Abstract
Introduction
It has been proven that ALK‐rearranged non‐small cell lung cancer (NSCLC) is sensitive to ALK inhibitors while the chemotherapy resistance is unavoidable. In this study, safety and antitumor activity of the novel ALK inhibitor (ALKi) CT‐707 were evaluated in Chinese patients with advanced ALK‐rearranged NSCLC.
Methods
This single‐center, open‐label phase I study recruited adult patients with ALK‐rearrangement (confirmed by fluorescence in situ hybridization and/or immunohistochemistry) of locally advanced/metastatic malignancies including NSCLC. This study consisted of two parts: dose escalation and dose expansion. CT‐707 was administered orally once a day for 21 days.
Results
A total of 13 patients who were treated with CT‐707 from 450 to 600 mg (in the dose increasing phase) were enrolled in this trial (two patients were previously treated with crizotinib). There were 12 patients diagnosed with lung adenocarcinoma and one patient with malignant pleural mesothelioma. After treatment, grade 3 diarrhea (600 mg once a day) was found as dose‐limiting toxicity (DLT).The most common adverse events included diarrhea (92%), elevated aspartate aminotransferase (61%), elevated alanine aminotransferase (54%), hair loss (38%), and vomiting (31%). The overall response rate was 77% (10/13). Among all patients, four of the five patients who did not receive any treatment, one of the two patients who had received treatments with crizotinib, and five of the six patients who received standard chemotherapy achieved partial response (PR). One patient reached a complete remission (CR).
Conclusions
This study indicated that CT‐707 is clinically effective as a new antitumor drug for Chinese lung adenocarcinoma patients with ALK rearrangement. It is safe and reliable and the dose‐expansion phase recruitment has started.
Keywords: ALK, CT‐707, NSCLC
Introduction
Anaplastic lymphoma kinase (ALK) was first discovered in 1994 in anaplastic large cell lymphoma (ALCL).1 Subsequent studies have found that inflammatory myofibroblastic tumors and neuroblastomas are associated with ALK gene mutations.2 In 2007, Soda et al. first reported ALK gene mutations in lung adenocarcinoma tumor tissues using proteomics techniques.3 ALK gene fusion exists in approximately 5% of non‐small‐cell lung carcinoma (NSCLC) patients, and EML4‐ALK fusion is the most common type,4 which occurs most frequently in non‐smoking, young women, adenocarcinoma, and EGFR wild‐type patients.5
Crizotinib is the first effective oral inhibitor that targets ALK, c‐MET, and ROS1 receptor tyrosine kinases. In several studies, the overall response rate (ORR) of patients with advanced ALK‐rearranged NSCLC was approximately 60%, with an 8–10 month median progressive‐free survival (mPFS). The PROFILE 1007 trial compared the efficacy and safety of crizotinib with chemotherapy (pemetrexed or docetaxel). The ORR of the two groups was 65% and 20%, respectively. The mPFS was 7.7 and 3.0 months, respectively.6 However, although crizotinib is effective, most patients develop drug resistance to crizotinib within 12 months.7 In this context, the development of new generation ALK inhibitors has emerged.
