Key Points
Question
What are the outcomes of different limbal stem cell transplant (LSCT) procedures?
Findings
In this meta-analysis that included 40 studies (2202 eyes), autologous LSCT had a significantly higher rate of restoration of the ocular surface and lower rate of complications than allogeneic LSCT. However, the criteria of LSCD grading and other efficacy outcome measures varied greatly among different studies.
Meaning
These findings support autologous LSCT in favor of allogenic LSCT, but randomized clinical trials using standardized efficacy measures are necessary to determine whether one approach is more effective than the other.
Abstract
Importance
Limbal stem cell transplant (LSCT) can be categorized as direct autologous limbal transplant (AULT), direct allogenic limbal transplant (ALLT), cultivated autologous limbal stem cells transplant (cAULT), and cultivated allogenic limbal stem cells transplant (cALLT). To our knowledge, there is no study directly comparing the outcomes and complications of these procedures.
Objective
To evaluate the outcomes of different LSCT procedures.
Data Source
We searched PubMed, EMBASE, Web of Science, and Cochrane without language filter for peer-reviewed articles about LSCT. The latest search was performed on June 30, 2019.
Study Selection
Clinical studies with the outcome of at least 20 eyes after LSCT were included. Animal studies and studies of other surgical interventions were excluded.
Data Extraction and Synthesis
Two reviewers independently abstracted the data from each study. Heterogeneity was evaluated with the I2 statistic, and a meta-analysis was performed using the random-effects model.
Main Outcomes and Measures
Outcome measures included the improvement of ocular surface, visual acuity (VA), and adverse events of recipient eyes and donor eyes.
Results
Forty studies (2202 eyes) with a mean (SD) follow-up of 31.3 (20.9) months met the inclusion criteria. The mean (SD) age of study participants was 38.4 (13.1) years, and men accounted for 74%. The number of eyes that underwent AULT, ALLT, cAULT, and cALLT were 505, 742, 771, and 184, respectively. Improvement of the ocular surface was achieved in 74.5% of all eyes, 85.7% of eyes after AULT (95% CI, 79.5%-90.3%), 84.7% after cAULT (95% CI, 77.2%-90.0%), 57.8% after ALLT (95% CI, 49.0%-66.1%), and 63.2% after cALLT (95% CI, 49.3%-75.2%). Autologous limbal transplantation resulted in a greater VA improvement rate (76%) than did the other 3 procedures (cAULT: 56.4%; ALLT: 52.3%; cALLT: 43.3%; all P < .001). The most common adverse events in all recipient eyes were recurrent/persistent epithelial erosion (10.5%; 95% CI, 7.2%-23.3%) and elevated intraocular pressure (intraocular pressure, 1.7%; 95% CI, 0.5%-7.8%). Patients who underwent ALLT had the highest rate of recurrent epithelial erosion (27.8%; 95% CI, 17.1%-41.9%) and intraocular pressure elevation (6.3%; 95% CI, 1.8%-19.4%).
Conclusions and Relevance
These findings suggest LSCT can improve or stabilize the corneal surface with a low rate of severe ocular complications and that autologous LSCT may have a higher success rate and fewer complications than allogenic LSCT.
This meta-analysis evaluates the outcomes of different limbal stem cell transplant procedures.
Introduction
Limbal stem cell deficiency (LSCD) is an ocular surface disease caused by a decrease in the population and/or function of limbal epithelial stem cells (LSCs), which leads to the inability to sustain the normal homeostasis of the corneal epithelium.1 The treatment of LSCD is challenging. Keratoplasty will fail if the normal function of LSCs is not restored first. Medication is only effective in partial LSCD.2 For eyes with severe or total LSCD, limbal stem cell transplantation (LSCT) is necessary to restore the population of LSCs. With a better understanding of the biology of LSCs and the advancement of microsurgery, substantial progress has been made in the surgical management of LSCD.
The surgeries involving LSCT can be divided into 2 groups: grafting (direct transplantation) of limbal tissues and transplantation of cultivated LSCs. The source of donor tissue can be autologous or allogeneic. The technique of direct transplantation can be further divided into conjunctival limbal autograft, conjunctival limbal allograft, keratolimbal allograft, and simple limbal epithelial transplantation (SLET). Transplantation of cultured LSCs mainly refers to cultivated limbal epithelial transplant. To our knowledge, no study has directly compared the outcomes and the complications of different types of LSCT. Although many studies indicate a higher success rate for autologous transplantation,3,4 previous reviews and meta-analysis of LSCT reported conflicting results.5,6,7,8 The purpose of this meta-analysis is to systematically evaluate the clinical outcomes and complications of autologous and allogeneic LSCT based on available literature.
Methods
Search Strategy
This study was approved by the institutional review board at the University of California, Los Angeles. We searched PubMed, EMBASE, Web of Science, and Cochrane to identify peer-reviewed, published articles that described relevant studies. The following search terms were used: “limbal stem cell deficiency” AND (“surgical treatment” OR “limbal transplantation” OR “cultivated limbal epithelial transplantation” OR “simple epithelial transplantation” OR “conjunctival limbal autograft” OR “conjunctival limbal allograft” OR “keratolimbal allograft”). We also reviewed the references from retrieved articles to identify additional related studies. Neither the language filter nor the publication time filter was used. The non-English articles were translated into English to obtain the needed information. The latest search was performed on June 30, 2019.
Eligibility Criteria
We sought prospective or retrospective interventional cohorts or case series, nonrandomized comparative or noncomparative studies, and randomized clinical trials. Studies that involved fewer than 20 eyes were excluded. Literature reviews, animal studies, laboratory studies without the assessment of clinical outcome, letters to the editor, correspondence, notes, editorials, and conference abstracts were also excluded. Studies of keratoprosthesis (Kpro), amniotic membrane transplant (AMT), optical keratoplasty (penetrating/deep anterior lamellar keratoplasty) after LSCT, and cultivated oral mucosal epithelial transplant were excluded. To compare the outcomes when different donor sources were used, studies were considered to be eligible only when the exact number of allografts and autografts, and their outcomes were provided separately in the publication. If multiple reports were published from the same authors at the same institutions, only the most recent studies with a larger number of patients and a longer follow-up were included to avoid redundant outcomes from an overlapping group of patients. (See details in eMethods in the Supplement).
Quality Assessment of Studies
A modified version of the Newcastle-Ottawa Scale was used to assess the quality of each cohort study.9 The methodologic quality were evaluated independently by two authors (Q.L. and T.C.). In cases of disagreement, a third author (S.X.D.) was included to reach a consensus (eMethods in the Supplement).
Data Extraction
Two authors (Q.L. and T.C.) independently extracted the following demographic and clinical data from each study: study design, sample size, demographic characteristics, surgery type, postoperative treatment, and follow-up. The outcomes extracted from studies contained 3 aspects: restoration of an intact corneal epithelium, vision improvement, and complications (eMethods in the Supplement).
Statistical Analysis
Mixed-effects logistic models were used to analyze dichotomized outcomes, such as clinical success, with studies as random effects. Robust meta-analysis techniques10,11 were used to estimate the change in LogMar VA before and after surgery. Study heterogeneity was quantified with I2 statistic and evaluated with likelihood ratio test. Contour-enhanced funnel plots were generated to inspect publication bias.12,13 A modified Macsskill test14,15 was performed to examine publication bias. All tests were 2-sided and P less than .05 was considered statistically significant. All statistical analyses were carried out with R software (the R Foundation; eMethods in the Supplement).
