Abstract
Programmed cell death‐1 immune checkpoint inhibitor (ICI) antibody has proven to be effective in advanced non‐small cell lung cancer (NSCLC) patients positive for programmed cell death‐1 ligand‐1. However, there are currently no reports which evaluate drug efficacy by continuous bronchoscopic observation. A 75‐year‐old man with complete right atelectasis was diagnosed with squamous cell carcinoma (SCC) of the right lower lobe (tumor proportion score: TPS 90%, cT4N3M0, stage 3C). For first‐line chemotherapy, carboplatin and nab‐paclitaxel were effective for the primary lesion and the right lung atelectasis improved. However, due to repeated febrile neutropenia with pneumonia, treatment was modified to pembrolizumab monotherapy. Bronchoscopic rebiopsy prior to second‐line treatment revealed high TPS, with a severe stenosis in the right main bronchus. After three courses of pembrolizumab, the right main bronchus opened completely, and no signs of malignancy were observed. Bronchoscopic narrow‐band and autofluorescence imaging also confirmed a complete endobronchial response. Subsequent bronchoscopic observation two years after the initial diagnosis showed a complete and continued response to treatment. ICIs can result in a drastic bronchoscopic response. In this case, the healing process was notable with minimal scarring, and resulted in continued locally bronchoscopic and complete pathological response to treatment compared to previous cytotoxic chemotherapy.
Keywords: Airway stenoses, bronchoscopy, immunotherapy, lung cancer treatment
Introduction
The development of immune checkpoint inhibitors (ICIs) has altered chemotherapy treatment for non‐small cell lung cancer (NSCLC) and other malignancies. In recent years, many ICIs have been developed and approved after promising results in clinical trials.1, 2, 3, 4, 5
Pembrolizumab is a humanized anti‐programmed cell death 1 (PD‐1) monoclonal antibody that inhibits PD‐1 from binding to programmed cell death‐1 ligand‐1 (PD‐L1). A phase 3 clinical trial (KEYNOTE‐024), which enrolled untreated high‐TPS NSCLC, revealed that pembrolizumab was more effective in progression‐free survival, overall survival, and a higher response rate than platinum‐based chemotherapy.4 While drastic clinical benefits have been proven in multicenter analysis, the interaction between ICI drugs and tumors remain unclear in each case. Moreover, in addition to computed tomography (CT) and other noninvasive imaging tools, there are few cases which have continuously observed the antitumor effects directly by bronchoscopy over an extended period of time. In this case, we confirmed the antitumor efficacy of pembrolizumab, not only by CT images, but also directly under long‐term bronchoscope observation.
Case report
A 75‐year‐old man complaining of dyspnea was referred to our department with complete right atelectasis on chest X‐ray which had been taken at a previous clinic (Fig 1a). Under bronchoscopy, the lung mass was diagnosed as a squamous cell carcinoma (SCC) originating in the right lower lobe (tumor proportion score; TPS: 90%, cT4N3M0, stage 3C, Fig 1b–e). For first‐line chemotherapy, carboplatin and nab‐paclitaxel every three weeks were effective for the primary lesion and his right lung atelectasis improved (Fig 2a). However, due to repeated febrile neutropenia with pneumonia, treatment was changed after three courses of cytotoxic chemotherapy to pembrolizumab monotherapy.
Figure 1.
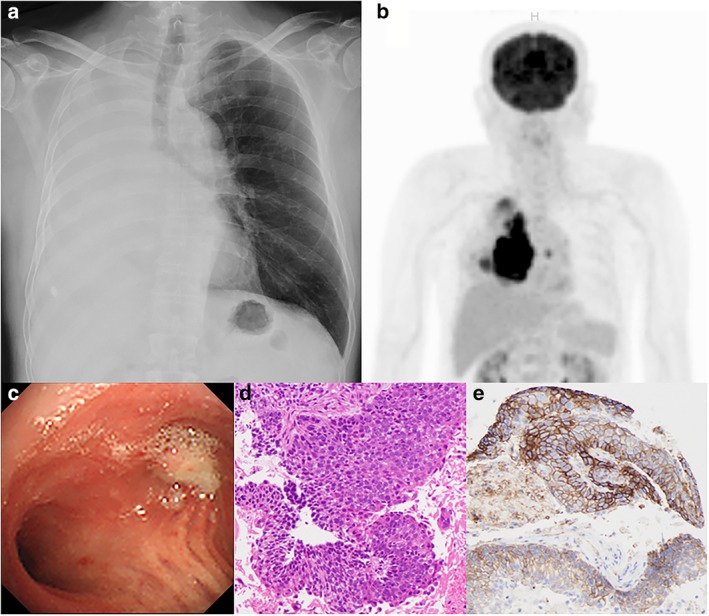
(a) Chest X‐ray on initial visit. (b) PET‐CT. (c) Bronchoscopic view during diagnostic examination. (d) Pathological findings with H&E staining. (e) Pathological findings with PD‐L1 staining.