Alectinib is a highly selective oral ALK inhibitor. In the ALEX study, the mPFS was 25.7 months in the treatment group and 10.4 months in the control group (HR = 0.50, P < 0.0001).8 Ceritinib is another highly selective and effective oral ALK inhibitor. In the ASCEND‐4 study, the mPFSs of the ceritinib and chemotherapy groups were 16.6 and 8.1 months (HR = 0.55), and the ORRs of the two groups were 72.5% and 26.7%, respectively. The median duration of response (DOR) was 23.9 months (ceritinib group) versus 11.1 months (chemotherapy group).9
These new general ALK inhibitors have shown excellent curative effects, but it is inevitable that the extremely high price of these drugs for the Chinese population pushes the trend of Chinese independent research toward the development of new ALK inhibitors. CT‐707 is a small‐molecule selective ALK inhibitor with a nanomolar intensity of action against ALK that can significantly inhibit the growth of ALK‐derived tumors at the cellular level and in animal models.10At the same time, CT‐707 has a good performance in terms of drug metabolism and safety and a large therapeutic window. Therefore, CT‐707 is a second‐generation clinical drug candidate targeting ALK for the treatment of ALK‐positive NSCLC with a significant curative effect and a strong safety profile. CT‐707 has been approved by the Chinese clinical trial committee as a new antitumor drug (batch number 2015L03333). This single‐center, open‐label, phase I study was conducted to determine the safety and efficacy of CT‐707 in patients with ALK‐rearranged NSCLC. Preliminary clinical trial results have been reported in the 2017 European Society for Medical Oncology Asia Congress.11
Methods
Study population
Adult patients (aged 18–65) with a locally advanced or metastatic malignancy harboring a genetic alteration in ALK, who had disease progression despite underlying standard chemotherapy or had no effective standard therapy, were included in this study. Patients had at least one measurable lesion (according to RECIST 1.1),12 and the tumor had to be ALK‐positive (ALK rearrangement was detected in more than 15% of cells, which was measured by the Ventana ALK IHC test or FISH using the Vysis break‐apart ALK probe). Partial ALK‐positive results, such as a lymph node needle biopsy, were also considered acceptable. The Eastern Cooperative Oncology Group (ECOG) score was less than two, the survival time was expected to be no less than 12 weeks, and patients had to have a relatively sufficient organ reserve function for treatment.
The main exclusion criteria were: (i) patients with symptomatic central nervous system (CNS) metastases who were neurologically unstable or required increasing doses of steroids or local CNS‐directed therapy to control their CNS symptoms; (ii) patients with impaired cardiac function or any clinically significant cardiac disease; (iii) patients with abnormal laboratory values that appeared during screening and on day 1 of predose; (iv) patients with an impairment in gastrointestinal (GI) function or GI disease that may significantly alter the absorption of CT‐707, and (v) patients with a history of pancreatitis or history of increased serum amylase or lipase that was due to pancreatic disease.
Study protocol
The study protocol and all amendments were reviewed by the ethics committee of Peking Union Medical College Hospital. This study was conducted according to the ethical principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonization. Institutional review board‐approved informed consent was obtained from each patient in writing at screening.
Study design
The primary objective of this single‐center study included the assessment of overall safety, tolerability, and antitumor efficacy of CT‐707.
CT‐707 is a single‐dose oral drug with one cycle every 21 days. Based on partial results of the CT‐707 Phase I clinical trial reported in the 2017 European Society for Medical Oncology Asia Congress, the drug was given once a day, and the dose was gradually increased from 50 to 600 mg. The disease control and partial response rates were 88.4% and 44.2%, respectively. The most common drug‐related adverse events (AEs) included diarrhea and elevated aspartate aminotransferase. Most AEs were 1–2 grade side effects, and no serious AEs were observed. Therefore, the initial dose of CT‐707 was chosen to be 450 mg once a day in this clinical trial. We also conducted a preliminary exploration of the CT‐707 mode of administration, recruiting two patients to receive CT‐707300 mg b.i.d., and more patients will be recruited during the dose expansion phase. The patients continued treatment until unacceptable toxicity, disease progression, or withdrawal of informed consent. If there was evidence of a clinical benefit (eg, reduction of primary lesions or improvement in symptoms), continued treatment was permitted after the disease had progressed.
Patient assessments
Dose‐limiting toxicities (DLTs) were evaluated during the first cycle of medication. AEs were assessed according to the Common Terminology Criteria for Adverse Events version 4.03.13 Tumor lesion response was assessed by computed tomography or magnetic resonance imaging (MRI) at baseline and on the first day of each odd cycle. According to the Response Evaluation Criteria in Solid Tumors version 1.1, the assessment was continued until disease progression. Because of the high incidence of brain and bone metastases, brain MRIs and bone scans were performed at baseline for all NSCLC patients. Computed tomography of the abdomen (including intact bilateral kidneys) and pelvic sites were performed to assess other possible metastatic lesions.
