Abstract
IMPORTANCE
As the US population ages, the number of operations performed on elderly patients will likely increase. Frailty predicts postoperative mortality and morbidity more than age alone, thus presenting opportunities to identify the highest-risk surgical patients and improve their outcomes.
OBJECTIVE
To examine the effect of the Frailty Screening Initiative (FSI) on mortality and complications by comparing the surgical outcomes of a cohort of surgical patients treated before and after implementation of the FSI.
DESIGN, SETTING, AND PARTICIPANTS
This single-site, facility-wide, prospective cohort quality improvement project studied all 9153 patients from a level 1b Veterans Affairs medical center who presented for major, elective, noncardiac surgery from October 1, 2007, to July 1, 2014.
INTERVENTIONS
Assessment of preoperative frailty in all patients scheduled for elective surgery began in July 2011. Frailty was assessed with the Risk Analysis Index (RAI), and the records of all frail patients (RAI score, ≥21) were flagged for administrative review by the chief of surgery (or designee) before the scheduled operation. On the basis of this review, clinicians from surgery, anesthesia, critical care, and palliative care were notified of the patient’s frailty and associated surgical risks; if indicated, perioperative plans were modified based on team input.
MAIN OUTCOMES AND MEASURES
Postoperative mortality at 30, 180, and 365 days.
RESULTS
From October 1, 2007, to July 1, 2014, a total of 9153 patients underwent surgery (mean [SD] age, 60.3 [13.5] years; female, 653 [7.1%]; and white, 7096 [79.8%]). Overall 30-day mortality decreased from 1.6% (84 of 5275 patients) to 0.7% (26 of 3878 patients, P < .001) after FSI implementation. Improvement was greatest among frail patients (12.2% [24 of 197 patients] to 3.8% [16 of 424 patients], P < .001), although mortality rates also decreased among the robust patients (1.2% [60 of 5078 patients] to 0.3% [10 of 3454 patients], P < .001). The magnitude of improvement among frail patients increased at 180 (23.9% [47 of 197 patients] to 7.7% [30 of 389 patients], P < .001) and 365 days (34.5% [68 of 197 patients] to 11.7% [36 of 309 patients], P < .001). Multivariable models revealed improved survival after FSI implementation, controlling for age, frailty, and predicted mortality (adjusted odds ratio for 180-day survival, 2.87; 95% CI, 1.98–4.16).
CONCLUSIONS AND RELEVANCE
Implementation of the FSI was associated with reduced mortality, suggesting the feasibility of widespread screening of patients preoperatively to identify frailty and the efficacy of system-level initiatives aimed at improving their surgical outcomes. Additional investigation is required to establish a causal connection.
Many patients older than 65 years undergo surgery.1 For some, surgery will confer substantial benefits (eg, extended life, improved quality of life). For others, surgery will confer burdens for patients, families, and society at large (eg, pain, distress, increased inpatient admissions, institutionalization, financial hardship, and increased health care costs).2,3 Thus, there is an imperative to identify patients at greatest risk for harm, ensure their decision-making process regarding surgery is patient centered, and provide tailored clinical care to improve surgical outcomes in high-risk patients.
Recent data indicate that frailty is a more powerful predictor of increased perioperative mortality, morbidity, and cost than predictions based on age or comorbidity alone.2–7 For example, when compared with robust patients, frail surgical patients are less likely to be discharged to home,6 more likely to be readmitted to the hospital within 30 days,3,7 and more likely to have substantially increased rates of perioperative mortality and complications.2,4,5,7,8 As such, measuring frailty substantially improves the receiver operating characteristic of predicting mortality and morbidity when compared with classic tools, such as American Society of Anesthesiologists (ASA) classification and the Lee criteria, which systematically underestimate mortality and morbidity in high-risk populations and are not suitable for rapid, system-level screening.5,7,9–11 However, to our knowledge, no published studies have examined facility-wide preoperative screening for frailty aimed at improving the care and outcomes of these vulnerable surgical patients.
The Surgical Service Line at the Veterans Affairs (VA) Nebraska–Western Iowa Health Care System (NWIHCS) in Omaha conducts 3600 operations annually, of which 41.8% are performed in those 65 years or older. Before 2011, preoperative risk assessment at the NWIHCS focused on traditional cardiopulmonary testing and evaluation by anesthesia. However, because of increasing postoperative mortality in 5 of 7 quarters before July 2011, the NWIHCS chief of surgery (J.M.J.) designed and implemented a quality improvement (QI) project called the Frailty Screening Initiative (FSI) aimed at improving postoperative survival. The FSI screened for frailty among all patients considering elective surgery, and for those identified as frail, the FSI reviewed the surgical decision making with surgeons, anesthesiologists, and palliative care physicians. Informed by the Standards for Quality Improvement Reporting Excellence 2 guidelines for reporting QI projects,12 we examined the effect of the FSI on mortality and complications by comparing the surgical outcomes of a cohort of surgical patients treated before and after implementation of the FSI.
