Abstract
Background
Heparin-induced thrombocytopenia (HIT) typically responds to heparin termination. Some types of HIT can persist after heparin discontinuation.
Case summary
A 95-year-old woman was referred to the cardiology from orthopaedics because of acute limb ischaemia (ALI) 1 day after surgery of a femoral neck fracture. Despite thrombectomy, ALI relapsed the next day. She had been treated with intravenous antibiotics with a diagnosis of aspiration pneumonia for 1 week until 3 days before surgery, together with heparin flush twice a day. Of note, no intra-/post-operative heparin was administered, no cell salvage device, central venous, nor arterial catheters were used before development of ALI. The patient and her family refused reattempting invasive therapies; consequently, the patient continued to worsen and died on post-operative day 3. Diagnosis of autoimmune HIT, which was prompted by surgery without re-exposure to heparin, was confirmed posthumously.
Discussion
This case emphasizes the significance of suspecting autoimmune HIT in any patient presenting with thrombosis, even if the heparin exposure dates back more than a few days or even without heparin exposure.
Keywords: Heparin-induced thrombocytopenia, Autoimmune, Thrombosis, Orthopaedic surgery, Case report
Learning points
Cardiologists should have the ability to differentiate and appropriately diagnose various thrombotic disorders in addition to the ability to manage the thrombosis.
Several type of heparin-induced thrombocytopenia can occur even if the heparin exposure dates back more than a few days or even without heparin exposure.
A high level of clinical suspicion should be paid in severe cases of thrombosis with unknown causes.
Introduction
In patients with arterial/venous thrombosis, heparin has been used as a first-line anticoagulant. Sometimes, heparin causes severe complications such as heparin-induced thrombocytopenia (HIT), which result in not only thrombocytopenia but also arterial/venous thrombosis. Heparin-induced thrombocytopenia is caused by Ig (immunoglobulin) G antibodies against complexes of platelet factor 4 (PF4) and heparin; these pathogenic antibodies against PF4/heparin complexes bind to and activate cellular FCγRIIa on platelets, resulting in a hypercoagulable state and thrombosis.1 Typically, HIT develops 5–10 days after the initiating heparin administration, which usually responds to the termination of heparin and the beginning of other anticoagulant therapy.2
Timeline
| Time | Events |
|---|---|
| Day 1 | A 95-year-old woman admitted to orthopaedic surgery because of left femoral neck fracture. |
| Day 4–11 | She was treated with intravenous antibiotics with a diagnosis of aspiration pneumonia, together with heparin flush twice a day. |
| Day 14 | She underwent total hip replacement. The platelet count suddenly declined to 22 000/μL immediately after surgery. |
| Day 15 | She developed acute limb ischaemia (ALI) which was treated with thrombectomy and endovascular therapy. During the procedure, 5000 IU of heparin was used. |
| Day 16 | The platelet count further decreased to 17 000/μL, and ALI relapsed. |
| Day 17 | The patient died. |
Case presentation
A 95-year-old woman was referred to the cardiology from orthopaedics with acute pain of the left leg 1 day after surgery of a femoral neck fracture. She had no prior exposure to heparin before this admission. Nevertheless, after admission, she had been treated with intravenous antibiotics with a diagnosis of aspiration pneumonia for 8 days until 3 days before surgery, together with heparin flush twice a day. Of note, no intra-/post-operative heparin was administered, no cell salvage device, central venous, nor arterial catheters were used. She started feeling pain and coldness at rest from the early morning on post-operative day (POD) 1. Initial vital signs were as follows: blood pressure, 153/71 mmHg; heart rate, 70 b.p.m.; body temperature, 37.0°C; and oxygen saturation, 100% at room air. The patient was in mild distress but awake and alert. Her wound was clear without signs of infections; however, her left lower thigh, calf and foot were cyanosed, cold and paralysed, her infra-inguinal arteries were not palpable, and the sensation was reduced. She had regular heart rhythm, no extra-sound and no murmur. Abdominal and neurological examinations were unremarkable, except for the left lower limb.
Her medical and surgical history comprised resection of colorectal cancer and insomnia. Her regular medication included magnesium-containing antacid and benzodiazepine. She had never smoked, had no other medical conditions predisposing to peripheral vascular disease, had no history of allergy to any drug.