Results
Literature Search
The original electronic database search identified 1159 nonduplicate articles, of which 1085 articles did not meet the inclusion criteria. The full text of the remaining 74 articles were reviewed. Forty studies3,4,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53 were eligible and included in this meta-analysis (eFigure 1 in the Supplement).
Characteristics of Included Studies
Details of all included studies are provided in eTable 1 in the Supplement. None of the included studies was a randomized clinical trial. Twenty-seven studies were retrospective or prospective cases series, 3 were retrospective cohort studies, and 10 did not have a clearly stated study design. Seventeen studies were comparative, and 23 were noncomparative. Three studies were conducted at multiple centers.
A total of 2202 eyes of 1999 patients were included for analysis (Table 1). Chemical burn and thermal injury were the leading indication for LSCT (1648 eyes [74.8%]) followed by chronic cicatricial ocular surface inflammation (Stevens-Johnson syndrome and mucous membrane pemphigoid; 208 eyes [9.4%]); and other etiologies in 346 eyes (15.7%). The extent of pretreatment LSCD involvement was reported for 1443 eyes (65.5%) in 23 studies. Total LSCD was diagnosed in 1232 eyes (85.4%), and partial LSCD in 211 eyes (14.6%). Only 18 studies (822 eyes [37.3%]) mentioned prior surgery before LSCT, and 78 eyes had prior failed LSCT.
Table 1. Demographic Characteristics of AULT, ALLT, cAULT, and cALLT.
| Characteristic | AULT | ALLT | cAULT | cALLT | P value |
|---|---|---|---|---|---|
| Studies, No. (%) | 16 (28) | 23 (40) | 12 (20) | 7 (12) | NA |
| Total sample size, No. (% of eyes) | 505 (23) | 742 (34) | 771 (35) | 184 (8) | NA |
| Mean sample size (range) | 31 (10-125) | 28 (6-165) | 45 (7-200) | 26 (8-80) | .19 |
| Sex, No. (%) | |||||
| Male | 297 (72) | 332 (67) | 577 (79) | 108 (80) | <.001 |
| Female | 118 (28) | 160 (33) | 156 (21) | 27 (20) | |
| Age, mean (IQR), y | 30.8 (15.2-62.5) | 41.8 (17-62.5) | 46.5 (14.7-54.8) | 36.8 (15.9-49) | .68 |
| Etiology of LSCD, No. (%) | |||||
| Chemical burn/thermal injury | 426 (84) | 407 (55) | 681 (88) | 134 (73) | <.001 |
| Chronic cicatricial ocular surface inflammation | 15 (3) | 152 (20) | 13 (2) | 28 (15) | |
| Others | 64 (13) | 183 (25) | 77 (10) | 22 (12) | |
| Range of LSCD, No. (%) | |||||
| Studies mentioned | 11 (78) | 10 (43) | 6 (50) | 5 (71) | .35 |
| Percentage of total LSCD eyes | (74.5) | (98.7) | (79.2) | (86.3) | .66 |
| Percentage of partial LSCD eyes | (25.5) | (1.3) | (21.8) | (13.7) | .14 |
| Prior surgery, No. (%) | |||||
| Studies mentioned | 9 (56) | 4 (17) | 8 (67) | 2 (29) | .02 |
| Percentage of eyes having prior surgery | (63.3) | (92.8) | (62) | (69.6) | .31 |
| Percentage of eyes having prior LSCT | (34.2) | (25.8) | (4.9) | (13.2) | .23 |
| Duration between Injury/onset of disease and surgery, mo (IQR) | 37.3 (34.6-40) | 42.3 (30.2-54.5) | 30.5 (28.5-32.5) | 36.3 (30.9-41.7) | .91 |
| Follow-up, mean (IQR), mo | 20 (6-47) | 31.2 (12-109.2) | 28.8 (9.7-96) | 28.5 (12-57.6) | .12 |
| Criteria used to define success, No. (%) | |||||
| Only “an intact epithelium and a stable ocular surface” | 0 | 3 (13) | 1 (8) | 0 | .23 |
| “An intact epithelium and a stable ocular surface” plus 1 additional criterion | 7 (44) | 7 (30) | 5 (42) | 6 (86) | |
| “An intact epithelium and a stable ocular surface” plus 2 additional criteria | 5 (31) | 11 (48) | 5 (42) | 0 | |
| “An intact epithelium and a stable ocular surface” plus 3 additional criteria | 4 (25) | 2 (9) | 1 (8) | 1 (14) |
Abbreviations: ALLT, allogenic direct limbal transplant; AULT, autologous direct limbal transplant; cALLT, allogenic cultured limbal stem cell transplant; cAULT, autologous cultured limbal stem cell transplant; IQR, interquartile range; LSCD, limbal stem cell deficiency; LSCT, limbal stem cell transplant; NA, not applicable.
All studies were divided into 4 subgroups based on surgical technique and donor source: autologous direct limbal transplantation (AULT; 505 eyes), allogenic direct limbal transplantation (ALLT; 742 eyes), autologous cultured LSC transplantation (cAULT; 771 eyes), and allogenic cultured LSC transplantation (cALLT; 184 eyes). Simple limbal epithelial transplantation was categorized as direct limbal transplantation because cell culture was not involved. The mean sample size, mean age, and mean length of follow-up were similar among the 4 subgroups (Table 1).
Clinical Outcomes
Clinical Success and Improvement
The criteria to define success and partial success varied greatly among studies. The criterion “the reconstruction of an intact epithelium and a stable ocular surface” was adopted by all studies. Nevertheless, 1 or more additional criteria were used to define success and partial success in 37 studies, which included “the absence/recession of corneal neovascularization” (30 studies; 81%), “vision improvement” (13 studies; 35%), “improvement of the cellular phenotype” (11 studies; 30%), and “improvement of ocular symptoms and/or vision-related quality of life” (10 studies; 27%).