Methods
Context
The FSI was conducted at the NWIHCS to address a clinical need of increased rates of case- and complexity-adjusted postoperative mortality. The NWIHCS is a large level 1b hospital at which 3600 operations are performed per year across 12 surgical service divisions (general surgery, vascular surgery, thoracic surgery, plastic surgery, urology, otolaryngology, ophthalmology, neurosurgery, orthopedics, oral maxillofacial, gynecology, and podiatry). Once a patient and surgeon agree to pursue surgery, the procedure is posted to the operating room schedule, and the patient is referred to the surgical evaluation unit (SEU) for perioperative risk assessment and management. The workup typically focuses on cardiopulmonary testing and optimization, but after implementing the FSI, it expanded to include frailty assessment.
The NWIHCS Institutional Review Board determined these procedures to be an operations activity not constituting research, and thus, per Veterans Health Administration policy (Handbook 1058.0513), the information presented in this article does not require informed consent or institutional review board approval.
Intervention
The FSI consisted of 2 parts: (1) screening for frailty with the goal of rapid assessment without need for patient medical record access and (2) review of surgical decision making. Beginning in July 2011, all patients presenting for elective surgical procedures at the NWIHCS were screened for frailty using the Risk Analysis Index (RAI) as part of the standard intake examination at the outpatient surgical clinics. The RAI is a 14-item questionnaire that takes less than 2 minutes to complete in a nonfrail patient, generates scores ranging from 0 to 81, and powerfully predicts postoperative mortality.14–17 To ensure adherence, the RAI score was required to schedule an operation.
Patients identified as frail (RAI score, ≥21) were flagged by the surgical quality nurse (G.P.) for administrative review by the chief of surgery (J.M.J.) or his designee. Reviewers included surgeons with a range of experience from senior staff to house officers. Reviewers examined the electronic medical record of each patient identified as frail to clarify decision making regarding surgery and optimize perioperative care. Interventions included informal discussions with the surgeons, anesthesiologists, and critical care physicians aimed at alerting them to the patient’s frailty, the attendant risks, and patient prognosis for 6-month mortality. When appropriate, formal preoperative palliative care consultation focused on clarifying goals and expectations for the surgery and postoperative recovery, including discussions regarding ventilator dependence, dialysis, and do-not-resuscitate or do-not-intubate status.
The goals of the FSI were clearly focused on assisting and enhancing the decision making shared by surgeon and patient, and the frailty score was never used to refuse an operation that the surgeon and patient wanted to pursue. However, it is likely that the frailty diagnosis occasionally changed the decision to operate, the choice of specific procedure, or the anesthetic plan, although because of the operational focus of this QI project, we were not able to capture qualitative or quantitative details about those changes. Nonetheless, the operative volume at the NWIHCS did not change substantively, suggesting that most operations proceeded as planned. In addition, and as described previously in a subgroup analysis of this cohort,15 the FSI significantly changed the pattern of palliative care consultation such that, after implementing the FSI, palliative care consultation was most often ordered before rather than after the operation and by a surgeon rather than an intensivist or hospitalist.
Statistical Analysis
To examine the effect of the FSI, we analyzed prospectively collected data from a cohort of patients treated at the NWIHCS from October 1, 2007, to July 1, 2014. These data were drawn from a QI database maintained by the NWIHCS that includes multiple quality variables, including all variables abstracted through the Veterans Affairs Surgical Quality Improvement Program (VASQIP). The database constitutes a representative sample of all the noncardiac, major elective surgical procedures conducted at the NWIHCS.18 We further linked these data to the US Department of Veterans Affairs vital statistics file to capture dates of death for all patients who had died.
To measure frailty in this cohort retrospectively, we mapped VASQIP variables to each of the 14 items of the RAI and calculated an RAI score as described elsewhere.14 We also c alculated the modified Frailty Index as previously described4,19 and used by others.20 We calculated the length of survival from the date of surgery to the date of death, presuming that patients without a date of death remained alive. Mortality rates before and after FSI implementation were compared using the Pearson χ2 tests. Two-sided P < .05 was considered significant. Multivariable logistic regression models examined the effect of the FSI on mortality, controlling for age and frailty. For illustrative purposes, we plotted Kaplan-Meier survival curves stratified by RAI score for the cohorts before and after FSI implementation, comparing the curves with pairwise Mantel-Cox log rank tests. All analyses were conducted using SPSS statistical software, version 23 (IBM Inc).
