Abstract
Alzheimer's disease (AD) is a common neurodegenerative disease. Aβ plays an important role in the pathogenesis of AD. Sodium butyrate (NaB) is a short-chain fatty acid salt that exerts neuroprotective effects such as anti-inflammatory, antioxidant, antiapoptotic, and cognitive improvement in central nervous system diseases. The aim of this study is to research the protective effects of NaB on neurons against Aβ toxicity and to uncover the underlying mechanisms. The results showed that 2 mM NaB had a significant improvement effect on Aβ-induced N2a cell injury, by increasing cell viability and reducing ROS to reduce injury. In addition, by acting on the GPR109A receptor, NaB regulates the expression of AD-related genes such as APP, NEP, and BDNF. Therefore, NaB protects N2a cells from Aβ-induced cell damage through activating GPR109A, which provides an innovative idea for the treatment of AD.
1. Introduction
Alzheimer's disease (AD) belongs to neurodegenerative diseases, which is characterized by extracellular amyloid deposition and intracellular neurofibrillary tangles [1–3]. Clinically, it shows through generalized dementia such as memory impairment, aphasia, disuse, loss of recognition, impairment of visuospatial skills, executive dysfunction, and personality or behavioral changes. Although there is still controversy about the pathogenesis of AD, the more accepted hypothesis is that the abnormal decomposition or production of the amyloid precursor protein (APP) leads to the deposition of neurotoxic β-amyloid (Aβ) oligomers, triggering an amyloid cascade reaction which finally leads to neurodegeneration [4–7]. Undisputed evidence says there are insoluble amyloid plaques formed by Aβ deposition in the brain of AD patients [8]. Although significant progress has been made in the study of its pathogenesis, the current clinical treatment methods can only delay the development of AD and cannot reverse the existing neuron damage. Therefore, it is the primary task to find effective therapeutic targets and therapeutic drugs effectively, to prevent damage to the nervous system, and to repair damaged nerve cells under the toxicity of Aβ plaques, thereby improving cognitive ability in early AD patients.
Butyrate is under a wide range of biological functions. Studies have shown that butyrate plays an active role in brain disorders in a variety of neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and Huntington's disease [9–12]. Sodium butyrate (NaB) is a common form of butyrate. A study by Sun et al. showed that NaB protects brain against amphetamine-induced oxidative stress in rats [13]. Some studies indicate that the Aβ-mediated oxidative stress is a major factor in the pathology of AD [14, 15]. In an AD mouse model, NaB significantly improves the associative memory of APPPS1-21 mice (Alzheimer's mice) [16].
G protein-coupled receptors are widely found in mammals and are encoded by approximately 800 genes. Typical natural ligands for G protein-coupled receptors include hormones, mediators, neurotransmitters, polypeptides, amino acids, and ions. Studies have demonstrated that in the process of energy metabolism, certain nutrients or their metabolites, which are the basic raw materials, activate G protein-coupled receptors to regulate metabolism [17]. The GPR109A receptor, otherwise known as niacin receptor 1, belongs to the G protein-coupled receptor family. Niacin is the major ligand for GPR109A, but the physiological concentration of niacin does not reach the threshold required to activate the receptor [18], while butyrate, as a suitable candidate ligand, has the potential in binding the GPR109A receptor at a low concentration [19]. As a Gi/G0-coupled receptor, pertussis toxin (PTX) blocks the effects of GPR109A.
Therefore, this study was aimed at investigating the protective effect of NaB on N2a cells induced by Aβ in vitro and at exploring the mechanisms on how GPR109A is involved.
2. Materials and Methods
2.1. Cell Culture
Mouse neuroblastoma N2a cells were donated by the pathology laboratory of the College of Veterinary Medicine, Jilin University. All cells were cultured in DMEM medium (Gibco, Grand Island, NY 14072, USA) containing 10% fetal bovine serum (FBS) (Clark Bioscience, USA) at 37°C in a humidified incubator with 5% CO2. N2a cells were cultured in a 60 mm × 15 mm cell culture dish (Life Science, Oneonta, USA).
2.2. Treatment of N2a Cells
Aβ25-35 (synthesized by Shanghai Sangon Biological Engineering Technology & Services Co.) was diluted in distilled water at a concentration of 16 mmol/L and was maintained at 37°C for 7 days to preage the peptide. N2a cells were divided into four groups: the control (NT) group, the NaB (2 mM) group, the Aβ25-35 (40 μmol/L) treatment group, and the Aβ25-35 (40 μmol/L) with NaB (2 mM) pretreatment group. Cells were starved for 4 h and then pretreated with 2 mM NaB for 2 h before Aβ25-35 was added. RNA or protein from N2a cells was extracted after 24 h. Each experiment was repeated three times.
