Abstract
Among the hundreds of reported Achillea species, A. membranacea (Labill.) DC. is one of the six that grow in Jordan. Many species of this genus are used in folk medicine to treat a variety of ailments and several biological and pharmacological activities have been ascribed to their essential oil (EO). For this study, the EO obtained from a specimen of A. membranacea grown in Jordan was analyzed by GC-MS. Ninety-six compounds were detected, of which oxygenated monoterpenes was the predominant class (47.9%), followed by non-terpene derivatives (27.9%), while sesquiterpenes represented 14.2% of the total composition. The most abundant compound in the EO was 1,8-cineole (21.7%). The cytotoxic activity of the EO was evaluated against three cancer cell lines (MCF7, A2780 and HT29), and one normal fibroblast cell line (MRC5) by MTT assay. Significant growth inhibition was observed in EO-exposed A2780 and HT29 cells (IC50 = 12.99 and 14.02 μg/mL, respectively), while MCF7 and MRC5 were less susceptible. The EO induced apoptosis and increased the preG1 events in A2780 cells. 1,8-Cineole, the major constituent of the EO, exhibited submicromolar cytotoxicity against A2780 cells, and was 42 times more selective against MRC5 cells. Its cytotoxicity against A2780 cells was comparable with that of doxorubicin, but 1,8-cineole was more selective for MRC5 normal cells. Interestingly, 1,8-cineole enhanced apoptosis in A2780, and caused a remarkable dose-dependent increase in preG1 events. Thus, 1,8-cineole has demonstrated promising cytotoxic and proapoptotic properties.
Keywords: Asteraceae; essential oil; GC-EI-MS; 1,8-cineole; cytotoxicity; apoptosis; cell cycle
1. Introduction
Medicinal plants and their constituents have countless biological properties, based mainly on their ability to interact with key enzymes linked to several ailments, including: cancer, diabetes, inflammation, hypertension and Alzheimer’s disease [1,2,3,4,5]. Recently, EOs have caught the attention of scientists due to their ability to prevent and treat cancer, as well as their state-of-the-art application in the most advanced and sophisticated technologies in nanoscience [6,7,8].
The genus Achillea includes over a hundred species and subspecies, which are distributed in the Northern hemisphere [9]. The use of the inflorescence, as well as the whole plant, of many of these species has a long-established role in folk medicine to treat a wide range of ailments that mainly affect the digestive system. It takes various application forms, which differ depending on the geographical area [10,11,12,13]. Besides their medicinal properties, some Achillea species are also cultivated as ornamental plants.
A. membranacea (Labill.) DC. is one of the six species reported in Jordan [14]: it is distributed in the Irano-Turanian phytogeographic region, which includes Jordan, Palestine, Syria, Lebanon, Iraq and Turkey [15]. A. membranacea is a perennial herb. It has numerous stems (25–50 cm), is lignified in the basal section, exhibits pannose, linear, sessile and pinnatisect (1.5–6 cm × 0.3–0.6 cm) leaves, and has a capitula up to 20 mm and yellow ray florets [15].
The EO of many Achillea species possesses several biological and pharmacological activities. It is antimicrobial, antimalarial, insecticidal, phytotoxic, anti-inflammatory and antihemorrhoidal [16,17,18,19,20]. Anticancer and cytotoxic effects were reported for the extracts of some Achillea species, including A. clavennae L. and A. millefolium L., the iso-seco-guaianolide, which was isolated from A. clavennae L., exhibited potent antiproliferative and apoptotic effects on U251 and glioma cell lines, with a potency comparable to that of cisplatin [21], mediated by its oxidative and mitotic activities. Another compound, casticin, showed multiple antitumor activities. Casticin, which was isolated from A. millefolium L. through HPLC, showed cytotoxic, early apoptosis, G2/M cell cycle arrest and consequently disruption of the mitotic spindle in two MCF7 sublines [22]. In a previous study, the methanol extract of A. membranacea exerted a moderate cytotoxic effect upon Jurkat cells (T-cell leukemia) using the MTT assay [23].
As a part of an ongoing research program on the Middle Eastern plants bioactivity [24], we have selected A. membranacea for this study with the aim to analyze the composition of the EO of A. membranacea collected in Jordan and to evaluate its cytotoxic activity against breast (MCF7), ovary (A2780) and colon (HT29) cancer cell lines, and non-tumorigenic human fetal lung fibroblasts (MRC5 cells). Moreover, its effects on apoptosis and cell cycle effect of the EO on A2780 cells were investigated. Beside the EO, we have tested the cytotoxicity, induction of apoptosis and cell cycle effect of its major constituent, 1,8-cineole, against A2780 cell line, disclosing the possible action mechanism of the EO and its major constituent.