Results
Development and testing of the FSI began in July 2010. Retrospective analysis of a cohort of patients with hip fracture revealed the RAI’s promising ability to predict postoperative morbidity and mortality. Pilot tests in small convenience samples of outpatients confirmed the RAI’s ease of clinical administration. On the basis of these data, we began screening patients with the RAI in the SEU in October 2010. This approach confirmed our ability to identify a limited group of frail patients at high risk, but we learned that screening in the SEU was not ideal because not all patients were evaluated by the SEU and diagnosis of frailty was delayed until after the decision for or against surgical treatment. We therefore moved the frailty screen upstream, deploying the RAI to select surgical clinics in January 2011, with increasing adoption during 2 quarters. Weekly assessment and feedback to clinics revealed increasing adherence in nearly 90% of elective surgical patients being assessed. Given the positive feedback from the effected surgical services, we made the RAI score mandatory in July 2011: the case scheduler was instructed to record the RAI score into the electronic medical record, thus achieving near 100% adherence for elective surgical procedures.
Administrative review of frail patients initially focused on clarifying the operative plan through discussion between the reviewer and the surgeon of record. During the first 6 months, the review rapidly expanded to include formal and informal consultation with anesthesiologists and critical care physicians to develop plans for intraoperative and postoperative care informed by geriatric care principles and strategies for early recognition and treatment of expected complications (eg, rescue therapy). In addition, the reviewer recommended preoperative palliative care consultation to the surgeon of record when the medical record did not document a clear discussion of the patient’s goals of care or the high risk of surgery in the setting of frailty.
The analysis includes data from a prospective cohort of 9153 patients who underwent surgery at the NWIHCS from October 1, 2007, to July 1, 2014 (mean [SD] age, 60.3 [13.5] years; 653 females [7.1%] and 7096 white [79.8%]). These patients included all those in the local VASQIP-related QI database who were also matched to the vital statistics file for long-term survival and mortality. Demographic characteristics of the patients treated before and after FSI implementation (July 2011) were similar with regard to age, sex, race, ASA classification, and comorbidity (Table 1). Most patients were not frail, with only 6.8% scoring 21 or higher on the RAI and only 11.1% scoring higher than 0.27 on the modified Frailty Index. Patient age and frailty were similar before and after FSI implementation (mean [SD] age of 60.3 [13.4] years before and 60.3 [13.7] years after, mean [SD] RAI score of 8.36 [4.86] before and 10.33 [7.38] after, and mean [SD] modified Frailty Index score of 0.20 [0.10] before and 0.20 [0.10] after). As expected, mortality rates increased with frailty (Table 2). For example, 180-day mortality increased from 1.6% (113 of 7217 patients) among those with the lowest RAI scores to 29.6% (16 of 54 patients) among those with the highest RAI scores.
Table 1.
Demographic Characteristics of 9153 Surgical Patients From 2007 to 2014a
| Characteristic | No. (%) of Patients | |
|---|---|---|
| Before FSI (n = 5275) | After FSI (n = 3878) | |
| Sex | ||
| Male | 4876 (92.4) | 3624 (93.5) |
| Female | 399 (7.6) | 254 (6.5) |
| Race (n = 8896) | ||
| American Indian or Alaska Native | 45 (0.9) | 45 (1.2) |
| Asian | 1 (0.0) | 0 (0.0) |
| Black or African American | 77 (1.5) | 39 (1.0) |
| Declined to answer | 305 (5.9) | 180 (4.8) |
| Native Hawaiian or Pacific Islander | 4 (0.1) | 6 (0.2) |
| White | 4042 (78.8) | 3054 (81.1) |
| Unknown by patient | 654 (12.8) | 444 (11.8) |
| ASA class (n = 9118) | ||
| 1 | 120 (2.3) | 73 (1.9) |
| 2 | 1015 (19.3) | 731 (18.9) |
| 3 | 3781 (72.0) | 2755 (71.3) |
| 4 | 334 (6.4) | 299 (7.7) |
| 5 | 5 (0.1) | 5 (0.1) |
| CHF | 27 (0.5) | 7 (0.2) |
| COPD | 987 (18.7) | 328 (8.5) |
| Renal insufficiency | 24 (0.5) | 22 (0.6) |
Abbreviations: ASA, American Society of Anesthesiologists; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; FSI, Frailty Screening Initiative.
Sample sizes change depending on race, ASA classification, and missing modified Frailty Index variables.
Table 2.
