Abstract
Background
The present article describes two cases of patients with coronary arteritis (CA) whose identification of CA diagnosis (late vs. early) resulted in different clinical courses and outcomes.
Case summary
Case 1 is a 53-year-old woman with multiple coronary risk factors who was admitted with acute coronary syndrome (ACS) and significant stenosis in the left main trunk (LMT). Although clues suggested arteritis (LMT lesion without any other stenosis, occlusion of left internal thoracic artery, etc.), the diagnosis of CA (coronary involvement of unclassified arteritis) was delayed and revascularization, including coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI), was performed under uncontrolled inflammatory status. As a result, Case 1 experienced repeated ACS episodes due to graft failure and in-stent restenosis, and repeatedly underwent PCI. Case 2 is a 76-year-old woman with no significant coronary risk factors who was admitted with ACS. This patient was successfully diagnosed with coronary involvement of Takayasu arteritis before revascularization. Coronary artery bypass grafting was performed after stabilizing inflammation with prednisolone, and the patient remains angina-free beyond 1-year post-CABG. In both cases, intravascular imaging clearly identified the localization and degree of inflammation related to CA by demonstrating specific findings (ambiguous typical three-layer structure of arterial wall and extended low-echoic areas within adventitia).
Discussion
Accurate and early diagnosis with meticulous diagnostic and therapeutic strategies appear to be important for favourable clinical outcomes in the medical treatment of patients with coronary involvement of arteritis. Intravascular imaging has the potential to contribute to optimizing clinical management of CA.
Keywords: Coronary arteritis, Takayasu arteritis, Unclassified arteritis, Intracoronary imaging, Coronary computed tomography angiography, Case series
Learning points
In patients with coronary arteritis (CA), timely diagnosis and controlling active inflammation before revascularization are important for achieving optimal therapies and improving clinical outcomes.
The integration of multimodality imaging data, such as computed tomography angiography, positron emission tomography–computed tomography, and intravascular imaging, may contribute greatly to the management of CA.
Introduction
Coronary arteritis (CA) is a rare but devastating complication of medium- to large-vessel vasculitis, such as Takayasu arteritis (TA), giant cell arteritis, and unclassified arteritis.1,2 When a patient with no significant coronary risk factors presents with coronary artery disease (CAD), it may be easier to consider CA as a differential diagnosis. However, CA can serve as an initial presentation of systemic vasculitis, even in elderly patients with multiple coronary risk factors.3,4 In this situation, it may be more difficult to ascertain the diagnosis of CA. Because the pathophysiology of CA is completely different from that of coronary atherosclerosis, an accurate diagnosis is essential to achieve optimal therapeutic strategies in CA patients. The present article describes two cases of patients with acute coronary syndromes (ACS) who suffered from the same disease (i.e. CA) but whose identifications of CA diagnosis (late vs. early) resulted in different clinical courses and outcomes.
Timeline
| Events | |
|---|---|
| Patient 1 | |
| Admission 1 | Acute coronary syndrome (ACS) and hospital admission |
| 6 January | Isolated left main trunk (LMT) disease, Coronary artery bypass grafting [CABG; aorta–radial artery (RA)–left anterior descending artery (LAD)] performed |
| 6 February | RA graft failure, plain old balloon angioplasty (POBA) performed |
| Admission 2 | ACS and hospital admission |
| 6 May | Total occlusion in RA graft and LMT disease progression Two sirolimus-eluting stent (SES) implanted from LMT to proximal LAD |
