 grade 3 adverse events: nausea and reflux.40 Like other studies, there were no clinical reports of hypoglycemia or lactic acidosis in association with metformin.39,40
No significant adverse events were attributed to mefloquine treatment. The final recommended dose of mefloquine in the doublet, triplet, and quadruplet treatment arms was the predefined target dose of 250 mg 3 times weekly. Notably, our study excluded patients who had clinically significant arrhythmia, prolonged QTc interval, or concurrent cardiac disease requiring β-blocker treatment. Abnormal ECG findings related to mefloquine occurred in 2 patients and were reversible.
Although the primary objective of this phase 1 study was to determine the tolerability of combinations of TMZ with the 3 repurposed drugs, efficacy data also were collected. With a median follow-up duration of >57 months, the median OS was 21 months, and the 2-year survival rate was 43%. Thirteen percent of our study population had IDH-mutant GBM, and MGMT testing was not performed for 88% of patients. In addition, this was a single institution, phase 1 trial and was not powered to evaluate efficacy; therefore, at this point, we cannot definitely declare improved treatment efficacy with these combinations. The efficacy results of our study should be interpreted with caution, taking into consideration the inclusion of a high percentage of patients with IDH-mutated GBM (13%), the unknown MGMT status in the majority of tumors, the high Karnofsky performance status (>80 in the majority of patients), and the enrollment after completion of chemoradiation with allowance of pseudoprogression events. Currently, a phase 1b/2 clinical trial of metformin and chloroquine is enrolling patients with IDH1/IDH2-mutant glioma.41 Future Phase 2 clinical trials with larger cohorts of patients who have GBM or other gliomas of other grades treated at homogeneous doses are needed to evaluate the efficacy of these drugs in combination with TMZ.
In conclusion, this phase 1 study demonstrated that combinations of TMZ with repurposed drugs as adjuvant treatment of newly diagnosed GBM are feasible and overall well tolerated, although at a lower dose than our initial target dose for memantine and metformin. We also have identified the doses of memantine, mefloquine, and metformin that can be used safely in combination with TMZ.
FUNDING SUPPORT
This study was supported by Departmental funds.
We acknowledge Kathy L. Hunter (Supervisor, Research Nurse), Be Lian Pei (Senior Research Nurse), and Sandra Ictech (Data Manager), all from the Department of Neuro-Oncology at The University of Texas MD Anderson Cancer Center. Editorial assistance was provided by Kathryn Hale (Department of Scientific Publications, The University of Texas MD Anderson Cancer Center).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Aaron G. Mammoser reports personal fees from NovoCure outside the submitted work. Ivo W. Tremont-Lukats reports honoraria and travel expenses from Novocure outside the submitted work. Barbara J. O’Brien reports personal fees from AbbVie Central Consultancy Group, nonfinancial support from Kadmon Corporation, and personal fees from Monteris Medical Corporation outside the submitted work. Vinay K. Puduvalli reports other from Gilead Biosciences, personal fees from SK Biosciences, personal fees from Orbus Therapeutics, outside the submitted work; honoraria from Nektar, Orbus Therapeutics, Foundation Medicine, and DePuy Companies SK Biosciences, all outside the submitted work; personal fees from Nektar, Threshold Pharmaceuticals, Novocure, and Ziopharm, all outside the submitted work; and stock and ownership interests in Giliad. Erick P. Sulman reports grants, personal fees, and nonfinancial support from Novocure; and grants and personal fees from AbbVie and Merck, all outside the submitted work. John F. de Groot reports grants from Sanofi-Aventis, AstraZeneca, EMD-Serono, Eli Lilly, Novartis, Deciphera Pharmaceuticals, and Mundipharma, all outside the submitted work; personal fees from Celldex, Deciphera Pharmaceuticals, AbbVie, FivePrime Therapeutics, Inc., GW Pharma, Eli Lilly, Boston Biomedical Inc., Kairos Venture Investments, Syneos Health, Monteris, Genentech, Celldex, Foundation Medicine, Inc., Novogen, Deciphera, AstraZeneca, Insys Therapeutics, Kadmon, Merck), and Eli Lilly, all out-side the submitted work; other financial or material interests in DSMB:VBL Therapeutics, DSMB: Novella, and VBI Vaccines, Inc., all outside the submitted work; employment with and stock ownership in Ziopharm Oncology; and stock ownership in Gilead. Marta Penas-Prado reports travel expenses from AGIOS and Lilly outside the submitted work. The remaining authors made no disclosures.