Prevalence of Frailty and Associated 30-Day Mortality as Measured by RAI and mFIa
| Variable | No.(%) inCohort | Mortality, % | ||
|---|---|---|---|---|
| 30 d (n = 9153 for RAI and 6639 for mFI) | 180 d (n = 8667 for RAI and 6638 for mFI) | 365 d (n = 8056 for RAI and 6638 for mFI) | ||
| RAI score stratum | ||||
| 0–10 | 7576 (82.8) | 0.4 | 1.6 | 2.6 |
| 11–15 | 550 (6.0) | 2.9 | 9.1 | 13.2 |
| 16–20 | 406 (4.4) | 5.7 | 14.4 | 18.9 |
| 21–25 | 368 (4.0) | 4.1 | 8.7 | 14.0 |
| 26–30 | 63 (0.7) | 9.5 | 20.3 | 32.0 |
| 31–35 | 132 (1.4) | 7.6 | 14.8 | 27.0 |
| 36–62 | 58 (0.6) | 15.5 | 29.6 | 32.7 |
| Overall | 9153 (100) | 1.2 | 3.3 | 4.9 |
| mFI score | ||||
| 0.09 | 1932 (29.1) | 0.3 | 1.3 | 1.5 |
| 0.18 | 2613 (39.4) | 0.7 | 2.5 | 3.8 |
| 0.27 | 1360 (20.5) | 2.4 | 5.7 | 8.0 |
| 0.36 | 510 (7.7) | 3.9 | 9.2 | 12.0 |
| 0.45 | 165 (2.5) | 9.1 | 16.4 | 23.6 |
| 0.55 | 45 (0.7) | 2.2 | 6.7 | 13.3 |
| >0.63 | 14 (0.2) | 14.3 | 21.4 | 21.4 |
| Overall | 6639 (100) | 1.4 | 3.7 | 5.3 |
Abbreviations: mFI, modified Frailty Index; RAI, Risk Analysis Index.
The RAI scores were calculated for all 9153 patients and sorted into categories. Because of missing data, the mFI scores were calculated for only 6639 of these patients and sorted into categories. For each category, the table reports the number of patients and the within-category mortality rate at 30, 180, and 365 days.
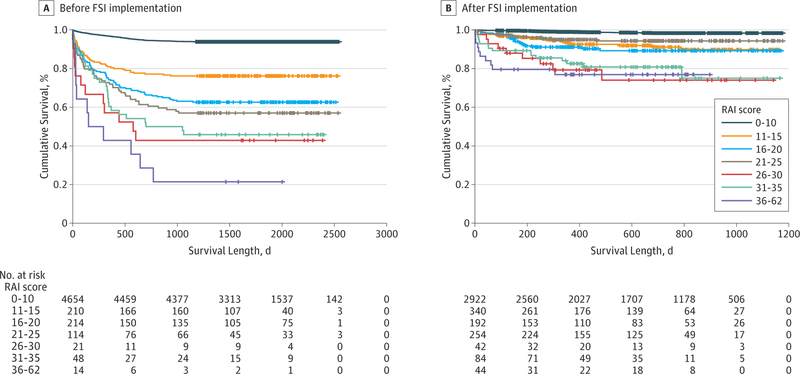
As reported in Table 3, overall 30-day mortality decreased from 1.6% (84 of 5275 patients) to 0.7% (26 of 3878 patients) (P < .001) after FSI implementation. Improvement was greatest among frail patients (12.2% [24 of 197 patients] to 3.8% [16 of 424 patients], P < .001), although mortality rates also decreased among robust patients (1.2% [60 of 5078 patients] to 0.3% [10 of 3454 patients], P < .001). The magnitude of improvement among frail patients increased at 180 (23.9% [47 of 197 patients] to 7.7% [30 of 389 patients], P < .001) and 365 days (34.5% [68 of 197 patients] to 11.7% [36 of 309 patients], P < .001). Kaplan-Meier curves (Figure) reveal that increasing frailty is associated with an increased risk of death but that these risks were significantly reduced after FSI implementation (P < .001).
Table 3.
Change in Mortality Before and After Implementing the FSIa
| 30-d Mortality |
180-d Mortality |
365-d Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BeforeFSI | After FSI | Total | Before FSI | After FSI | Total | Before FSI | After FSI | Total | |
| Overall | |||||||||
| No. dead | 84 | 26 | 110 | 223 | 66 | 289 | 320 | 78 | 398 |
| No. at risk | 5275 | 3878 | 9153 | 5275 | 3392 | 8667 | 5275 | 2781 | 8056 |
| Mortality rate, % | 1.6 | 0.7 | 1.2 | 4.2 | 1.9 | 3.3 | 6.1 | 2.8 | 4.9 |
| Nonfrail | |||||||||
| No. dead | 60 | 10 | 70 | 176 | 36 | 212 | 252 | 42 | 294 |
| No. at risk | 5078 | 3454 | 8532 | 5078 | 3003 | 8081 | 5078 | 2472 | 7550 |
| Mortality rate, % | 1.2 | 0.3 | 0.8 | 3.5 | 1.2 | 2.6 | 5.0 | 1.7 | 3.9 |
| Frail | |||||||||
| No. dead | 24 | 16 | 40 | 47 | 30 | 77 | 68 | 36 | 104 |
| No. at risk | 197 | 424 | 621 | 197 | 389 | 586 | 197 | 309 | 506 |
| Mortality rate, % | 12.2 | 3.8 | 6.4 | 23.9 | 7.7 | 13.1 | 34.5 | 11.7 | 20.6 |
Abbreviation: FSI, Frailty Screening Initiative.
Differences between mortality before and after implementing the FSI were tested using the Pearson χ2 test. Differences were significant at every time horizon and in every group (frail, nonfrail, and overall) at P < .001. Patients with a Risk Analysis Index of 21 or higher were considered frail. The number (percentage) of frail patients was 621 (6.8%) at 30 days, 586 (6.8%) at 180 days, and 506 (6.3%) at 365 days.