| Admission 3 | ACS and hospital admission |
| 6 December | Severe in-stent restenosis (ISR; SES), another SES implanted Diagnosed unclassified arteritis and started prednisolone (PSL) |
| Admission 4 | ACS and hospital admission |
| 12 May | Severe ISR (SES), POBA performed |
| Admission 5 | ACS and hospital admission |
| 12 December | ISR in LMT and LAD, and de novo lesion in left circumflex artery (LCX) ostium |
| POBA performed in ISR in LAD; everolimus-eluting stent (EES) implanted in LMT-LCX | |
| Admission 6 | ACS and hospital admission |
| 17 June | LMT bifurcation ISR, drug-coated balloon (DCB) performed |
| Admission 7 | ACS and hospital admission |
| 18 January | ISR (in both SES and EES), DCB performed |
| Outpatient | |
| 18 May–present | Stable (no coronary event) |
| Patient 2 | |
| Admission 1 | ACS and hospital admission |
| 17 November | Diagnosed Takayasu arteritis (TA) and started PSL, stenoses in right coronary artery (RCA), LMT, and LCX |
| CABG (left subclavian artery-saphenous vein graft-LAD-posterolateral branch, gastroepiploic artery-RCA) performed | |
| Outpatient | |
| 18 August– present | Stable (no coronary event) |
Case presentation
Case 1
A 53-year-old woman with history of hypertension, hyperlipidaemia, diabetes mellitus, and synovitis–acne–pustulosis–hyperostosis–osteomyelitis (SAPHO) syndrome presented to our hospital with worsening chest pain. The electrocardiogram showed ST-segment depression in leads V4–V6. The echocardiogram detected hypokinesis of the anterior wall. There was no significant elevation of cardiac troponin I (<0.04 ng/mL) on admission and at 6 h later. The C-reactive protein (CRP) level on admission was 2.927 mg/dL (normal range: <0.14 mg/dL). She was admitted with diagnosis of ACS. Coronary angiogram (CAG) revealed a significant stenosis in the proximal left main trunk (LMT) (Supplementary material online, Figure S1A). Although computed tomography angiography (CTA) observed no inflammatory thickening of the aorta with major-branch involvement, there was occlusion of left internal thoracic artery (LITA). The patient underwent coronary artery bypass grafting (CABG) with free radial artery graft to revascularize the left anterior descending artery (LAD) because intraoperative findings also demonstrated no abnormal appearance of the aorta. However, the patient underwent numerous percutaneous coronary interventions (PCIs) with balloon dilation and/or drug-eluting balloon, sirolimus-eluting stents (SES), and everolimus-eluting stents, because of graft failure and frequent restenosis due to the progression of coronary involvement of arteritis (seven PCI during 12-year follow-up period) (Timeline).
After several restenoses, the patient was diagnosed with coronary involvement of unclassified arteritis based on the clinical course: (i) isolated LMT disease and de novo occlusion of LITA with no inflammatory findings of the aorta and its major branches3; (ii) persistently elevated CRP (2.927–5.463 mg/dL during 1 year after the first admission) and erythrocyte sedimentation rate; (iii) the presence of SAPHO syndrome which has been related to arteritis5; and (iv) repeat revascularization with unusual findings of CAG and intravascular imaging. Coronary angiogram observed diffuse bead-like pattern of multiple focal stenosis and remarkably proliferated neovascularization6 (Figure 1A and B and Supplementary material online, Videos S1 and S2). Optical coherence tomography showed concentric neointimal thickening with multiple microvessels communicating with lumen and peri-strut low-intensity area; intravascular ultrasound (IVUS) demonstrated ambiguous three-layered structure of intima, media and adventitia, various degrees of low-echoic areas within adventitia (peri-arterial low-echoic area: PLEA) and peri-arterial small vessels within stents and adjacent stent edges (Figure 1C–F), suggesting increased inflammatory status of coronary arteries.7
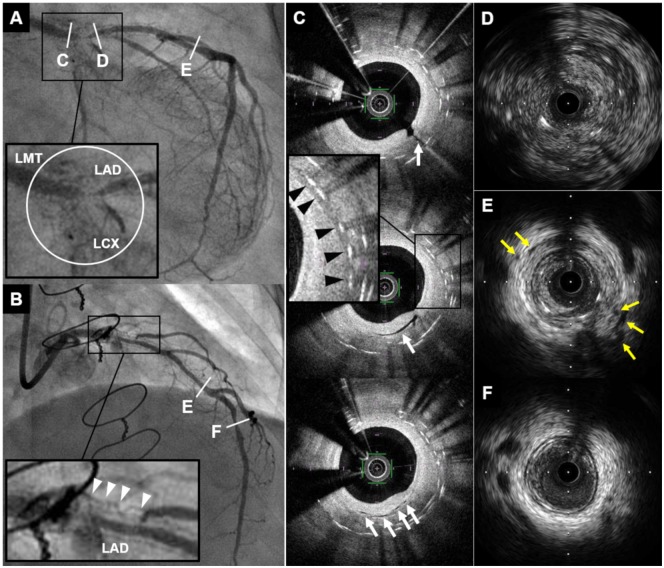
Figure 1.
