Abstract
Background
Cardiac masses (CM) encompass a broad set of lesions that can be either neoplastic or non-neoplastic. A stepwise diagnostic strategy through multimodality imaging evaluation is the cornerstone for the appropriate approach.
Case summary
We report the case of an 83-year-old man presenting at the emergency department for acute heart failure showing bilateral atrial masses without unequivocal aetiological aspects at several imaging techniques, emphasizing the critical aspects in the differential diagnosis.
Discussion
In the complex field of CM, a proper differential diagnosis is very important in order to start the appropriate treatment; however, sometimes it could be challenging despite a multimodality imaging approach, therefore still requiring histologic examination.
Keywords: Cardiac masses, Atrial thrombi, Imaging of intracardiac masses, Case report
Learning points
To be able to acknowledge critical aspects and limitations of the multimodality imaging techniques in the assessment of intracardiac masses;
To understand the importance of a stepwise diagnostic strategy in order to start the appropriate treatment in cardiac masses considering limitations imposed by clinical practice;
To highlight that vascularization and thrombosis could coexist making cardiac imaging techniques misleading;
Even in the multimodality imaging era ‘in vivo’ anatomical aspects sometimes are needed to reach the correct diagnosis.
Introduction
Cardiac masses (CM) encompass a broad set of lesions that can be either neoplastic or non-neoplastic. The estimated prevalence for primary cardiac tumours is 1:2000 and for secondary tumours 1:100 autopsies, with a secondary/primary ratio of 20:1. Approximately 10% of primary cardiac tumours are malignant and 90% benign, among benign the majority are myxomas. Thrombi are the most common CM.1. This article reports the case of an 83-year-old male presenting at the emergency department (ED) for acute heart failure showing bilateral atrial masses emphasizing the critical aspects of differential diagnosis between CM.
Timeline
| Day 1 | Admission to the emergency department for acute heart failure, evidence of bi-atrial masses at the urgent ultrasound scan. |
| Day 2 | Transthoracic echocardiography: confirmation of bi-atrial masses. |
| Day 3 | Transoesophageal echocardiography: vacuolated and inhomogeneous areas, cardiac myxomas are suspected. |
| Day 5 | Computed tomography evaluation: morphologically compatible with myxomas, but they did not enhance iodine contrast agent as a typical thrombus behaviour. |
| Day 6 | Contrast echocardiography: mild late contrast enhancement of left atrial mass suggesting the diagnostic hypothesis of bilateral myxomas. |
| Day 9 | Surgical resection: inspection of the masses was suggestive for neoplastic origin. |
| Day 11 | Histological examination: aspect of vascularized thrombotic material. |
Case presentation
An 83-year-old man with left ventricular dysfunction due to hypertensive cardiomyopathy was admitted to the ED for acute heart failure. Although in chronic atrial fibrillation, he was not in anticoagulation therapy because of neurological contraindication due to the presence of cerebral venous angiomas found several years before.
The urgent ultrasound scan performed at the ED showed bilateral intra-atrial masses mainly involving the left atrium. Haemodynamic impairment caused by the size of the left atrial mass could not be excluded, subsequently, in accordance with the neurologist, unfractionated intravenous (IV) heparin was started and the patient was admitted to the cardiology ward.
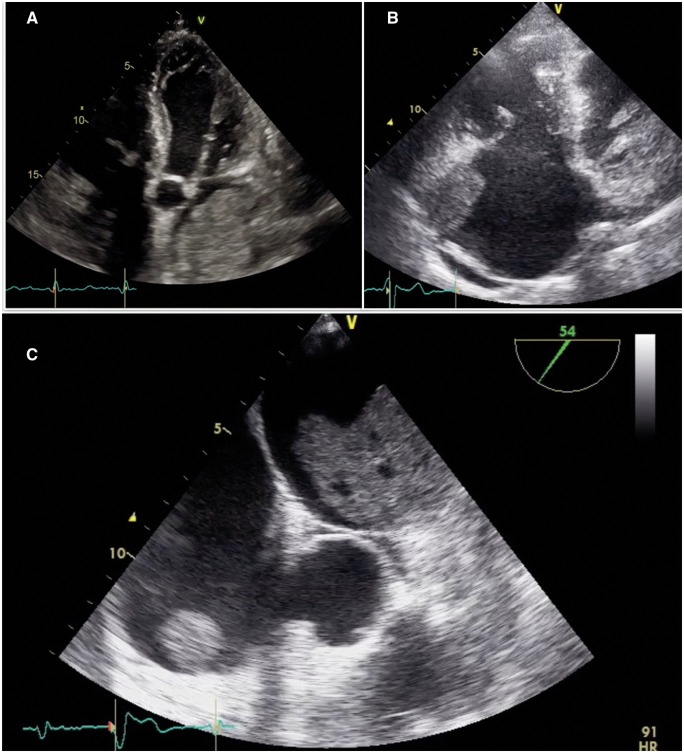
The day after, a complete transthoracic echocardiography (TTE) was performed (Figure 1A) showing within the left atrium an isoechoic and not pedunculated mass with lobulated margins attached to the anterior wall of the left atrium and extended also inside the left atrial appendage. The mass was not mobile, without evidence of haemodynamic impairment on mitral inflow. A smaller mass with the same characteristics was attached to the anterior wall of the right atrium (Figure 1B). There was no pericardial effusion and left ventricular ejection fraction was 40% with a normal right ventricular function.