Figure.
The sample included all 9153 patients (5275 before FSI implementation and 3878 after FSI implementation). Mantel-Cox log rank tests for differences in the survival distribution are as follows (P < .001 for overall difference before and after FSI implementation). Before FSI implementation, the lowest 2 strata of frailty were different from each other and from all the other strata (all P < .001). There was no difference between the 16 to 20 and 21 to 25 Risk Analysis Index (RAI) strata (P = .31), although the 16 to 20 RAI stratum was different from the highest 3 strata of frailty (all P < .05). The 21 to 25 RAI stratum was not different from the 26 to 30 (P = .16) or the 31 to 35 (P = .24) RAI stratum, but it was different from the 36 to 62 RAI stratum (P = .004). Although the lines of the highest 3 strata diverge, the differences did not reach statistical significance (all P > .05); however, this is likely attributable to the low numbers in these RAI strata. After FSI implementation, the lowest frailty stratum was different from all others (P < .001), but there was no difference between the next RAI strata (eg, 11–15, 16–20, and 21–25; all P > .20), although these 3 were different from the top 3 strata (all P < .03). There was no difference between the top 3 strata (eg, 26–30, 31–35, and 36–62; all P > .50), but they were all different from each of the lowest 3 strata (all P < .05). Hash marks indicate censored data.
Multivariable models controlling for age and RAI score revealed that postoperative survival improved at each time horizon (Table 4). To examine what portion of the effect was attributable to the intervention, we added to our model an interaction between FSI implementation and frailty (eg, RAI score >21). At 30 days, the interaction was not a significant predictor of mortality (P = .66), but the interaction predicted survival at 180 and 365 days (Table 4). Finally, for all but 418 patients, our data included the probability of death predicted by the VASQIP algorithms based on patient- and procedure-related risk factors. Adding this to our model diminished the magnitude of the main effect slightly, but the overarching findings of the analysis remained robust after this control (Table 4), confirming the independent role of the screening initiative in decreasing mortality.
Table 4.
Multivariable Models Testing the Association of FSI Implementation With Survival, Controlling for Age, Frailty, and Predicted Mortalitya
| Model | 30-d Survival |
180-d Survival |
365-d Survival |
|||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Model 1 | ||||||
| FSI implementation | 4.86 (2.94–8.04) | <.001 | 4.02 (2.93–5.53) | <.001 | 4.11 (3.07–5.52) | <.001 |
| Age | 0.94 (0.92–0.96) | <.001 | 0.94 (0.93–0.95) | <.001 | 0.95 (0.94–0.96) | <.001 |
| RAI score | 0.89 (0.88–0.91) | <.001 | 0.90 (0.89–0.92) | <.001 | 0.90 (0.89–0.91) | <.001 |
| Model 2 | ||||||
| FSI implementation | 4.39 (2.53–8.54) | <.001 | 3.11 (2.17–4.48) | <.001 | 3.22 (2.31–4.48) | <.001 |
| Age | 0.94 (0.92–0.96) | <.001 | 0.94 (0.93–0.95) | <.001 | 0.95 (0.94–0.96) | <.001 |
| RAI score | 0.89 (0.87–0.91) | <.001 | 0.90 (0.88–0.91) | <.001 | 0.89 (0.88–0.90) | <.001 |
| FSI implementation × frailty | 1.24 (0.48–3.23) | .66 | 2.10 (1.12–3.92) | .02 | 2.08 (1.16–3.73) | .01 |
| Model 3 | ||||||
| FSI implementation | 3.55 (1.80–7.02) | <.001 | 2.87 (1.98–4.16) | <.001 | 2.97 (2.11–4.19) | <.001 |
| Age | 0.94 (0.92–0.96) | <.001 | 0.95 (0.93–0.96) | <.001 | 0.95 (0.94–0.96) | <.001 |
| RAI score | 0.90 (0.88–0.93) | <.001 | 0.90 (0.89–0.92) | <.001 | 0.90 (0.89–0.92) | <.001 |
| FSI implementation × frailty | 1.41 (0.49–4.08) | .53 | 2.19 (1.11–4.32) | .02 | 1.88 (1.00–3.55) | .051 |
| Predicted mortality | 0.06 (0.01–0.33) | .001 | 0.04 (0.01–0.13) | <.001 | 0.03 (0.01–0.09) | <.001 |
Abbreviations: FSI, Frailty Screening Initiative; OR, odds ratio; RAI, Risk Analysis Index.
For the interaction between FSI implementation and frailty, patients were considered frail if they had RAI scores of 21 or higher. Sample sizes for models 1 and 2 were 9153, 8667, and 8056 for the 30-day, 180-day, and 365-day survival groups, respectively. Because 418 patients were missing data on the predicted mortality based on Veterans Affairs Surgical Quality Improvement Program algorithms, the sample sizes for model 3 were 8735, 8249, and 7638 for the 30-day, 180-day, and 365-day survival groups, respectively.