2.3. Cell Counting Kit-8 Assay
N2a cells were centrifuged and seeded in 96-well plates at a density of 2 × 104 cells/mL. 200 μL of PBS was added, and the cells were incubated at 37°C. After 24 hours, the complete medium was removed, and 200 μL prewarmed DMEM basal medium was added for cell starvation. Subsequently, NaB was added after 4 h to pretreat the cells, and after 2 h, Aβ25-35 was added for stimulation. There were 5 replicates for each test group. After 24 h of treatment, 10 μL CCK8 (Saint-Bio, Shanghai, China) was added to each well. After 1 h, absorbance (OD) was measured at 450 nm in a microplate reader.
2.4. Reactive Oxygen Species Assay Kit
N2a cells were seeded in a 96-well plate at a liquid volume of 2 × 104 cells/mL, and each treatment group was set to five repeats. After culturing for 24 h, replace with DMEM incomplete medium to starve the cells for 4 h. Then, 2 mM NaB was added for preprotection for 2 h. And the cells were stimulated with Aβ25-35 at a dose of 40 μM/well for 24 h. Thereafter, DCFH-DA was diluted with noncomplete medium at a ratio of 1 : 2000 and cultured for 40 min. The detection wavelength is 488 nm excitation wavelength and 525 nm emission wavelength.
2.5. Real-Time qPCR
RNA extraction, reverse transcription, and RT-PCR were performed following our previously published procedure [20]. The primer sequences are shown in Table 1.
Table 1.
Primers for real-time PCR.
| Gene | Sequences | Length (bp) |
|---|---|---|
| GPR109A | Forward: 5′-CCGTCGTGTAGTCTGTCTCGTG-3′ | 119 |
| Reverse: 5′-GCTGCGGTTATTGTTGGACT-3′ | ||
|
| ||
| APP | Forward: 5′-CCAAGGAGGGCATCTTGCAG-3′ | 138 |
| Reverse: 5′-TGTGGGTGTGTGTCTTGCAC-3′ | ||
|
| ||
| BDNF | Forward: 5′-CGACGACATCACTGGCTGAC-3′ | 124 |
| Reverse: 5′-AGCATCACCCGGGAAGTGTA-3′ | ||
|
| ||
| NEP | Forward: 5′-CTA CCG GCC AGA GTA TGC AG-3′ | 133 |
| Reverse: 5′-TTG CGG CAA TGA AAG GCA TC-3′ | ||
|
| ||
| GAPDH | Forward: 5′-GCCATCACTGCCACCCAGAA-3′ | 153 |
| Reverse: 5′-GCCAGTGAGCTTCCCGTTGA-3′ | ||
2.6. Western Blot
Total proteins were extracted from N2a cells following the procedure we described previously [20]. Subsequently, western blotting was performed according to the standard protocol [20]. Antibody information is as follows: specific antibodies including tubulin (1 : 4000), APP (1 : 5000; A8717, Sigma-Aldrich), NEP (1 : 1500; 18008-1-AP, Proteintech), BDNF (1 : 1000; 28205-1-AP, Proteintech), GPR109A (1 : 500; NBP1-92180,NOVUS), goat anti-rabbit IgG-HRP (1 : 3000; Santa Cruz Biotechnology), and goat anti-mouse IgG-HRP antibody (1 : 3000; Santa Cruz Biotechnology).
2.7. Statistical Analysis
The results are expressed as the mean ± SD (or mean ± SEM). Statistical analysis was performed using Student's t-test or one-way ANOVA followed by Tukey's post hoc test using Prism5.0 (GraphPad Software). P values < 0.05 were considered as statistically significant.
3. Result
3.1. NaB Regulates a Variety of AD-Related Genes in N2a Cells
According to our previous results, we found that NaB has effects on multiple cells in regulating gene expression. In order to examine the effect of NaB on N2a cells and obtain the optimal concentration, we detected AD-related genes in N2a cells by RT-PCR, under the treatment of 1, 2, and 3 mM NaB. Compared with the control group, 2 mM NaB had the most significant inhibitory effect on APP (Figure 1(a)) and the promotion effect on NEP and BDNF (Figures 1(b) and 1(c)). In the subsequent experiments, we chose 2 mM as the appropriate concentration of NaB. In addition, we found that NaB also significantly increases the expression of GPR109A (Figure 1(d)).
Figure 1.