Figure 1.
(A) Transthoracic echocardiography showing a large left atrial mass involving left atrial appendage. (B) Transthoracic echocardiography showing stratified right atrial mass involving right atrial appendage. (C) Transoesophageal echocardiography showing lobulated aspects of the bi-atrial masses and hypoechoic zones within the left atrial mass.
A transoesophageal echocardiogram confirmed these aspects and showed also vacuolated and inhomogeneous areas inside the lobulated masses resembling the morphology of cardiac myxomas; no valvular abnormalities nor atrial septal defects were found (Figure 1C).
To further evaluation, cardiac magnetic resonance (CMR) was proposed but could not be performed due to patient claustrophobia and sedative refusal.
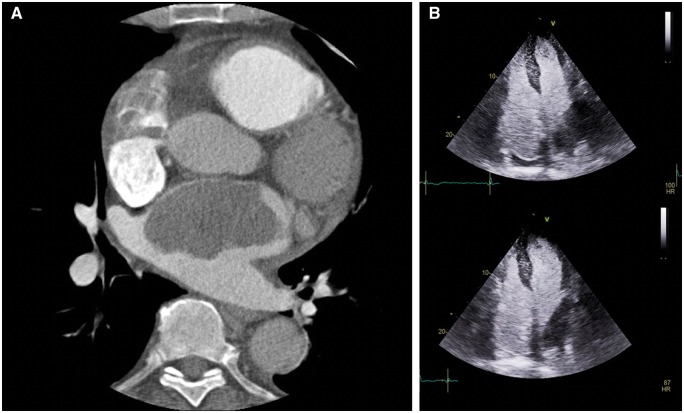
Therefore, electrocardiographic gated cardiac computed tomography (CCT) was carried out for a better differential diagnosis between thrombi vs. myxomas vs. malignant tumours as well as to look for possible embolization or primary tumours (in the hypothesis of cardiac metastases). Considering the atrial masses, they appeared morphologically compatible with myxomas, but they didn’t enhance iodine contrast agent as a typical thrombus behaviour (Figure 2A). No systemic emboli nor extracardiac tumours were found.
Figure 2.
(A) Absence of contrast enhancement at the level of the left atrial mass at cardiac computed tomography scan. (B) Mild late contrast enhancement of left atrial mass with IV Sonovue (contrast-transthoracic echocardiography).
In order to further analyse the vascularization of the masses, contrast-TTE with 1 mL of IV Sonovue was accomplished. Interestingly, a mild late contrast enhancement of left atrial mass was seen, thus suggesting the diagnostic hypothesis of bilateral myxomas (Figure 2B).
In the meantime, considering also the hypothesis of intra-atrial thrombi, the treatment with IV heparin was continued and the masses were monitored with transthoracic echocardiography. However, after more than 1 week of IV heparin treatment, the masses volume did not change.
In this context, we decided to perform transvenous biopsy of right atrial mass under intracardiac echocardiographic guidance in order to clarify the nature of the masses. The biopsy samples from the outer wall of the right atrial mass showed mild thrombotic features without excluding an underlying myxoma.
After heart team consultation, with a diagnostic and curative purpose, the patient underwent to surgical resection of the masses through atrial approach.
