Dear Sirs,
The coronavirus disease-2019 (COVID-19) pandemic originated in Wuhan (China) on December 2019. So far, more than 1,500,000 cases have been confirmed worldwide (> 150,000 cases in Italy) [1]. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is a systemic disorder typically presenting with fever, fatigue and prevalent respiratory disturbances (dry cough, dyspnea, chest pain, interstitial pneumonia) [2], although neurological manifestations are increasingly reported [3].
Guillain-Barré syndrome (GBS) is an acute/subacute immune-mediated polyradiculoneuropathy characterized by varying degrees of limbs or cranial-nerves weakness, loss of deep tendon reflexes, sensory and dysautonomic symptoms due to peripheral nerves and roots demyelination and/or axonal damage [4]. According to a recent review about two-third of all GBS are preceded by upper respiratory infection or enteritis [4]. Here we describe a case of GBS following a clinically resolved paucisymptomatic COVID-19.
A 70-year-old-woman was referred to our emergency department (ED) on Mar-28 complaining of asthenia, hands and feet paresthesia and gait difficulties progressing within 1 day. On Mar-4 she had developed fever (body temperature—BT = 38.5 °C) and dry cough. A day later she had been tested positive for SARS-CoV-2-RNA on RT-PCR with a nasopharyngeal swab. Symptoms of COVID-19 had resolved in a few days. The epidemiological survey had revealed a previous hospital visit to an inpatient in an area with high incidence for COVID-19 (Piacenza, Italy) on Feb-28, 29.
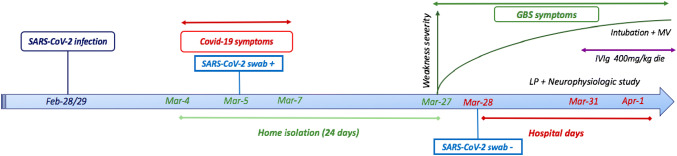
At the ED admission BT was 36.5 °C, oxygen saturation was 98% on room air. Arterial blood gas analysis showed pO2 = 76 mmHg with normal p/f ratio (= 363). Hematological investigations revealed slightly increased white blood cells (10.41 × 109/L, normal = 4–10 × 109/L) with 8.15 × 109/L neutrophils (normal = 2–8 × 109/L) and lymphocytes in the normal range. D-dimer, creatine phosphokinase, blood glucose, hepatic and renal function were normal, as well as c-reactive protein, erythrocyte sedimentation rate, folate and vitamin B12 blood levels. A chest high-resolution computed tomography revealed some small “ground glass” areas in both lungs. A repeated nasopharyngeal swab for SARS-CoV-2-RNA was negative. Mycoplasma Pneumoniae and Cytomegalovirus (CMV) serology (IgM and IgG), Legionella Pneumophila and Streptococcus Pneumoniae urinary tests were unrevealing. The neurological examination disclosed moderate (Medical Research Council grade 4/5) symmetric distal upper and lower limbs weakness, loss of deep tendon reflexes, preserved light touch and pinprick sensation. On Mar-31 a lumbar puncture was performed. The cerebrospinal fluid (CSF) analysis revealed slight albumino-cytological dissociation (CSF proteins = 48 mg/dL, normal = 0–40 mg/dL, white blood cells = 1 × 106/L, normal = 0–8 × 106/L). Microbiologic testing on CSF was negative (including herpes simplex virus, varicella zoster virus, Epstein-Bar virus, CMV, HIV-1, Borrelia Burgdorferi IgM and IgG). Neurophysiologic findings were consistent with a diagnosis of GBS (Table 1), according to current criteria [5]. A trial with 400 mg/die intravenous immunoglobulin (IVIg) for 5 days was started. On Apr-1 the patient was intubated and mechanical ventilation was applied, because of respiratory failure due to the worsening of muscle weakness. Figure 1 displays timeline of main clinical events and diagnostic investigations.
Table 1.
