Abstract
Background
To compare the diagnostic yield of peripheral pulmonary lesions (PPLs) with and without navigation system.
Methods
Studies dating from January 1990 to October 2019 were collected from databases. Diagnostic yield of navigation bronchoscopy and non‐navigation bronchoscopy was extracted from comparative studies. Subgroup analysis was adopted to test diagnostic yield variation by lesion size, lobe location of the lesion, distance from the hilum, bronchus sign and nature of the lesion.
Results
In total, 2131 patients from 10 studies were enrolled into the study. Diagnostic yield of navigation bronchoscopy was statistically higher than non‐navigation bronchoscopy for PPLs (odds ratio [OR] 1.69, 95% confidence interval [CI] 1.32, 2.18, P < 0.001), particularly for PPLs in the peripheral third lung (OR 2.26, 95% CI 1.48, 3.44, P < 0.001) and for bronchus sign positive PPLs (OR 2.26, 95% CI 1.21, 4.26, P = 0.011). Navigation bronchoscopy had better performance than non‐navigation bronchoscopy when PPLs were ≤ 20 mm (OR 2.09, 95% CI 1.44, 3.03, P < 0.001). It also elevated diagnostic yield of malignant PPLs (OR 1.67, 95% CI 1.26, 2.22, P < 0.001) and PPLs in the bilateral upper lobes (OR 1.50, 95% CI 1.09, 2.08, P = 0.014).
Conclusions
Navigation bronchoscopy enhanced diagnostic yield when compared to non‐navigation bronchoscopy, particularly for PPLs in the peripheral third lung, PPLs being bronchus sign positive, PPLs ≤ 20 mm, malignant PPLs and PPLs in the bilateral upper lobes.
Key points
The current study provided systematic evaluation on the diagnostic value of navigation bronchoscopy by comparing it with non‐navigation bronchoscopy, and exploring the factors affecting the diagnostic yield.
Keywords: Diagnostic yield, electromagnetic navigation bronchoscopy (ENB), peripheral pulmonary lesions (PPLs), transbronchial lung biopsy (TBLB), virtual bronchoscopic navigation (VBN)
Introduction
Early diagnosis of pulmonary lesions is of great importance to reduce mortality due to lung cancer.1 When endobronchial lesions can be directly visualized by flexible bronchoscopes, peripheral pulmonary lesions (PPLs), generally defined as lesions surrounded by normal pulmonary parenchyma without any computed tomography (CT) evidence of endobronchial abnormalities, are unlikely to be detected by ordinary bronchoscopes.2, 3 Transthoracic needle aspiration (TTNA) has been recommended for nonsurgical diagnosis of PPLs with a sensitivity of 90%, but the relatively high risk of pneumothorax and other complications has limited its application, in particular when PPLs are small or located far from the chest.3, 4, 5, 6 Flexible bronchoscopic biopsy has a lower risk of occurrence of complications; however, the overall sensitivity of PPLs has previously been reported to be only 69%.5 Therefore, multi‐instruments have been developed to improve the performance of bronchoscopy for PPL diagnosis. For example, ultrathin bronchoscopy (UTB) enables a bronchoscopist to access PPLs at the distal bronchus.7 Endobronchial ultrasound (EBUS) confirms the arrival of biopsy instruments on site.8, 9 Navigation bronchoscopy, including virtual bronchoscopic navigation (VBN), electromagnetic navigation (ENB) and other navigation instruments, facilitates PPL diagnosis by directing a bronchoscope to its intended target via visualized three‐dimensional lung models.10, 11 All have been reported to increase diagnostic yield.12 Although there have been contradictory findings which report that navigation bronchoscopy only shortens the operation time of bronchoscopy instead of improving the diagnostic yield,13 ENB has even been associated with a lower diagnostic yield.14 In our analysis, in order to give a clear outline on the efficacy of navigation bronchoscopy, we pooled all the studies that directly compared diagnostic yield of bronchoscopy with or without navigation assistance.
Methods
Search strategy
Two reviewers independently searched records from PubMed, Ovid, Embase, Web of Science, Scopus, Science Direct, with a published date ranging from January 1990 to October 2019. The search strategy was as follows: “virtual bronchoscopic navigation” or “electromagnetic navigation bronchoscopy” and “peripheral pulmonary lesions:” their analogues were also included, and all the records were then summarized.
Study selection and quality assessment
Inclusion criteria: (i) Clinical studies which reported the diagnostic yield of navigation bronchoscopy for PPL diagnosis, including VBN, ENB and other navigational instruments for bronchoscopy, as well as the diagnostic yield of comparative non‐navigation bronchoscopy. (ii) The search was limited to reports published in English and humans. (iii) When data were presented in more than one article, the latest update details were extracted. Exclusion criteria: (i) Conference, reviews, case reports, and pilot studies. (ii) Studies with less than 30 subjects in each group.
Data extracted from records
Information extracted from the records included first author's family name, publication date, study design, nationality, bronchoscopy, navigation system, biopsy instruments and other auxiliaries. Overall diagnostic yield and diagnostic yield by lesion size, lobe location of the lesion, distance from hilum, bronchus sign, and nature of the lesion (malignant or benign) were also distilled. All the information was recorded independently by two reviewers. A divergence of views was discussed until an agreement was reached. The quality of included studies was assessed with the quality assessment of diagnostic accuracy studies tool‐2 (QUADAS‐2).