Statistical analysis
This test was performed according to the initial dose and the dose at the end of treatment. The measurement data of each visit were statistically described as the mean ± standard deviation and median (minimum, maximum). The count data were statistically described using the frequency (composition ratio).
Safety analysis
Inductive analysis of safety data for patients who received at least one dose of CT‐707 was performed. The frequency and corresponding percentage of AEs are listed.
Curative effect analysis
The ORR and disease control rates were summarized, along with the exact (Clopper–Pearson) 95% confidence intervals. The data cutoff date was 1 January 2020 for safety and efficacy data.
Results
Patient disposition
A total of 13 patients were enrolled and treated in the dose escalation stage of this study (in Peking Union Medical cCllege hospital). The CT‐707 doses were 450 mg once a day (n = 7), 300 mg b.i.d. (n = 2), and 600 mg once a day (n = 4). At the cutoff date of data collection, three patients (23%) were still receiving CT‐707 treatment. The most common cause of cessation was disease progression (six patients [46%], including patients with treatment response before the disease progressed). AEs led to the discontinuation of the drug in another two patients (15%). One patient discontinued treatment due to drug‐induced liver injury and was recorded as DLT. The other patient discontinued treatment due to severe diarrhea, which was considered to be related to CT‐707. Two patients died during the study due to disease progression. Neither deaths were considered to be related to the study drug. One patient died because of the disease progression of ALK‐rearranged pleural mesothelioma. The two patients died because of respiratory failure.
Demographics and baseline disease characteristics
Overall, the median age of the 13 patients was 49. Of these patients, 54% were male, and 77% of patients had an ECOG score of 0 (Table 1). A total of 12 patients (92%) had lung adenocarcinoma, and one patient (8%) had malignant pleural mesothelioma. ALK positivity in patients with mesothelioma was confirmed by immunohistochemistry. All 12 lung adenocarcinoma patients had confirmed ALK rearrangement, which was determined by the FISH method. There were five patients with distant metastases, two patients with bone metastases and two patients with brain metastases. Two patients had previously received crizotinib, six patients received platinum‐containing chemotherapy, and four patients had not received any treatment. All patients had measurable lesions according to the RECIST v1.1 criteria.
Table 1.
Baseline characteristics
| CT‐707 dose | ||||
|---|---|---|---|---|
| 300 mg b.i.d. | 450 mg once a day | 600 mg once a day | All patients | |
| Demographic variable | (n = 2) | (n = 7) | (n = 4) | (n = 13) |
| Age, year | ||||
| Median (range) | 55 (50–60) | 45 (32–60) | 52 (46–58) | 49 (32–60) |
| <50, n (%) | 0 | 4 (57) | 2 (50) | 6 (46) |
| ≥50, n (%) | 2 (100) | 3 (43) | 2 (50) | 7 (54) |
| Sex, n (%) | ||||
| Female | 2 (100) | 3 (43) | 1 (25) | 6 (46) |
| Male | 0 | 4 (57) | 3 (75) | 7 (54) |
| ECOG performance status, n (%) | ||||
| 0 | 2 (100) | 5 (71) | 3 (75) | 10 (77) |
| 1 | 0 | 2 (29) | 0 | 2 (15) |
| 2 | 0 | 0 | 1 (25) | 1 (8) |
| Baseline brain metastases, n (%) | 0 | 1 (17) | 2 (50) | 3 (23) |
| Smoking status, n (%) | ||||
| Never smoked | 2 (100) | 6 (86) | 4 (100) | 12 (92) |
| Former smoker | 0 | 1 (14) | 0 | 1 (8) |
| Type of ALK‐rearranged tumors | ||||
| Lung adenocarcinoma | 2 (100) | 7 (100) | 3 (75) | 12 (92) |
| Pleural mesothelioma | 0 | 0 | 1 (25) | 1 (8) |
| Prior therapy, n (%) | ||||
| Crizotinib only | 0 | 0 | 2 (50) | 2 (15) |
| Pemetrexed + Platinum | 2 (100) | 4 (57) | 0 | 6 (46) |
| No treatment | 0 | 3 (43) | 2 (50) | 5 (39) |
| TNM stage, n (%) | ||||
| III A | 0 | 1 (14) | 0 | 1 (8) |
| III B | 1 (50) | 3 (43) | 1 (25) | 5 (39) |
| IV | 1 (50) | 3 (43) | 3 (75) | 7 (54) |
Dose escalation and toxicity
One dose‐limiting toxicity (600 mg once daily) of grade 3 diarrhea was reported during the escalation phase. The patient's symptoms did not improve after symptomatic treatment until the permanent discontinuation of CT‐707. Based on these findings, the MTD of CT‐707 in Chinese patients was initially determined to be 600 mg. The posterior probability of the DLT rate falling in the excessive toxicity interval (≥33% to ≤100%) at the 600 mg once a day dose was 10.4%, which fulfilled the EWOC principle (<25%).
