Abstract
Granulomatous dermatitis (GD) is the most common among a variety of skin reactions that may occur in the varicella‐zoster virus (VZV) reactivation area. It is thought that the formation of granulomas may be the result of a delayed hypersensitivity reaction to viral envelope glycoproteins. Immune checkpoint inhibitors (ICIs), such as nivolumab stimulate T cells and promote hypersensitivity reactions, leading to the formation of granulomas in VZV wrapping proteins, thus triggering VZV‐GD. Few cases of the use of ICIs in patients diagnosed with VZV‐GD have been reported in the literature. Here, we report the clinical case of a patient with metastatic lung cancer which was treated with nivolumab who subsequently developed VZV‐GD. Accurate clinical diagnosis and prompt treatment with antiviral agents have resulted in a complete resolution of the clinical picture.
Key points
Significant findings
Treatment with ICIs may result in VZV reactivation.
Accurate differential diagnosis and early treatment led to the resolution of VZV‐GD.
What this study adds
Few cases of ICI and VZV reactivation have been reported in the literature.
Full and timely resolution of VZV‐GD allowed the continuation of ICI treatment.
Keywords: dermatologic adverse events, herpes zoster, immune checkpoint inhibitors, metastatic lung cancer, nivolumab
Introduction
Varicella‐zoster virus (VZV‐GD) is a cutaneous reaction that can appear in the area in which a reactivation of the VZV takes place. It may occur during treatment with ICIs but very few cases are described in the literature.1, 2
A differential diagnosis of dermatological adverse events (dAEs) related to treatment with ICIs should be carried out. dAEs occur in 34%–45% of patients treated with ICIs.2 They may present as a rash, pruritus, hypopigmentation/vitiligo, but also as xerosis, alopecia, stomatitis, urticaria, a photosensitivity reaction, hyperhidrosis and skin exfoliation.3 Management depends on classification of skin signs and symptoms and their severity.2
Here, we report the clinical case of a patient with metastatic lung cancer which was treated with nivolumab who subsequently developed VZV‐GD. Accurate clinical diagnosis and prompt treatment with antiviral agents have resulted in a complete resolution of the clinical picture.
Case report
A 65‐year‐old woman presented to the clinic, following the appearance of supraclavicular lymphadenopathy with a diameter of 20 mm. A biopsy was performed, with a subsequent diagnosis of lung adenocarcinoma with mutation Exon 19 EGFR, ALK and ROS not rearranged, PDL‐1 negative.
Positron emission tomography (PET) and computed tomography (CT) scans were performed which indicated multiple mediastinal lymphadenopathy. Treatment with Afatinib was initiated and the disease was subsequently controlled for seven months. Following progression of the disease, no T790M mutation was detected in the circulating DNA, or after a new biopsy of the lesion.
The patient then commenced chemotherapy with six cycles of pemetrexed and cisplatin and whilst complete metabolic remission of a very short duration was achieved, it was followed by a rapidly evolving relapse. A PET/CT scan showed diffuse adenopathies, right adrenal loggia nodularity (SUV 12.1) right iliac adenopathies and cruralw inguin (SUV 13.7). The patient commenced treatment with nivolumab and achieved a complete response which was documented by PET scan. After six months of treatment, there was widespread erythema evident at the level of the humeral‐scapula articulation, with severe itching and pain. Subsequently, 2–3 days later, maculae and papules appeared which evolved into vesicles and then pustules. The area was affected throughout by severe itching and pain.
Dermatological diagnosis was a grade 3 dAE due to VZV‐GD, with interesting scapular and supraclavicular cutaneous areas (Figs 1a and 1b). Histopathology of the skin biopsy confirmed it was VZV infection (Figs 2a and 2b). Treatment with nivolumab was subsequently temporarily discontinued. The patient commenced treatment with valaciclovir, 1000 mg three times a day for seven days in addition to fusidic acid cream which was applied twice a day to the damaged skin.
Figure 1.