Results of neurophysiologic study
| Motor nerve conductions | Distal latency (ms) | Amplitude (mV) | Conduction velocity (m/s) | F waves |
|---|---|---|---|---|
| Median nerve | ||||
| Wrist-abductor pollicis brevis | R = NE; L = 4.5 (n.v. < 4.2) | R = 1.3; L = 4.5 (n.v. > 6) | R = A; L = A | |
| Antecubital fossa-wrist | R = NE; L = 12.3 | R = 0.8; L = 2.5 | R = 38.0; L = 30.8 (n.v. > 50) | |
| Ulnar nerve | ||||
| Wrist-abductor digiti minimi | R = 4.6; L = 1.5 (n.v. < 3.2) | R = 2.8; L = 2.3 (n.v. > 7) | R = A; L = A | |
| Below elbow-wrist | R = 9.7; L = 9.8 | R = 2.5; L = 1.7 | R = 43.1; L = 30.1 (n.v. > 52) | |
| Tibial nerve | ||||
| Medial malleolus-abductor hallucis brevis | R = 8.7; L = 8.5 (n.v. < 5) | R = 1.3; L = 1.1 (n.v. > 5) | R = A; L = A | |
| Popliteal fossa-medial malleolus | R = 19.5; L = 18.9 | R = 0.8; L = 0.1 | R = 38.0; L = 38.5 (n.v. > 41) | |
| Peroneal nerve | ||||
| Ankle-extensor digitorum brevis | R = NE; L = NE (n.v. < 5.5) | R = NE; L = NE (n.v. > 3) | R = A; L = A | |
| Below fibula-ankle | R = NE; L = NE | R = NE; L = NE | R = NE; L = NE (n.v. > 40) | |
| Orthodromic sensory nerve conduction studies | Amplitude (mV) | Conduction velocity (m/s) | ||
| Left median nerve | ||||
| Digit 3-wrist | NE (n.v. > 4) | NE (n.v. > 45) | ||
| Ulnar nerve | ||||
| Digit 5-wrist | R = 0.4; L = NE (n.v. > 3.5) | R = 37.5; L = NE (n.v. > 45) | ||
| Superficial fibular nerve | ||||
| Lateral calf—lateral ankle | R = NE; L = NE (n.v. > 3) | R = NE; L = NE (n.v. > 40) | ||
| Sural nerve | ||||
| Posterior ankle—calf | R = 4.6; L = 5.2 (n.v. > 5) | R = 55.0; L = 66.7 (n.v. > 40) | ||
Soleus H reflex was absent bilaterally. Evocable distal compound muscle action potentials (CMAPs) showed reduced amplitude because of temporal dispersion due to demyelination. Needle electromyography disclosed reorganization of motor units with polyphasic and long-duration potentials, without signs of acute denervation
R right, L left, n.v. normal values adjusted for age (65–75 years), NE not evocable, A absent
Fig. 1.
Timeline of clinical events and diagnostic investigations. COVID-19 coronavirus disease-2019, GBS Guillain-Barré syndrome, LP lumbar puncture, IVIg intravenous immunoglobulin, MV mechanical ventilation
To date, only a prior case of GBS concomitant with SARS-CoV-2 infection and a parainfectious profile has been reported [6]. In our patient, the temporal evolution of neurological manifestations resembles that of a postinfectious etiology, although a single repeated negative nasopharyngeal swab was available. Therefore, we may speculate an association between the acute polyradiculopathy and SARS-CoV-2 infection. This hypothesis is supported by the notion of 24-days home isolation before the onset of neurological symptoms and by the comprehensive exclusion of most common infectious agents related to GBS (negative IgM/IgG for CMV and Mycoplasma Pneumoniae, negative history for enteritis). However, we could not rule out with certainty a COVID-19 parainfectious neurological syndrome, given the reported suboptimal sensitivity of the RT-PCR for swab due to laboratory error or insufficient viral material in the specimen [7]. Moreover, other less common infectious agents (eg. West Nile and Toscana viruses), which were not tested but are endemic in Northern Italy, might be responsible for the present clinical picture [8]. A shortcoming of our report is the lack of antiganglioside antibody testing to identify specific targets of the autoimmune GBS process [9]. Furthermore, we did not perform a complete paraneoplastic/autoimmune screening in the acute phase including testing for serum onconeural and vasculitis-related antibodies (e.g. antineutrophil cytoplasmic antibodies—ANCA). Hence, we could not exclude the possibility of an autoimmune or paraneoplastic polyradiculoneuropathy mimicking GBS [10]. Nevertheless, the postinfectious onset, the acute clinical course and the typical neurophysiologic findings (polyradiculoneuropathy with predominant demyelination of both motor and sensory fibers, sural sparing pattern), together with the negative history for autoimmune, neoplastic or neurologic antecedences, make these alternative diagnoses less suitable. Another major limitation relies on the unavailability of SARS-CoV-2 serology and CSF validated test (e.g. PCR on CSF) in our center.