2. Results
2.1. A. membranacea Essential Oil Composition
The complete composition of the essential oil (EO) hydrodistilled from A. membranacea aerial parts is reported in Table 1. A total of 96 compounds were identified, accounting for 92.0% of the total composition, which was chiefly made up of monoterpenes. In particular, over 45% of the total was constituted by oxygenated monoterpenes, of which 1,8-cineole was the most represented: exhibiting a relative abundance of 21.7%, it was also the most abundant compound in the EO. Other quantitatively relevant compounds of this chemical class were borneol and trans-pinocarveol, whose relative concentrations were 4.3% and 3.1%, respectively. Non-terpene derivatives accounted for up to 22.4%, making them the second most abundant detected chemical class of compounds in the EO. Among them, aldehydes prevailed (12.7%), with decanal and (Z)-4-decenal being the most abundant; hydrocarbons followed, accounting for up to 5.0%. Sesquiterpenes were almost equally represented by their hydrocarbon and oxygenated forms (6.7% and 6.9%, respectively). Among the former, α-cadinene was the most represented, while spathulenol was the most abundant of the latter class. Apocarotenes and phenylpropanoids followed, with relative concentrations of 4.2% and 3.9%, respectively.
Table 1.
Complete composition of A. membranacea essential oil (EO).
| Constituents | l.r.i.a | Relative Abundance (%) |
|---|---|---|
| (E)-2-hexenal | 855 | 1.7 |
| 1-hexanol | 868 | 0.4 |
| heptanal | 900 | 0.4 |
| (E,E)-2,4-hexadienal | 910 | tr b |
| α-thujene | 931 | tr |
| α-pinene | 939 | 0.7 |
| camphene | 953 | tr |
| (Z)-2-heptenal | 959 | 0.2 |
| benzaldehyde | 961 | 0.8 |
| 1-heptanol | 969 | tr |
| sabinene | 976 | tr |
| 1-octen-3-ol | 978 | 0.2 |
| 6-methyl-5-hepten-2-one | 985 | 0.7 |
| 2,3-dehydro-1,8-cineole | 991 | 1.3 |
| octanal | 1002 | 1.6 |
| α-phellandrene | 1005 | 0.4 |
| (E,E)-2,4-heptadienal | 1015 | 0.4 |
| α-terpinene | 1018 | 0.2 |
| p-cymene | 1027 | 0.3 |
| 1,8-cineole | 1035 | 21.7 |
| (Z)-β-ocimene | 1041 | tr |
| 3-octen-2-one | 1042 | tr |
| phenyl acetaldehyde | 1045 | 1.5 |
| (E,E)-3,5-octadien-2-one | 1090 | 0.4 |
| linalool | 1099 | 1.1 |
| α-thujone | 1102 | 2.3 |
| cis-p-menth-2-en-1-ol | 1123 | 2 |
| α-campholenal | 1127 | tr |
| cis-p-mentha-2,8-dien-1-ol | 1139 | tr |
| trans-pinocarveol | 1140 | 3.1 |
| camphor | 1145 | 1.6 |
| (E)-2-nonenal | 1162 | 0.7 |
| pinocarvone | 1164 | 1.0 |
| borneol | 1166 | 4.3 |
| 4-terpineol | 1178 | 0.9 |
| p-cymen-8-ol | 1185 | 0.3 |
| cryptone | 1186 | tr |
| α-terpineol | 1190 | 1.4 |
| (Z)-4-decenal | 1194 | 2.2 |
| myrtenal | 1195 | 0.8 |
| safranal | 1200 | 0.4 |
| decanal | 1205 | 2.5 |
| trans-carveol | 1219 | tr |
| β-cyclocitral | 1223 | 0.3 |
| isobornyl formate | 1233 | 0.3 |
| piperitone | 1254 | 2.1 |
| linalyl acetate | 1258 | 0.7 |
| cis-chrysanthenyl acetate | 1263 | tr |
| geranial | 1272 | tr |
| isobornyl acetate | 1286 | 0.3 |
| thymol | 1291 | 0.3 |
| carvacrol | 1300 | 0.4 |