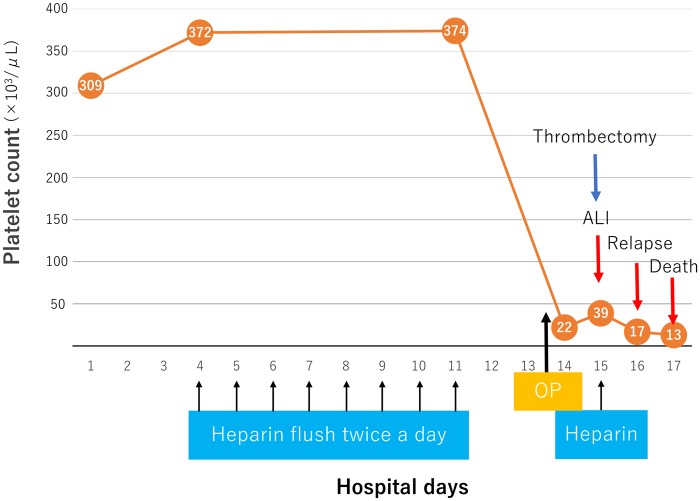
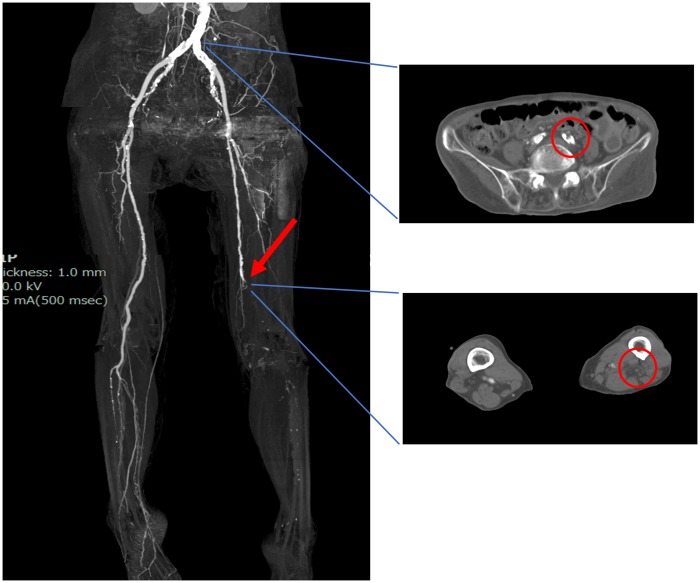
The electrocardiogram revealed a normal sinus rhythm. In addition, complete blood count revealed leucocytosis, with the white blood cell count of 13 600/μL [normal range (NR) 3500–9900/μL]. Platelets were 39 000/μL (NR 120 000–400 000/μL), which was dropped from the baseline value of 309 000/μL at admission (timeline; Figure 1). The patient had marginally increased creatine kinase level [468 IU/L (NR 35–200 IU/L)]. The clotting test revealed the following: international normalized ratio of prothrombin time, 1.19 (NR 0.85–1.15); activated partial thromboplastin time, 46.6 s (NR 24–39 s); fibrin degradation products, 79.3 μg/mL (NR 0.0–5.0 μg/mL); and d-dimer, 28.4 μg/mL (NR 0.0–0.9 μg/mL). Contrast-enhanced computed tomography (CT) of the lower limb exhibited multiple thrombotic occlusion at the left iliac artery, superficial femoral artery (SFA), and tibial arteries (TA); CT also showed calcification of infra-renal aorta but not showed a lot of thrombus nor significant atherosclerotic plaque (Figure 2).
Figure 1.
The clinical course of the patient and the platelet count. The patient was treated with intravenous antibiotics and heparin flush was used until 3 days before surgery. The platelet count suddenly declined to 22 000/μL immediately after surgery. Post-operative day 1, acute limb ischaemia developed and thrombectomy was performed. During the procedure, 5000 IU of heparin was used. Post-operative day 2, the platelet count further decreased <20 000/μL, and acute limb ischaemia relapsed. Post-operative day 3, the patient died. ALI, acute limb ischaemia; OP, surgical operation.
Figure 2.
Computed tomographic angiography. Computed tomographic angiography demonstrated the total occlusion of the left common iliac artery and distal superficial femoral artery.