Statistical analysis
Since both randomized control trials (RCT) and case‐control studies were included in this analysis, we applied odds ratio (OR) to evaluate diagnostic yield variance between bronchoscopy with and without navigation. The OR of diagnostic yield was analyzed by Stata (version 14.0). A statistical test with P < 0.05 was considered statistically significant. Publication bias was explored by Begg's test. Duval's trim and fill analysis was adopted to adjust the result when prominent publication bias was displayed.15 A random effect model was applied when heterogeneity was prominent (I‐square > 25%); if not, a fixed effect model was adopted. Sensitivity analysis and meta‐regression were conducted to analyze the heterogeneity. Subgroup analysis was carried out to review the diagnostic yield changes according to lesion size, lobe location of the lesion, distance from the hilum, bronchus sign and nature of the lesion.
Results
Study selection
A total of 1117 records were collected from PubMed, Ovid, Embase, Web of science, Scopus, Science Direct from January 1990 to October 2019. After checking for duplicates, 588 records were removed. By screening titles and abstracts, 28 records were found. By analyzing the full text, 14 comparative studies which provided information for analysis were determined. Among these studies, four, including two with patients with less than 30 in each group,16, 17 one with prominent selection bias beyond adjustment,14 and one with a latest update,18, 19 were excluded from the analysis (Fig 1).
Figure 1.

Study search and selection flow.
Characteristics of included studies
In total, 10 clinical trials met the inclusive criteria,13, 19, 20, 21, 22, 23, 24, 25, 26, 27 of which, nine trials compared diagnostic yield of bronchoscopes with and without VBN, and only one study elevated the diagnostic value of ENB. In this study, when the control group was assisted by EBUS‐GS, the navigation group incorporated ENB with EBUS. These two groups were comparable because an extending working channel (EWC) of ENB performed almost equal to a thick guide sheath, as they had the same outer diameter and compatible biopsy channel. More details on bronchoscopies, navigation systems, biopsy methods and other auxiliaries are listed in Table 1.
Table 1.
Basic characteristics of studies
| Study | Type | Design | Ration | Bronchoscope | NB | Brand | Other auxiliaries | Biopsy |
|---|---|---|---|---|---|---|---|---|
| Bo et al. 201913 | Prospective | RCT | China | Not mentioned | VBN | DirectPath | EBUS‐GS | Forceps |
| Asano et al. 201519 | Prospective | RCT | Japan | P260F | VBN | Bf‐NAVI | EBUS‐GS, X‐ray | Forceps, brush, lavage |
| Asano 2013 et al. 20 | Prospective | RCT | Japan | XP260F, XP40 | VBN | Bf‐NAVI | X‐ray | Forceps, brush, lavage |
| Oshige et al. 201121 | Prospective | Non‐RCT | German | P260F, 1T‐260R | VBN | Bf‐NAVI | EBUS‐GS | Forceps, brush |
| Diez‐Ferrer et al. 201922 | Ambispective | Case–control | Spain | XP160F, XP190 | VBN | LungPoint | X‐ray | Forceps, brush, lavage |
| Kato et al. 201823 | Prospective | Non‐RCT | Japan | P260F | VBN | LungPoint | CT | Forceps, lavage |
| Xu et al. 201924 | Prospective | RCT | China | P260F | VBN | DirectPath | EBUS | Forceps |
| Miyoshi et al. 201925 | Retrospective | Case–control | Japan | 1T260 | VBN | SYNAPSE VINCENT | X‐ray | Forceps, brush |
| Matsumoto et al. 201726 | Retrospective | Case–control | Japan | Varied | VBN | Ziostation | EBUS‐GS, X‐ray, ROSE | Forceps, brush, TBNA |
| Eberhardt et al. 200727 | Prospective | RCT | American, German | 1T160 | ENB | SuperDimension | EBUS/EBUS‐GS | Forceps |
CT, computed tomography; EBUS, endobronchial ultrasound; ENB, electromagnetic navigation bronchoscopy; GS, guided sheath; NB, navigation bronchoscopy; non‐RCT, randomized control trial be disturbed; RCT, randomized control trial; ROSE, rapid on‐site evaluation; TBNA, transbronchial needle aspiration; VBN, virtual bronchoscopic navigation.
The quality of included studies assessed with QUADAS‐2 is shown in Figures S1 and S2. Risk of bias came primarily from patients who dropped out of the study and not all patients received surgery or other biopsy as a reference standard for diagnosis.
Overall, 1037 patients were enrolled in navigation bronchoscopy, whose pooled diagnostic yield was 73.58%. In total, 1094 patients were enrolled in non‐navigation bronchoscopy, with a pooled diagnostic yield of 62.80%. When a random effect model was adopted for the test, the overall OR of diagnostic yield of navigation bronchoscopy to non‐navigation bronchoscopy was 1.69 (95% confidence interval [CI] 1.32, 2.18), which was statistically significant (P < 0.001, Fig 2).
Figure 2.

Forest plot of diagnostic OR of navigation bronchoscopy to non‐navigation bronchoscopy. CI, confidence interval; NB, navigation bronchoscopy; NON‐NB, non‐navigation bronchoscopy; OR, odds ratio.