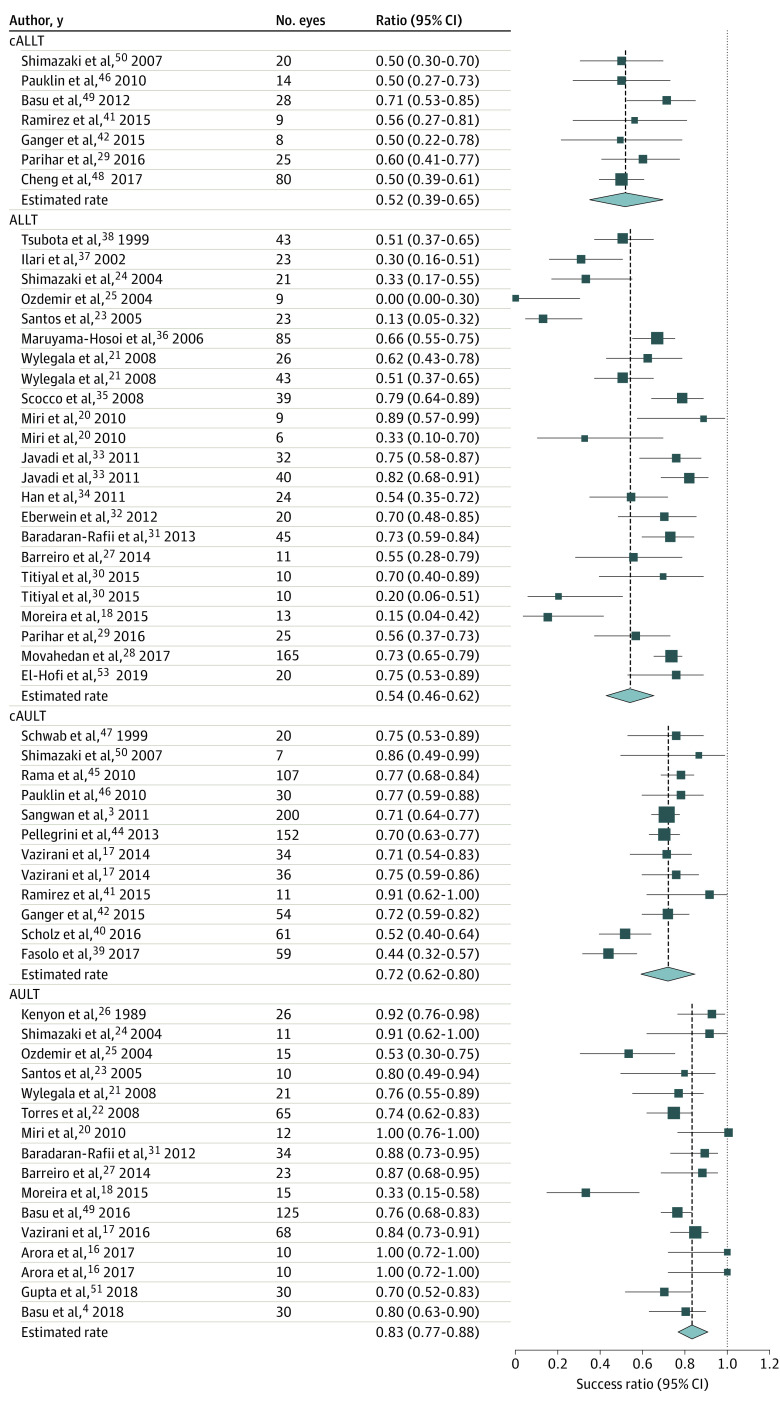
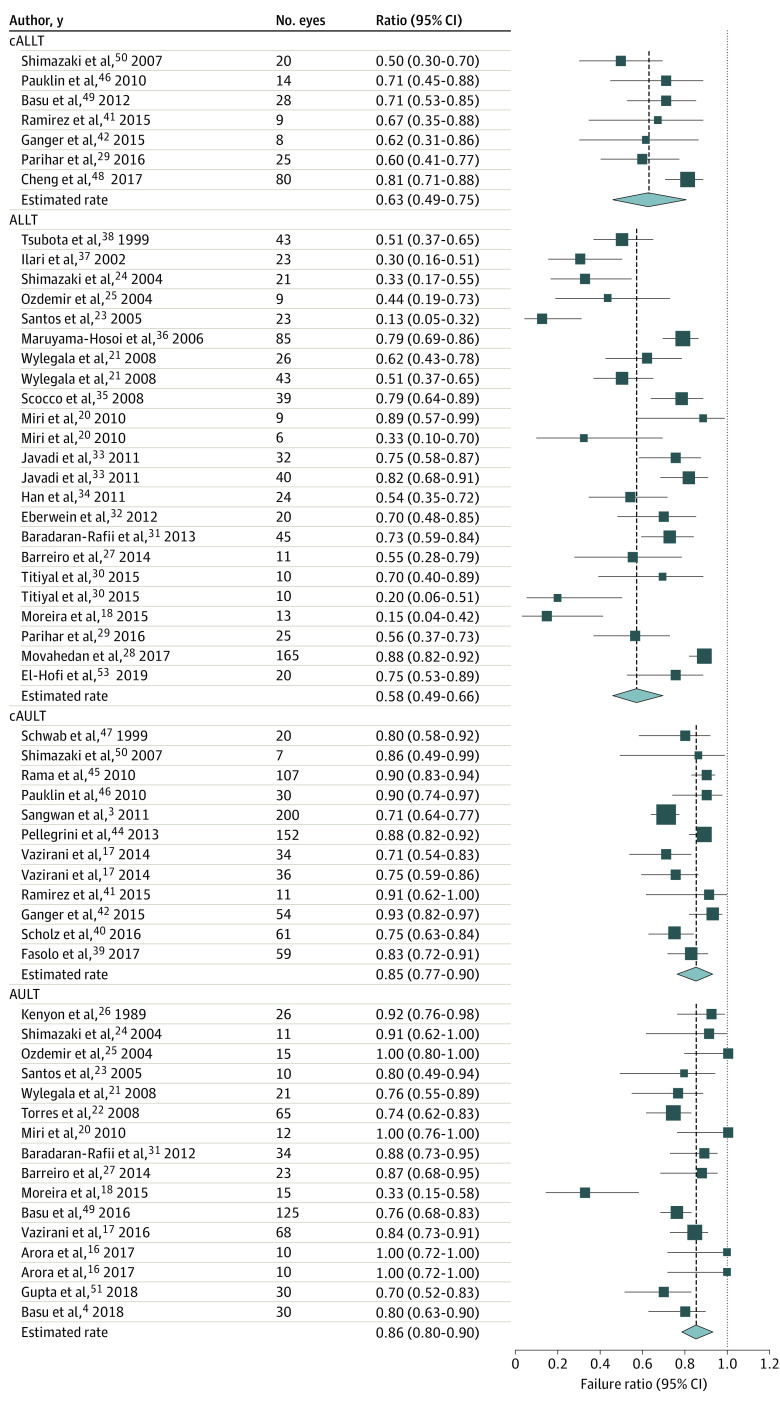
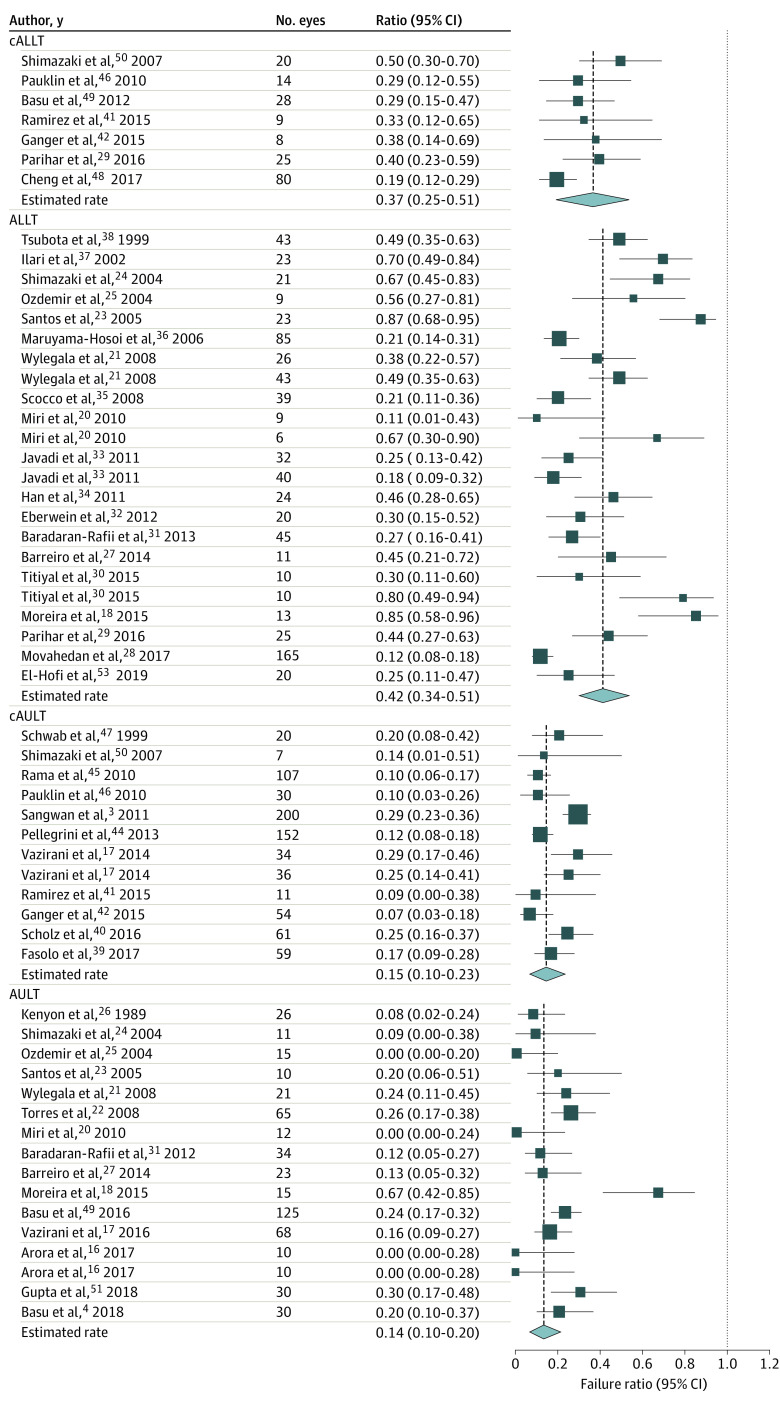
The overall success rate of all LSCT was 67.4% (95% CI, 62.1%-72.3%), and the overall improvement rate of the ocular surface was 74.5% (95% CI, 69.3%-79.2%), with a mean (SD) follow-up of 31.3 (20.9) months. The highest success rates were achieved after AULT (83.2%; 95% CI, 76.7%-88.1%) followed by cAULT (71.8%; 95% CI, 62.2%-79.9%, P = .02). The success rates after ALLT (53.9%; 95% CI, 45.6%-62.1%) and cALLT (52.1%; 95% CI, 39.1%-65.0%) were significantly lower than that after AULT (both P < .001) and cAULT (cAULT [83.2%] vs ALLT [53.9%]; P = .005; cAULT [83.2%] vs cALLT [52.1%]; P = .004, respectively; Figure 1). The surface improvement rates of AULT (85.7%; 95% CI, 79.5%-90.3%) and cAULT (84.7%; 95% CI, 77.2%-90.0%) were similar (P = .79; Figure 2). Although the overall failure rate after all LSCT was only 25.5% (95% CI, 20.8%-30.7%), this rate was significantly higher for allogeneic transplantation (ALLT: 42.2%; 95% CI, 33.9%-51.0%; cALLT: 36.8%; 95% CI, 24.8%-50.7%) than for autologous transplantation graft (AULT: 14.3%; 95% CI, 9.7%-20.5%; cAULT: 15.3%; 95% CI, 9. 9%-22.8%; all P < .001; Figure 3).
Figure 1. Forest Plots for Success Rate of Limbal Stem Cell Transplant.
The highest success rates were achieved after direct autologous limbal transplant (AULT) followed by cultivated autologous limbal stem cells transplant (cAULT). The success rates after direct allogenic limbal transplant (ALLT) and cultivated allogenic limbal stem cells transplant (cALLT) were lower than that after AULT.
Figure 2. Forest Plots for Improvement Rate of Limbal Stem Cell Transplantation.
The surface improvement rates of after direct autologous limbal transplant (AULT) and autologous limbal stem cells transplant (cAULT) were similar to the success rates shown in Figure 1.
Figure 3. Forest Plots for Failure Rate of Limbal Stem Cell Transplantation.
The failure rate was significantly higher for direct allogenic limbal transplant (ALLT) and cultivated allogenic limbal stem cells transplant (cALLT) than for direct autologous limbal transplant (AULT)and cultivated autologous limbal transplant (cAULT).
Visual Outcome