| (E,E)-2,4-decadienal | 1316 | 0.2 |
| methyl decanoate | 1326 | tr |
| hexyl tiglate | 1333 | 0.7 |
| trans-piperitol acetate | 1346 | tr |
| α-terpinyl acetate | 1351 | tr |
| eugenol | 1358 | tr |
| neryl acetate | 1367 | tr |
| α-copaene | 1376 | tr |
| (E)-β-damascenone | 1382 | 0.9 |
| n-tetradecane | 1400 | 0.2 |
| methyl eugenol | 1403 | tr |
| dodecanal | 1408 | tr |
| β-caryophyllene | 1418 | 0.4 |
| (E)-α-ionone | 1428 | 0.3 |
| cabreuva oxide A | 1447 | tr |
| (E)-geranyl acetone | 1454 | 1.7 |
| β-santalene | 1462 | 1.2 |
| cabreuva oxide D | 1480 | 0.8 |
| germacrene D | 1485 | 0.9 |
| (E)-β-ionone | 1485 | 0.6 |
| cis-β-guaiene | 1492 | 0.9 |
| bicyclogermacrene | 1494 | 0.4 |
| n-pentadecane | 1500 | tr |
| tridecanal | 1518 | 0.3 |
| myristicin | 1520 | 3.3 |
| 7-epi-a-selinene | 1522 | 0.3 |
| α-cadinene | 1538 | 2.6 |
| ledol | 1565 | 0.3 |
| trans-nerolidol | 1566 | 0.5 |
| spathulenol | 1576 | 2.7 |
| caryophyllene oxide | 1581 | 0.8 |
| n-hexadecane | 1600 | 0.4 |
| β-oplopenone | 1606 | 0.4 |
| dill apiole | 1621 | 0.6 |
| τ-cadinol | 1641 | 0.4 |
| β-eudesmol | 1649 | 0.7 |
| intermedeol | 1667 | 0.3 |
| n-heptadecane | 1700 | tr |
| pentadecanal | 1717 | 0.2 |
| hexahydrofarnesylacetone | 1845 | tr |
| (3Z)-cembrene A | 1959 | 0.4 |
| linoleic acid ethyl ester | 2160 | 2.3 |
| 1-pentacosene | 2400 | 3.8 |
| n-pentacosane | 2500 | 0.6 |
| Monoterpene hydrocarbons | 1.6 | |
| Oxygenated monoterpenes | 45.9 | |
| Sesquiterpene hydrocarbons | 6.7 | |
| Oxygenated sesquiterpenes | 6.9 | |
| Diterpene hydrocarbons | 0.4 | |
| Apocarotenes | 4.2 | |
| Phenylpropanoids | 3.9 | |
| Other non-terpene derivatives | 22.4 | |
| Total identified (%) | 92.0 | |
a Linear retention indices on a DB-5 capillary column; b Traces, < 0.1%.
2.2. Cytotoxic Activity
The cytotoxic activity of A. membranacea EO against the tested cell lines is reported in Table 2. It induced significant growth inhibition in ovarian (A2780) and colorectal (HT29) cells (IC50 = 12.99 and 14.02 μg/mL, respectively). Negligible toxicity was noticed, however, in treated breast cancer (MCF7) and human fetal lung fibroblast (MRC5) cells (IC50 = 50.86 μg/mL and IC50 = 49.25 μg/mL, respectively). The EO was four times more selective for A2780 and HT29 cell lines compared to MRC5 cells (IC50 = 49.25 μg/mL). The major component, 1,8-cineole, was selected for further cytotoxicity assessments. 1,8-Cineole showed remarkable cytotoxicity compared to A. membranacea EO in A2780 cells (0.26 μM); its selectivity was 42 times higher for A2780 compared to MRC5 cells (10.5 μM). Using the same cell lines, the selectivity profile of 1,8-cineole was better compared to doxorubicin, as the latter showed only a twofold improvement in selectivity (Table 3).
Table 2.
Cytotoxic activity of A. membranacea essential oil against three cancer cell lines and one normal fibroblast (MTT 72 h, IC50 ± SD μg/mL).
| MCF7 | A2780 | HT29 | MRC5 |
|---|---|---|---|
| 50.86 ± 10.14 | 12.99 ± 2.96 | 14.02 ± 4.89 | 49.25 ± 1.27 |
Table 3.