Publication bias and heterogeneity
The existence of publication bias was revealed by the asymmetric funnel plot graphed by Begg's test (Fig 3a, Begg's test, P > 0.020). As a result, Duval's trim and filled analysis was applied to correct for bias. By Duval's trim and filled analysis, three estimated potentially unreported studies were automatically included in the analysis, which turned the funnel plot symmetric (Fig 3b) and shifted the value of pooled OR to 1.46 (95% CI 1.12, 1.93, P = 0.006), but the pooled OR did not change significantly. The outcome indicated that the previous result of overall OR was stable, despite publication bias. Heterogeneity between studies was evaluated by omitting one specific study at a time and calculating pooled OR changes. No prominent heterogeneity was found in any specific study (Fig S3). Heterogeneity of included studies was further analyzed by meta‐regression according to publication year, nationality, protocol design, navigation system and the particular bronchoscopy that navigation system combined. However, none of the across study differences were the major contributors that introduced heterogeneity into the test.
Figure 3.

Funnel plot. (a) Funnel plot of 10 studies included in analysis of diagnostic OR of navigation bronchoscopy to non‐navigation bronchoscopy graphed by Begg's test. (b) Funnel plot adjusted by Duval's trim and filled test. ( ) Included studies and (
) Included studies and ( ) filled studies.
) filled studies.
Diagnostic yield by lesion size
Subgroup analysis was adopted to test OR of bronchoscopic diagnostic yield of PPLs by lesion size with or without navigation assistance, and in total nine studies were included in the analysis.13, 19, 20, 21, 22, 23, 24, 25, 27 Diagnostic yield of navigation bronchoscopy (64.09%) was statistically higher than that of non‐navigation bronchoscopy (48.67%) for PPLs ≤ 20 mm (OR 2.09, 95% CI 1.44, 3.03, P < 0.001). In PPLs > 20 mm, there was no statistical difference between pooled diagnostic yield of navigation bronchoscopy (79.27%) and non‐navigation bronchoscopy (yield 76.42%, OR 1.15, 95% CI 0.74, 1.78, P = 0.527) (Fig 4).
Figure 4.

Forest plot of diagnostic OR of navigation bronchoscopy to non‐navigation bronchoscopy by lesion size.
Diagnostic yield by lobe location of the lesion
Six studies provided information on bronchoscopic diagnostic yield according to the pulmonary lobe containing the lesion.13, 19, 20, 24, 25, 27 Whilst five studies grouped PPLs by different pulmonary lobes, Miyoshi et al. classified PPLs in the right middle lobe (RML) and left lingula lobe of the lung into the same group, and this study was therefore excluded from the analysis.25 Pooled diagnostic yield of navigation bronchoscopy and non‐navigation bronchoscopy for PPLs at bilateral upper lobes (BULs), RML and bilateral lower lobes (BLLs) was 75.56%, 89.23%, 70.74% and 67.28%, 82.46%, 65.90%, respectively. Diagnostic yield of navigation bronchoscopy was statistically better than non‐navigation bronchoscopy at BULs (OR 1.50, 95% CI 1.09, 2.08, P = 0.014). However, the diagnostic difference of bronchoscopy with and without navigation was not statistically significant at RML (OR 1.73, 95% CI 0.61, 4.95, P = 0.303) or BULs (OR 1.21, 95% CI 0.84, 1.75, P = 0.313) (Fig 5).
Figure 5.

Forest plot of diagnostic OR of navigation bronchoscopy to non‐navigation bronchoscopy by the lobe location of the lesion. BLLs, bilateral lower lobes; BULs, bilateral upper lobes; RML, right middle lobe.
Diagnostic yield by distance from the hilum
Location of lesions were classified into center, intermediate, and peripheral third according to the distance from the hilum.28 In this analysis, PPLs in the center and intermediate third lung were included in one group and compared with PPLs in the peripheral third. Four studies were adopted for this analysis.19, 20, 22, 23 Navigation bronchoscopy yielded 70.59% and was statistically better than non‐navigation bronchoscopy which yielded 50.36% in the peripheral third lung (OR 2.26, 95% CI 1.48, 5.92, P < 0.001). When PPLs were located at the center and intermediate thirds, diagnostic yield of navigation bronchoscopy (68.97%) and non‐navigation bronchoscopy (67.36%) did not differ statistically (OR 0.93, 95% CI 0.34, 2.54, P = 0.890) (Fig 6).
Figure 6.
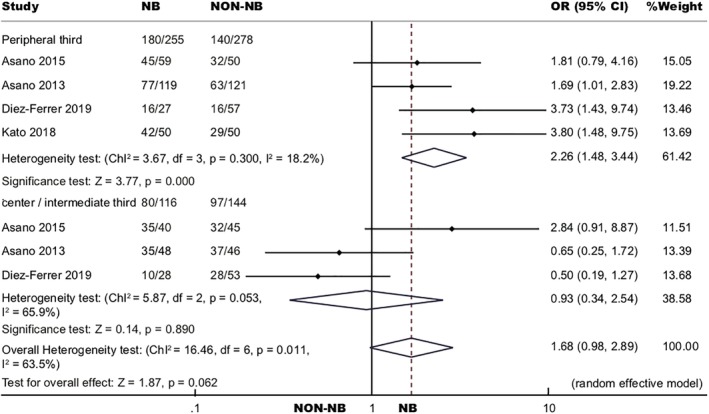
Forest plot of diagnostic OR of navigation bronchoscopy to non‐navigation bronchoscopy by distance from the lesion to the hilum.