Coronary angiogram and intracoronary imaging findings of Case 1. (A and B) Coronary angiogram demonstrated severe in-stent restenosis with remarkable proliferated neovascularization around the stented segment (white circle and white arrowheads) in distal left main trunk to ostium of left anterior descending artery and left circumflex coronary artery. (C) Optical coherence tomography revealed multiple microvessels communicating with lumen (white arrows) and peri-strut low intensity area (black arrowheads) within neointima. (D–F) Intravascular ultrasound showed ambiguous three-layered structure within the stented and non-stented segments (D), and various degrees of peri-arterial low-echoic area and peri-arterial small vessels (yellow arrows) within stents and near distal stent edge (E). In contrast, these findings were not apparent in distal left anterior descending artery (F). CAG, coronary angiography; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex coronary artery; LMT, left main trunk; OCT, optical coherence tomography; PLEA, peri-arterial low-echoic area.
In addition to intensive anti-atherosclerotic treatments [low-density lipoprotein cholesterol (LDL-C) was maintained around 70 mg/dL during the clinical course], prednisolone (PSL) treatment (half-dose methylprednisolone pulses followed by 40 mg/day of oral PSL) was started on Admission 2 to stabilize inflammation, and then meticulous dose adjustment (4–30 mg/day) was performed according to the inflammatory status during the clinical course. The patient continued to take PSL and to be stable over one year since the last admission (Admission 8) (CRP level was 0.085 mg/dL under PSL 14 mg/day).
Case 2
A 76-year-old woman with neither significant coronary risk factors nor past medical history presented with new onset of chest pain and was admitted with ACS. Troponin I level was within normal range but inflammatory markers on admission mildly elevated (CRP 0.648 mg/dL). The electrocardiogram showed Q and negative T waves in leads III and aVF and the echocardiogram demonstrated segmental asynergy of the inferior wall. Coronary CTA revealed a severe stenosis with aneurysm in the mid-right coronary artery (RCA), and calcified stenoses in both LMT and LCX (Figure 2A–D). There was also concentric wall thickening with high-attenuation on plain CT and with low-attenuation on contrast-enhanced CTA (Figures 2E–G), as well as ‘double ring enhancement’ on delayed phase contrast-enhanced CTA due to poorly enhanced edematous intima and enhanced outer ring with inflammation in media and adventitia (Figure 2H) in the ascending aorta, which are typical findings of early TA.8 Additionally, CTA showed a mild stenosis in the proximal segment of the left subclavian artery (LSCA) near the orifice of LITA. Positron emission tomography-CT (PET-CT) identified increased F-18 fluorodeoxyglucose (FDG) uptake in the wall of ascending aorta and proximal left and right coronary arteries (Figure 2I). Following these non-invasive imaging tests, CAG confirmed significant stenoses in RCA, LMT, and LCX. As in Case 1, IVUS observed extended PLEA and/or obscured three-layered structure in both LMT and proximal LAD, where increased FDG uptakes were observed on PET-CT (Figure 3A–D). The patient was diagnosed with TA according to the guideline from Japanese Circulation Society and started on PSL treatment (oral PSL 20 mg/day). After stabilization of inflammatory status (CRP 0.013 mg/dL), CABG was performed using saphenous vein graft (SVG) and gastroepiploic artery graft. Saphenous vein graft was sutured to the distal part of LSCA because no significant inflammatory changes were seen either on PET-CT or visual assessment, and sequential SVG anastomosis to the mid-LAD and posterolateral (PL) branch was performed. Gastroepiploic artery graft was also anastomosed to the distal RCA segment. Intraoperative findings demonstrated a white pottery-like appearance (suggestive of inflammation) in the wall of ascending aorta (Supplementary material online, Figure S2A). The patient responded well to PSL and was discharged after confirming patency of all grafts on CTA (Supplementary material online, Figure S2B). At 9-month post-CABG, both contrast-enhanced CTA and PET-CT demonstrated improved inflammatory status in ascending aorta and coronary arteries; the patient remains angina-free beyond 1 year after CABG under PSL (6.5 mg/day) treatment.