Figure 2.
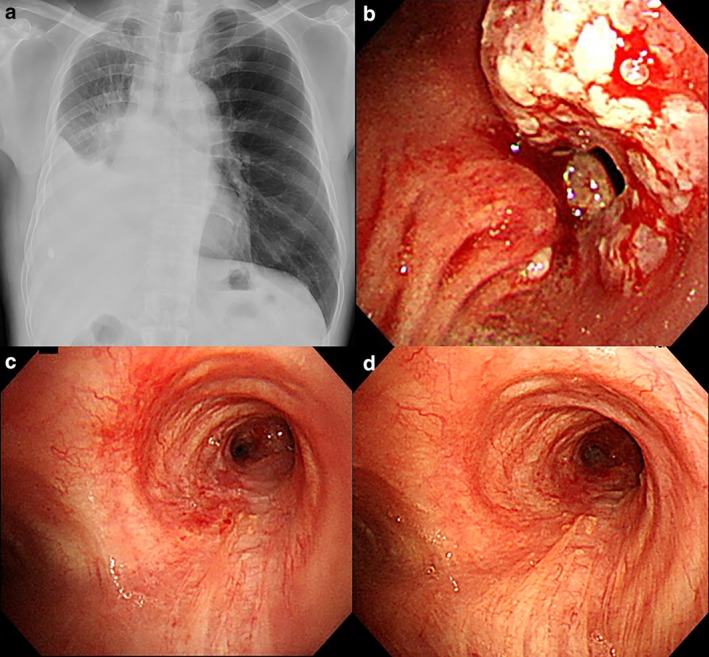
(a) Chest X‐ray after administration of cytotoxic chemotherapy. (b) Bronchoscopic view after administration of cytotoxic chemotherapy. (c) Bronchoscopic view after three courses of pembrolizumab administration. (d) Bronchoscopic view after 26 courses of pembrolizumab administration.
Bronchoscopic examination prior to second‐line treatment revealed that residual cancer cells appeared circumferentially at the right main bronchus and a severe stenosis was still present (Fig 2b). Rebiopsy samples confirmed that TPS was also high. After three courses of pembrolizumab administration, the right main bronchus opened completely and no malignant findings were observed (Figs 2c and 3a). Narrow‐band imaging and autofluorescence imaging also confirmed a complete endobronchial response (Fig 3b–d). Subsequent PET‐CT and bronchoscopic observation showed a stabilized response two years after the first diagnosis and an improvement in capillary vessel distribution in the affected bronchus (Fig 2d). This complete response lasted after pembrolizumab had been administered more than 30 times, with a grade 2 abnormal thyroid function test reported as the only immune‐related adverse event. Repeat rebiopsy using forceps and a cryoprobe was carried out in the right main bronchus (Fig 4a) and subsequent pathology revealed no malignant cell recurrence (Fig 4b), with complete regeneration of the bronchial epithelium (Fig 4c). Fibroblastic proliferation and infiltration of lymphocytes were observed in the submucosal space (Fig 4d).
Figure 3.