Cytotoxic activity of 1,8-cineole and doxorubicin against A2780 and MRC5 cells (MTT 72 h, IC50 ± SD μM).
| A2780 | MRC5 | |
|---|---|---|
| 1,8-Cineole | 0.26 ± 0.04 | 10.50 ± 1.70 |
| doxorubicin | 0.14 ± 0.02 | 0.21 ± 0.03 |
2.3. Induction of Cell Apoptosis
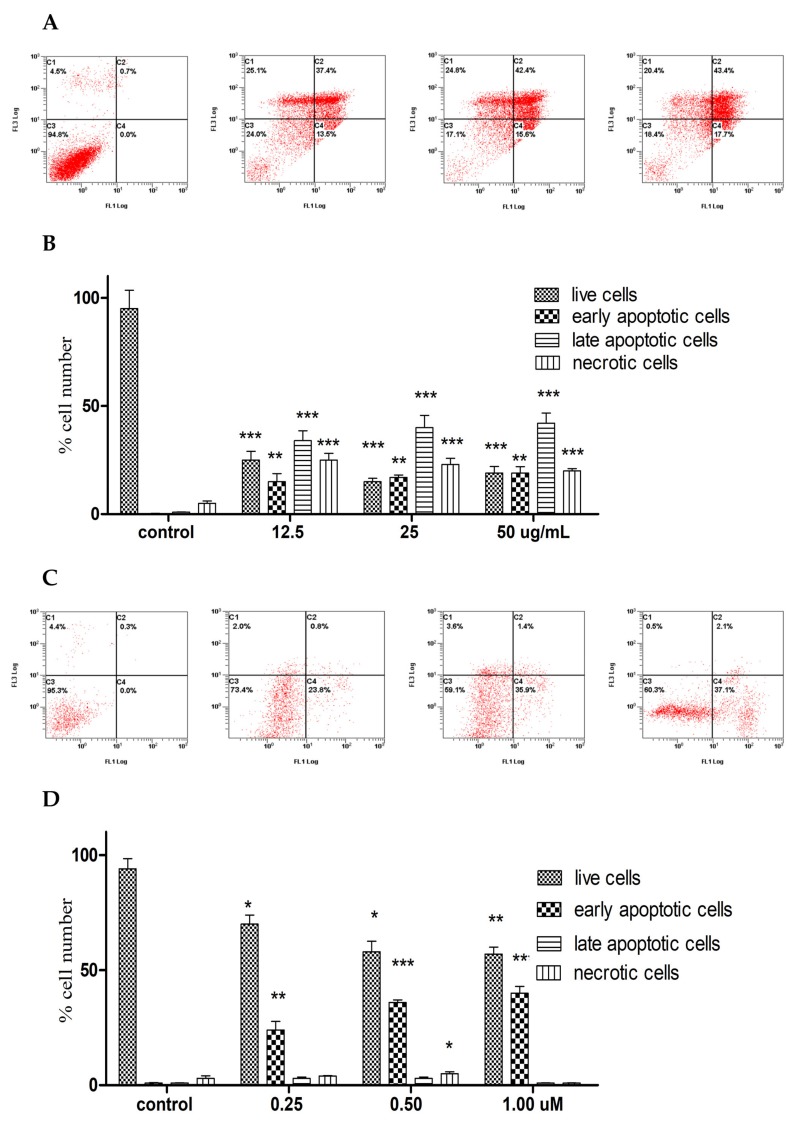
In order to explore the mechanism of action, the induction of apoptosis by A. membranacea EO on A2780 cells was investigated at the same time-point of testing the cytotoxicity (72 h) to confirm its apoptotic vs necrotic properties. The EO showed a dose-dependent increase of combined early and late apoptosis in A2780 cells, although its effect at the 25 and 50 µg/mL was close. The EO caused mainly a late apoptosis compared to early apoptosis in A2780 cells. There was also an increase in the necrotic events from 4% to 20% at the three doses (Figure 1A,B). The situation was inverted when 1,8-cineole was used alone in A2780 cells at the same time-point, as it caused a dose-dependent increase in early apoptosis up to 37% at 1 µM, with only 2% late apoptosis and negligible necrotic effect (Figure 1C,D).
Figure 1.
Detection of early and late apoptosis in A2780 cells (72 h) treated with A. membranacea essential oil (A,B), and 1,8-cineole (C,D). Dot histograms (A,C): x-axis: annexin V, y-axis: propidium iodide. C1: necrotic cells, C2: late apoptotic cells, C3: live cells, C4: early apoptotic cells. Data shown are % mean ± SD (n = 3). All experiments were performed three times. Statistical differences, compared to untreated control cells, were assessed by a one-way ANOVA with the Tukey’s post-hoc multiple comparison test. p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) were taken as significant.