Taking together all these findings, the causal association between GBS and COVID-19 remains speculative, but more probable, given that GBS and Bickerstaff’s encephalitis have been already described as postinfectious complications of other coronavirus, sharing similarities with SARS-CoV-2 (Middle East respiratory syndrome, MERS-CoV) [11]. If our hypothesis will be confirmed in larger case series, neurologists and other clinicians should be aware of the important early recognition and treatment of the potential neuromuscular and autonomic worsening leading to cardio-respiratory failure in patients with GBS and mild or controlled pulmonary COVID-19 Notwithstanding the causative relationship remains unproved, we believe that our case description provide further evidence to the heterogenous and multi-systemic complications associated with SARS-CoV-2. Future researches and data acquisition are needed to clarify the possible pathophysiological correlation, as well as to characterize the clinical/electrophysiological pattern of new cases of GBS observed in the context of COVID-19 pandemic.
Availability of data and material
Deidentified data and material inherent to the case report and not included in the manuscript are available on request to the corresponding author by any qualified investigator.
Author contributions
PM: acquisition of data, analysis and interpretation of data, drafted the manuscript for intellectual content. MV, AGM, PMG, ARS, QP: acquisition of data, revised the manuscript for intellectual content. CC: acquisition of data, table creation, revised the manuscript for intellectual content. FM: study concept and design, acquisition of data, figure creation, analysis and interpretation of data, study supervision, drafted and revised the manuscript for intellectual content.
Funding
Not targeted funding reported.
Compliance with ethical standards
Conflicts of interest
The Authors declare no conflicts of interest relevant to the manuscript.
Ethical approval
This article does not contain any studies involving human participants performed by any of the Authors.
Informed consent
Written informed consent was collected from the patient for the inclusion of deidentified clinical data in a scientific publication, in accordance with the Declaration of Helsinki.
References
- 1.WHO (2020) Coronavirus disease 2019 (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Wang M, Chen S, et al. (2020) Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. MedRxiv 2020:2020.02.22.20026500.
- 4.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 5.Uncini A, Kuwabara S. The electrodiagnosis of Guillain-Barré syndrome subtypes: where do we stand? Clin Neurophysiol. 2018;129:2586–2593. doi: 10.1016/j.clinph.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Zhao H, Shen D, Zhou H, et al (2020) Guillain-Barré syndrome associated with SARS CoV-2 infection: causality or coincidence? Lancet Neurol. [DOI] [PMC free article] [PubMed]
- 7.Xie X, Zhong Z, Zhao W, et al. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020;26:200642. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okar SV, Bekircan-Kurt CE, Hacıoğlu S, et al. Toscana virus associated with Guillain-Barré syndrome: a case-control study. Acta Neurol Belg. 2020 doi: 10.1007/s13760-020-01279-5. [DOI] [PubMed] [Google Scholar]
- 9.Wanleenuwat P, Iwanowski P, Kozubski W. Antiganglioside antibodies in neurological diseases. J Neurol Sci. 2020;408:116576. doi: 10.1016/j.jns.2019.116576. [DOI] [PubMed] [Google Scholar]
- 10.Wakerley BR, Yuki N. Mimics and chameleons in Guillain-Barré and Miller Fisher syndromes. Pract Neurol. 2015;15(2):90–99. doi: 10.1136/practneurol-2014-000937. [DOI] [PubMed] [Google Scholar]
- 11.Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of Middle East respiratory syndrome. J Clin Neurol. 2010;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data and material inherent to the case report and not included in the manuscript are available on request to the corresponding author by any qualified investigator.