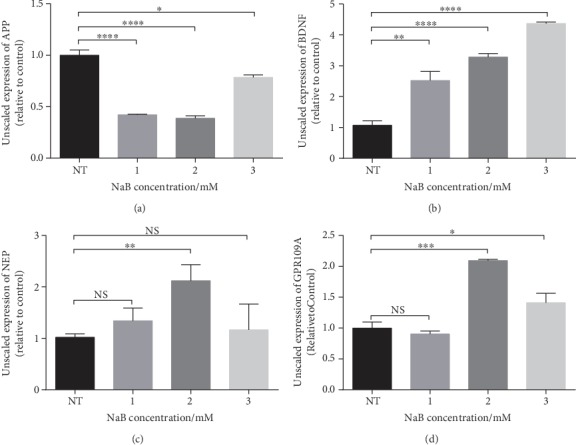
NaB regulates a variety of AD-related genes in N2a cells. N2a cells were treated with 0, 1, 2, and 3 mM NaB for 24 hours. (a–d) The mRNA expressions of APP, BENF, NEP, and GPR109A were assessed by RT-PCR (n = 3, means ± SD, Student's t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001). The effect of NaB on N2a cells is most obvious when the concentration is 2 mM.
3.2. NaB Shows Protective Effect on Aβ-Induced N2a Cell Viability Decrease
Aβ oligomer is cytotoxic and can significantly reduce cell vitality. When the concentration of Aβ reached 40 μM, Aβ caused a significant decrease in cell viability (Figure 2(a)). To investigate the protective effect of NaB on N2a cells, we used CCK-8 to detect cell viability under Aβ oligomer incubation. The results show that compared with the control group, Aβ dramatically decreased cell viability, while NaB had no significant effect on cell viability (Figure 2(b)). Compared with Aβ treatment, NaB protected N2a cells on cell viability significantly under Aβ oligomer incubation (Figure 2(b)).
Figure 2.
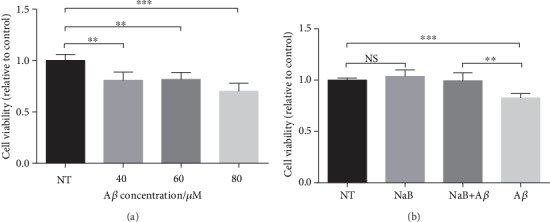
NaB shows protective effect on Aβ-induced N2a cell viability decrease. (a) Effect of Aβ25-35 on N2a cell viability. Cells were incubated with 0, 40, 60, or 80 μM Aβ25-35 for 24 hours (n = 4, means ± SD, Student's t-test, ∗∗P < 0.01, and ∗∗∗P < 0.001). (b) Effect of 2 mM NaB pretreatment on cell viability challenged by 40 μM Aβ25-35 for 24 hours. Cells were starved for 4 h and then pretreated with 2 mM NaB for 2 h before Aβ25-35 was added. Cell viability was analyzed by the CCK-8 assay (n = 4, means ± SD, Student's t-test, ∗∗P < 0.01, and ∗∗∗P < 0.001). When the concentration of Aβ is 40 μM, it had caused obvious damage to the N2a cells. After pretreatment with 2 mM NaB, cell viability was restored.
3.3. NaB Attenuates Aβ Toxicity and Maintains Mitochondrial Respiratory Function in N2a Cells
ROS are mainly produced in the mitochondrial electron-transport chain. The accumulation of ROS reflects the damage degree of mitochondrial function. Using a ROS assay kit, we found that Aβ made N2a cells produce a large amount of ROS (Figure 3), while treating with NaB significantly inhibited the production of Aβ-induced ROS in N2a cells (Figure 3).
Figure 3.
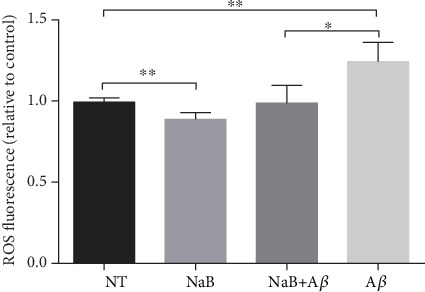
NaB attenuates Aβ toxicity and maintains mitochondrial respiratory function in N2a cells. Effect of 2 mM NaB pretreatment on ROS challenged by 40 μM Aβ25-35 for 24 hours. Cells were starved for 4 h and then pretreated with 2 mM NaB for 2 h before Aβ25-35 was added. The ROS was assessed by a ROS assay kit (n = 4, means ± SD, Student's t-test, ∗P < 0.05, and ∗∗P < 0.01). When the concentration of Aβ is 40 μM, it had led to increased ROS in N2a cells. After pretreatment with 2 mM NaB, ROS was reduced to normal levels.