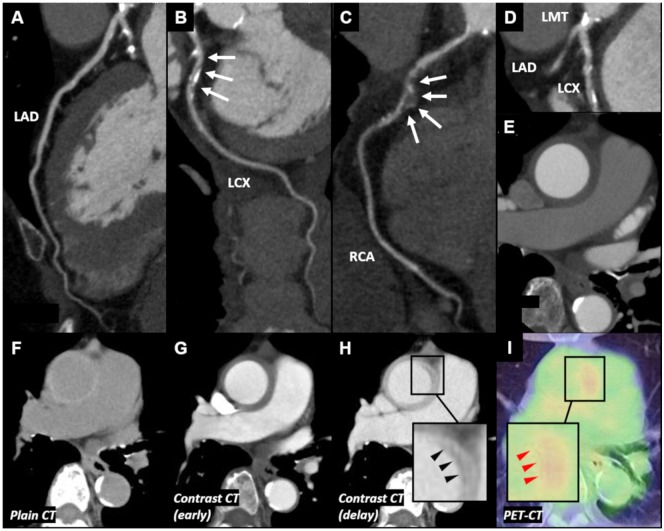
Figure 2.
Computed tomography, computed tomography angiography, and positron emission tomography–computed tomography findings of Case 2. (A) No significant stenosis was observed in left anterior descending artery. (B) Significant stenosis was observed in left circumflex coronary artery. (C) Significant stenosis with aneurysm was observed in mid-right coronary artery. (D) Significant stenosis with calcification was observed in left main trunk. (E) Concentric wall thickening of the ascending aorta was observed. (F) Plain computed tomography showed concentric thickened high-attenuation wall of ascending aorta. (G) Contrast-enhanced computed tomography showed that the thickened wall appeared with low-attenuation. (H) Delayed phase contrast-enhanced computed tomography showed ‘double ring enhancement’ (black arrowheads). (I) Positron emission tomography–computed tomography revealed increased F-18 fluorodeoxyglucose uptake in the wall of ascending aorta, especially a part of ‘double ring enhancement’ (red arrowheads). CAG, coronary angiography; CT, computed tomography; CTA, computed tomography angiography; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex coronary artery; LMT, left main trunk; OCT, optical coherence tomography; PET, positron emission tomography; PLEA, peri-arterial low-echoic area; RCA, right coronary artery.
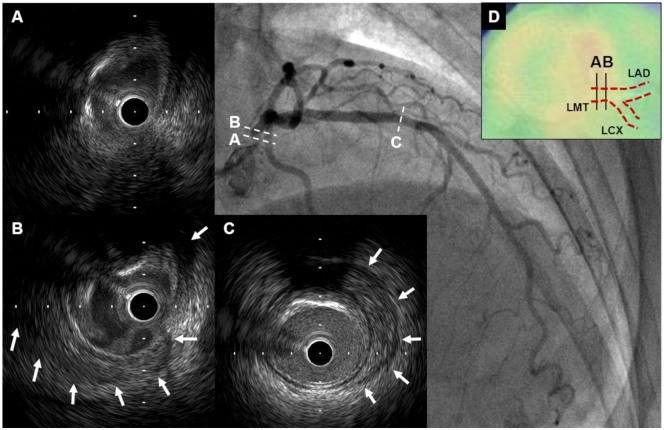
Figure 3.
Intravascular ultrasound findings of Case 2. (A and B) Intravascular ultrasound revealed obscured three-layered structure and peri-arterial low-echoic area (white arrows) in left main trunk. (C) Peri-arterial low-echoic area (white arrows) was observed at the site of mild plaque in mid-left anterior descending artery segment. (D) On PET-CT, there was increased F-18 fluorodeoxyglucose uptake in left main trunk where abnormal IVUS findings were seen. CT, computed tomography; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex coronary artery; LMT, left main trunk; PET, positron emission tomography.