Herpes zoster infection with (a) necrotizing scapular and (b) supraclavicular cutaneous areas.
Figure 2.
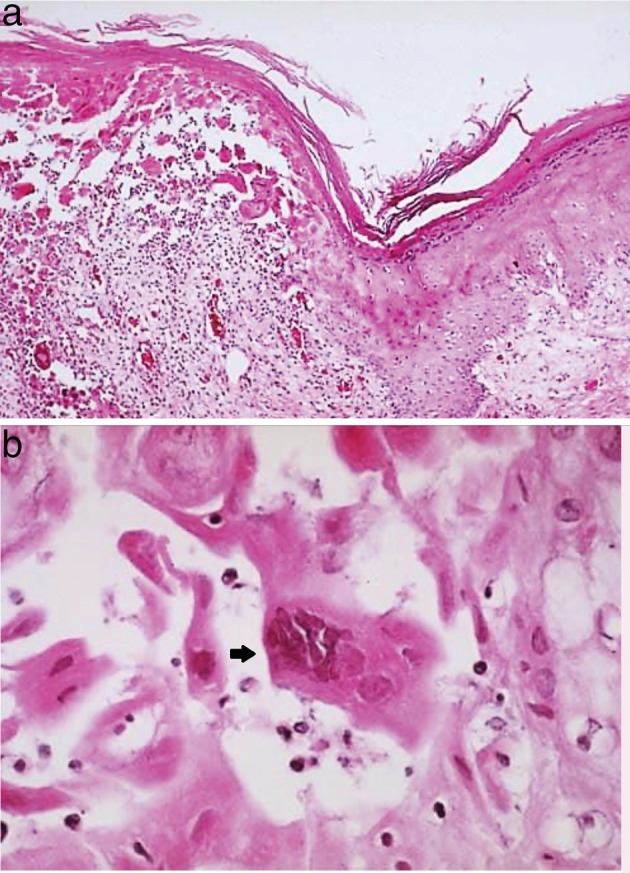
Histopathological analysis following skin biopsy. (a) High power intraepidermal vesicles with acantholysis indicative of herpesvirus infection (x200). (b) Swollen pale keratinocytes with enlarged slate‐grey nuclei and multinucleated cells (arrow) (x400).
A clinical reassessment after three weeks from the diagnosis of the infection documented a good resolution of the clinical picture with improvement in the cutaneous erythema, the rash had dried with the formation of crusts and almost complete disappearance of the symptoms of itching.
At the time of writing this report, the patient is continuing treatment with nivolumab with excellent disease control.
Discussion
The primary infection of VZV is chickenpox, manifested by viremia with a diffuse rash and seeding of multiple sensory ganglia where the virus establishes life‐long latency.4 The herpes zoster (HZ) virus is caused by the reactivation of latent VZV by the cranial nerve, or by ganglia of the dorsal root with spread of the virus along the sensory nerve to the dermatome. It is generally a mild, self‐limiting condition, but in some cases complications such as encephalitis, post‐herpetic neuralgia and Ramsay Hunt syndrome may occur, which can seriously affect the quality of life.5
There are more than one million cases of HZ in the United States each year with an annual rate of three to four cases per 1000 people. Most cases of HZ can be diagnosed clinically.4 Post VZV‐GD is the most frequent type within a variety of cutaneous reactions that can appear in the area in which a reactivation of this virus takes place.1, 2, 3, 4, 5, 6, 7 The formation of granulomas may be because of a delayed hypersensitivity reaction to viral envelope glycoproteins. These reactions have occurred in patients with lymphoproliferative syndromes, related in these cases to alterations in the number and function of immunoglobulins, cellular immunity and the increase in hypersensitivity reactions.1
Nivolumab is a human immunoglobulin monoclonal antibody, which binds to programmed death‐1 receptor (PD‐1) and blocks its interaction with the PD‐L1 and the PD‐L2. Interaction of the PD‐1 with the PD‐L1 and PD‐L2 ligands, which are expressed by the antigen‐presenting cells, cancer cell or by other cells in tumor microenvironment, involves inhibition of T cell proliferation and cytokine secretion. Nivolumab strengthens T cell responses, including antitumor responses.6 Some authors1 have postulated that nivolumab stimulated T cells, which promoted hypersensitivity reactions and the formation of granulomas to VZV envelope glycoproteins, triggering VZV‐GD.
