Abstract
Background
Mycotic aneurysms of coronary vein grafts are rare and associated with high mortality. They are most commonly a result of surgical or percutaneous intervention, and present with complications including myocardial infarction (MI), infective endocarditis. A recent literature review identified 97 cases of mycotic coronary aneurysms in total.
Case summary
A 49-year-old man with a history of coronary artery bypass grafting and septic arthrithis presented with chest pain and fevers and ST elevation on electrocardiogram. Urgent angiogram showed an aneurysmal saphenous vein graft from the PL branch to PDA—no acute intervention was performed due to concern about bacteraemia. Methicillin-sensitive Staphylococcus aureus was grown in urine and blood but no focus of infection was identified. Despite treatment with antibiotics and antiplatelets, the patient returned with evidence of expansion of the SVG aneurysm requiring surgical resection.
Discussion
This case highlights the difficulty in treating acute coronary syndromes involving mycotic aneurysms. Multimodal imaging approaches are useful to identify suspected infection, but false negatives occur. Due to high risk of rupture or haemorrhage, there are limited options for urgent reperfusion in cases of MI with mycotic aneurysm, demonstrating the need for an individualized approach and close follow-up.
Keywords: Case reports, Mycotic aneurysm, Myocardial infarction, Saphenous vein graft
Learning points
Mycotic aneurysms of coronary vein grafts are rare and associated with high mortality.
In patients presenting with ST-elevation myocardial infarction and mycotic anueyrm urgent reperfusion with percutaneous coronary intervention or thrombolysis pose the risk of rupture, surgical resection is often required.
A multimodal approach with a coronary angiogram, echocardiography, and other modalities, such as fluorodeoxyglucose positron emission tomography and labelled white scan, improve diagnosis in mycotic aneurysms but are not 100% sensitive and a high degree of suspicion is required.
Introduction
Mycotic aneurysms of coronary vein grafts are rare and associated with significant morbidity and mortality.1 There are few publications available regarding diagnosis and management to guide treatment. Staphylococcus aureus is the most common causative organism, identified in over half the cases, followed by Streptococcal species, accounting for 18%.1
Timeline
| 14 years earlier | Myocardial infarction and coronary artery bypass graft |
| 10 years earlier | Methicillin-sensitive Staphylococcus aureus (MSSA) arthritis and femoral pseudoaneurysm—repaired. |
| 1 week prior | Knee pain, fevers, and stops aspirin. |
| Presentation | Dull chest pain, ST elevation. |
| Angiogram—SVG aneurysm—medical management | |
| 6 hours later | Persistent chest pain. Fever. |
| Positive urinalysis. MSSA in urine and BC. Flucloxacillin | |
| Negative transthoracic and transoesophageal echocardiogram | |
| Day 7 | Negative white cell scan |
| Day 12 | Discharged on IV flucloxacillin and dual antiplatelet therapy |
| Day 16 | Represents with pleuritic chest pain, negative blood cultures |
| Repeat transthoracic and transoesophageal echocardiogram demonstrates mycotic aneurysm | |
| Day 31 | CTx perform redo CABG, identifying and resecting mycotic aneurysm |
| Day 365 | Living independently in the community without assistance |
Case presentation
A 49-year-old man presented to our hospital complaining of dull, central chest pain after 1 week of subjective fever. Fourteen years prior to this presentation, the patient underwent coronary artery bypass grafting, and 10 years prior suffered right knee septic arthritis due to Methicillin-sensitive Staphylococcus aureus (MSSA) which was complicated by a mycotic right femoral pseudoaneurysm. The patient had no immunosuppressive medications or medical conditions.
In the week prior to presentation, the patient had taken non-steroidal anti-inflammatory drugs for right knee pain after a mechanical injury and had ceased aspirin due to concerns about gastrointestinal haemorrhage. The patient had experienced subjective fevers and knee pain, however, had full range of movement with no tenderness, warmth, or erythaema.