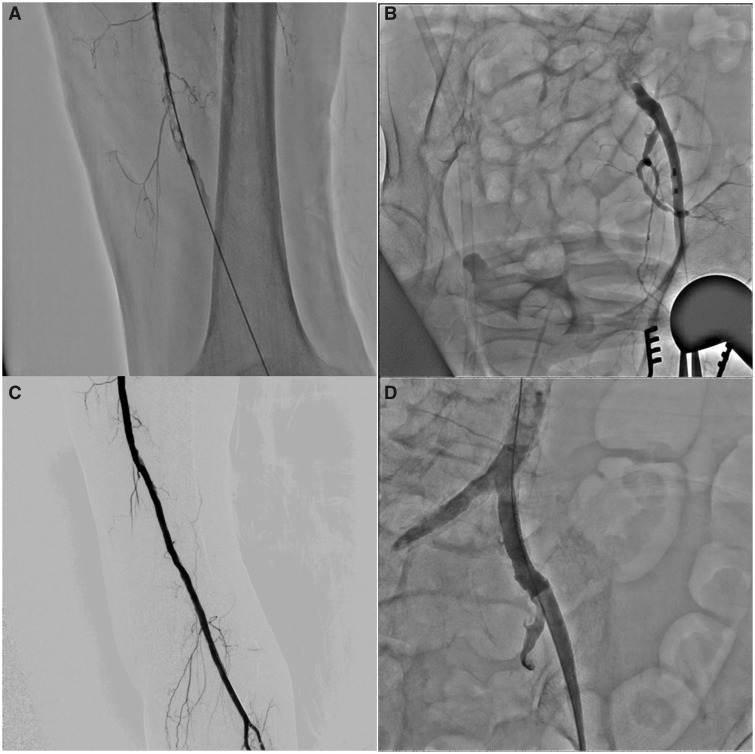
The initial assessment was acute limb ischaemia (ALI) because of disseminated intravascular coagulation after surgery. Initially, we performed thrombectomy using the Fogarty catheter for SFA and TA (Figure 3A). Then, thrombectomy for the iliac artery was also attempted; however, the Fogarty catheter could not pass the iliac artery occlusion (Figure 3B). As we suspected that the iliac lesion was atheromatous chronic total occlusion (CTO), endovascular therapy (EVT) using 0.018-inch wire system and implantation of a bare-metal stent (Express SD 7.0/37 mm; Boston Scientific, Marlborough, MA, USA) was performed. Finally, patency was established through to the end of the TA (Figure 3C and D). During thrombectomy and EVT, 5000 IU heparin was used as an anticoagulant. One day after thrombectomy (POD 2 after orthopaedic surgery), the platelet counts further dropped to 17 000/μL, and the symptom of ALI relapsed. The patient exhibited reocclusion of the left superficial femoral–popliteal arteries on CT scanning. The diagnosis of HIT was suspected, which aroused discussion on the use of argatroban; however, the severe thrombocytopenia and bleeding signs, such as subcutaneous bleedings, discouraged the use of it. The patient and her family refused reattempting invasive therapies; consequently, the patient continued to worsen and died on POD 3. Heparin-induced thrombocytopenia antibodies (IgG/IgA/IgM) measured on POD 2 by latex agglutination turbidimetry were later known to be positive with an optical density of 2.20 (strongly positive).
Figure 3.
Invasive angiography and thrombectomy. (A) The angiography after one session of thrombectomy using the Fogarty catheter to the superficial femoral artery. (B) The occlusion of the common iliac artery. (C) The final angiography of the superficial femoral artery. (D) The angiography after stent implantation to the common iliac artery.
Discussion
Previous case reports have presented patients who were sensitized by surgery and developed HIT (spontaneous HIT).3 Here, we present a case of HIT that was sensitized by a minimal amount of heparin (heparin flush) and prompted by orthopaedic surgery.
Based on the onset timing of thrombocytopenia and sensitization, HIT can be categorized as follows2,4,5: (i) typical-onset HIT (70% of cases) develops gradual thrombocytopenia, at least 5 days after initiating heparin administration and while continuing to receive heparin; (ii) rapid-onset HIT (25% of cases) develops within 24 h of heparin re-exposure because of the already circulating HIT antibodies that resulted from the recent use of heparin (within previous 90 days, especially within 30 days)—in this type, HIT is often complicated by an anaphylactoid reaction within 30 min after a heparin bolus1; (iii) delayed-onset HIT (<5% of cases) develops thrombocytopenia and thrombosis several days (from 5 days up to 3 weeks) after discontinuing heparin; and (iv) spontaneous HIT develops in patients without any history of heparin exposure. Delayed-onset HIT is caused by very high titers of platelet-activating antibodies that recognize PF4 and could activate platelets in the absence of heparin.4,6 Spontaneous HIT occurs because of antibodies induced by complexes of PF4 with other polyanions than heparin, such as bacterial surfaces and nucleic acids, the properties of which are consistent with HIT antibodies.7 Thus, most patients who develop spontaneous HIT have proximate episodes of infection or major surgery.8 In delayed-onset/spontaneous HIT, the heparin cessation, or even lack of heparin treatment misleads clinicians to another diagnosis, accounting for marked delays in the diagnosis. Furthermore, as in our case, misdiagnosis often results in the use of heparin for the treatment of thrombosis, further exacerbating thrombosis.