All patients experienced one or more drug‐related AE. The most common AEs were diarrhea (92%), elevated aspartate aminotransferase (61%), elevated alanine aminotransferase (54%), nausea (38%), and vomiting (31%) (Table 2). Two patients (15%) reported grade 3/4 AEs associated with CT‐707, which were diarrhea, elevated alkaline phosphatase, and elevated glutamyl transpeptidase (Table 2). Grade 3 elevated alkaline phosphatase and glutamyl transpeptidase (>2.0–5.0 × upper normal [ULN]) occurred in a patient who was treated with CT‐707450 mg once a day. After liver protection treatment, the laboratory indicators returned to normal, and CT‐707 treatment was not interrupted. Except for the DLT, no other drug‐related AEs led to the discontinuation of the study. One patient (8%) experienced a dose reduction, and three patients (23%) had at least one medication interruption due to AEs. Overall, CT‐707 was well tolerated in Chinese patients, and there were no reports of treatment‐related deaths in this study.
Table 2.
Adverse events in the study considered to be drug related
| CT‐707 dose | ||||||||
|---|---|---|---|---|---|---|---|---|
| 300 mg b.i.d. (n = 2) | 450 mg once a day (n = 7) | 600 mg once a day (n = 4) | All patients (n = 13) | |||||
| Preferred term | All grades, n (%) | Grade 3/4, n (%) | All grades, n (%) | Grade 3/4, n (%) | All grades, n (%) | Grade 3/4, n (%) | All grades, n (%) | Grade 3/4, n (%) |
| Diarrhea | 2 (100) | 0 | 7 (100) | 0 | 3 (75) | 1 (25) | 12 (92) | 1 (8) |
| Increased aspartate aminotransferase | 1 (50) | 0 | 6 (86) | 0 | 1 (25) | 0 | 8 (61) | 0 |
| Increased alanine aminotransferase | 0 | 0 | 6 (86) | 0 | 1 (25) | 0 | 7 (54) | 0 |
| Nausea | 2 (100) | 0 | 2 (28) | 0 | 1 (25) | 0 | 5 (38) | 0 |
| Vomiting | 0 | 0 | 3 (43) | 0 | 1 (25) | 0 | 4 (31) | 0 |
| Increased serum creatinine | 1 (50) | 0 | 3 (43) | 0 | 0 | 0 | 4 (31) | 0 |
| Increased Glutamyltranspeptidase | 1 (50) | 0 | 3 (43) | 1 (17) | 0 | 0 | 4 (31) | 1 (8) |
| Increased creatine kinase | 1 (50) | 0 | 2 (28) | 0 | 0 | 0 | 3 (23) | 0 |
| Increased uric acid | 0 | 0 | 2 (28) | 0 | 1 (25) | 0 | 3 (23) | 0 |
| Increased alkaline phosphatase | 1 (50) | 0 | 3 (43) | 1 (17) | 0 | 0 | 3 (23) | 1 (8) |
| Loss of appetite | 1 (50) | 0 | 1 (14) | 0 | 1 (25) | 0 | 3 (23) | 0 |
| Stomach ache | 0 | 0 | 2 (28) | 0 | 0 | 0 | 2 (15) | 0 |
| Increased fasting blood glucose | 0 | 0 | 1 (14) | 0 | 0 | 0 | 1 (8) | 0 |
| Increased glycated hemoglobin | 1 (50) | 0 | 1 (14) | 0 | 0 | 0 | 1 (8) | 0 |
| Increased total bile acids | 0 | 0 | 0 | 0 | 1 (25) | 0 | 1 (8) | 0 |
| Increased eosinophil absolute value | 1 (50) | 0 | 0 | 0 | 0 | 0 | 1 (8) | 0 |
Efficacy
Tumor response