2.4. Perturbation of the Cell Cycle
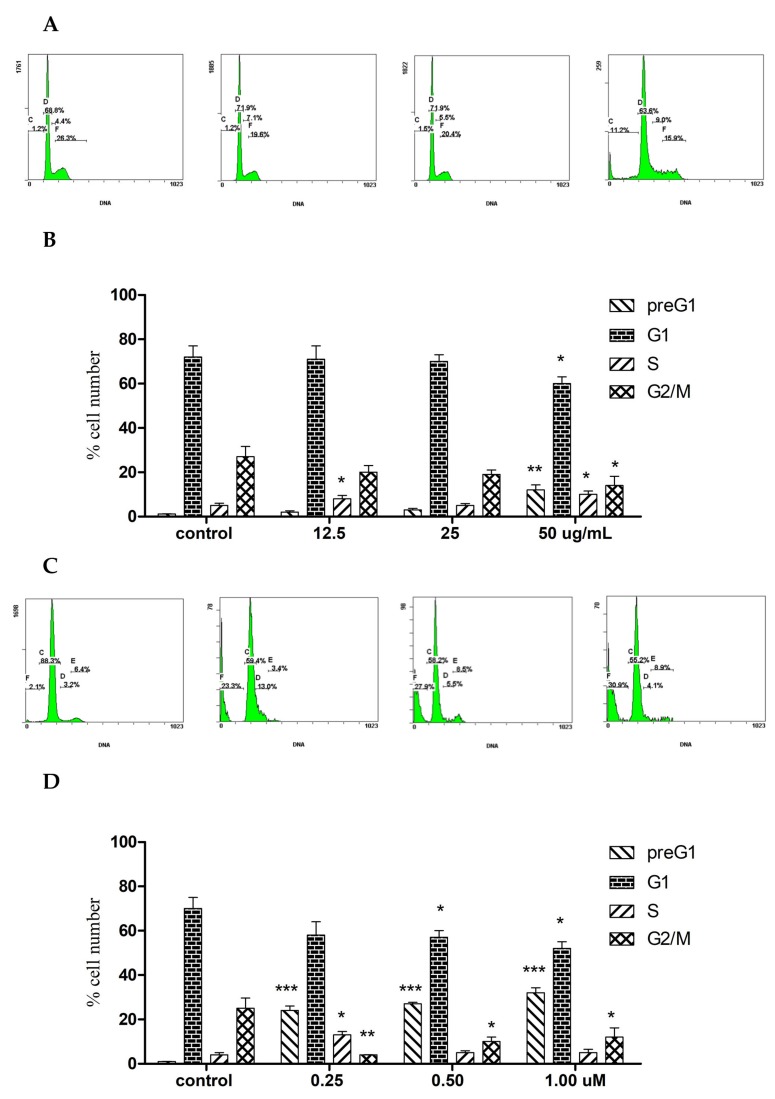
The cell-cycle analysis on A. membranacea EO treated A2780 cells was performed (72 h), showing a non-dose dependent slight S arrest at 12.5 and 50 μg/mL. Moreover, the EO caused a 10% increase in the preG1 at the expense of G1 and G2/M decreased events (Figure 2A,B). 1,8-Cineole also caused S phase arrest only at 12.5 μg/mL, but it induced a 15-20-fold sharp dose dependent increase in the preG1 apoptotic events in A2780 cells (Figure 2C,D).
Figure 2.
Flow cytometry showing the effect of A. membranacea EO (A,B) and 1,8-cineole (C,D) on cell cycle distribution after 72 h treatment in A2780 cells. A, C: X-axis: DNA content of 20,000 events, y axis: % cell number (n = 3, experiments were repeated 3×). Statistical differences, compared to untreated control cells, were assessed by a one-way ANOVA with the Tukey’s post-hoc multiple comparison test. p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) were taken as significant.
3. Discussion
The main constituent of the A. membranacea EO analyzed in this study was 1,8-cineole (over 20% of the total). This monoterpene was also found to be a major constituent of some other Achillea spp., such as A. santolina L. (syn. of Achillea tenuifolia Lam.; 16.7%) [25], A. tomentosaL. (56.1%) [26], A. wilhelmsii (syn. of Achillea santolinoides subsp. wilhelmsii (K.Koch) Greuter), A. thracica Velen. (7.46%–35.72%) [27] and A. millefolium L. (1.2%–19.8%) [28]. Previous studies on 1,8 cineole, or on EOs containing it, reported apoptotic effects on a variety of cancer cell lines, including: SK-MEL-28 (human melanoma); A549 (human lung carcinoma); Colo-205 (Human Caucasian colon adenocarcinoma); (SiHa) cells (human cervical carcinoma); Hep-G2 (hepatocellular carcinoma); MCF-7, T47D, MDA-MB-231 (human breast adenocarcinoma); RKO (Human colon carcinoma); Caco-2 (human Caucasian colon adenocarcinoma); A431 (squamous cell carcinoma); MG-63 (osteosarcoma) and P815 (murine mastocytoma) [29,30,31,32,33,34,35,36,37]. This study has enriched the list of those cancer cell lines susceptible to 1,8-cineole and EO rich in1,8-cineole, and has demonstrated its potent effect on the ovarian cancer cell line (A2780).