Diagnostic yield by bronchus sign
Bronchus sign was evaluated by CT to describe the positional relationship of a PPL relative to the nearby bronchus.29 No bronchus found near the PPL was termed as bronchus sign negative. If a bronchus was found adjacent to, or within, the PPL, it was defined as bronchus sign positive. Five studies were included in our analysis.19, 20, 22, 23, 25 In the bronchus sign positive subgroup, overall OR of diagnostic yield of navigation bronchoscopy (75.00%) to non‐navigation bronchoscopy (59.73%) was 2.26, 95% CI (1.21, 4.26), which was statistically significant (P = 0.011). In the bronchus sign negative subgroup, pooled diagnostic yield of navigation bronchoscopy (39.24%) was similar to non‐navigation bronchoscopy (31.03%), and the overall OR was 1.49 (95% CI 0.77, 2.98, P = 0.234) (Fig 7).
Figure 7.
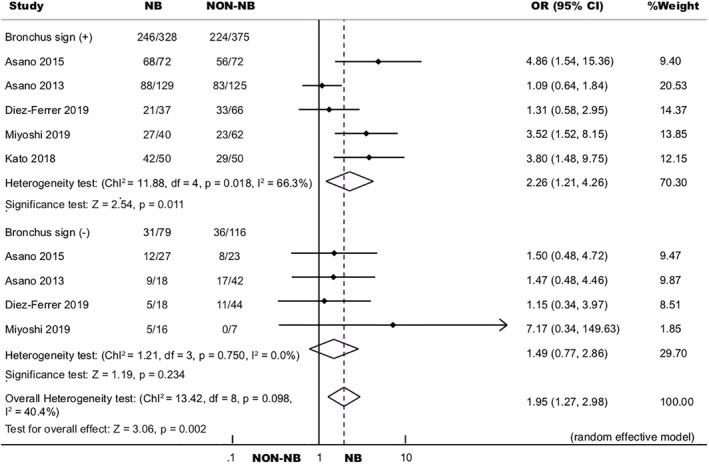
Forest plot of diagnostic OR of navigation bronchoscopy to non‐navigation bronchoscopy by bronchus sign.
Diagnostic yield by nature of the lesion
Seven studies were included in the analysis.13, 20, 21, 22, 24, 25, 27 In total, 498 malignant PPLs detected by navigation bronchoscopy yielded 77.91%, and 596 malignant PPLs, detected by non‐navigation bronchoscopy, yielded 65.94%. The pooled OR was 1.67 (95% CI 1.26, 2.22, P < 0.001). Pooled diagnostic yield of navigation bronchoscopy for benign PPLs was 63.10% (159/252), Diagnostic yield of non‐navigation bronchoscopy for benign PPLs was 55.17% (128/232), and the pooled OR was 1.40 (95% CI 0.97, 2.03, P = 0.075) (Fig 8).
Figure 8.

Forest plot of diagnostic OR of navigation bronchoscopy to non‐navigation bronchoscopy by nature of the lesion.
We further analyzed bronchoscopic diagnostic yield by different characteristics of the lesions (size, lobe location, distance from the hilum, bronchus sign and nature). Results of the diagnostic OR covariant by navigation bronchoscopy and non‐navigation bronchoscopy were as follows: overall bronchoscopic diagnostic yield for PPLs > 20 mm was statistically higher than PPLs ≤ 20 mm (OR 2.55, 95% CI 1.59, 4.10, P < 0.001). Non‐navigation bronchoscopy had better performance at the inner two thirds of the lung compared to the peripheral third (OR 2.52, 95% CI 1.42, 4.46, P = 0.002). Overall, bronchoscopic diagnostic yield for PPLs at BULs was almost the same as PPLs at the RML and BLLs (OR 1.06, 95% CI 0.74, 1.50, P = 0.763). Bronchosopic diagnostic yield of bronchus sign positive PPLs was greater than bronchus sign negative PPLs (OR 4.22, 95% CI 2.62, 6.81, P < 0.001), irrespective of whether they were navigation assisted or not. Diagnostic yield of malignant lesions was statistically higher than benign lesions, both in the navigation group (OR 2.57, 95% CI 1.76, 3.74, P < 0.001) and non‐navigation group (OR 2.63, 95% CI 1.82, 3.81, P < 0.001) (Table 2).
Table 2.