Based on the investigator's assessment of all dose groups, the ORR and disease control rates among patients were 10 of 13 (77%) and 11 of 13 (85%), respectively (Table 3). Four of the five patients with ALK‐rearranged tumors who were not previously treated had a confirmed PR. The patient with malignant pleural mesothelioma in the 600 mg group developed disease progression after one cycle of CT‐707 and died because of tumor progression. Two patients with ALK‐rearranged NSCLC had previously received crizotinib, which was discontinued due to cancer progression. PR was confirmed in one of the two patients. Six patients with ALK‐rearranged NSCLC had previously received chemotherapy with platinum‐based dual‐agent regimens to achieve PR, and then the chemotherapy was discontinued due to disease progression. One of the six patients was confirmed to have achieved a CR, four patients were confirmed PR, and one patient was confirmed to have stable disease (SD). Measurable lesions were present at baseline in 13 patients, and 11 subjects showed reduced target lesions (Fig 1).
Table 3.
Best overall response with ceritinib in patients with ALK‐rearranged NSCLC (RECIST Version 1.1)
| CT‐707 dose | ||||
|---|---|---|---|---|
| 300 mg b.i.d. | 450 mg once a day | 600 mg once a day | All patients | |
| Best overall response, n (%) | n = 2 | n = 7 | n = 4 | n = 13 |
| CR | 0 | 1 | 0 | 1 |
| PR | 1 | 6 | 2 | 9 |
| SD | 1 | 0 | 0 | 1 |
| PD | 0 | 0 | 2 | 2 |
| ORR (CR + PR) | 1 (50) | 7 (100) | 2 (50) | 10 (77) |
| DCR (CR + PR + SD) | 2 (100) | 7 (100) | 2 (50) | 11 (85) |
Figure 1.
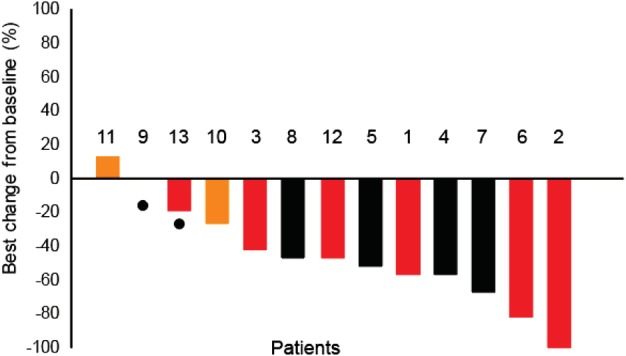
All patients target lesion changes from baseline (%).  Crizotinib,
Crizotinib,  Platinum,
Platinum,  NO, and
NO, and  Dead.
Dead.
Patient 2002 was administered a dose of 450 mg CT‐707 once a day. The best effect in this patient was CR, and the duration of treatment exceeded 26 months. Figure 2 shows the CT images of patient 2002 before CT‐707 treatment, three months after treatment, 11 months after treatment, and 29 months after treatment. Patients 2001, 2003, and 2004 reached PR, and the current duration of treatment exceeded 18 months for all three patients. CT‐707 was also effective against craniocerebral metastases. Patient 2006 took 450 mg of CT‐707 once a day, and the brain metastases were significantly reduced. PR was maintained for more than 11 months, but the observation was stopped because the drug was discontinued for more than 14 days by the patient. Patient 2010 had a temporal lobe metastasis, and the metastasis was significantly reduced after taking CT‐707600 mg once a day for one week. After 12 months of treatment, a new brain lesion appeared, whereas the chest lesion remained stable. After the clinical trial, this patient continued to take CT‐707, and the head lesion was given local treatment.