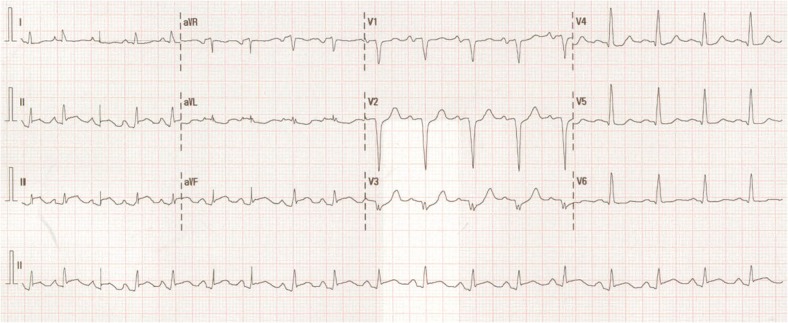
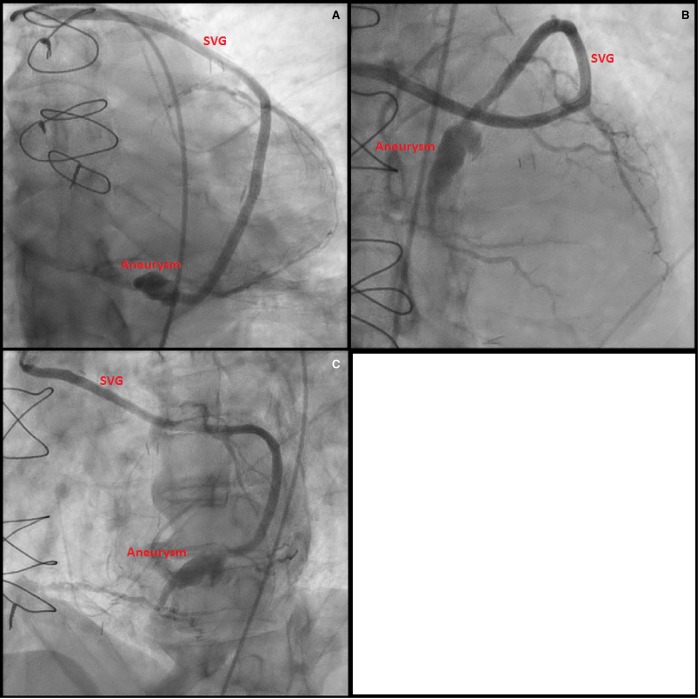
On arrival, his electrocardiogram (ECG) showed sinus tachycardia with inferior ST elevation (Figure 1). Physical examination revealed a blood pressure of 128/88 mmHg, heart rate of 112 b.p.m. and temperature of 36.7°C. Cardiovascular examination was unremarkable and examination of the right knee demonstrated normal range of motion without pain and no effusion. An urgent coronary angiogram was performed demonstrating severe native triple vessel disease, a patent left internal mammary artery graft and an aneurysmal but patent saphenous vein graft from the posterolateral branch to posterior descending branch of the right coronary artery (PDA) (Figure 2). The Saphenous vein graft skip to the PDA was believed to be the infarct-related artery. No intervention was performed and the patient was managed with aspirin, clopidogrel, beta-blockade, angiotensin receptor antagonist, spironolactone, and intravenous nitrate infusion. Pathology is available in Table 1.
Figure 1.
Electrocardiogram on presentation (Day 0) with sinus tachycardia and inferioposterior ST-elevation myocardial infarction.
Figure 2.
(A) Angiogram at Day 0, LAO 4, CAU 40, injection of the saphenous vein graft to obtuse marginal, skip to posterior descending artery. Aneurysm of the graft proximal to PDA touchdown; (B) angiogram at Day 0, LAO 4, CRA 34, injection of the saphenous vein graft to obtuse marginal, skip to posterior descending artery. Aneurysm of the graft proximal to PDA touchdown; (C) angiogram at Day 0, LAO 37, CAU 1, injection of the saphenous vein graft to obtuse marginal, skip to posterior descending artery. Aneurysm of the graft proximal to PDA touchdown.
Six hours later, the patient developed fever (38.4°C) with no localizing signs of infection identified; a septic screen was performed. Leucocytosis on the urinalysis led to commencement of IV augmentin (1000 mg + 200 mg QID) and gentamicin (320 mg), which were then changed to flucloxacillin (2 g QID) after blood and urine cultures grew MSSA.