3.4. NaB Suppresses APP Expression and Increases BDNF/NEP Level in GPR109A-Dependent Manner
We have proven that NaB has a positive effect on Aβ-induced neurocyte injury. To investigate whether this effect is mediated by GPR109A, we treated N2a cells with PTX, which inhibits the G protein and its physiological function in the signaling pathway. We found both in the mRNA level (Figure 4) and in the protein level (Figure 5), compared with the NaB group; after PTX treatment, the inhibitory effect of NaB on APP and the promoting effect on NEP and BDNF were alleviated. Meanwhile, NaB significantly increased GPR109A gene expression. These results indicate that the GPR109A is necessary for mediating AD-related gene expression regulated by NaB.
Figure 4.
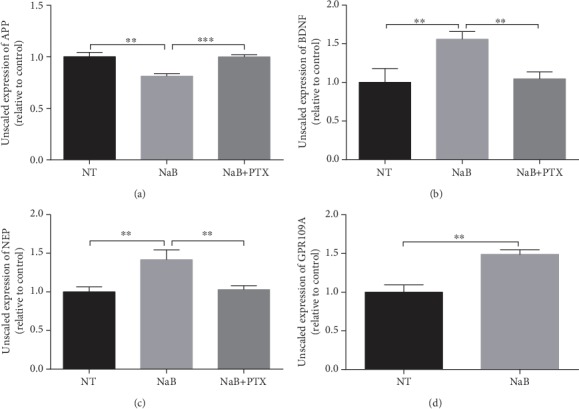
NaB suppresses APP expression and increases the BDNF/NEP level in a GPR109A-dependent manner in the mRNA level. The mRNA expressions of APP, BENF, NEP, and GPR109A were assessed by RT-PCR. The N2a cells were pretreated for 1 hour with 1 mM PTX and then incubated for 24 hours with 2 mM NaB. (a–d) PTX inhibited NaB-mediated change in APP, BDNF, NEP, and GPR109A mRNA expression (n = 3, means ± SD, Student's t-test, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001). 2 mM NaB reduced transcription of APP and increased transcription of BDNF and NEP. At the same time, NaB promoted the transcription of the GPR109A.
Figure 5.
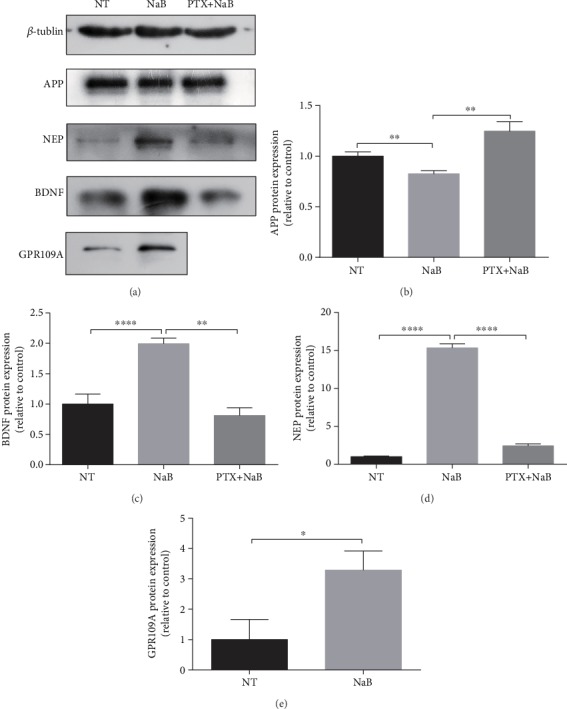
NaB suppresses APP expression and increases the BDNF/NEP level in a GPR109A-dependent manner in the protein level. The N2a cells were pretreated for 1 hour with 1 mM PTX and then incubated for 24 hours with 2 mM NaB. (a) Protein expression of APP, NEP, BDNF, and GPR109A in N2a cells was assessed by western blotting (n = 3); β-tubulin was used as an internal loading control. (b–e) Quantitative analysis of the expression of APP, BDNF, NEP, and GPR109A of no treatment (NT), NaB-treated (NaB), and PTX and NaB-treated (PTX+NaB) (n = 3, means ± SD, Student's t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001). 2 mM NaB reduced expression of APP and increased expression of BDNF and NEP. Simultaneously, NaB promoted the expression of the GPR109A.