Figure 2.
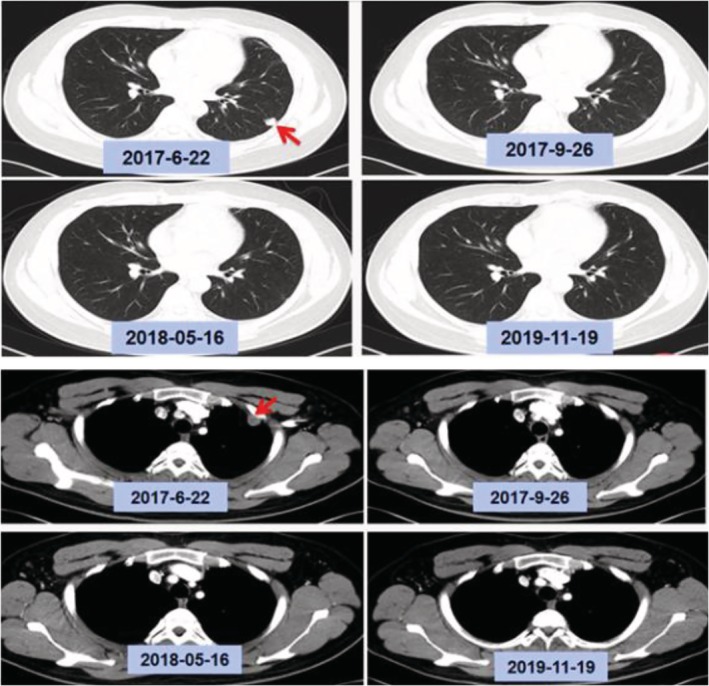
The CT images of patient 2002 before CT‐707 treatment, three months after treatment, 11 months after treatment, and 29 months after treatment.
Duration of response and progression‐free survival
In the 12 patients with lung adenocarcinoma who were given at least 450 mg CT‐707 per day, 58% of the treatment durations were 11 months or longer (Fig 3), indicating that patients with a PR can maintain an effective response over a longer period of time. The median progression‐free survival was 13 months (95% CI: 9.98–16.00) (Fig 4). The median progression‐free survival was not achieved in the subgroup of six patients with NSCLC who were previously treated with platinum‐based chemotherapy. The median progression‐free survival was 10.4 months in the subgroup of five patients with NSCLC who had not previously received any treatment. At the cutoff date of data collection, the overall survival data were not yet completed. The overall survival rate of 12 months was observed in 85% of patients, which has not yet reached the median overall survival.
Figure 3.
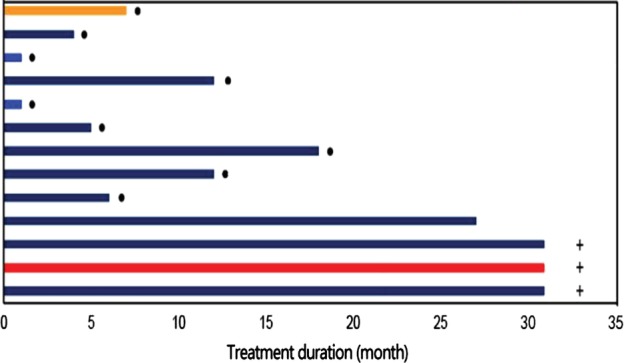
Duration of treatment.  SD,
SD,  PR,
PR,  PD,
PD,  CR,
CR,  Disease progression, and
Disease progression, and  Treatment ongoing.
Treatment ongoing.
Figure 4.