Thirty-one studies (1654 eyes) used 2-line improvement of Snellen VA, presurgery and postsurgery logMar VA, or both to describe visual improvement. Nine studies (548 eyes) did not report visual outcomes.18,22,23,27,32,33,36,39,44 Five hundred and sixty-seven eyes underwent penetrating/lamellar keratoplasty or cataract surgery after LSCT.
Among 1576 eyes from 28 studies,3,4,17,20,21,24,26,28,29,30,31,34,35,37,38,40,41,42,43,45,46,47,48,49,50,51,52,53 922 eyes (58.5%) obtained 2-line improvement of Snellen VA after LSCT. Fifteen studies provided the details of preoperative and postoperative VA (eFigure 2 in the Supplement).16,19,21,25,26,31,34,38,40,43,45,46,47,50,53 Mean (SD) LogMar VA improved from 2.1 (0.2) before LSCT to 0.7 (0.2) after the surgery (P < .001). Of the 955 eyes for which detailed VA results were available, 582 eyes (60.9%; 95% CI, 50.5%-73.8%) retained functional VA (Snellen VA ≥20/200 or LogMar VA ≤1.0) at the final visit. Vision declined in 63 eyes (6.6%).
Autologous limbal transplant resulted in the highest rate of 2-line visual improvement (76.6%; 95% CI, 66.3%-84.4%), and this rate was greater than those of cAULT (56.4%; 95% CI, 45.0%-67.1%; P = .008), ALLT (52.3%; 95% CI, 43.1%-61.3%; P < .001), and cALLT (43.3%; 95% CI, 31.4%-56.1%; P < .001). Functional VA at the final follow-up was worse after ALLT (51.3%; 95% CI, 46.0%-56.5%) than after AULT (68.8%; 95% CI, 59.5%-76.8%; P = .001), cAULT (64.7%; 95% CI, 60.0%-69.2%; P = .002), and cALLT (64.5%; 95% CI, 51.9%-75.4%; P = .03).
Complications
Recipient Eye
The most common complication after LSCT was recurrent/persistent epithelial erosion at 10.5% (95% CI, 7.2%-23.3%). The rate of recurrent or persistent epithelial erosion after LSCT was higher with allogeneic transplants (ALLT, 28.8%; cALLT, 18.5%) than after autologous transplants (AULT, 4.3%; cAULT, 3.4%; ALLT [28.8%] vs AULT [4.3%]; P <.001; ALLT [28.8%] vs cAULT [3.4%]; P <.001; cALLT [18.5%] vs AULT [4.3%]; P = .006; cALLT [18.5%] vs cAULT [3.4%]; P = .02). The overall rate of IOP elevation after LSCT was 1.7% (95% CI, 0.5%-7.8%). The rate of IOP elevation was also higher after ALLT (6.3%; 95% CI, 1.8%-19.4%) than after AULT (0.8%; 95% CI: 0.1%-4.2%; P = .002) and cAULT (0.3%; 95% CI, 0.04%-3.9%; P = .02). Direct allogenic limbal transplant had a higher rejection rate (27.6%; 95% CI, 20.3%-36.4%) than cALLT did (5.2%; 95% CI, 1.9%-13.9%; P < .001).