Due to ongoing chest pain, intervention with covered stents to the aneurysmal graft was considered but decided against because of the persistent bacteraemia and fever.2 Without revascularization, the patient developed inferior hypokinesis on echocardiogram and Q waves in the inferior leads on ECG. He remained persistently febrile until Day 5 of IV flucloxacillin.
There were no other symptoms or signs to suggest another source of infection. The abdomen was soft and non-tender. As MSSA was detected in urine during the initial work up a renal tract US was performed which revealed echogenic kidneys, and the treating cardiology and infectious disease teams attributed this to the systemic sepsis.
Transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE) showed no evidence of infective endocarditis. Vasculitis and rheumatological screens were negative. Given the strong suspicion of a mycotic aneurysm, a labelled white cell scan was performed on Day 7 post-presentation, which failed to demonstrate an infective focus, or infection within the aneurysm.
The patient was discharged on IV flucloxacillin for 6 weeks, and dual antiplatelet therapy with a view to cardiology and infectious disease follow-up for consideration of treatment of aneurysm with percutaneous treatment in the future.
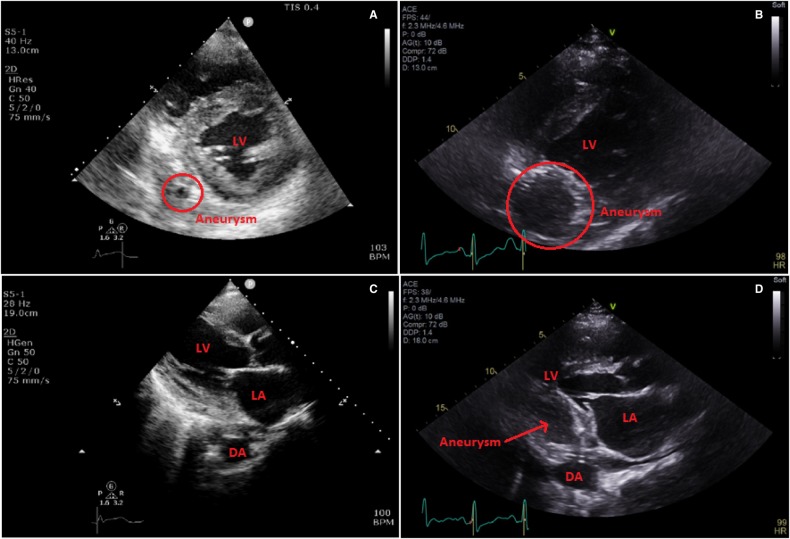
Four days after discharge, the patient represented with left-sided pleuritic chest pain. Blood cultures were persistently negative. His repeat echocardiogram demonstrated moderately reduced left ventricular (LV) function, with thin walled, akinetic inferior and posterior walls consistent with a completed inferior infarct. The expanding aneurysm of the saphenous vein graft was now visible (on TTE and TOE) as a large pulsatile chamber along the posterior and inferior wall, with flow principally seen in diastole, and compressing the inferoposterior LV wall during diastole consistent with expanding aneurysm of the saphenous vein graft (Figure 3).
Figure 3.
(A) Transthoracic echocardiogram Day 1, parasternal short axis, aneurysm circled. (B) Transthoracic echocardiogram Day 16, parasternal short axis, rapidly enlarging aneurysm circled. (C) Transthoracic echocardiogram Day 1, parasternal long axis. (D) Transthoracic echocardiogram Day 1, parasternal long axis, off axis with large aneurysm. DA, descending aorta; LA, left atrium; LV, left ventricle.
The cardiothoracic surgery team performed a redo coronary artery bypass grafting with successful identification and resection of the mycotic aneurysm. Cultures of the operative specimen were negative, though the histological microscopic appearance confirmed the presence of the mycotic aneurysm.
The patient’s post-operative recovery was complicated by one admission for management of heart failure, after which he has remained stable and independent in the community as of August 2019. The most recent echocardiograph revealed normal LV systolic function with infarcted inferior and posterior walls, severe left atrial enlargement, and mild mitral regurgitation.