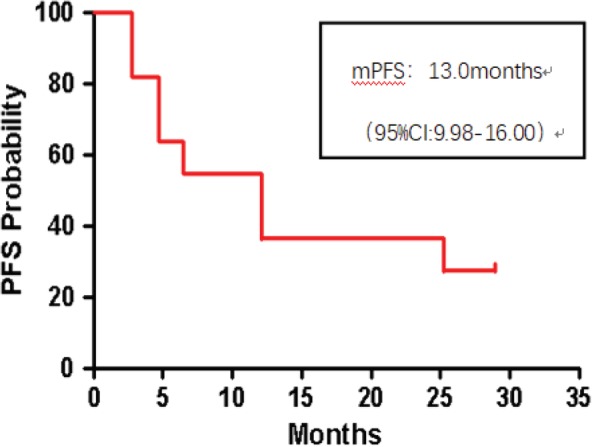
Progression‐free survival curve of 13 patients.
Discussion
The results of this single‐center study initially determined that the MTD of CT‐707 in patients with ALK‐rearranged lung adenocarcinoma in China was set to 600 mg once a day. A total of 12 NSCLC patients and one IMT patient were recruited in this study. One of the 12 NSCLC patients (8%) confirmed CR and five (42%) achieved PR; however, the patient with pleural mesothelioma did not receive a disease response.
The main adverse events were mostly grade 1 or 2 diarrhea. Most patients did not require dose adjustment after proper symptomatic treatment. According to previous studies, GIAEs (nausea, diarrhea, and vomiting) are also common in patients taking crizotinib (39%–56%). The incidence of drug‐related grade 3/4 AEs reported in this study was 23%, which was consistent with the 24% of grade 3/4 AEs observed with crizotinib.6 Overall, taking the absence of treatment‐related deaths and the AE data into consideration, the safety of CT‐707 was acceptable.
The ORR of CT‐707 was 77% in patients with ALK‐rearranged tumors in China, which was higher than the ORR values of crizotinib in PROFILE 1001 (ORR: 60.8%)6 and PROFILE 1005 (ORR: 60.8%) trails.14 However, the ORR of CT‐707 was lower than the PROFILE 1029 trial (ORR: 88%) of crizotinib in the East Asian population. This may be due to the small number of our subjects, or because there are differences in baseline characteristics between Asian populations and Chinese NSCLC patients.
Preclinical studies have shown that CT‐707 has a higher inhibitory activity against mutant kinases than crizotinib (CT‐707 IC50 = 3.8 nM; crizotinibIC50 = 15.9 nM). The kinase inhibition studies of four ALK mutant kinases expressed by eukaryotic insect expression systems revealed that CT‐707 more effectively inhibits four common crizotinib‐resistant ALK kinase mutants, including ALK L1196M, ALK F1174L, ALK G1296S, and ALK R1275Q in vitro. In particular, the inhibitory effect of CT‐707 on ALK L1196M is 10 times that of crizotinib.15, 16, 17 One limitation of this study was that only two NSCLC patients with brain metastases who were resistant to crizotinib treatment were involved. One patient had a persistent response to CT‐707 with a PFS of more than 12 months, but observation was stopped because of new lesions in the brain. However, the primary lesion remained PR, CT‐707 treatment was continued, and the cerebral metastases were given radiotherapy. The other patient developed disease progression after only one month of treatment. It is expected that more crizotinib‐resistant patients can be enrolled during the dose expansion phase to further confirm the activity of CT‐707 in patients with crizotinib resistance.
The precise treatment plan for malignant tumors based on molecular pathology rather than traditional pathology (basket plan) will be a future direction of cancer treatment. The first clinical trial of the basket plan, NCI‐MATCH, is still in progress, and the results of this clinical trial have not yet been published.18 This study included a patient with ALK‐rearranged malignant pleural mesothelioma. Disease progression occurred only after one month of CT‐707 treatment, which might be related to the poor physical condition of the patient. It is expected that patients with other kinds of ALK‐positive tumors can be recruited during the dose amplification phase to further assess the efficacy of CT‐707 for ALK‐rearranged tumors.
In conclusion, the results of this study suggest that CT‐707 is effective in Chinese patients with tumors harboring ALK rearrangements. In addition, it has a reliable safety profile and clinical application value.