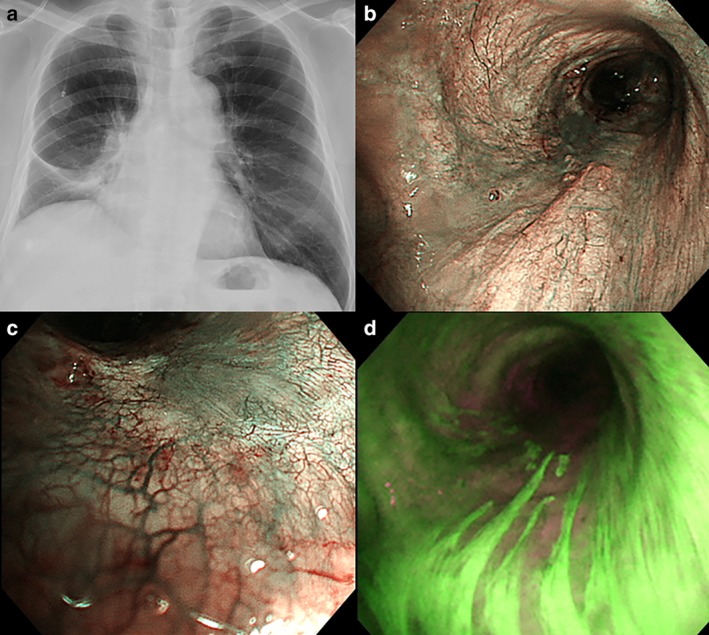
(a) Chest X‐ray after administration of pembrolizumab. (b) Bronchoscopic distant view of narrow‐band imaging. (c) Bronchoscopic close‐up view of narrow‐band imaging. (d) Bronchoscopic autofluorescence imaging.
Figure 4.
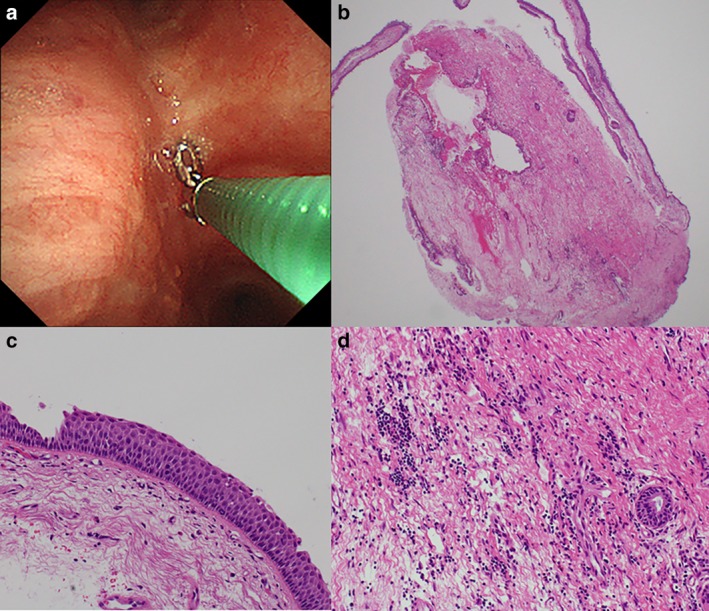
(a) Rebiopsy after 30 times of pembrolizumab administration. (b) Pathologically, there was no malignant cell recurrence in the cryobiopsy sample. (c) The bronchial epithelium had completely regenerated. (d) Fibroblastic proliferation and infiltration of lymphocytes were observed in the submucosal space.