Discussion
This study reveals the feasibility of facility-wide frailty screening in elective surgical populations. It also suggests the potential to improve postoperative survival among the frail through systematic administrative screening, review, and optimization of perioperative plans. The absolute reduction in 180-day mortality among frail patients was more than 19%, with improvement remaining robust even after controlling for age, frailty, and predicted mortality.
Although the initiative was aimed primarily at frail patients, improvements were noted among both frail and robust patients. This finding is likely because of a Hawthorne effect.21,22 However, improvement was greatest among frail patients at 180 and 365 days (odds ratios, 2.19 and 1.88, respectively) but not at 30 days. This finding is significant because the RAI was designed to predict medium-term mortality at 180 days. By identifying frail patients (eg, RAI score ≥21) and targeting interventions based on geriatric domains, it appears that the FSI effectively mitigated the longer-term risks associated with frailty itself. This finding also suggests that it takes more than 30 days to detect the effect of these interventions, further delineating the limitations of 30-day outcomes noted by others.23,24
The ultimate cause of the survival benefit is likely multifactorial, including changes in preoperative decision making, intraoperative management, and postoperative rescue. A consensus panel outlined several potential targets for improving the perioperative management of frail patients, including frailty-specific anesthetic plans, clarified goals of care identified in the preoperative setting, and improved postoperative management.25 Postoperative rescue therapy deserves specific consideration. Research reveals that major complications occur in 40% of frail patients after major operations, and thus frail patients frequently require attempts at rescue from those complications.20 By identifying frail patients at greatest risk for complications, the FSI may have raised the vigilance of clinicians to recognize those complications earlier and treat them more effectively. In addition, we suspect that increasing reliance on preoperative palliative care consultation and formal, preoperative documentation of goals may have improved the rate of rescue by better delineating the patient’s expectations regarding rescue therapies, such as ventilator management or dialysis in the immediate postoperative period. In fact, on more than one occasion, NWIHCS clinicians described situations when a patient became incapacitated from a complication in the postoperative period: the surrogate decision maker initiated conversations about withdrawal of care, but the palliative care consultant’s note clearly stated the patient’s intention to pursue aggressive rescue therapies for at least a time-limited trial. Having these expectations well documented by somebody other than the surgeon may help build consensus among patients’ families and clinicians, thus giving rescue therapies adequate time to treat some of the survivable complications that frail patients predictably incur. Finally, on the basis of improvement in survival not only at 30 days but also at 180 and 365 days, we suspect post-discharge care and social support were also improved through engagement of the family in the entire operative process, including long-term recovery.
Although our data cannot quantify how the FSI changed perioperative decision making, it is likely that some frail patients did not undergo surgery and are thus not included in this analysis. This potential selection bias could explain some of the effect. However, the mean frailty of the cohort after FSI implementation was actually higher than that in the cohort before FSI implementation (mean RAI scores, 10.33 vs 8.36), suggesting that many frail patients continued to seek and secure surgical treatment—even in light of preoperatively diagnosed frailty. In a previously published subgroup analysis of 310 of these patients receiving palliative care consultations, we controlled for whether patients underwent surgery, and the survival benefit remained robust (odds ratio of dying after FSI implementation, 0.37; 95% CI, 0.22–0.62).15
These results also provide estimates of the likely rates of postoperative mortality over time at different levels of frailty, and these estimates have potential to inform the shared decision making between surgeons and patients. For example, patients with RAI scores between 26 and 30 have associated mortality risks of 20.3% at 6 months, increasing to 32.0% at 1 year. The associated 30-day mortality of only 9.5% might be perceived as a better than 90% chance of survival, and thus surgeons and patients alike might persevere with surgical treatment. However, with these reliable estimates of longer-term mortality, it is likely that some patients will consider the risks too great and forgo surgery. Such longer-term mortality estimates are critical for shared decision making when the time to treatment equipoise26 for the proposed surgery approaches or exceeds the patient’s expected life span.
From a systems perspective, frailty screening with the RAI has many advantages. First, because the RAI is based on the deficit accumulation frailty model, it is easier to operationalize than functional assessments, such as the Fried frailty phenotype.27 However, because it includes 10 elements of the Canadian Study of Health and Aging Frailty Index and because a similarly abbreviated version of the Canadian Study of Health and Aging Frailty Index had excellent predictive ability and discrimination compared with the Fried frailty phenotype,27 it is likely that the RAI will also perform on par with functional assessments of frailty. Second, because the RAI encompasses multiple domains of frailty (comorbidity, functional status, nutrition, and cognition), it represents a more comprehensive frailty assessment than the modified Frailty Index, the other deficit accumulation model of frailty validated in surgical populations that only includes domains of comorbidity and functional status. Third, the RAI is the only surgical frailty measure to look beyond 30 days, predicting longer-term outcomes to 1 year. Fourth, the current study reveals the ability to screen entire populations of surgical patients with a precision that makes it flexible for clinical use.