4. Discussion
Butyrate reportedly exerts numerous beneficial effects on the gut, immune system, central nervous system, and cardiovascular system [21]. Previous studies have shown that butyrate has a positive effect on both astrocytes [22] and microglia [23–26] of the central nervous system. Moreover, previous research in our laboratory shows that β-hydroxybutyric acid as a short-chain fatty acid, the same with butyrate, protected dopaminergic neurons through inhibiting microglia-mediated neuroinflammation both in vitro and in vivo [27] and limited the excessive activation of microglia and inhibited the production of inflammatory cytokines in 5XFAD mice [28]. However, the effects and mechanism of butyrate on neuronal cell regulation, especially under AD conditions and Aβ toxicity, need to be studied well and clarified. Our results suggest that NaB reduces Aβ-induced N2a cell damage by reducing ROS. NaB inhibits the expression of APP, promotes the expression of NEP and BDNF in N2a cells by activating G proteins, especially GPR109A. These results suggest that NaB may be a candidate for the cure of AD.
Aβ is produced by the cleavage of APP by β- and γ-secretase [29]. Previous studies have found that the overexpression of APP led to the increase of Aβ, which is closely related to the occurrence of AD [30–43]. It has been shown that BHBA can improve cognitive behavior in an Alzheimer's disease mouse model. In the course of its action, the expression of APP decreased [28]. On the one hand, NEP can effectively disintegrate Aβ and slow down the development of amyloidosis [44–46]. Therefore, the dysregulation of NEP activity can promote the deposition of Aβ and affect the development of AD [47–49]. According to the research of Wei et al., it is shown that pratensein can promote the expression of NEP and reduce the deposition of Aβ [50]. In addition, early studies have shown that during the occurrence and development of AD, the expression of BDNF is reduced both in mRNA and protein levels [51–53]. Increased BDNF in the blood slowed AD development and cognitive decline [51, 53–55]. Studies have shown that the expression of BDNF is significantly reduced in an Aβ1-42-induced rat model, while pratensein can reverse the decrease of BDNF expression [50]. In our study, NaB can reduce the production of APP in mRNA and protein levels, thereby reducing the formation of Aβ. Simultaneously, NaB can also promote the generation of NEP, sequentially promoting the degradation of Aβ. In addition, NaB can also promote the production of BDNF and reduce Aβ-induced cell damage.
It has been proved that the deposition of Aβ in mitochondria and the promotion of ROS under the action of various metal ions are important features of AD during the development of AD [56–58]. The analysis of mouse models and autopsy of AD patients showed that mitochondrial dysfunction led to increased ROS, further exacerbating mitochondrial dysfunction, which further resulted in the deposition of Aβ. In AD pathology, with the accumulation of ROS, Aβ deposition caused mitochondrial dysfunction, which can lead to neuronal cell death [59]. Our study shows that NaB can reduce the production of ROS, thereby maintaining mitochondrial function and reducing Aβ-induced cell damage.
We showed the strong evidence that NaB inhibits the expression of APP, promotes the expression of NEP and BDNF, reduces the accumulation of ROS, and reverses the decrease of cell activity caused by Aβ, by activating G protein, especially GPR109A, which may provide new ideas for the treatment of AD.
Acknowledgments
The authors acknowledge the financial supported by the National Nature Science Foundation of China (Project No. 31872442) and the Graduate Innovation Fund of Jilin University (Project No. 201910183730).
Contributor Information
Yongxin Luan, Email: yongxinluan@163.com.
Wei Wang, Email: wang_wei99@jlu.edu.cn.
Data Availability
“The data used to support the findings of this study are available from the corresponding author upon request.”
Conflicts of Interest
None of the authors or funding sources have conflict of interest.
Authors' Contributions
JX Sun and BY Yuan performed most of the experiments, analyzed the results, and wrote the manuscript. W Wang and YX Luan conceived and designed the study and analyzed the data. All authors have read and approved the final manuscript. JX Sun and BY Yuan contributed equally to this work.