Pooled OR of bronchoscopic diagnostic yield listing navigation bronchoscopy subgroup, non‐navigation bronchoscopy subgroup and overall
| Group | Yielda | Yieldb | OR | 95% CI | P‐value |
|---|---|---|---|---|---|
| PPLs >20 mma/PPLs <20 mmb | |||||
| NB | 79.27% (371/468) | 61.69% (256/415) | 1.89 | (0.88, 4.06) | 0.105 |
| Non‐NB | 76.42% (376/492) | 47.61% (209/439) | 3.36 | (1.82, 6.18) | <0.001* |
| Overall | 77.81% (747/960) | 54.45% (465/854) | 2.55 | (1.59, 4.10) | <0.001* |
| Center and intermediate thirdsa/peripheral thirdb | |||||
| NB | 68.97% (80/116) | 67.32% (138/205) | 1.10 | (0.43, 2.80) | 0.850 |
| Non‐NB | 67.36% (97/144) | 48.68% (111/228) | 2.52 | (1.42, 4.46) | 0.002* |
| Overall | 65.6% (177/270) | 57.51% (249/433) | 1.70 | (0.95, 3.05) | 0.075 |
| BULsa/RML and BLLsb | |||||
| NB | 75.56% (272/360) | 74.44% (249/335) | 1.19 | (0.70, 2.00) | 0.524 |
| Non‐NB | 67.28% (255/379) | 68.87% (219/318) | 0.95 | (0.55, 1.66) | 0.863 |
| Overall | 71.31% (527/739) | 71.67% (468/653) | 1.06 | (0.74, 1.50) | 0.763 |
| Bronchus sign positivea/negativeb | |||||
| NB | 73.38% (204/278) | 39.24% (31/79) | 4.96 | (1.89, 13.05) | 0.001* |
| Non‐NB | 60.00% (195/325) | 31.03% (36/116) | 3.61 | (2.24, 5.80) | <0.001* |
| Overall | 66.17% (399/603) | 34.36% (67/195) | 4.22 | (2.62, 6.81) | <0.001* |
| Malignanta/benignb | |||||
| NB | 77.91% (388/498) | 63.10% (159/252) | 2.57 | (1.76, 3.74) | <0.001* |
| Non‐NB | 65.94% (393/596) | 55.17% (128/232) | 2.44 | (1.70,3.50) | <0.001* |
| Overall | 71.39% (781/1094) | 59.30% (287/484) | 2.50 | (1.93,3.24) | <0.001* |
P < 0.05.
BLLs, bilateral lower lobes; BULs, bilateral upper lobes; CI, confident index; NB, navigation bronchoscopy; Non‐NB, non‐navigation bronchoscopy; OR, odds ratio; PPLs, peripheral pulmonary lesions; RML, right middle lobe; yielda, diagnostic yield of groupa; yieldb, diagnostic yield of groupb.
Complications
A total of seven studies included in the analysis reported complications.13, 19, 20, 23, 24, 26, 27 Prevalence of complications reported in navigation bronchoscopy and non‐navigation bronchoscopy was 3.22% and 2.67%, respectively. There was no significant difference between onset of complications of the above two groups (OR 1.28, 95% CI 0.73, 2.25, P = 0.397). Pneumothorax and hemorrhage were the most common complications reported. There was 1.73% pneumothorax, 1.38% hemorrhage, 0.11% others happened in the navigation group, and 1.51% pneumothorax, 0.93% hemorrhage, 0.23% others happened in the non‐navigation group.
Discussion
Diagnostic yield of bronchoscopy for pulmonary lesions is affected by multiple factors, such as prevalence of malignancy,28, 30 lesion size,8, 14, 31 localization14 and bronchus sign.32, 33 Other than all the variations, such as experienced bronchoscopist,34 different bronchoscopes,7 auxiliary instruments,35, 36 and sampling technics,37 all these factors will have some influence on transbronchial lung biopsy. After sampling of the lesions, rapid on‐site evaluation (ROSE) and histological staining to confirm the diagnosis will also divert diagnostic yield.38, 39 It is a very difficult task to determine whether navigation bronchoscopy improved diagnostic accuracy when compared to non‐navigation bronchoscopy according to the results of different studies. Therefore, in our meta‐analysis, we adjusted navigational bronchoscopy and non‐navigational bronchoscopy performance under the same condition to make the diagnostic yield comparable.
According to our meta‐analysis, diagnostic yield of navigation bronchoscopy for PPLs was 1.69 times higher than that of non‐navigation bronchoscopy. The value of OR did not vary between publication year, nationality, design, navigation system and particular bronchoscopes combined with navigation system. The reason why navigation bronchoscopy accumulated diagnostic yield may be because the navigation system enabled a bronchoscopist to find a precise bronchial route to access the PPLs, thus increasing the possibility of achieving proper samples.25
Bronchoscopic diagnostic yield was prominently elevated by the navigation system in the peripheral third lung, but not in the inner two thirds. Lesions in the peripheral third lung challenged the precise direction because it needed to pass several generations of bronchi to reach the intended lesions, which made the biopsy route become a complex labyrinth. As a result, navigation assistance was of great importance in these circumstances.20 Some researchers have defined PPLs as lesions located in the peripheral third of the lung without bronchoscopic evidence of endobronchial abnormalities, and this narrowed definition may make navigation bronchoscopy superior in the diagnosis of PPLs.25, 40
In accordance with previous records, both navigation bronchoscopy and non‐navigation bronchoscopy had a relatively higher diagnostic yield for bronchus sign positive PPLs than bronchus sign negative PPLs,8, 29, 32, 41, 42 yet navigation bronchoscopy still yielded higher than non‐navigation bronchoscopy when the bronchus sign was presented. The improvement in diagnostic yield was attributed to the improved precision in arriving at the intended bronchi. What is more, the fact that bronchoscopy was poor at diagnosing bronchus sign negative PPLs, irrespective of whether it was navigation‐assisted or not, may indicated that TTNA or surgery should be applied in this case.29, 43
Diagnostic yield of navigation bronchoscopy was statistically higher than non‐navigation bronchoscopy for PPLs ≤ 20 mm, but the elevation did not reach statistical significance for PPLs > 20 mm. It should be noted that the number of subjects enrolled in the two groups were parallel, providing evidence that navigation bronchoscopy is more superior than non‐navigation bronchoscopy in the diagnosis of smaller lesions. Previous research proved endobronchial pathway selection to the lesion to be a major possible error in detecting a peripheral lesion.44 In contrast with large lesions, which could have several right routes reaching out to intended lesions, it is much more difficult to find a proper biopsy pathway for a small nodule, so navigation assistance in this instance is essential.