Most frequent adverse events during treatment with ICIs are dAEs, which may range from vesicular, maculopapular, follicular, papular to exfoliative lesions or lupus‐like reactions.2
According to the European Society for Medical Oncology guidelines on the management of dAEs, the first requirement is to rule out any other etiology of the skin problem (Table 1). 8 In our case, accurate clinical diagnosis and prompt treatment resulted in a complete resolution of the clinical symptoms and the patient continues treatment with nivolumab with good disease control.
Table 1.
ESMO guidelines on the management of dermatological adverse events (dAEs)
| G | Macules/papules | Therapy |
|---|---|---|
| 1 | Covering <10% BSA | ICIs can be continued. TE, OA and TC |
| 2 | Covering 10%–30% BSA limiting ADL | ICIs can be continued. If not resolved, treatment should be interrupted until adverse skin event has reverted to grade 1. TE, OA and TC |
| 3 | Covering >30% BSA limiting selfcare ADL | Immediate interruption of ICIs, until these are back to grade 1. TE, OA and TC Systemic corticosteroids 0.5–1 mg/kg can be considered |
| 4 | Papulo‐pustular rash associated with life‐threatening superinfection Stevens‐Johnson syndrome TEN and bullous dermatitis covering >30% of BSA | Intensive care unit admission ICIs should be interrupted. Intravenous (methyl) prednisolone 1–2 mg/kg with tapering when toxicity resolves |
ADL, activities of daily living; BSA, body surface area; G, guideline; ICIs, immune checkpoint inhibitors; OA, oral antihistamines; TC, topical corticosteroids; TE, topical emollients; TEN, toxic epidermal necrolysis.
Disclosure
No authors report any conflict of interest.
Contributor Information
Elisa Gozzi, Email: vittoria.eli@virgilio.it.
Luigi Rossi, Email: dr.rossi@ymail.com.
References
- 1. Martín‐Carrasco P, Pérez‐Ruiz C, Zulueta‐Dorado T, Conejo‐Mir J. Post‐herpetic granulomatous dermatitis in a man treated with Nivolumab. Actas Dermosifiliogr 2017; 108 (8): 783–4. [DOI] [PubMed] [Google Scholar]
- 2. Assi T, Danu A, Mateus C et al Post‐shingles granulomatous dermatosis related to anti‐programmed cell death 1. Immunotherapy . 2019; 11 (7): 591–8. 10.2217/imt-2018-0169. [DOI] [PubMed] [Google Scholar]
- 3. Belum VR, Benhuri B, Postow MA et al Characterisation and management of dermatologic adverse events to agents targeting the PD‐1 receptor. Eur J Cancer 2016; 60: 12–25. 10.1016/j.ejca.2016.02.010 Epub 2016 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013; 369 (3): 255–63. 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forbes H, Douglas I, Finn A et al Risk of herpes zoster after exposure to varicella to explore the exogenous boosting hypothesis: Self controlled case series study using UK electronic healthcare data. BMJ 2020; 368: l6987 10.1136/bmj.l6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Official Nivolumab EMA data sheet . Available from URL: https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_it.pdf.
- 7. Ferenczi K, Rosenberg AS, McCalmont TH et al Herpes zoster granulomatous dermatitis: histopathologic findings in a case series. J Cutan Pathol 2015; 42 (10): 739–45. 10.1111/cup.12531. [DOI] [PubMed] [Google Scholar]
- 8. Haanen J, Carbonnel F, Robert C et al Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2017; 28 (suppl 4): iv119–42. [DOI] [PubMed] [Google Scholar]