References
- 1.Aron L., Yankner B. A. Neural synchronization in Alzheimer's disease. Nature. 2016;540(7632):207–208. doi: 10.1038/540207a. [DOI] [PubMed] [Google Scholar]
- 2.Hui Z., Zhijun Y., Yushan Y., et al. The combination of acyclovir and dexamethasone protects against Alzheimer’s disease-related cognitive impairments in mice. Psychopharmacology. 2020;27 doi: 10.1007/s00213-020-05503-1. [DOI] [PubMed] [Google Scholar]
- 3.Roher A., Wolfe D., Palutke M., KuKuruga D. Purification, ultrastructure, and chemical analysis of Alzheimer disease amyloid plaque core protein. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(8):2662–2666. doi: 10.1073/pnas.83.8.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briggs R., Kennelly S. P., O'Neill D. Drug treatments in Alzheimer’s disease. Clinical Medicine. 2016;16(3):247–253. doi: 10.7861/clinmedicine.16-3-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiss A. B., Arain H. A., Stecker M. M., Siegart N. M., Kasselman L. J. Amyloid toxicity in Alzheimer’s disease. Reviews in the Neurosciences. 2018;29(6):613–627. doi: 10.1515/revneuro-2017-0063. [DOI] [PubMed] [Google Scholar]
- 6.Tanzi R. E., Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Wisniewski T., Goni F. Immunotherapeutic approaches for Alzheimer’s disease. Neuron. 2015;85(6):1162–1176. doi: 10.1016/j.neuron.2014.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hickman R. A., Faustin A., Wisniewski T. Alzheimer disease and its growing epidemic: risk factors, biomarkers, and the urgent need for therapeutics. Neurologic Clinics. 2016;34(4):941–953. doi: 10.1016/j.ncl.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante R. J., Kubilus J. K., Lee J., et al. Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. The Journal of Neuroscience. 2003;23(28):9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva Y. P., Bernardi A., Frozza R. L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Frontiers in Endocrinology. 2020;11:p. 25. doi: 10.3389/fendo.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Srivastav S., Neupane S., Bhurtel S., et al. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. The Journal of Nutritional Biochemistry. 2019;69:73–86. doi: 10.1016/j.jnutbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Stilling R. M., van de Wouw M., Clarke G., Stanton C., Dinan T. G., Cryan J. F. The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain axis? Neurochemistry International. 2016;99:110–132. doi: 10.1016/j.neuint.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Sun B., Jia Y., Yang S., et al. Sodium butyrate protects against high-fat diet-induced oxidative stress in rat liver by promoting expression of nuclear factor E2-related factor 2. British Journal of Nutrition. 2019;122(4):400–410. doi: 10.1017/s0007114519001399. [DOI] [PubMed] [Google Scholar]
- 14.Pohanka M. Oxidative stress in Alzheimer disease as a target for therapy. Bratislavské Lekárske Listy. 2018;119(9):535–543. doi: 10.4149/BLL_2018_097. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield D. A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nature Reviews. Neuroscience. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernando W., Martins I. J., Morici M., et al. Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer’s disease transgenic mouse model at an early disease stage. Journal of Alzheimer’s Disease. 2020;74(1):91–99. doi: 10.3233/JAD-190120. [DOI] [PubMed] [Google Scholar]
- 17.Amisten S., Neville M., Hawkes R., Persaud S. J., Karpe F., Salehi A. An atlas of G-protein coupled receptor expression and function in human subcutaneous adipose tissue. Pharmacology & Therapeutics. 2015;146:61–93. doi: 10.1016/j.pharmthera.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Wanders D., Graff E. C., Judd R. L. Effects of high fat diet on GPR109A and GPR81 gene expression. Biochemical and Biophysical Research Communications. 2012;425(2):278–283. doi: 10.1016/j.bbrc.2012.07.082. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y., Lin C., Zhang Y., Deng Y., Liu C., Yang Q. Probiotic mixture of Lactobacillus and Bifidobacterium alleviates systemic adiposity and inflammation in non-alcoholic fatty liver disease rats through Gpr109a and the commensal metabolite butyrate. Inflammopharmacology. 2018;26(4):1051–1055. doi: 10.1007/s10787-018-0479-8. [DOI] [PubMed] [Google Scholar]
- 20.Feng W., Wu Y., Chen G., et al. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in Colon in a GPR109A-dependent manner. Cellular Physiology and Biochemistry. 2018;47(4):1617–1629. doi: 10.1159/000490981. [DOI] [PubMed] [Google Scholar]