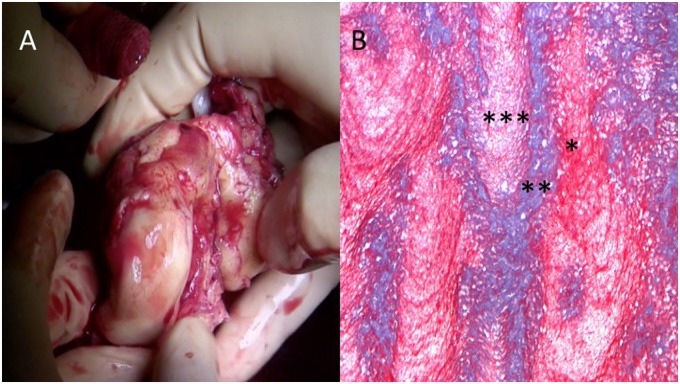
The surgical inspection of the masses was suggestive for neoplastic origin (Figure 3A). However, histological examination, unexpectedly, showed only thrombotic material with several phases of organization and mild aspects of neo-angiogenesis combined with mild lymphocyte infiltration and necrotic areas (Figure 3B). Before discharge, a total-body CT scan excluded tumours located in other districts.
Figure 3.
(A) Reddish-grey 69 g of weight, friable cardiac mass from the left atrium. (B) Azan Mallory (10×). Histological sample showing stratified thrombotic material with different chronological phases (***new; **intermediate; *old).
Discussion
Transthoracic echocardiography remains the first-line diagnostic procedure and permits to evaluate size, contours, mobility, site of attachment, and haemodynamic impact of the mass. Myxomas appear with finger-like projections or with a smooth surface, usually are attached by a stalk to the atrial septum in the fossa ovalis and could present inhomogeneous areas of hyperechogenicity due to calcification.2 Conversely thrombi have frequently homogenous echogenicity, usually are not highly mobile and are often found in the atrial appendage and in the context of atrial fibrillation.
In order to help to distinguish vascular tumour from thrombi, contrast-TTE has been used to assess the perfusion of the CM. Malignant tumours are frequently highly vascularized and present greater contrast enhancement than the adjacent myocardium, whereas, myxomas demonstrate partial perfusion on visual inspection and quantitatively less perfusion than the surrounding myocardium; thrombi, being avascular show complete absence of perfusion.3
However, as in our case, contrast-TTE may lead to uncertain information and this is the reason why CCT and CMR are often utilized synergistically with echocardiography.
Cardiac magnetic resonance is often the preferred imaging modality for CM assessment4 but in our case it was not possible to be performed.
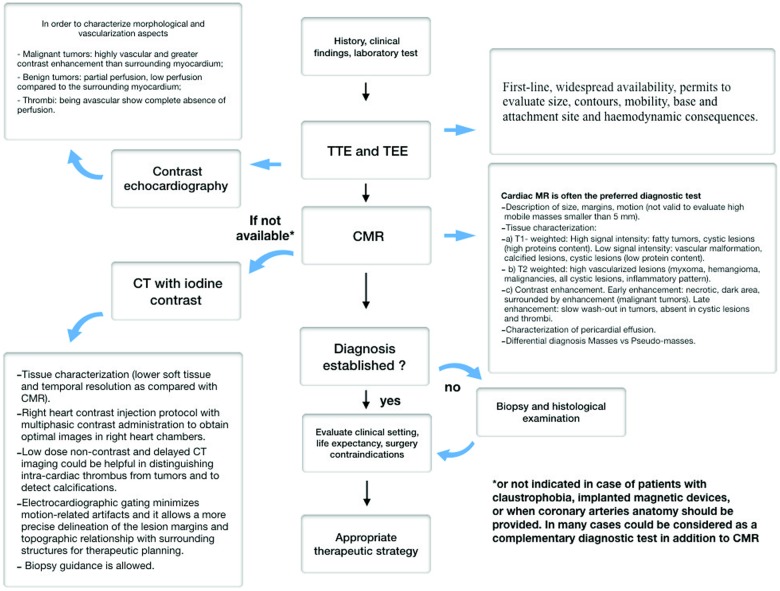
Cardiac computed tomography is an alternative imaging modality to assess CM, particularly in patients with contraindications to CMR, and contrast-enhanced CT may be useful to distinguish between a thrombus and a cardiac tumour. On CT, myxoma appears as intracavitary filling defects with heterogeneous contrast enhancement whereas thrombus typically appears as a filling defect within the cardiac chamber as showed by delayed post-contrast imaging. Thrombus may be differentiated from primary and secondary tumours by knowledge of predisposing risk factors, attachment location, shape, and lack of mobility.5,6 (Figure 4—diagnostic flow-chart; Supplementary material online, Figure S1—CM classification).
Figure 4.
Recommended diagnostic flow-chart.