Discussion
In the present case series, both patients suffered from CA but followed significantly different clinical courses, offering several important messages. First, the accurate diagnosis of CA appears to play a key role in achieving optimal therapies and leading to favourable clinical outcome as in Case 2. In contrast, delayed diagnosis might result in the chronic admission/readmission cycles as in Case 1. Notably, the present case series suggest that intracoronary imaging can offer valuable data to diagnose CA because increased inflammatory status and atypical disease progression were clearly identified by demonstrating obscured three-layered structure with increased vascularity and PLEA.9,10 These findings appear consistent with results of previous pathohistological studies of TA,7 thereby may help us consider CA as a differential diagnosis. Second, controlling inflammation is essential for revascularization in CA patients. In this context, early initiation of PSL with meticulous dose adjustment is crucial to control active inflammation. The combination of PSL with other immunosuppressive drugs should also be considered in patients with intractable CA like Case 1. Moreover, optimal medical therapy (e.g. aggressive lipid-lowering therapy) for CAD may contribute to achieving favourable outcomes because inflammation plays pivotal roles in the development of coronary atherosclerosis.11 Third, the types of bypass grafts and anastomosis sites should be carefully determined when performing CABG in CA patients.12 In Case 2, SVG (a better choice in patients with arteritis13) was sutured from the distal LSCA through the mid-LAD to PL because all sites were considered to have no significant inflammation from analysis of CTA, PET-CT, and IVUS. IVUS can clearly identify localization and degree of inflammation and may be useful to select distal anastomosis sites. Finally, stent implantation remains challenging in CA patients, even in the DES era.14,15 Multiple studies have reported that SES can induce chronic local inflammation related to its durable polymer in arterial walls.16–18 The present case series also observed SES-related inflammatory changes in arterial walls. These local inflammatory responses possibly accelerated disease activity of CA (as a persistent trigger of inflammation), contributing to frequent restenoses in Case 1.
Conclusion
The treatment of coronary involvement of arteritis remains challenging. The present case series suggests that accurate diagnosis of CA before revascularization, appropriate timing of revascularization with controlling active inflammation, meticulous selection of bypass grafts and anastomosis sites when performing CABG, and avoiding implanting a foreign body (i.e. stent) as much as possible are all important factors for achieving optimal therapies and ultimately improving clinical outcomes in patients with CA. The integration of data obtained through multimodality imaging, such as CTA, PET-CT, and intravascular imaging, contributed greatly to the management of this CA patient.
Lead author biography

Dr Shinnosuke Kikuchi graduated Yokohama City University School of Medicine in 2008 and began his medical training at the Yokohama City University Medical Center. He then continued his practical training in the field of Cardiology and is currently working as an interventional cardiologist at the Yokohama City University Medical Center.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors appreciate Heidi N. Bonneau, RN, MS, CCA for her review and editing advice.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, Lightfoot RW, Masi AT, McShane DJ, Mills JA, Stevens MB, Wallace SL, Zvaifler NJ.. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 2010;33:1129–1134. [DOI] [PubMed] [Google Scholar]