REFERENCES
- 1.Johnson DR, Omuro AMP, Ravelo A, et al. Overall survival in patients with glioblastoma before and after bevacizumab approval. Curr Med Res Opin. 2017;34:813–820. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318:2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantziarka P, Bouche G, Meheus L, Sukhatme V, Sukhatme VP, Vikas P. The Repurposing Drugs in Oncology (ReDO) Project [serial online]. Ecancermedicalscience. 2014; 8:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rundle-Thiele D, Head R, Cosgrove L, Martin JH. Repurposing some older drugs that cross the blood-brain barrier and have potential anticancer activity to provide new treatment options for glioblastoma. Br J Clin Pharmacol. 2016;81:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groves MD, Puduvalli VK, Gilbert MR, et al. Two phase II trials of temozolomide with interferon-alpha2b (pegylated and nonpegylated) in patients with recurrent glioblastoma multiforme. Br J Cancer. 2009;101:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groves MD, Puduvalli VK, Chang SM, et al. A North American Brain Tumor Consortium (NABTC 99–04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81:271–277. [DOI] [PubMed] [Google Scholar]
- 8.Jaeckle KA, Hess KR, Yung WK, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium study. J Clin Oncol. 2003;21:2305–2311. [DOI] [PubMed] [Google Scholar]
- 9.Groves MD, Puduvalli VK, Hess KR, et al. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20:1383–1388. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert MR, Gonzalez J, Hunter K, et al. A phase I factorial design study of dose-dense temozolomide alone and in combination with thalidomide, isotretinoin, and/or celecoxib as postchemoradiation adjuvant therapy for newly diagnosed glioblastoma. Neuro Oncol. 2010;12:1167–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penas-Prado M, Hess KR, Fisch MJ, et al. Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro Oncol. 2015;17:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA. 2001;98:6372–6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stepulak A, Luksch H, Gebhardt C, et al. Expression of glutamate receptor subunits in human cancers. Histochem Cell Biol. 2009;132:435–445. [DOI] [PubMed] [Google Scholar]
- 14.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7:1010–1015. [DOI] [PubMed] [Google Scholar]
- 15.Noch E, Khalili K. Molecular mechanisms of necrosis in glioblastoma: the role of glutamate excitotoxicity. Cancer Biol Ther. 2009;8:1791–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59:4383–4391. [PubMed] [Google Scholar]
- 17.Ishiuchi S, Yoshida Y, Sugawara K, et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J Neurosci. 2007;27:7987–8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009;27:4155–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim EL, Wustenberg R, Rubsam A, et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro Oncol. 2010;12:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng Y, Kohli L, Klocke BJ, Roth KA. Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro Oncol. 2010;12:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briceno E, Calderon A, Sotelo J. Institutional experience with chloroquine as an adjuvant to the therapy for glioblastoma multiforme. Surg Neurol. 2007;67:388–391. [DOI] [PubMed] [Google Scholar]
- 22.Chang YL, Sheu WH, Lin SY, Liou WS. Good glycaemic control is associated with a better prognosis in breast cancer patients with type 2 diabetes mellitus. Clin Exp Med. 2018;18:383–390. [DOI] [PubMed] [Google Scholar]
- 23.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isakovic A, Harhaji L, Stevanovic D, et al. Dual antiglioma action of metformin: cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalving M, Gietema JA, Lefrandt JD, et al. Metformin: taking away the candy for cancer? Eur J Cancer. 2010;46:2369–2380. [DOI] [PubMed] [Google Scholar]
- 26.Byron SA, Tran NL, Halperin RF, et al. Prospective feasibility trial for genomics-informed treatment in recurrent and progressive glioblastoma. Clin Cancer Res. 2018;24:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. [DOI] [PubMed] [Google Scholar]
- 28.Wick W, Steinbach JP, Kuker WM, Dichgans J, Bamberg M, Weller M. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62:2113–2115. [DOI] [PubMed] [Google Scholar]
- 29.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SJ, Rolston JD, Molinaro AM, et al. Phase II trial of 7 days on/7 days off temozolomide for recurrent high-grade glioma. Neuro Oncol. 2014;16:1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Starck M, Albrecht H, Pollmann W, Straube A, Dieterich M. Drug therapy for acquired pendular nystagmus in multiple sclerosis. J Neurol. 1997;244:9–16. [DOI] [PubMed] [Google Scholar]
- 33.Boudreau EF, Fleckenstein L, Pang LW, et al. Mefloquine kinetics in cured and recrudescent patients with acute falciparum malaria and in healthy volunteers. Clin Pharmacol Ther. 1990;48:399–409. [DOI] [PubMed] [Google Scholar]
- 34.Charles BG, Blomgren A, Nasveld PE, et al. Population pharmacokinetics of mefloquine in military personnel for prophylaxis against malaria infection during field deployment. Eur J Clin Pharmacol. 2007;63:271–278. [DOI] [PubMed] [Google Scholar]
- 35.Pennie RA, Koren G, Crevoisier C. Steady state pharmacokinetics of mefloquine in long-term travellers. Trans R Soc Trop Med Hyg. 1993;87:459–462. [DOI] [PubMed] [Google Scholar]
- 36.Bassil N, Thaipisuttikul P, Grossberg GT. Memantine ER, a once-daily formulation for the treatment of Alzheimer’s disease. Expert Opin Pharmacother. 2010;11:1765–1771. [DOI] [PubMed] [Google Scholar]
- 37.Starck M, Albrecht H, Pollmann W, Dieterich M, Straube A. Acquired pendular nystagmus in multiple sclerosis: an examiner-blind cross-over treatment study of memantine and gabapentin. J Neurol. 2010;257:322–327. [DOI] [PubMed] [Google Scholar]
- 38.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Gong C, Wang Z, et al. A randomized phase II study of aromatase inhibitors plus metformin in pretreated postmenopausal patients with hormone receptor positive metastatic breast cancer. Oncotarget. 2017;8:84224–84236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parikh AB, Kozuch P, Rohs N, Becker DJ, Levy BP. Metformin as a repurposed therapy in advanced nonsmall cell lung cancer (NSCLC): results of a phase II trial. Invest New Drugs. 2017;35:813–819. [DOI] [PubMed] [Google Scholar]
- 41.Molenaar RJ, Coelen RJ, Khurshed M, et al. Study protocol of a phase IB/II clinical trial of metformin and chloroquine in patients with IDH1-mutated or IDH2-mutated solid tumours [serial online]. BMJ Open. 2017;7:e014961. [DOI] [PMC free article] [PubMed] [Google Scholar]