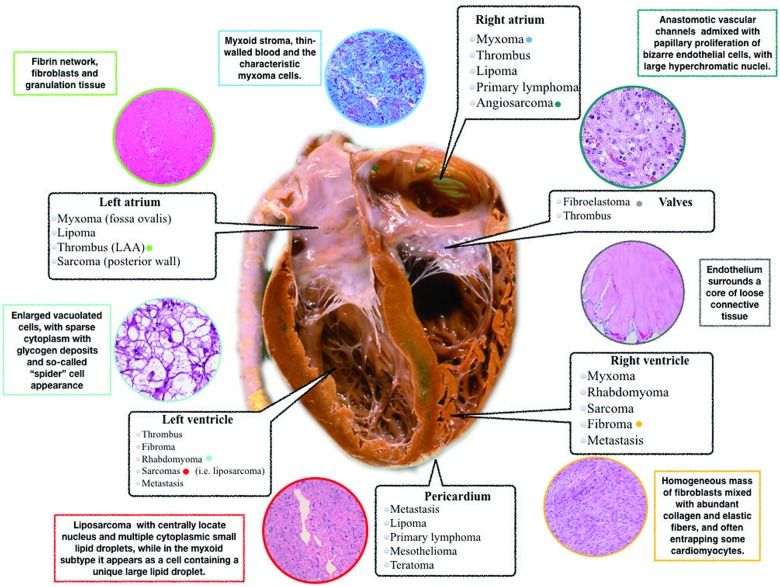
Histopathological characterization remains the diagnostic gold standard in any resected CM and, as we have shown, it has the main role in the differential diagnosis. The typical aspects of thrombotic material are the evidence of fibrin network, blood cells, fibroblasts, and granulation tissue depending on thrombus ‘age’. Conversely, the main aspects of myxomas are spindle cells, vascular structure, myxoid matrix, and sometimes with haemorrhagic and calcified areas1 (Figure 5—localization and histopathological aspects of CM).
Figure 5.
Localization, pathophysiology, and histopathological aspects of cardiac masses.
In our case, despite a multimodality imaging approach, the histological examination after surgical removal had a key diagnostic role showing organized thrombotic material. We hypothesized that the histological features of the lesion and the presence of small vessels are probably related to atrial tissue. These characteristics in addition to the aspects of vacuolization, could explain the mild late enhancement at contrast-TTE simulating the features of cardiac tumour.
Conclusion
In summary, in the complex field of CM a proper differential diagnosis is extremely important in order to start the appropriate treatment, however, sometimes it could be challenging, and the definite diagnosis may require histologic examination.
Lead author biography

Matteo Castrichini graduated from the University of Parma Medical School in 2016 and moved to Trieste in 2017 to undertake his clinical and research training at the specialization school of cardiovascular disease directed by Prof. Sinagra G. He has a keen interest in Cardiac Imaging and is currently involved in several research projects in heart failure and cardiomyopathies.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Basso C, Valente M, Poletti A, Casarotto D, Thiene G.. Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardiothorac Surg 1997;12:730–737; discussion 737–738. [DOI] [PubMed] [Google Scholar]
- 2. Maleszewski JJ, Bois MC, Bois JP, Young PM, Stulak JM, Klarich KW.. Neoplasia and the Heart. J Am Coll Cardiol 2018;72:202–227. [DOI] [PubMed] [Google Scholar]
- 3. Bhattacharyya S, Khattar R, Senior R.. Characterisation of intra-cardiac masses by myocardial contrast echocardiography. Int J Cardiol 2013;163:e11–e13. [DOI] [PubMed] [Google Scholar]
- 4. O’Donnell DH, Abbara S, Chaithiraphan V, Yared K, Killeen RP, Cury RC, Dodd JD.. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. Am J Roentgenol 2009;193:377–387. [DOI] [PubMed] [Google Scholar]
- 5. Anavekar NS, Bonnichsen CR, Foley TA, Morris MF, Martinez MW, Williamson EE, Glockner JF, Miller DV, Breen JF, Araoz PA.. Computed tomography of cardiac pseudotumors and neoplasms. Radiol Clin North Am 2010;48:799–816. [DOI] [PubMed] [Google Scholar]
- 6. Bittencourt MS, Achenbach S, Marwan M, Seltmann M, Muschiol G, Ropers D, Daniel WG, Pflederer T.. Left ventricular thrombus attenuation characterization in cardiac computed tomography angiography. J Cardiovasc Comput Tomogr 2012;6:121–126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.