Disclosure
The authors declare there are no conflicts of interest.
Acknowledgments
The authors thank the participating patients, their families, all study coinvestigators, and research coordinators.
References
- 1. Morris SW, Kirstein MN, Valentine MB et al Fusion of a kinase gene ALK to a nucleolar protein gene NPM in non‐Hodgkin's lymphoma. Science 1994; 263 (5151): 1281–4. [DOI] [PubMed] [Google Scholar]
- 2. Mossé YP, Laudenslager M, Longo L et al Identification of ALK as a major familial neurobla ‐stoma predisposition gene. Nature 2008; 455 (7215): 930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soda M, Choi YL, Enomoto M et al Identification of the transforming EML4‐ALK fusion genein non‐small‐cell lung cancer. Nature 2007; 448 (7153): 561–6. [DOI] [PubMed] [Google Scholar]
- 4. Tsao AS, Scagliotti GV, Bunn PA et al Scientific advances in lung cancer 2015. J Thorac Oncol 2016; 11 (5): 613–38. [DOI] [PubMed] [Google Scholar]
- 5. Shaw AT, Yeap BY, Mino‐Kenudson M et al Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009; 27 (26): 4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Camidge DR, Bang Y‐J, Kwak EL et al Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: Updated results from a phase 1 study. Lancet Oncol 2012; 13 (10): 1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katayama R, Shaw AT, Khan TM et al Mechanisms of acquired crizotinib resistance in ALK‐rearranged lung cancers. Sci Transl Med 2012; 4: 120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters S, Camidge DR, Shaw AT et al. Alectinib versus Crizotinib in Untreated ALK‐Positive Non‐Small‐Cell Lung Cancer. N Engl J Med. 2017; 377(9): 829–838. [DOI] [PubMed] [Google Scholar]
- 9. Soria JC, Tan DSW, Chiari R et al First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): A randomised, open‐label, phase 3 study. Lancet 2017; 389 (10072): 917–29. [DOI] [PubMed] [Google Scholar]
- 10. Peng Y, Sun Y, Huang Y et al Development of a novel ALK targeted tyrosine kinase inhibitor CT‐707 for non‐small cell lung cancer patients In: 2014 Frontiers of Medical Sciences and the Third Symposium on New Trends in Individualized Therapy and Antitumor Drug Research. Shenzhen, China: Chinese Academy of Engineering Faculty of Medicine and Health; 2015; 66–7. [Google Scholar]
- 11. Shi Y, Hao X, Xing P et al Phase I study of safety and pharmacokinetics for CT‐707 in ALK‐positive advanced non‐small cell lung cancer. Ann Oncol 2017; 28: XI32. [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45 (2): 228–47. [DOI] [PubMed] [Google Scholar]
- 13. National Cancer Institute, National Institutes of Health, U.S . Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. [last accessed March 16, 2015];NIH publication # 09–7473. Published May 29, 2009; Revised Version 4.03 June 14, 2010. Available from URL: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5%C3%977.pdf.
- 14. Shaw AT, Kim D‐W, Nakagawa K et al Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med 2013; 368 (25): 2385–94. [DOI] [PubMed] [Google Scholar]
- 15. Wang D, Chen Y, Chen Z et al CT‐707, a novel FAK inhibitor, synergizes with cabozantinib to suppress hepatocellular carcinoma by blocking cabozantinib‐induced FAK activation. Mol Cancer Ther 2016; 15: 2916–25. [DOI] [PubMed] [Google Scholar]
- 16. Wu J, Savooji J, Liu D et al Second‐and third‐generation ALK inhibitors for non‐small cell lung cancer. J Hematol Oncol 2016; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davare MA, Vellore NA, Wagner JP et al Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors. Proc Natl Acad Sci U S A 2015; 112 (39): E5381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conley BA, Doroshow JH. Molecular analysis for therapy choice: NCIMATCH. Semin Oncol 2014; 41 (3): 297–9. [DOI] [PubMed] [Google Scholar]