The pathogenesis of our patient could be simply classified into delayed-onset HIT because of the onset after the cessation of heparin. Nevertheless, the sudden decline of platelets right after surgery and thrombosis on POD 1 indicates the correlation between the HIT development and surgery. Hence, we speculate that the surgery itself could trigger this immune response, where heparin-like molecules, such as glycosaminoglycans, were released intraoperatively, triggering the activation of anti-PF4/polyanion antibodies. Our case was unique in that orthopaedic surgery serves as not a generator but activator of HIT antibodies. Another thought-provoking issue in our patient is the development of thrombosis in the same limb as the surgical site. Some studies have reported the placement of the apheresis catheter or peripherally inserted central catheter line as likely predisposing features of venous thrombosis in the same limb as the catheters.9 However, our patient received neither central venous catheter insertion nor tourniquet use. We assumed that local inflammation because of fracture and surgery, and arterial flow disturbance because of the existence of CTO in the iliac artery could have contributed to the development of thrombosis in the left lower limb of our patient.
Recently, Greinacher et al.10 proposed new criteria for ‘autoimmune’ HIT syndrome, which includes delayed-onset HIT, persisting HIT, spontaneous HIT, fondaparinux-associated HIT, and heparin flushes HIT. Sera from these patients contain a subset of heparin-independent antibodies bounding much more strongly to PF4–heparin–polyanion complexes than did heparin-dependent HIT antibodies.10 Perhaps, our patient also belonged to this subset of rare HIT syndrome, whose pathogenesis was the mixture of delayed-onset and spontaneous HIT.
This case emphasizes the significance of suspecting autoimmune HIT in any patient presenting with thrombotic disease, even if the heparin exposure dates back more than a few days or even without heparin exposure. Cardiologists should be more familiar with these HIT syndromes, as patients with thrombosis often first present to the cardiology.
Lead author biography

Dr Ruka Yoshida graduated from the Nagoya University Medical School. After completion of his Internal Medicine training, he is currently working as an Interventional cardiologist at Japanese Red Cross Nagoya Daini Hospital as well as visiting research scholar at Nagoya University Graduate School of Medicine.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
The authors thank Mr Jonathan Leung for his assistant with English proofreading.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med 2015;373:252–261. [DOI] [PubMed] [Google Scholar]
- 2. Warkentin TE, Kelton JG.. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med 2001;344:1286–1292. [DOI] [PubMed] [Google Scholar]
- 3. Jay RM, Warkentin TE.. Fatal heparin-induced thrombocytopenia (HIT) during warfarin thromboprophylaxis following orthopedic surgery: another example of ‘spontaneous’ HIT? J Thromb Haemost 2008;6:1598–1600. [DOI] [PubMed] [Google Scholar]
- 4. Warkentin TE, Kelton JG.. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med 2001;135:502–506. [DOI] [PubMed] [Google Scholar]
- 5. Warkentin TE, Basciano PA, Knopman J, Bernstein RA.. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood 2014;123:3651–3654. [DOI] [PubMed] [Google Scholar]
- 6. Padmanabhan A, Jones CG, Bougie DW, Curtis BR, McFarland JG, Wang D, Aster RH.. Heparin-independent, PF4-dependent binding of HIT antibodies to platelets: implications for HIT pathogenesis. Blood 2015;125:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krauel K, Pötschke C, Weber C, Kessler W, Fürll B, Ittermann T, Maier S, Hammerschmidt S, Bröker BM, Greinacher A.. Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia. Blood 2011;117:1370–1378. [DOI] [PubMed] [Google Scholar]
- 8. Greinacher A. Me or not me? The danger of spontaneity. Blood 2014;123:3536–3538. [DOI] [PubMed] [Google Scholar]
- 9. Mian H, Warkentin TE, Sheppard J-AI, MacDonald A, Linkins L-A, Benger A, Foley R.. Autoimmune HIT due to apheresis catheter heparin flushes for stem cell harvesting before autotransplantation for myeloma. Blood 2017;130:1679–1682. [DOI] [PubMed] [Google Scholar]
- 10. Greinacher A, Selleng K, Warkentin TE.. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost 2017;15:2099–2114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.