Navigation bronchoscopy elevated diagnostic yield when lesions were malignant. Benign lesions were much more difficult to distinguish than malignant diseases, since the biopsy specimens were usually nonspecific.45 Navigation bronchoscopy was found to enhance the diagnostic yield of PPLs at BULs, but not at RML and BLLs, because navigation bronchoscopy makes it possible for bronchoscopists to arrange the best biopsy route so as to avoid sharp angles and the complex structures of the upper lobes.27 Bronchi in the upper lobe, seldom affected by respiratory motion and heart beats, further facilitated virtual image formation.20 However, the constant movement in lesion position with respiratory motion during biopsy disturbed the performance of ENB at BLLs.46
Historical rate of complications reported for bronchoscopy was 0%–5%,8, 27, 47 and the outcome of our meta‐analysis coincides with previous studies. Combined navigation bronchoscopy accumulated diagnostic yield without increasing the risk of complications, confirming that it was a safe and effective approach.
There are several limitations in our study. First, both case‐control studies and studies that were not fully randomized were included in the analysis, and would therefore introduce selection bias. Second, only one study included in the analysis evaluated efficacy of ENB, so its representativeness for ENB may be open to doubt. Third, some subgroup analysis was not very convincing due to limited sample size. When analyzing diagnostic yield by size, some studies did not group lesions by PPLs ≤ 20 mm and PPLs >20 mm, and therefore different classification of lesions on the edge of 20 mm may affect the results. Fourth, in this article, diagnostic yield of navigation bronchoscopy affected by characteristic of the lesions itself (size, lobe location, distance from the hilum, bronchus sign and nature) were the main considerations. However, changes caused by different bronchoscopy and biopsy methods (forceps, brush, lavage) were neglected, and require further study.
In conclusion, navigation bronchoscopy is a safe and effective approach that improves diagnostic accuracy without increasing incidence of complications. It accumulates diagnostic yield of PPLs in the peripheral third lung, PPLs being bronchus sign positive, PPLs ≤ 20 mm, malignant PPLs and PPLs in the bilateral upper lobes. A series of multicenter, large‐scale studies are needed to further confirm the results of our study.
Disclosure
The authors have no conflicts of interest to declare.
Supporting information
Figure S1 Risk of bias and applicability concerns graph: review authors' judgments about each domain for each included study.
Figure S2 Risk of bias and applicability concerns graph: review authors' judgments about each domain presented as percentages across included studies.
Figure S3 Sensitive analysis by omitting given named study from the overall analysis and estimating diagnostic odds ratio (OR) of navigation bronchoscopy to non‐navigation bronchoscopy for peripheral pulmonary lesions (PPLs).
Acknowledgments
This work was supported by National Key R&D Program of China (grant number 2017YFC0112700), Shanghai Municipal Health and Medical Talents Training Program (grant number 2018BR09), Shanghai Municipal Education Commission‐Gaofeng Clinical Medicine Grant Support (grant number 20181815). The authors thank Dr Sakae Homma from the Department of Respiratory Medicine, Toho University Graduate School of Medicine (Tokyo, Japan) and Dr Liyan Bo from the Department of Respiratory and Critical Care Medicine, Tangdu Hospital. Air Force Medical University (Xi'an, China) provided further explanation of their articles for the analysis.
Contributor Information
Simin Jiang, Email: jsm0000@163.com.
Jiayuan Sun, Email: xkyyjysun@163.com.