Limitations
Our findings are limited in several ways. Most important, although we were able to control for frailty, we were unable to account for patients who screened as frail and did not undergo surgery. This limitation may be a source of significant selection bias, although it is clear that surgeons continued to operate on frail patients. Further research using a randomized controlled design will be necessary to establish the causal connection between the FSI and mortality outcomes. In addition, our QI intervention did not adhere to a formal postoperative intervention or prehabilitation protocol, thus limiting our ability to infer the causative factor behind improvement. Further research is required to standardize the approach and discern which parts of the intervention are essential and influential. The generalizability of our findings is limited to a single Veterans Affairs medical center, although the characteristics of the cohort appear comparable to other Veterans Affairs populations. Last, although survival improved, we know little about the quality of the surviving life. Future studies must confirm this assumption by assessing patient-centered outcomes, such as quality of life and the patient-centeredness of decisions.
Conclusions
To our knowledge, this is one of the first studies to document the feasibility of facility-wide screening of frailty and how implementation of an FSI is associated with improved survival at 30, 180, and 365 days. We also found that the RAI predicts postoperative mortality, with an initial calibration of the RAI to predict mortality among elective surgical patients within a Veterans Affairs population. Depending on the threshold chosen, the RAI identifies 5% to 20% of the population as potentially frail, and although further research is needed, there are several plausible interventions to improve outcomes among the frail through prehabilitation,28,29 patient-centered decision making,15 and rescue therapy. This project is timely in the changing health care environment, which incentivizes value-based care deployed to enhance population health. Hospitals and surgeons are looking for replicable models that can efficiently use existing resources and improve the quality and safety of surgery in a rapidly aging population. This study builds a platform for further investigation into the causal connections and mechanisms behind improved survival after systematic frailty screening in preoperative populations. The sustainability of FSI in the long term and implementation in different settings will depend on integration with clinical work-flow, use of electronic medical records, and standardization of intervention for frail patients.
Key Points.
Question Can surgical outcomes of frail patients be improved by facility-wide frailty screening and subsequent administrative review of perioperative surgical decision making?
Findings After implementing a quality improvement project called the Frailty Screening Initiative in a prospective cohort of 9153 patients who underwent surgery, postoperative mortality decreased significantly at 30, 180, and 365 days. Multivariate models revealed a 3-fold survival benefit after controlling for age, frailty, and predicted mortality.
Meaning Frailty screening of preoperative patients is feasible and may be an effective tool for improving surgical outcomes for an aging and increasingly frail US population.
Acknowledgments
Funding/Support: This investigation was supported by grant CDA 08–281 from the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development (Dr Hall).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Meeting Presentation: This paper was presented in part at the 2015 Clinical Congress of the American College of Surgeons; October 7, 2015; Chicago, Illinois.
Footnotes
Conflict of Interest Disclosures: Dr Hall reported serving as a consultant to the University of Pennsylvania Medical Center on frailty. Dr Johanning reported holding intellectual property on frailty through FutureAssure LLC. No other disclosures were reported.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the US Department of Veterans Affairs or the US government.
Contributor Information
Daniel E. Hall, Center for Health Equity Research and Promotion, Veterans Affairs Pittsburgh Healthcare System, Pittsburgh, Pennsylvania; Department of Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Shipra Arya, Surgical Service Line, Atlanta Veterans Affairs Medical Center, Atlanta, Georgia; Division of Vascular and Endovascular Therapy, Department of Surgery, Emory University, Atlanta, Georgia.
Kendra K. Schmid, Department of Biostatistics, University of Nebraska Medical Center, Omaha.
Mark A. Carlson, Department of Genetics, Cell Biology, and Anatomy, University of Nebraska Medical Center, Omaha; Department of Surgery, University of Nebraska Medical Center, Omaha; Department of Surgery, VA Nebraska–Western Iowa Health Care System, Omaha.
Pierre Lavedan, Department of Extended Care and Rehabilitation, VA Nebraska–Western Iowa Health Care System, Omaha.
Travis L. Bailey, Department of Surgery, VA Nebraska–Western Iowa Health Care System, Omaha; Department of Orthopaedic Surgery, University of Utah School of Medicine, Salt Lake City.
Georgia Purviance, Department of Surgery, VA Nebraska–Western Iowa Health Care System, Omaha.
Tammy Bockman, Department of Surgery, VA Nebraska–Western Iowa Health Care System, Omaha.
Thomas G. Lynch, Office of Health Operations and Management, Department of Veterans Affairs, Veterans Health Administration,Washington, DC; Department of Surgery, George Washington University School of Medicine, Washington, DC.
Jason M. Johanning, Department of Surgery, University of Nebraska Medical Center, Omaha; Department of Surgery, VA Nebraska–Western Iowa Health Care System, Omaha.