In a previous published study, we reported the marginal cytotoxic effect of the methanol extract of the aerial parts of A. membranacea on Jukart cells, with an IC50 value of 60 ± 5.1 μg/mL [15]. That extract, however, did not exert any cytotoxic effect on MCF7 or A2780 cancer cell lines (IC50 > 200 μg/mL) [23]. The growth of A2780 cells in this study was, however, significantly inhibited (IC50 = 12.99 μg/mL) by A. membranacea EO, since the natural product components are generally considered cytotoxic when their IC50 is ≤ 20 μg/mL [38]. The tested EO resulted in the cytotoxicity of the A2780 cell line, while its effect on MCF7was negligible (IC50 = 50.86 μg/mL). In this study, both the EO and 1,8-cineole induced apoptosis in A2780 cells; but 1,8-cineole induced more early apoptosis with minimal late apoptosis and necrosis, compared to a major induction of late apoptosis by the EO at the same time point. The EO also caused 4%–20% necrotic events in A2780 cells compared to control cells, indicating the possible effect of other components. The improved cytotoxic and apoptotic activities of 1,8-cineole compared to A. membranacea indicates the possible involvement of one major mechanism of action in A. membranacea EO, while the substantial difference between the activities of the EO and the methanol extract of the aerial parts could be due to the difference in the constituents of each extract.
Furthermore, the cell cycle perturbation results of the present work were similar to those observed in a previous study conducted on wild-type p53 H460 cells (but not mutant p53 H460 cells) after the treatment with Achillea millefolium hydroalcoholic extract [39]. Pereira et al. (2018) demonstrated the induction of apoptosis and S-phase arrest, with an increased level of p53 and p21 indicating the different mechanisms involved in the Achillea millefolium extract-induced growth arrest. Likewise, a previous study reported that casticin, a flavonoid extracted from Achillea millefolium, caused G2/M arrest in HCT116 colon cancer cells, which was confirmed by the induction of p21 and down-regulation of CDK1[14]. However, 1,8 cineole was reported to induce cell cycle arrest in the G2/M phase in the HCT116 cell line [30], while the studied A. membranacea EO and 1,8 cineole induced preG1 increase, which indicate DNA fragmentation and confirm the pro-apoptotic effect in A2780 cells. Further mechanistic studies of the activities of A. membranacea components and 1,8 cineole against an array of cancer cell lines are worthwhile, as well as in vivo studies, which will provide more support for their application.
4. Materials and Methods
4.1. Plant Material
The aerial parts of A. membranacea were collected during the spring in the Dab’a desert reserve (50 km South of Amman), Jordan (GPS coordinates: 31° 31’ 58” N, 36° 01’ 20” E). The plant was identified by Prof. A. Bader. A voucher specimen was deposited in the herbarium of the Pharmacognosy Lab (Number Jo-It 2018/2), at Umm Al-Qura University, Makkah, Saudi Arabia.
4.2. Hydrodistillation of the Essential Oil
The hydrodistillation of A. membranacea was performed with a Clevenger-type apparatus equipped with an electric mantle heater (Tecnovetro, Pisa, Italy). A sample (100 g) of air-dried material was hydrodistilled for 3 h. The extraction yield was 0.09 % w/w.
4.3. Gas Chromatography–Mass Spectrometry Analyses and Compounds Identification
The hydrodistilled EO of A. membranacea was diluted to 5% in HPLC-grade n-hexane and then injected into a Varian CP-3800 GC-MS apparatus (GC-EI-MS, Varian, Inc. Palo Alto, CA, USA) equipped with a DB-5 capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and a Varian Saturn 2000 ion trap mass detector (Varian, Inc. Palo Alto, CA, USA). Analytical conditions were as previously reported [40]. The identification of the constituents was based on the comparison of the retention times with those of authentic samples, comparing their linear retention indices relative to the series of n-hydrocarbons, commercial libraries such as NIST 14 and ADAMS and a homemade mass-spectral library of pure compounds were used to compare the mass spectra [41,42].
4.4. Chemicals and Reagents
1,8-Cineole was kindly gifted by Mr. Mohammed Khair Al Halabi, United Tetra Group for Medical and Scientific Supplies, Jordan. Doxorubicin and all reagents were purchased from Sigma–Aldrich, St. Louis, MO, USA.