The rates of complications after AULT and cAULT were lower than or similar to those after ALLT and cALLT (Table 2). The only exception was hemorrhage underneath amniotic membrane after cAULT in which amniotic membrane served as the cell carrier (9.2%; 95% CI, 3.5%-22.1%). The rates of other complications were low and similar among the 4 types of LSCT.
Table 2. Rates of Adverse Events Among AULT, ALLT, cAULT, and cALLT.
| Event | Rate (95% CI) | P value | ||||
|---|---|---|---|---|---|---|
| Overall | AULT | ALLT | cAULT | cALLT | ||
| Recipient eyes | ||||||
| Recurrent/persistent epithelial erosion | 10.5 (7.2-23.3) | 4.3 (2.0-9.2) | 28.8 (17.1-41.9) | 3.4 (1.0-11.1) | 18.5 (7.7-38.0) | ALLT vs AULT: P < .001; ALLT vs cAULT: P < .001; cALLT vs AULT: P = .006; cALLT vs cAULT: P = .02 |
| Infectious keratitis | 2.3 (1.8-4.4) | 2.9 (1.1-7.2) | 4.2 (2.2-7.8) | 2.1 (0.8-5.3) | 1.3 (0.2-10.1) | NA |
| Corneal melting/perforation | 2.6 (1.5-4.6), | 1.7 (0.6-5) | 5.6 (3-10.1) | 1.9 (0.7-5.1) | 4.2 (1.1-14.5) | AULT vs ALLT: P = .02 |
| Symblepharon | 0.4 (0.01-4.2) | 1.3 (0.1-10.3) | 0.11 (0.01-1.8) | 0.5 (0.05-4.9) | 0.7 (0.04-13.0) | NA |
| Rejection | 3.5 (1.2-9.3) | 0 (0-0.6) | 27.6 (20.3-36.4) | 0 (0-1) | 5.3 (1.9-13.9) | ALLT vs AULT: P < .001; ALLT vs cAULT: P < .001; cALLT vs AULT: P < .001; ALLT vs cALLT: P < .001; cALLT vs cAULT: P < .001 |
| Hemorrhage under amniotic membrane | 0.3 (0.04-3.6) | 1.8 (0.5-5.7) | 0.16 (0.02-1.4) | 9.2 (3.5-22.1) | 0 (0-7.1) | cAULT vs AULT: P = .03; cAULT vs ALLT: P < .001; cAULT vs cALLT: P = .04 |
| Necrosis/loss of transplant | 0.4 (0.1-2.1) | 0.9 (0.1-7.6) | 0.4 (0.04-3.2) | 0.2 (0.04-5.1) | 0 (0-7.1) | NA |
| Elevated IOP | 1.7 (0.5-7.8) | 0.8 (0.1-4.2) | 6.3 (1.8-19.4) | 0.3 (0.04-3.9) | 2.1 (0.01-45.3) | ALLT vs AULT: P = .002; ALLT vs cAULT: P = .02 |
| Others | 1.3 (0.4-4.8) | 0.5 (0.01-3.6) | 5.4 (1.4-18.6) | 1.4 (0.14-12.1) | 6.5 (0.6-44.7) | AULT vs ALLT: P = .004 |
| Donor eyes | ||||||
| Hemorrhage | 0.18 (0-12.3) | 0.3 (0.04-7.3) | 0 (0-16.8) | 0.1 (0-6.2) | NA | NA |
| LSCD | 0.04‰ (0.01‰-0.6) | 0.2 (0.04-1.4) | 0 (0-16.8) | 0 (0-0.5) | NA | NA |
Abbreviations: ALLT, allogenic direct limbal transplant; AULT, autologous direct limbal transplant; cALLT, allogenic cultured limbal stem cell transplant; cAULT, autologous cultured limbal stem cell transplant; IOP, intraocular pressure; LSCD, limbal stem cell deficiency; NA, not applicable.
Donor Eye
The most common adverse event of donor eyes was hemorrhage at the donor site (AULT: 0.31%; 95% CI, 0.04%-7.3%; cAULT: 0.1%; 95% CI, 0%-6.2%). The overall rate of iatrogenic LSCD was extremely low (0.004%; 95% CI, 0.01‰-0.55%). Of the 1276 autologous transplants, only 1 case of iatrogenic LSCD at the donor site was reported in the AULT group (Table 2).
Other Factors Associated With Outcomes
Systemic immunosuppressive therapy often consists of 1 or more of the following medications: corticosteroids, tacrolimus, cyclosporine, mycophenoloate mofetil, and azathioprine.23,24,28,31,32,33,34,36,37,38,49,50,53 Among 25 studies that used immunosuppression regimen, 5 studies in which a 3-medication regimen was used showed a higher success rate (81.2%; 95% CI, 65.0%-91.0%) than did those studies in which fewer immunosuppressants were used (odds ratio [OR], 3.844; 95% CI, 1.6-9.1; P = .002). However, the number and dose of medications were not associated with the improvement rate (OR, 2.575; 95% CI, 1.0-6.9; P = .05). Only long-term use of immunosuppressants yielded a higher success rate (74.9%; 95% CI, 57.3%-86.9%) and improvement rate (81.6%; 95% CI, 66.1%-91.0%; eTable 2 in the Supplement). Neither the dosage nor the duration of systemic corticosteroid therapy were associated with the clinical outcome.
In the 17 studies that evaluated cultivated LSCs, the cultivation methods without 3T3 feeder cells (327 eyes; 63%) resulted in a lower improvement rate than did the cultivation methods that used feeder cells (376 eyes [77%]; OR, 1.931; 95% CI, 1.3-2.9; P = .001). However, the use of 3T3 feeder cells did not affect the success rate. Human leukocyte antigen–matched allografts, the use of amniotic membrane in AULT and ALLT, the use of serum during LSC culture, and the substrate on which LSC sheets were cultivated did not show association with the success rate and improvement rate (eTable 2 in the Supplement).
Heterogeneity Analysis and Publication Bias
The heterogeneity analysis showed the I2 value was 33% for the success rate, 36% for the improvement rate, and 36% for the failure rate; thus, the between-study heterogeneity was not significant. Analyses of publication bias regarding the success rate and the improvement rate were based on both the absolute rate and log-odds. Contour-enhanced funnel plots (eFigure 3 in the Supplement) showed a symmetrical plot distribution, indicating an absence of publication bias.
Discussion
Both our study and previous systemic review54 confirmed that LSCT can restore a stable ocular surface in most eyes. The success rate and improvement rate were both significantly higher for autologous transplants than for allogeneic transplants, with an average follow-up of 31 months. Two 2019 studies55,56 reported that ocular surface stability was achieved in 71% to 78% of eyes at up to 72 months after autologous LSCT, confirming its long-term efficacy. The success rate of AULT was slightly higher than that of cAULT, as previously reported.57 The improvement rates of AULT and cAULT were similar, probably because some studies distinguished success from partial success while the others used success rate to account for both.