- 2. Armellin L, Sammel AM, Ng B, Sarathy K, Lambros J, Amir-Nezami T, Thomas SD, Highton J, Damodaran A.. Coronary artery stenting in acute coronary syndrome associated with giant cell arteritis. J Cardiol Cases 2017;16:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kihara M, Kimura K, Yakuwa H, Minamisawa K, Hayashi S, Umemura S, Nihei T, Shionoiri H, Ishii M.. Isolated left coronary ostial stenosis as the sole arterial involvement in Takayasu’s disease. J Intern Med 1992;232:353–355. [DOI] [PubMed] [Google Scholar]
- 4. Watanabe Y, Miyata T, Tanemoto K.. Current clinical features of new patients with takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation 2015;132:1701–1709. [DOI] [PubMed] [Google Scholar]
- 5. Shirai T, Hanaoka R, Goto Y, Kojima I, Ishii Y, Hoshi Y, Fujita Y, Shirota Y, Fujii H, Ishii T, Harigae H.. Takayasu arteritis coexisting with sclerosing osteomyelitis. Intern Med 2018;57:1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez F, Degnan KO, Nagpal P, Blankstein R, Gerhard-Herman MD.. Insidious: takayasu arteritis. Am J Med 2015;128:1288–1291. [DOI] [PubMed] [Google Scholar]
- 7. Matsubara O, Kuwata T, Nemoto T, Kasuga T, Numano F.. Coronary artery lesions in Takayasu arteritis: pathological considerations. Heart Vessels 1992;7:26–31. [DOI] [PubMed] [Google Scholar]
- 8. Hartlage GR, Palios J, Barron BJ, Stillman AE, Bossone E, Clements SD, Lerakis S.. Multimodality imaging of aortitis. JACC Cardiovasc Imaging 2014;7:605–619. [DOI] [PubMed] [Google Scholar]
- 9. Jin S-A, Lee J-H, Park J-H, Oh JK, Kim MS, Park YK, Kim JH, Kang SW, Kim SS.. Endovascular treatment in a patient with left main coronary and pulmonary arterial stenoses as an initial manifestation of takayasu’s arteritis. Heart Lung Circ 2015;24:e26–e30. [DOI] [PubMed] [Google Scholar]
- 10. Ishiyama Y, Eguchi K, Yokota K, Ikemoto T, Kario K.. New-onset takayasu’s arteritis as acute myocardial infarction. Intern Med 2018;57:1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalkman DN, Aquino M, Claessen BE, Baber U, Guedeney P, Sorrentino S, Vogel B, de Winter RJ, Sweeny J, Kovacic JC, Shah S, Vijay P, Barman N, Kini A, Sharma S, Dangas GD, Mehran R.. Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur Heart J 2018;39:4101–4108. [DOI] [PubMed] [Google Scholar]
- 12.JCS Joint Working Group. Guideline for management of vasculitis syndrome (JCS 2008). Japanese Circulation Society. Circ J 2011;75:474–503. [DOI] [PubMed] [Google Scholar]
- 13. Endo M, Tomizawa Y, Nishida H, Aomi S, Nakazawa M, Tsurumi Y, Kawana M, Kasanuki H.. Angiographic findings and surgical treatments of coronary artery involvement in Takayasu arteritis. J Thorac Cardiovasc Surg 2003;125:570–577. [DOI] [PubMed] [Google Scholar]
- 14. Wang X, Dang A, Lv N, Cheng N, Cheng X, Yang Y, Song Y.. Long-term outcomes of coronary artery bypass grafting versus percutaneous coronary intervention for Takayasu arteritis patients with coronary artery involvement. Semin Arthritis Rheum 2017;47:247–252. [DOI] [PubMed] [Google Scholar]
- 15. Yang Y, Tian T, Yang K, Zhang Y, Meng X, Fan P, Feng L, Mu C, Gao L, Zhou X.. Outcomes of percutaneous coronary intervention and coronary artery bypass grafting in patients with Takayasu arteritis. Int J Cardiol 2017;241:64–69. [DOI] [PubMed] [Google Scholar]
- 16. Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R.. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 2006;48:193–202. [DOI] [PubMed] [Google Scholar]
- 17. Cook S, Ladich E, Nakazawa G, Eshtehardi P, Neidhart M, Vogel R, Togni M, Wenaweser P, Billinger M, Seiler C, Gay S, Meier B, Pichler WJ, JüNi P, Virmani R, Windecker S.. Correlation of intravascular ultrasound findings with histopathological analysis of thrombus aspirates in patients with very late drug-eluting stent thrombosis. Circulation 2009;120:391–399. [DOI] [PubMed] [Google Scholar]
- 18. Nishimiya K, Matsumoto Y, Shindo T, Hanawa K, Hasebe Y, Tsuburaya R, Shiroto T, Takahashi J, Ito K, Ishibashi-Ueda H, Yasuda S, Shimokawa H.. Association of adventitial vasa vasorum and inflammation with coronary hyperconstriction after drug-eluting stent implantation in pigs in vivo. Circ J 2015;79:1787–1798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.