- 21.Furusawa Y., Obata Y., Fukuda S., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 22.Yang T., Rodriguez V., Malphurs W. L., et al. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiological Reports. 2018;6(14, article e13732) doi: 10.14814/phy2.13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patnala R., Arumugam T. V., Gupta N., Dheen S. T. HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Molecular Neurobiology. 2017;54(8):6391–6411. doi: 10.1007/s12035-016-0149-z. [DOI] [PubMed] [Google Scholar]
- 24.Park M. J., Sohrabji F. The histone deacetylase inhibitor, sodium butyrate, exhibits neuroprotective effects for ischemic stroke in middle-aged female rats. Journal of Neuroinflammation. 2016;13(1):p. 300. doi: 10.1186/s12974-016-0765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaworska J., Ziemka-Nalecz M., Sypecka J., Zalewska T. The potential neuroprotective role of a histone deacetylase inhibitor, sodium butyrate, after neonatal hypoxia-ischemia. Journal of Neuroinflammation. 2017;14(1):p. 34. doi: 10.1186/s12974-017-0807-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P., Zhang Y., Gong Y., et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiology of Disease. 2018;111:12–25. doi: 10.1016/j.nbd.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Fu S. P., Wang J. F., Xue W. J., et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. Journal of Neuroinflammation. 2015;12(1):p. 9. doi: 10.1186/s12974-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Y., Gong Y., Luan Y., et al. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. The FASEB Journal. 2020;34(1):1412–1429. doi: 10.1096/fj.201901984R. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Zhou X., Li G., Zhang Y., Wu Y., Song W. Modifications and trafficking of APP in the pathogenesis of Alzheimer’s disease. Frontiers in Molecular Neuroscience. 2017;10:p. 294. doi: 10.3389/fnmol.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H., Li S., Li Z., et al. Intranasal delivery of 9-cis retinoic acid reduces beta-amyloid deposition via inhibiting astrocyte-mediated inflammation. Aging. 2020;12 doi: 10.18632/aging.102970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wójtowicz S., Strosznajder A. K., Jeżyna M., Strosznajder J. B. The novel role of PPAR alpha in the brain: promising target in therapy of Alzheimer’s disease and other neurodegenerative disorders. Neurochemical Research. 2020;13 doi: 10.1007/s11064-020-02993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei C., Fan J., Sun X., et al. Acetyl-11-keto-β-boswellic acid ameliorates cognitive deficits and reduces amyloid-β levels in APPswe/PS1dE9 mice through antioxidant and anti-inflammatory pathways. Free Radical Biology & Medicine. 2020;150:96–108. doi: 10.1016/j.freeradbiomed.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Wang N., Wang H., Li L., et al. β-Asarone Inhibits Amyloid-β by Promoting Autophagy in a Cell Model of Alzheimer's Disease. Frontiers in Pharmacology. 2020;10:p. 1529. doi: 10.3389/fphar.2019.01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiwari S., Atluri V., Kaushik A., Yndart A., Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. International Journal of Nanomedicine. 2019;14:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tambini M. D., Norris K. A., D'Adamio L. Opposite changes in APP processing and human Aβ levels in rats carrying either a protective or a pathogenic APP mutation. Elife. 2020;9 doi: 10.7554/elife.52612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song W.-J., Song E.-A. C., Jung M.-S., et al. Phosphorylation and inactivation of glycogen synthase kinase 3β (GSK3β) by dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A) Journal of Biological Chemistry. 2015;290(4):2321–2333. doi: 10.1074/jbc.M114.594952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seki T., Kanagawa M., Kobayashi K., et al. Galectin 3-binding protein suppresses amyloid-β production by modulating β-cleavage of amyloid precursor protein. Journal of Biological Chemistry. 2020;295(11):3678–3691. doi: 10.1074/jbc.RA119.008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng Z., Luo Y., Xiao Z. Y. Angiopoietin-1 accelerates Alzheimer's disease via FOXA2/PEN2/APP pathway in APP/PS1 mice. Life Sciences. 2020;246:p. 117430. doi: 10.1016/j.lfs.2020.117430. [DOI] [PubMed] [Google Scholar]
- 39.Kumar D., Sharma A., Sharma L. A comprehensive review of Alzheimer’s association with related proteins: pathological role and therapeutic significance. Current Neuropharmacology. 2020;18 doi: 10.2174/1570159x18666200203101828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esquerda-Canals G., Montoliu-Gaya L., Güell-Bosch J., Villegas S. Mouse models of Alzheimer’s disease. Journal of Alzheimer's Disease. 2017;57(4):1171–1183. doi: 10.3233/JAD-170045. [DOI] [PubMed] [Google Scholar]
- 41.Dorszewska J., Prendecki M., Oczkowska A., Dezor M., Kozubski W. Molecular basis of familial and sporadic Alzheimer’s disease. Current Alzheimer Research. 2016;13(9):952–963. doi: 10.2174/1567205013666160314150501. [DOI] [PubMed] [Google Scholar]