Discussion
Aneurysms of coronary vein grafts are uncommon and mycotic aneurysms are even more scarce.1 A recent literature review of mycotic aneurysms found just 6 of 97 cases involved saphenous vein grafts, usually occurring in the perioperative period.3 Myocotic aneurysms of native coronary arteries typically occur as a result of PCI or infective endocarditis. Similar to infective endocarditis, the organisms involved are most commonly staphylococcal and streptococcal species.1 In our case, the previous distant episode MSSA pseudoaneurysm associated with septic arthritis suggested ongoing residual, low-grade asymptomatic infection, undetectable on imaging.
Myocardial infarction is the most common complication of mycotic aneurysms, with other complications including purulent or haemorrhagic pericardial effusion and rupture.1
In conjunction with angiogram and echocardiogram, other imaging modalities, including fluorodeoxyglucose positron emission tomography and labelled white cell scan, are increasingly used to identify myocotic aneurysms, with labelled white cell scan being the most specific modality. These modalities improve diagnosis of infective endocarditis and mycotic aneurysms, but false negatives do occur, such as in our case.4,5
European guidelines for the management of infective endocarditis suggest urgent intervention in ruptured aneurysms, and delayed intervention for those that persist or expand after antibiotic therapy. Due to their rare nature, there is no evidence base for the guidance of management decisions.6
In patients presenting with acute coronary syndromes due to mycotic aneurysms, it is unclear whether to proceed with intervention and risk stent infection or to treat medically and control infection first. Most approaches involve long-term antibiotic therapy and resection of the infected vessel together with bypass.1,7
Conclusions
This case demonstrates the complex management issues in patients with mycotic coronary artery aneurysms.
Standard acute reperfusion approaches are limited in this context by the potential for rupture with thrombolysis, and infection of implants with percutaneous intervention; an individualized approach with expert infectious disease, cardiothoracic and cardiology input should be used.
Close monitoring and follow-up are warranted as mycotic aneurysms may grow rapidly, with important sequalae.
Lead author biography

Max Ray is a cardiology advanced trainee at John Hunter Hospital, Australia with an interest in cardiac imaging and health economics.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Table 1.
Biochemistry results from presentation
| Value | Day 0 | Peak | Reference |
|---|---|---|---|
| White blood cell | 15.6 | 19.3 | 4.0–11.0 10^9/L |
| Neutrophils | 13.4 | 17.4 | 2.0–8.0 10^9/L |
| Platelets | 244 | 449 | 150–400 10^9/L |
| HS troponin I | 267 | 14 796 | <26 ng/L |
| C-reactive protein | 200 | 210 | <2 mg/L |
HS, high sensitivity.
Supplementary Material
References
- 1. Baker DW, Whitehead NJ, Barlow M.. Mycotic coronary aneurysms. Heart Lung Circ 2020;29:128–136. [DOI] [PubMed] [Google Scholar]
- 2. Khan A, Brienesse S, Boyle A, Collins N.. Percutaneous treatment of saphenous vein graft aneurysm: contemporary procedural considerations. Catheter Cardiovasc Interv 2019;93:927–932. [DOI] [PubMed] [Google Scholar]
- 3. Hirsch GA, Johnston PV, Conte JV Jr, Achuff SC.. Mycotic aortocoronary saphenous vein graft aneurysm presenting with unstable angina pectoris. Ann Thorac Surg 2004;78:1456–1458. [DOI] [PubMed] [Google Scholar]
- 4. Erba PA, Conti U, Lazzeri E, Sollini M, Doria R, De Tommasi SM, Bandera F, Tascini C, Menichetti F, Dierckx RA, Signore A, Mariani G.. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med 2012;53:1235–1243. [DOI] [PubMed] [Google Scholar]
- 5. Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, Casalta J-P, Gouriet F, Riberi A, Avierinos J-F, Collart F, Mundler O, Raoult D, Thuny F.. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol 2013;61:2374–2382. [DOI] [PubMed] [Google Scholar]
- 6. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 7. Goldblatt J, Doi A, Negri J, Nanayakkara S, McGiffin D.. Mycotic pseudoaneurysms of the coronary arteries. J Card Surg 2015;30:555–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.