REFERENCES
- 1.Kwok AC, Semel ME, Lipsitz SR, et al. The intensity and variation of surgical care at the end of life: a retrospective cohort study. Lancet. 2011;378 (9800):1408–1413. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg. 2013;206(4):544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202(5):511–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams P, Ghanem T, Stachler R, Hall F, Velanovich V, Rubinfeld I. Frailty as a predictor of morbidity and mortality in inpatient head and neck surgery. JAMA Otolaryngol Head Neck Surg. 2013; 139(8):783–789. [DOI] [PubMed] [Google Scholar]
- 5.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. [DOI] [PubMed] [Google Scholar]
- 6.Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg. 2011;213(1):37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13(8):2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dwyer JG, Reynoso JF, Seevers GA, et al. Assessing preoperative frailty utilizing validated geriatric mortality calculators and their association with postoperative hip fracture mortality risk. Geriatr Orthop Surg Rehabil. 2014;5(3):109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anaya DA, Becker NS, Abraham NS. Global graying, colorectal cancer and liver metastasis: new implications for surgical management. Crit Rev Oncol Hematol. 2011;77(2):100–108. [DOI] [PubMed] [Google Scholar]
- 10.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145(9):646–653. [DOI] [PubMed] [Google Scholar]
- 11.Colorectal Cancer Collaborative Group. Surgery for colorectal cancer in elderly patients: a systematic review. Lancet. 2000;356(9234):968–974. [PubMed] [Google Scholar]
- 12.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veterans Health Administration. Handbook 1058.05: VHA Operations Activities That May Constitute Research. Washington, DC: Veterans Health Administration; October 28, 2011. [Google Scholar]
- 14.Hall DE, Arya S, Schmid KK, et al. Development and initial validation of the Risk Analysis Index for measuring frailty in surgical populations [published online November 23, 2016]. JAMA Surg. doi: 10.1001/jamasurg.2016.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernst KF, Hall DE, Schmid KK, et al. Surgical palliative care consultations over time in relationship to systemwide frailty screening. JAMA Surg. 2014;149(11):1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson MS, Bailey TL, Schmid KK, Lydiatt WM, Johanning JM. A frailty index identifies patients at high risk of mortality after tracheostomy. Otolaryngol Head Neck Surg. 2014;150(4):568–573. [DOI] [PubMed] [Google Scholar]
- 17.Melin AA, Schmid KK, Lynch TG, et al. Preoperative frailty Risk Analysis Index to stratify patients undergoing carotid endarterectomy. J Vasc Surg. 2015;61(3):683–689. [DOI] [PubMed] [Google Scholar]
- 18.Khuri SF, Daley J, Henderson W, et al. ; National VA Surgical Quality Improvement Program. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. Ann Surg. 1998;228(4):491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsiouris A, Hammoud ZT, Velanovich V, Hodari A, Borgi J, Rubinfeld I. A modified frailty index to assess morbidity and mortality after lobectomy. J Surg Res. 2013;183(1):40–46. [DOI] [PubMed] [Google Scholar]
- 20.Arya S, Kim SI, Duwayri Y, et al. Frailty increases the risk of 30-day mortality, morbidity, and failure to rescue after elective abdominal aortic aneurysm repair independent of age and comorbidities. J Vasc Surg. 2015;61(2):324–331. [DOI] [PubMed] [Google Scholar]
- 21.Leonard K, Masatu MC. Outpatient process quality evaluation and the Hawthorne Effect. Soc Sci Med. 2006;63(9):2330–2340. [DOI] [PubMed] [Google Scholar]
- 22.McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarze ML, Brasel KJ, Mosenthal AC. Beyond 30-day mortality: aligning surgical quality with outcomes that patients value. JAMA Surg. 2014;149 (7):631–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wancata LM, Hinshaw DB. Rethinking autonomy: decision making between patient and surgeon in advanced illnesses. Ann Transl Med. 2016;4(4):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anaya DA, Johanning J, Spector SA, et al. Summary of the panel session at the 38th Annual Surgical Symposium of the Association of VA Surgeons: what is the big deal about frailty? JAMA Surg. 2014;149(11):1191–1197. [DOI] [PubMed] [Google Scholar]
- 26.Noorani A, Hippelainen M, Nashef SA. Time until treatment equipoise: a new concept in surgical decision making. JAMA Surg. 2014;149(2):109–111. [DOI] [PubMed] [Google Scholar]
- 27.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007;62(7):738–743. [DOI] [PubMed] [Google Scholar]
- 28.Varghese TK. Strong for surgery. http://www.becertain.org/strong_for_surgery. Accessed January 11, 2016. [Google Scholar]
- 29.Englesbe MJ, Lussiez AD, Friedman JF, Sullivan JA, Wang SC. Starting a surgical home. Ann Surg. 2015;262(6):901–903. [DOI] [PubMed] [Google Scholar]