Even with the use of immunosuppressive therapy in most studies, a mean of 42.2% cases had total surface failure after allogeneic transplantation. A progressive decline of allograft survival and ambulatory vision with time was oberseved.7,8,56 However, 3 meta-analyses5,54,58 did not find a difference in success rates between autografts and allografts. These studies only focused on the outcome of cultivated limbal epithelial transplantation, with a mean length of follow-up less than 2 years. Moreover, these studies included many small cases series with sample sizes of less than 10 patients. Selection bias caused by a small sample size and a shorter length of follow-up might be the main reasons for their finding of similar outcomes between autologous and allogenic cultivated limbal epithelial transplantation.
Similar success rates and improvement rates were found between the HLA-matched and unmatched allografts. A 2018 systematic review59 drew the conclusion that the current literature did not show which regimen or allograft type was most efficacious in treating the different etiologies of LSCD. Our study confirms that immunosuppression regimens varied significantly in the selection, combination, and dosage of different immunosuppressive agents. A higher rate of elevated intraocular pressure occurred after ALLT, which was likely caused by systemic and topical use of steroids. Randomized controlled studies are necessary to demonstrate the efficacy, safety, and the length of treatment of different immunosuppressive regimens.
The safety of limbal biopsy of the donor eye is a major concern associated with autologous LSCT, especially AULT with an exception of SLET, because the biopsy carries the potential risk of iatrogenic LSCD in the donor eye. Previous studies reported that iatrogenic LSCD was found in the donor eyes with a history of contact lens wear.60,61 To our knowledge, only 1 study62 investigates the rate of iatrogenic LSCD in donor eye. The true rate of iatrogenic LSCD is unknown.
Our study found that 60.9% of eyes achieve a best-corrected VA of at least 20/200 after LSCT with a mean follow-up of 31.3 months. Boston type I Kpro (KproI) has been used to treat eyes with LSCD. A systematic review63 of the outcome of KproI for the treatment of LSCD after chemical injury reported that 64.1% of eyes achieved a best-corrected VA of at least 20/200, with a mean follow-up period of 25 months after cases of Kpro extrusion (12%) were excluded. Glaucomatous optic neuropathy was the most common cause for best-corrected VA less than 20/200 in eyes (66.7%) that retained the Kpro.63 In contrast, intraocular pressure elevation was only found in 1.7% eyes after LSCT. Other complications such as corneal necrosis/melt far rarely occurred after LSCT than after KproI. Posterior complications such as retinal detachment, endophthalmitis, sterile vitritis, and cystoid macular edema occurred in 21.6% of eyes after KproI while none was reported after LSCT. Limbal stem cell transplantation appears to have far fewer complications than KproI.
There was a lack of standardized criteria to stage the severity of LSCD and to define success, partial success, and failure; thus, comparison of the actual outcomes among different treatments was challenging. Only 26% of studies used diagnostic tests, such as impression cytology and/or in vivo confocal microscopy, to confirm the diagnosis of LSCD.64 The limitation of using clinical signs in the diagnosis of LSCD needs to be recognized.65,66 The International LSCD Working Group has established a consensus on the diagnosis, classification, and staging of LSCD, which will serve as a guideline for future clinical studies.1 Although visual improvement has been used as an additional criterion to define success in many studies, a lack of vision improvement does not necessarily indicate LSCT failure because LSCT restores LSC function but does not treat the residual corneal stromal opacity.
Limitations
This study has limitations. First, to our knowledge, there is no randomized clinical trial comparing different LSCT. Therefore, the efficacy of each approach could not be evaluated. Second, among 25 studies using immunosuppression treatment, only 5 studies used 3 immunosuppressive medications. Considering that the subgroup analysis revealed a significantly higher success rate and improvement rate when pooling data from these 5 studies, the results of immunosuppression need to be interpreted with caution.
Conclusions
In summary, this meta-analysis supports that LSCT improves the corneal surface with a low rate of severe ocular complications and that autologous LSCT may have a higher success rate and fewer complications than allogenic LSCT. However, lack of standardization of efficacy measures precludes determining whether one approach is superior to the other. Randomized clinical trials are necessary to compare the efficacy of different LSCT.
eMethods.
eTable 1. Characteristics of eligible studies and quality assessment
eTable 2. Comparisons on postoperative systemic immunosuppressive therapy and other factors affecting the success rate and the improvement rate
eFigure 1. Flowchart of study selection
eFigure 2. Visual acuity (LogMar) before and after limbal stem cell transplantation
eFigure 3. Funnel plots of publication bias analyses for the success rate (A, B) and improvement rate (C, D).
References
- 1.Deng SX, Borderie V, Chan CC, et al. ; and The International Limbal Stem Cell Deficiency Working Group . Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea. 2019;38(3):364-375. doi: 10.1097/ICO.0000000000001820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121(10):2053-2058. doi: 10.1016/j.ophtha.2014.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95(11):1525-1529. doi: 10.1136/bjophthalmol-2011-300352 [DOI] [PubMed] [Google Scholar]
- 4.Basu S, Sureka SP, Shanbhag SS, Kethiri AR, Singh V, Sangwan VS. Simple limbal epithelial transplantation: long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology. 2016;123(5):1000-1010. doi: 10.1016/j.ophtha.2015.12.042 [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Ma L. Systematic review and meta-analysis on transplantation of ex vivo cultivated limbal epithelial stem cell on amniotic membrane in limbal stem cell deficiency. Cornea. 2015;34(5):592-600. doi: 10.1097/ICO.0000000000000398 [DOI] [PubMed] [Google Scholar]
- 6.Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol. 2007;52(5):483-502. doi: 10.1016/j.survophthal.2007.06.013 [DOI] [PubMed] [Google Scholar]
- 7.Shanbhag SS, Saeed HN, Paschalis EI, Chodosh J. Keratolimbal allograft for limbal stem cell deficiency after severe corneal chemical injury: a systematic review. Br J Ophthalmol. 2018;102(8):1114-1121. [DOI] [PubMed] [Google Scholar]
- 8.Yin J, Jurkunas U. Limbal stem cell transplantation and complications. Semin Ophthalmol. 2018;33(1):134-141. doi: 10.1080/08820538.2017.1353834 [DOI] [PubMed] [Google Scholar]