4.5. Cell Culture
Three human cancerous (MCF7, A2780 and HT29) and one fibroblast (MRC5) cell lines were used in this study. Cancer cells were maintained in RPMI-1640, and MRC5 cells were maintained in EMEM media. All media were supplemented with FBS (10%), sodium pyruvate (1 mM), L-glutamine (2 mM) and penicillin/streptomycin (1%). Cell cultures were kept on 5% CO2 in humidified incubator at 37 °C.
4.6. Cytotoxicity Assay
Cells were seeded in 96-well plates and exposed to the essential oil of A. membranacea at concentrations up to 100 μg/mL for 72 h. 1,8-cineole and doxorubicin concentrations were made up to 50 µM also for 72 h. MTT assay was then performed as previously reported [43]. Absorbances were measured using a multi-plate reader at a wavelength 550 nm. IC50 values were determined by calculated the oil concentration-induced 50% reduction in the absorbance compared to untreated control.
4.7. Induction of Apoptosis Assay
The induction of apoptosis activity of A. membranacea EO and 1,8-cineole was investigated by annexin V FITC/Propidium iodide protocol as described in the literature [44]. Briefly, A2780 cells were seeded at 1 × 105 cells/well in 6-well plate overnight before treatment with either A. membranacea EO or 1,8-cineole at four concentrations, ranging from 0 to 50 μg/mL, or 0–1 µM, respectively (representing IC50: 0, ×1, ×2 and ×4). Following treatment, cells were collected (including the supernatant), washed with ice-cold PBS and then incubated for 2 min in binding buffer (100 µL) and annexin V FITC (10 μL), at room temperature in the dark. Additional 400 μL of binding buffer and 10 μL PI were then added. The early apoptotic, late apoptotic, and necrotic cell populations were analyzed by flow cytometry (FC500, Beckman Coulter, Brea, CA, USA), based on 20,000 events per sample.
4.8. Perturbation of the Cell Cycle
A2780 cells were seeded at 1 × 105 cells/well in 6-wellplate overnight before being treated with either A. membranacea or 1,8-cineole essential oils at four concentrations ranged from 0 to 50 μg/mL, or 0 to 1 µM, respectively. Cells were then suspended by trypsin-EDTA and spun at 200× g for 5 min. Collected cell pellets were washed in ice-cold PBS before they were fixed in 70% ice-cold ethanol overnight. The cell cycle analysis was then performed and analyzed for 20,000 events per sample, using flow cytometry (FC500, BC, USA), following the protocol described elsewhere [45,46].
4.9. Statistical Analysis
Statistical differences of samples, compared to untreated control cells, were assessed by a one-way ANOVA with the Tukey’s post-hoc multiple comparison test (GraphPad Prism Version 5, GraphPad Software, San Diego, CA, USA). p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) were taken as significant.
Author Contributions
Conceptualization, A.B.; methodology, A.N.A., G.F., R.A. and A.B.; software, R.A.; validation, Q.M.A.A. and U.S.; formal analysis, G.F., R.A.; investigation, A.N.A., Q.M.A.A, G.F. and R.A.; resources, U.S; data curation, M.I.S.A.; writing—original draft preparation, A.N.A, G.F., R.A. and A.B.,; writing—review and editing, G.F., R.A. and M.M.B.; supervision, A.B.; project administration, A.B.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH) King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, award number (12-MED2310-10) through Science and Technology Unit (STU) at Umm Al-Qura University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Sample Availability: The starting plant material is available from the authors.