Discussion
This is the first case report, to the best of our knowledge, to visually investigate the efficacy of an ICI in a NSCLC patient over a two‐year period using bronchoscopy. Recently, ICIs have become common therapy for NSCLC patients to prolong progression‐free survival and overall survival compared to previous systemic chemotherapy treatments. However, due to pseudo‐progression of the tumor sometimes seen on chest CT, it can be difficult to evaluate the efficacy of ICIs.6
In this case, we were able to compare the healing process after first‐line cytotoxic chemotherapy and subsequent second‐line ICI therapy. After first‐line administration of cytotoxic chemotherapy, residual cancer cells remained circumferentially at the right main bronchus, and rebiopsy tissue showed high TPS indicating squamous cell carcinoma. Fragile tumor‐related vessels covered the surface of the right main bronchus and bleeding occurred as the bronchoscope made contact with the lesion (Fig 2b). There are few reports concerning bronchoscopic observation or pathological changes after treatment, except in bronchoscopic rebiopsy cases with EGFR‐mutant adenocarcinoma7 or neoadjuvant ICI/chemotherapy cases comparing major pathological response (MPR).8 In a recent study, sequential TPS change depended on previous therapies; molecular‐targeted therapy, cytotoxic chemotherapy and radiation therapy.9
In contrast, after ICI administration, residual tumor cells diminished and the original bronchial structure was maintained. Under bronchoscopic narrow‐band imaging (NBI), the proliferation of capillary vessels was observed subepithelially (Fig 3b,c), suggesting a history of previous inflammation due to an invasive mass. Bronchoscopic autofluorescence imaging (AFI) can be useful as it distinguishes the tumor lesion as a magenta‐color.10 In this case, the bronchial membranous portion showed that the magenta‐color area was linear with a thickened epithelium (Fig 3d), with no sign of tumor progression or invasion. Repeated re‐biopsy also supported a complete pathological response and tissue regeneration (Fig 4). We recommend bronchoscopic observation because complete response might predict a durable ICI effect at an invasive location. Even if there is regrowth of the tumor at the stenotic bronchus, bronchoscopic observation will be needed for the adoption of radiotherapy or stent insertion in clinical practice.
ICIs have proven to be effective by inhibiting several steps of the tumor progression cascade.11 This is especially true for immunogenic cell death (ICD), for the recognition of tumor antigens by T cells.12 ICD might have been effectively induced in this case, suggesting good compatibility with prior systemic chemotherapy (CBDCA+nab‐PTX). The combination of ICIs plus systemic chemotherapy is expected to have a synergistic effect as reported in recent studies.13, 14
In conclusion, treatment with ICIs can show a drastic bronchoscopic response, as seen in this case where the healing process was notable with minimal scarring. Furthermore, this case has continued to retain locally bronchoscopic and complete pathological response to treatment compared to previous cytotoxic chemotherapy.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
The authors also thank Mr Jason Tonge from St Marianna University School of Medicine for the linguistic review of this manuscript.
References
- 1. Garon EB, Rizvi NA, Hui R et al Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015; 372 (21): 2018–28. 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373 (17): 1627–39. 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373 (2): 123–35. 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW et al Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016; 387: 1540–50. Available from URL: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)01281-7/fulltext. [DOI] [PubMed] [Google Scholar]
- 6. Wang GX, Kurra V, Gainor JF et al Immune checkpoint inhibitor cancer therapy: Spectrum of imaging findings. Radiographics 2017; 37 (7): 2132–44. 10.1148/rg.2017170085. [DOI] [PubMed] [Google Scholar]
- 7. Morikawa K, Kurimoto N, Kakinuma K, Furuya N, Miyazawa T, Mineshita M. Three cases of an unsuccessful histopathological diagnosis of bronchoscopic re‐biopsy specimens from EGFR‐mutant adenocarcinoma patients after EGFR‐TKI therapy. Jpn J Lung Cancer 2016; 56: 219–26. Available from URL: https://www.jstage.jst.go.jp/article/haigan/56/3/56_219/_pdf/-char/ja. [Google Scholar]
- 8. Forde PM, Chaft JE, Smith KN et al Neoadjuvant PD‐1 blockade in resectable lung cancer. N Engl J Med 2018; 378 (21): 1976–86. 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lingzhi H, Seyedeh D, Marcelo VN et al Spatial and temporal heterogeneity of PD‐L1 and its impact on benefit from immune checkpoint blockade in non‐small cell lung cancer (NSCLC). J Clin Oncol 2019; 37 (Suppl. abstr 9017). 10.1200/JCO.2019.37.15_suppl.9017. [DOI] [Google Scholar]
- 10. Chiyo M, Shibuya K, Hoshino H et al Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung Cancer 2005; 48 (3): 307–13. 10.1016/j.lungcan.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 11. Chen DS, Mellman I. Oncology meets immunology: The cancer‐immunity cycle. Immunity 2013; 39 (1): 1–10. 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 12. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31: 51–72. 10.1146/annurev-immunol-032712-100008#article-denial. [DOI] [PubMed] [Google Scholar]
- 13. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018; 378 (22): 2078–92. 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 14. Paz‐Ares L, Luft A, Vicente D et al Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018; 379 (21): 2040–51. 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]