References
- 1. de Koning HJ, Meza R, Plevritis SK et al Benefits and harms of computed tomography lung cancer screening strategies: A comparative modeling study for the U.S. preventive services task force. Ann Intern Med 2014; 160: 311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacomelli M, Demarzo SE, Cardoso PFG, Palomino ALM, Figueiredo VR. Radial‐probe EBUS for the diagnosis of peripheral pulmonary lesions. J Bras Pneumol 2016; 42: 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest 2013; 143: e142S–65S. [DOI] [PubMed] [Google Scholar]
- 4. Bai C, Choi CM, Chu CM et al Evaluation of pulmonary nodules: Clinical practice consensus guidelines for Asia. Chest 2016; 150: 877–93. [DOI] [PubMed] [Google Scholar]
- 5. Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: Summary of published evidence. Chest 2003; 123: 115S–28S. [DOI] [PubMed] [Google Scholar]
- 6. Heerink WJ, de Bock GH, de Jonge GJ, Groen HJM, Vliegenthart R, Oudkerk M. Complication rates of CT‐guided transthoracic lung biopsy: Meta‐analysis. Eur Radiol 2017; 27: 138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oki M, Saka H, Ando M et al Ultrathin bronchoscopy with multimodal devices for peripheral pulmonary lesions. A randomized trial. Am J Respir Crit Care Med 2015; 192: 468–76. [DOI] [PubMed] [Google Scholar]
- 8. Ali MS, Trick W, Mba BI, Mohananey D, Sethi J, Musani AI. Radial endobronchial ultrasound for the diagnosis of peripheral pulmonary lesions: A systematic review and meta‐analysis. Respirology 2017; 22: 443–53. [DOI] [PubMed] [Google Scholar]
- 9. Wang Memoli JS, Nietert PJ, Silvestri GA. Meta‐analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012; 142: 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang W, Chen S, Dong X, Lei P. Meta‐analysis of the diagnostic yield and safety of electromagnetic navigation bronchoscopy for lung nodules. J Thorac Dis 2015; 7: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan KA, Nardelli P, Jaeger A, O'Shea C, Cantillon‐Murphy P, Kennedy MP. Navigational bronchoscopy for early lung cancer: A road to therapy. Adv Ther 2016; 33: 580–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishiwata T, Gregor A, Inage T, Yasufuku K. Bronchoscopic navigation and tissue diagnosis. Gen Thorac Cardiovasc Surg 2019. 10.1007/s11748-019-01241-0. [DOI] [PubMed] [Google Scholar]
- 13. Bo L, Li C, Pan L et al Diagnosing a solitary pulmonary nodule using multiple bronchoscopic guided technologies: A prospective randomized study. Lung Cancer 2019; 129: 48–54. [DOI] [PubMed] [Google Scholar]
- 14. Ost DE, Ernst A, Lei X et al Diagnostic yield and complications of bronchoscopy for peripheral lung lesions: Results of the AQuIRE registry. Am J Respir Crit Care Med 2016; 193: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duval S, Tweedie R. Trim and fill: A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000; 56: 455–63. [DOI] [PubMed] [Google Scholar]
- 16. Haidong H, Yunye N, Wei Z et al Multiple guided technologies based on radial probe endobronchial ultrasound for the diagnosis of solitary peripheral pulmonary lesions: A single‐center study. J Cancer 2017; 8: 3514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong K‐Y, Tse H, Pak KKT et al Integrated use of virtual bronchoscopy and endobronchial ultrasonography on the diagnosis of peripheral lung lesions. J Bronchology Interv Pulmonol 2014; 21: 14–20. [DOI] [PubMed] [Google Scholar]
- 18. Ishida T, Asano F, Yamazaki K et al Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: A randomised trial. Thorax 2011; 66: 1072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asano F, Shinagawa N, Ishida T et al Virtual bronchoscopic navigation improves the diagnostic yield of radial‐endobronchial ultrasound for peripheral pulmonary lesions with involved bronchi on CT. Intern Med 2015; 54: 1021–5. [DOI] [PubMed] [Google Scholar]
- 20. Asano F, Shinagawa N, Ishida T et al Virtual bronchoscopic navigation combined with ultrathin bronchoscopy. A randomized clinical trial. Am J Respir Crit Care Med 2013; 188: 327–33. [DOI] [PubMed] [Google Scholar]
- 21. Oshige M, Shirakawa T, Nakamura M et al Clinical application of virtual bronchoscopic navigation system for peripheral lung lesions. J Bronchology Interv Pulmonol 2011; 18: 196–202. [DOI] [PubMed] [Google Scholar]
- 22. Diez‐Ferrer M, Morales A, Tebé C et al Ultrathin bronchoscopy with and without virtual bronchoscopic navigation: Influence of segmentation on diagnostic yield. Respiration 2019; 97: 252–8. [DOI] [PubMed] [Google Scholar]
- 23. Kato A, Yasuo M, Tokoro Y et al Virtual bronchoscopic navigation as an aid to CT‐guided transbronchial biopsy improves the diagnostic yield for small peripheral pulmonary lesions. Respirology 2018; 23: 1049–54. [DOI] [PubMed] [Google Scholar]
- 24. Xu C, Yuan Q, Wang Y et al Usefulness of virtual bronchoscopic navigation combined with endobronchial ultrasound guided transbronchial lung biopsy for solitary pulmonary nodules. Medicine (Baltimore) 2019; 98: e14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyoshi S, Isobe K, Shimizu H et al The utility of virtual bronchoscopy using a computed tomography workstation for conducting conventional bronchoscopy: A retrospective analysis of clinical practice. Respiration 2019; 97: 52–9. [DOI] [PubMed] [Google Scholar]