- 9.Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad Med. 2015;90(8):1067-1076. doi: 10.1097/ACM.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 10.Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1(1):39-65. doi: 10.1002/jrsm.5 [DOI] [PubMed] [Google Scholar]
- 11.Hedges LV, Tipton E, Johnson MC. Erratum: robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1(2):164-165. doi: 10.1002/jrsm.17 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein H, Rasbash J. Improved approximations for multilevel models with binary responses. J Roy Stat Soc a Sta. 1996;159:505-513. doi: 10.2307/2983328 [DOI] [Google Scholar]
- 13.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991-996. doi: 10.1016/j.jclinepi.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 14.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676-680. doi: 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- 15.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20(4):641-654. doi: 10.1002/sim.698 [DOI] [PubMed] [Google Scholar]
- 16.Arora R, Dokania P, Manudhane A, Goyal JL. Preliminary results from the comparison of simple limbal epithelial transplantation with conjunctival limbal autologous transplantation in severe unilateral chronic ocular burns. Indian J Ophthalmol. 2017;65(1):35-40. doi: 10.4103/0301-4738.202312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vazirani J, Ali MH, Sharma N, et al. Autologous simple limbal epithelial transplantation for unilateral limbal stem cell deficiency: multicentre results. Br J Ophthalmol. 2016;100(10):1416-1420. doi: 10.1136/bjophthalmol-2015-307348 [DOI] [PubMed] [Google Scholar]
- 18.Moreira PB, Magalhães RS, Pereira NC, Oliveira LA, Sousa LB. Limbal transplantation at a tertiary hospital in Brazil: a retrospective study. Arq Bras Oftalmol. 2015;78(4):207-211. doi: 10.5935/0004-2749.20150054 [DOI] [PubMed] [Google Scholar]
- 19.Baradaran-Rafii A, Eslani M, Jamali H, Karimian F, Tailor UA, Djalilian AR. Postoperative complications of conjunctival limbal autograft surgery. Cornea. 2012;31(8):893-899. doi: 10.1097/ICO.0b013e31823f095d [DOI] [PubMed] [Google Scholar]
- 20.Miri A, Al-Deiri B, Dua HS. Long-term outcomes of autolimbal and allolimbal transplants. Ophthalmology. 2010;117(6):1207-1213. doi: 10.1016/j.ophtha.2009.10.028 [DOI] [PubMed] [Google Scholar]
- 21.Wylegala E, Dobrowolski D, Tarnawska D, et al. Limbal stem cells transplantation in the reconstruction of the ocular surface: 6 years experience. Eur J Ophthalmol. 2008;18(6):886-890. doi: 10.1177/112067210801800605 [DOI] [PubMed] [Google Scholar]
- 22.Torres J, Fernández I, Quadrado MJ, et al. Limbal transplantation: multicenter retrospective case series analysis. Arch Soc Esp Oftalmol. 2008;83(7):417-422. doi: 10.4321/S0365-66912008000700005 [DOI] [PubMed] [Google Scholar]
- 23.Santos MS, Gomes JA, Hofling-Lima AL, Rizzo LV, Romano AC, Belfort R Jr. Survival analysis of conjunctival limbal grafts and amniotic membrane transplantation in eyes with total limbal stem cell deficiency. Am J Ophthalmol. 2005;140(2):223-230. doi: 10.1016/j.ajo.2005.03.022 [DOI] [PubMed] [Google Scholar]
- 24.Shimazaki J, Shimmura S, Tsubota K. Donor source affects the outcome of ocular surface reconstruction in chemical or thermal burns of the cornea. Ophthalmology. 2004;111(1):38-44. doi: 10.1016/j.ophtha.2003.02.003 [DOI] [PubMed] [Google Scholar]
- 25.Ozdemir O, Tekeli O, Ornek K, Arslanpençe A, Yalçindağ NF. Limbal autograft and allograft transplantations in patients with corneal burns. Eye (Lond). 2004;18(3):241-248. doi: 10.1038/sj.eye.6700640 [DOI] [PubMed] [Google Scholar]
- 26.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96(5):709-722. doi: 10.1016/S0161-6420(89)32833-8 [DOI] [PubMed] [Google Scholar]
- 27.Barreiro TP, Santos MS, Vieira AC, de Nadai Barros J, Hazarbassanov RM, Gomes JA. Comparative study of conjunctival limbal transplantation not associated with the use of amniotic membrane transplantation for treatment of total limbal deficiency secondary to chemical injury. Cornea. 2014;33(7):716-720. doi: 10.1097/ICO.0000000000000139 [DOI] [PubMed] [Google Scholar]
- 28.Movahedan A, Cheung AY, Eslani M, Mogilishetty G, Govil A, Holland EJ. Long-term outcomes of ocular surface stem cell allograft transplantation. Am J Ophthalmol. 2017;184:97-107. doi: 10.1016/j.ajo.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 29.Parihar JKS, Parihar AS, Jain VK, Kaushik J, Nath P. Allogenic cultivated limbal stem cell transplantation versus cadaveric keratolimbal allograft in ocular surface disorder: 1-year outcome. Int Ophthalmol. 2017;37(6):1323-1331. [DOI] [PubMed] [Google Scholar]
- 30.Titiyal JS, Sharma N, Agarwal AK, Prakash G, Tandon R, Vajpayee R. Live related versus cadaveric limbal allograft in limbal stem cell deficiency. Ocul Immunol Inflamm. 2015;23(3):232-239. doi: 10.3109/09273948.2014.902076 [DOI] [PubMed] [Google Scholar]
- 31.Baradaran-Rafii A, Eslani M, Djalillian AR. Complications of keratolimbal allograft surgery. Cornea. 2013;32(5):561-566. doi: 10.1097/ICO.0b013e31826215eb [DOI] [PubMed] [Google Scholar]
- 32.Eberwein P, Böhringer D, Schwartzkopff J, Birnbaum F, Reinhard T. Allogenic limbo-keratoplasty with conjunctivoplasty, mitomycin C, and amniotic membrane for bilateral limbal stem cell deficiency. Ophthalmology. 2012;119(5):930-937. doi: 10.1016/j.ophtha.2011.10.039 [DOI] [PubMed] [Google Scholar]
- 33.Javadi MA, Jafarinasab MR, Feizi S, Karimian F, Negahban K. Management of mustard gas-induced limbal stem cell deficiency and keratitis. Ophthalmology. 2011;118(7):1272-1281. doi: 10.1016/j.ophtha.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 34.Han ES, Wee WR, Lee JH, Kim MK. Long-term outcome and prognostic factor analysis for keratolimbal allografts. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1697-1704. doi: 10.1007/s00417-011-1760-3 [DOI] [PubMed] [Google Scholar]
- 35.Scocco C, Kwitko S, Rymer S, Marinho D, Bocaccio F, Lindenmeyer R. HLA-matched living-related conjunctival limbal allograft for bilateral ocular surface disorders: long-term results. Arq Bras Oftalmol. 2008;71(6):781-787. doi: 10.1590/S0004-27492008000600003 [DOI] [PubMed] [Google Scholar]
- 36.Maruyama-Hosoi F, Shimazaki J, Shimmura S, Tsubota K. Changes observed in keratolimbal allograft. Cornea. 2006;25(4):377-382. doi: 10.1097/01.ico.0000176608.65708.27 [DOI] [PubMed] [Google Scholar]
- 37.Ilari L, Daya SM. Long-term outcomes of keratolimbal allograft for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109(7):1278-1284. doi: 10.1016/S0161-6420(02)01081-3 [DOI] [PubMed] [Google Scholar]
- 38.Tsubota K, Satake Y, Kaido M, et al. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med. 1999;340(22):1697-1703. doi: 10.1056/NEJM199906033402201 [DOI] [PubMed] [Google Scholar]
- 39.Fasolo A, Pedrotti E, Passilongo M, et al. Safety outcomes and long-term effectiveness of ex vivo autologous cultured limbal epithelial transplantation for limbal stem cell deficiency. Br J Ophthalmol. 2017;101(5):640-649. doi: 10.1136/bjophthalmol-2015-308272 [DOI] [PubMed] [Google Scholar]