- 42.Alsunusi S., Kumosani T. A., Glabe C. G., Huwait E. A., Moselhy S. S. In vitro study of the mechanism of intraneuronal β-amyloid aggregation in Alzheimer’s disease. Archives of Physiology and Biochemistry. 2020:1–8. doi: 10.1080/13813455.2020.1722707. [DOI] [PubMed] [Google Scholar]
- 43.Ali W., Ikram M., Park H. Y., et al. Oral administration of alpha linoleic acid rescues Aβ-induced Glia-mediated neuroinflammation and cognitive dysfunction in C57BL/6N mice, Cells. 2020;9(3):p. 667. doi: 10.3390/cells9030667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campos C. R., Kemble A. M., Niewoehner J., Freskgård P. O., Urich E. Brain shuttle neprilysin reduces central Amyloid-β levels. PLoS One. 2020;15(3, article e0229850) doi: 10.1371/journal.pone.0229850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimmer T., Goldhardt O., Yakushev I., et al. Associations of neprilysin activity in CSF with biomarkers for Alzheimer’s disease. Neurodegenerative Diseases. 2019;19(1):43–50. doi: 10.1159/000500811. [DOI] [PubMed] [Google Scholar]
- 46.Klein C., Roussel G., Brun S., et al. 5-HIAA induces neprilysin to ameliorate pathophysiology and symptoms in a mouse model for Alzheimer’s disease. Acta Neuropathologica Communications. 2018;6(1):p. 136. doi: 10.1186/s40478-018-0640-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baranello R. J., Bharani K. L., Padmaraju V., et al. Amyloid-beta protein clearance and degradation (ABCD) pathways and their role in Alzheimer’s disease. Current Alzheimer Research. 2015;12(1):32–46. doi: 10.2174/1567205012666141218140953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornung K., Zampar S., Engel N., et al. N-terminal truncated Aβ4-42 is a substrate for neprilysin degradation in vitro and in vivo. Journal of Alzheimer's Disease. 2019;67(3):849–858. doi: 10.3233/JAD-181134. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto N., Ishikuro R., Tanida M., Suzuki K., Ikeda-Matsuo Y., Sobue K. Insulin-signaling pathway regulates the degradation of amyloid β-protein via astrocytes. Neuroscience. 2018;385:227–236. doi: 10.1016/j.neuroscience.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Wei L., Lv S., Huang Q., et al. Pratensein attenuates Aβ-induced cognitive deficits in rats: enhancement of synaptic plasticity and cholinergic function. Fitoterapia. 2015;101:208–217. doi: 10.1016/j.fitote.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Giuffrida M. L., Copani A., Rizzarelli E. A promising connection between BDNF and Alzheimer’s disease. Aging. 2018;10(8):1791–1792. doi: 10.18632/aging.101518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song J. H., Yu J. T., Tan L. Brain-derived neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Molecular Neurobiology. 2015;52(3):1477–1493. doi: 10.1007/s12035-014-8958-4. [DOI] [PubMed] [Google Scholar]
- 53.Tanila H. The role of BDNF in Alzheimer's disease. Neurobiology of Disease. 2017;97(part B):114–118. doi: 10.1016/j.nbd.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Choi S. H., Bylykbashi E., Chatila Z. K., et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361(6406):p. eaan8821. doi: 10.1126/science.aan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pang J., Hou J., Zhou Z., et al. Safflower yellow improves synaptic plasticity in APP/PS1 mice by regulating microglia activation phenotypes and BDNF/TrkB/ERK signaling pathway. NeuroMolecular Medicine. 2020;11, article 8591 doi: 10.1007/s12017-020-08591-6. [DOI] [PubMed] [Google Scholar]
- 56.Chang K. W., Zong H. F., Ma K. G., et al. Activation of α7 nicotinic acetylcholine receptor alleviates Aβ1-42-induced neurotoxicity via downregulation of p38 and JNK MAPK signaling pathways. Neurochemistry International. 2018;120:238–250. doi: 10.1016/j.neuint.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 57.Kowalska M., Wize K., Prendecki M., Lianeri M., Kozubski W., Dorszewska J. Genetic variants and oxidative stress in Alzheimer’s disease. Current Alzheimer Research. 2020;17 doi: 10.2174/1567205017666200224121447. [DOI] [PubMed] [Google Scholar]
- 58.Li B., Liu J., Gu G., Han X., Zhang Q., Zhang W. Impact of neural stem cell‐derived extracellular vesicles on mitochondrial dysfunction, sirtuin 1 level and synaptic deficits in Alzheimer’s disease. Journal of Neurochemistry. 2020;(article e15001) doi: 10.1111/jnc.15001. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad W., Ijaz B., Shabbiri K., Ahmed F., Rehman S. Oxidative toxicity in diabetes and Alzheimer’s disease: mechanisms behind ROS/RNS generation. Journal of Biomedical Science. 2017;24(1, article 379):p. 76. doi: 10.1186/s12929-017-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
“The data used to support the findings of this study are available from the corresponding author upon request.”