- 26. Matsumoto Y, Izumo T, Sasada S, Tsuchida T, Ohe Y. Diagnostic utility of endobronchial ultrasound with a guide sheath under the computed tomography workstation (ziostation) for small peripheral pulmonary lesions. Clin Respir J 2017; 11: 185–92. [DOI] [PubMed] [Google Scholar]
- 27. Eberhardt R, Anantham D, Ernst A, Feller‐Kopman D, Herth F. Multimodality bronchoscopic diagnosis of peripheral lung lesions: A randomized controlled trial. Am J Respir Crit Care Med 2007; 176: 36–41. [DOI] [PubMed] [Google Scholar]
- 28. Baaklini WA, Reinoso MA, Gorin AB, Sharafkaneh A, Manian P. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117: 1049–54. [DOI] [PubMed] [Google Scholar]
- 29. Gaeta M, Pandolfo I, Volta S et al Bronchus sign on CT in peripheral carcinoma of the lung: Value in predicting results of transbronchial biopsy. Am J Roentgenol 1991; 157: 1181–5. [DOI] [PubMed] [Google Scholar]
- 30. Sun J, Garfield DH, Lam BJ et al The value of autofluorescence bronchoscopy combined with white light bronchoscopy compared with white light alone in the diagnosis of intraepithelial neoplasia and invasive lung cancer: A meta‐analysis. J Thorac Oncol 2011; 6: 1336–44. [DOI] [PubMed] [Google Scholar]
- 31. McWilliams A, Tammemagi MC, Mayo JR et al Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013; 369: 910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seijo LM, De Torres JP, Lozano MD et al Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: Results from a prospective study. Chest 2010; 138: 1316–21. [DOI] [PubMed] [Google Scholar]
- 33. Evison M, Crosbie PAJ, Morris J, Martin J, Barber PV, Booton R. Can computed tomography characteristics predict outcomes in patients undergoing radial endobronchial ultrasound‐guided biopsy of peripheral lung lesions? J Thorac Oncol 2014; 9: 1393–7. [DOI] [PubMed] [Google Scholar]
- 34. Wahidi MM, Silvestri GA, Coakley RD et al A prospective multicenter study of competency metrics and educational interventions in the learning of bronchoscopy among new pulmonary fellows. Chest 2010; 137: 1040–9. [DOI] [PubMed] [Google Scholar]
- 35. Asano F, Ishida T, Shinagawa N et al Virtual bronchoscopic navigation without X‐ray fluoroscopy to diagnose peripheral pulmonary lesions: A randomized trial. BMC Pulm Med 2017; 17: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsushima K, Sone S, Hanaoka T, Takayama F, Honda T, Kubo K. Comparison of bronchoscopic diagnosis for peripheral pulmonary nodule under fluoroscopic guidance with CT guidance. Respir Med 2006; 100: 737–45. [DOI] [PubMed] [Google Scholar]
- 37. Sasada S, Ogata Y, Kobayashi M et al Angle forceps used for transbronchial biopsy in which standard forceps are difficult to manipulate: A comparative study. Chest 2006; 129: 725–33. [DOI] [PubMed] [Google Scholar]
- 38. Diacon AH, Koegelenberg CFN, Schubert P et al Rapid on‐site evaluation of transbronchial aspirates: Randomised comparison of two methods. Eur Respir J 2010; 35: 1216–20. [DOI] [PubMed] [Google Scholar]
- 39. Steinfort DP, Leong TL, Laska IF, Beaty A, Tsui A, Irving LB. Diagnostic utility and accuracy of rapid on‐site evaluation of bronchoscopic brushings. Eur Respir J 2015; 45: 1653–60. [DOI] [PubMed] [Google Scholar]
- 40. Dhillon SS, Harris K. Bronchoscopy for the diagnosis of peripheral lung lesions. J Thorac Dis 2017; 9: S1047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shinagawa N, Yamazaki K, Onodera Y et al Factors related to diagnostic sensitivity using an ultrathin bronchoscope under CT guidance. Chest 2007; 131: 549–53. [DOI] [PubMed] [Google Scholar]
- 42. Tay JH, Irving L, Antippa P, Steinfort DP. Radial probe endobronchial ultrasound: Factors influencing visualization yield of peripheral pulmonary lesions. Respirology 2013; 18: 185–90. [DOI] [PubMed] [Google Scholar]
- 43. Chao TY, Te Chien M, Lie CH, Chung YH, Wang JL, Lin MC. Endobronchial ultrasonography‐guided transbronchial needle aspiration increases the diagnostic yield of peripheral pulmonary lesions: A randomized trial. Chest 2009; 136: 229–36. [DOI] [PubMed] [Google Scholar]
- 44. Dolina MY, Cornish DC, Merritt SA et al Interbronchoscopist variability in endobronchial path selection. Chest 2008; 133: 897–905. [DOI] [PubMed] [Google Scholar]
- 45. Wilson RK, Fechner RE, Greenberg SD, Estrada R, Stevens PM. Clinical implications of a ‘nonspecific’ transbronchial biopsy. Am J Med 1978; 65: 252–6. [DOI] [PubMed] [Google Scholar]
- 46. Al‐Jaghbeer M, Marcus M, Durkin M, McGuire FR, Iftikhar IH. Diagnostic yield of electromagnetic navigational bronchoscopy. Ther Adv Respir Dis 2016; 10: 295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Folch EE, Pritchett MA, Nead MA et al Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: One‐year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol 2019; 14: 445–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Risk of bias and applicability concerns graph: review authors' judgments about each domain for each included study.
Figure S2 Risk of bias and applicability concerns graph: review authors' judgments about each domain presented as percentages across included studies.
Figure S3 Sensitive analysis by omitting given named study from the overall analysis and estimating diagnostic odds ratio (OR) of navigation bronchoscopy to non‐navigation bronchoscopy for peripheral pulmonary lesions (PPLs).