- 40.Scholz SL, Thomasen H, Hestermann K, Dekowski D, Steuhl KP, Meller D. Long-term results of autologous transplantation of limbal epithelium cultivated ex vivo for limbal stem cell deficiency. Ophthalmologe. 2016;113(4):321-329. doi: 10.1007/s00347-015-0110-y [DOI] [PubMed] [Google Scholar]
- 41.Ramírez BE, Sánchez A, Herreras JM, et al. Stem cell therapy for corneal epithelium regeneration following good manufacturing and clinical procedures. Biomed Res Int. 2015;2015:408495. doi: 10.1155/2015/408495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganger A, Vanathi M, Mohanty S, Tandon R. Long-term outcomes of cultivated limbal epithelial transplantation: evaluation and comparison of results in children and adults. Biomed Res Int. 2015;2015:480983. doi: 10.1155/2015/480983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazirani J, Basu S, Kenia H, et al. Unilateral partial limbal stem cell deficiency: contralateral versus ipsilateral autologous cultivated limbal epithelial transplantation. Am J Ophthalmol. 2014;157(3):584-90.e1, 2. doi: 10.1016/j.ajo.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 44.Pellegrini G, Rama P, Matuska S, et al. Biological parameters determining the clinical outcome of autologous cultures of limbal stem cells. Regen Med. 2013;8(5):553-567. doi: 10.2217/rme.13.43 [DOI] [PubMed] [Google Scholar]
- 45.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147-155. doi: 10.1056/NEJMoa0905955 [DOI] [PubMed] [Google Scholar]
- 46.Pauklin M, Fuchsluger TA, Westekemper H, Steuhl KP, Meller D. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57-70. doi: 10.1159/000315020 [DOI] [PubMed] [Google Scholar]
- 47.Schwab IR. Cultured corneal epithelia for ocular surface disease. Trans Am Ophthalmol Soc. 1999;97:891-986. [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng J, Zhai H, Wang J, Duan H, Zhou Q. Long-term outcome of allogeneic cultivated limbal epithelial transplantation for symblepharon caused by severe ocular burns. BMC Ophthalmol. 2017;17(1):8. doi: 10.1186/s12886-017-0403-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basu S, Fernandez MM, Das S, Gaddipati S, Vemuganti GK, Sangwan VS. Clinical outcomes of xeno-free allogeneic cultivated limbal epithelial transplantation for bilateral limbal stem cell deficiency. Br J Ophthalmol. 2012;96(12):1504-1509. doi: 10.1136/bjophthalmol-2012-301869 [DOI] [PubMed] [Google Scholar]
- 50.Shimazaki J, Higa K, Morito F, et al. Factors influencing outcomes in cultivated limbal epithelial transplantation for chronic cicatricial ocular surface disorders. Am J Ophthalmol. 2007;143(6):945-953. doi: 10.1016/j.ajo.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 51.Gupta N, Joshi J, Farooqui JH, Mathur U. Results of simple limbal epithelial transplantation in unilateral ocular surface burn. Indian J Ophthalmol. 2018;66(1):45-52. doi: 10.4103/ijo.IJO_602_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basu S, Mohan S, Bhalekar S, Singh V, Sangwan V. Simple limbal epithelial transplantation (SLET) in failed cultivated limbal epithelial transplantation (CLET) for unilateral chronic ocular burns. Br J Ophthalmol. 2018;102(12):1640-1645. doi: 10.1136/bjophthalmol-2017-311506 [DOI] [PubMed] [Google Scholar]
- 53.El-Hofi AH, Helaly HA. Evaluation of limbal transplantation in eyes with bilateral severe ocular surface damage secondary to chemical injury. Clin Ophthalmol. 2019;13:383-390. doi: 10.2147/OPTH.S192316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baylis O, Figueiredo F, Henein C, Lako M, Ahmad S. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. J Cell Biochem. 2011;112(4):993-1002. doi: 10.1002/jcb.23028 [DOI] [PubMed] [Google Scholar]
- 55.Eslani M, Cheung AY, Kurji K, Pierson K, Sarnicola E, Holland EJ. Long-term outcomes of conjunctival limbal autograft in patients with unilateral total limbal stem cell deficiency. Ocul Surf. 2019;17(4):670-674. doi: 10.1016/j.jtos.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 56.Borderie VM, Ghoubay D, Georgeon C, et al. Long-term results of cultured limbal stem cell versus limbal tissue transplantation in stage iii limbal deficiency. Stem Cells Transl Med. 2019;8(12):1230-1241. doi: 10.1002/sctm.19-0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shanbhag SS, Nikpoor N, Rao Donthineni P, Singh V, Chodosh J, Basu S. Autologous limbal stem cell transplantation: a systematic review of clinical outcomes with different surgical techniques. Br J Ophthalmol. 2020;104(2):247-253. [DOI] [PubMed] [Google Scholar]
- 58.Mishan MA, Yaseri M, Baradaran-Rafii A, Kanavi MR. Systematic review and meta-analysis investigating autograft versus allograft cultivated limbal epithelial transplantation in limbal stem cell deficiency. Int Ophthalmol. 2019;39(11):2685-2696. doi: 10.1007/s10792-019-01092-x [DOI] [PubMed] [Google Scholar]
- 59.Ballios BG, Weisbrod M, Chan CC, et al. Systemic immunosuppression in limbal stem cell transplantation: best practices and future challenges. Can J Ophthalmol. 2018;53(4):314-323. doi: 10.1016/j.jcjo.2017.10.040 [DOI] [PubMed] [Google Scholar]
- 60.Jenkins C, Tuft S, Liu C, Buckley R. Limbal transplantation in the management of chronic contact-lens-associated epitheliopathy. Eye (Lond). 1993;7(Pt 5):629-633. doi: 10.1038/eye.1993.145 [DOI] [PubMed] [Google Scholar]
- 61.Tan DT, Ficker LA, Buckley RJ. Limbal transplantation. Ophthalmology. 1996;103(1):29-36. doi: 10.1016/S0161-6420(96)30737-9 [DOI] [PubMed] [Google Scholar]
- 62.Cheung AY, Sarnicola E, Holland EJ. Long-term ocular surface stability in conjunctival limbal autograft donor eyes. Cornea. 2017;36(9):1031-1035. doi: 10.1097/ICO.0000000000001260 [DOI] [PubMed] [Google Scholar]
- 63.Shanbhag SS, Saeed HN, Paschalis EI, Chodosh J. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical corneal injury: A systematic review. Ocul Surf. 2018;16(3):272-281. doi: 10.1016/j.jtos.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 64.Le Q, Chauhan T, Deng SX. Diagnostic criteria for limbal stem cell deficiency before surgical intervention: a systematic literature review and analysis. Surv Ophthalmol. 2020;65(1):32-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chan E, Le Q, Codriansky A, Hong J, Xu J, Deng SX. Existence of normal limbal epithelium in eyes with clinical signs of total limbal stem cell deficiency. Cornea. 2016;35(11):1483-1487. doi: 10.1097/ICO.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Q, Samson CM, Deng SX. A case of corneal neovascularization misdiagnosed as total limbal stem cell deficiency. Cornea. 2018;37(8):1067-1070. doi: 10.1097/ICO.0000000000001631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Characteristics of eligible studies and quality assessment
eTable 2. Comparisons on postoperative systemic immunosuppressive therapy and other factors affecting the success rate and the improvement rate
eFigure 1. Flowchart of study selection
eFigure 2. Visual acuity (LogMar) before and after limbal stem cell transplantation
eFigure 3. Funnel plots of publication bias analyses for the success rate (A, B) and improvement rate (C, D).