References
- 1.Kwan Y.P., Saito T., Ibrahim D., Al-Hassan F.M., Ein Oon C., Chen Y., Jothy S.L., Kanwar J.R., Sasidharan S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2016;54:1223–1236. doi: 10.3109/13880209.2015.1064451. [DOI] [PubMed] [Google Scholar]
- 2.Iannuzzi A.M., Camero C.M., D’Ambola M., D’Angelo V., Amira S., Bader A., Braca A., De Tommasi N., Germanò M.P. Antiangiogenic Iridoids from Stachys ocymastrum and Premna resinosa. Planta Med. 2019;85:1034–1039. doi: 10.1055/a-0889-0412. [DOI] [PubMed] [Google Scholar]
- 3.Marsik P., Kokoska L., Landa P., Nepovim A., Soudek P., Vanek T. In vitro inhibitory effects of thymol and quinones of Nigella sativa seeds on cyclooxygenase-1- and -2-catalyzed prostaglandin E2 biosynthese. Planta Med. 2005;71:739–742. doi: 10.1055/s-2005-871288. [DOI] [PubMed] [Google Scholar]
- 4.Zengin G., Sarıkürkçü C., Aktümsek A., Ceylan R. Antioxidant potential and inhibition of key enzymes linked to Alzheimer’s diseases and diabetes mellitus by monoterpene-rich essential oil from Sideritis galatica Bornm. Endemic to Turkey. Rec. Nat. Prod. 2015;10:195–206. [Google Scholar]
- 5.De Andrade T.U., Brasil G.A., Endringer D.C., Da Nóbrega F.R., De Sousa D.P. Cardiovascular activity of the chemical constituents of essential oils. Molecules. 2017;22:1539. doi: 10.3390/molecules22091539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugier D., Sugier P., Jakubowicz-Gil J., Winiarczyk K., Kowalski R. Essential oil from Arnica montana L. Achenes: Chemical characteristics and anticancer activity. Molecules. 2019;24:4158. doi: 10.3390/molecules24224158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attallah O.A., Shetta A., Elshishiny F., Mamdouh W. Essential oil loaded pectin/chitosan nanoparticles preparation and optimization: Via Box-Behnken design against MCF-7 breast cancer cell lines. RSC Adv. 2020;10:8703–8708. doi: 10.1039/C9RA10204C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joyce Nirmala M., Durai L., Gopakumar V., Nagarajan R. Anticancer and antibacterial effects of a clove bud essential oil-based nanoscale emulsion system. Int. J. Nanomed. 2019;14:6439–6450. doi: 10.2147/IJN.S211047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth E., Bernath J. Biological Activities of Yarrow Species (Achillea spp.) Curr. Pharm. Des. 2008;14:3151–3167. doi: 10.2174/138161208786404281. [DOI] [PubMed] [Google Scholar]
- 10.Jarić S., Popović Z., Mačukanović-Jocić M., Djurdjević L., Mijatović M., Karadžić B., Mitrović M., Pavlović P. An ethnobotanical study on the usage of wild medicinal herbs from Kopaonik Mountain (Central Serbia) J. Ethnopharmacol. 2007;111:160–175. doi: 10.1016/j.jep.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Kültür Ş. Medicinal plants used in Kırklareli Province (Turkey) J. Ethnopharmacol. 2007;111:341–364. doi: 10.1016/j.jep.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Aburjai T., Hudaib M., Tayyem R., Yousef M., Qishawi M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007;110:294–304. doi: 10.1016/j.jep.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Lev E. Reconstructed materia medica of the Medieval and Ottoman al-Sham. J. Ethnopharmacol. 2002;80:167–179. doi: 10.1016/S0378-8741(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 14.Al-Eisawi D.M. List of Jordan Vascular Plants. Mitt. Bot. Staatssamml. München. 1982;18:79–182. [Google Scholar]
- 15.Arabaci T., Yildiz B. Taxonomy and threatened categories of three Achillea L. (Asteraceae-Anthemideae) Species Previously cited in the Data Deficient (DD) category. Turk. J. Bot. 2008;32:311–317. [Google Scholar]
- 16.Bader A., Panizzi L., Cioni P., Flamini G. Achillea ligustica: Composition and antimicrobial activity of essential oils from the leaves, flowers and some pure constituents. Open Life Sci. 2007;2:206–212. doi: 10.2478/s11535-007-0020-3. [DOI] [Google Scholar]
- 17.Özek T., Tabanca N., Demirci F., Wedge D.E., Başer H.C. Enantiomeric distribution of some linalool containing essential oils and their biological activities. Rec. Nat. Prod. 2010;4:180–192. [Google Scholar]
- 18.Çakır A., Özer H., Aydın T., Kordali Ş., Çavuşoglu A.T., Akçin T., Mete T.E., Akçin A. Phytotoxic and insecticidal properties of essential oils and extracts of four Achillea species. Rec. Nat. Prod. 2015;10:154–167. [Google Scholar]
- 19.Chagas-Paula D., Oliveira T., Faleiro D., Oliveira R., Da Costa F. Outstanding Anti-inflammatory Potential of Selected Asteraceae Species through the Potent Dual Inhibition of Cyclooxygenase-1 and 5-Lipoxygenase. Planta Med. 2015;81:1296–1307. doi: 10.1055/s-0035-1546206. [DOI] [PubMed] [Google Scholar]
- 20.Caliskan U.K., Aka C., Oz M.G. Plants used in Anatolian traditional medicine for the treatment of hemorrhoid. Rec. Nat. Prod. 2017;11:235–250. [Google Scholar]
- 21.Trifunović S., Isaković A., Isaković A., Vučković I., Mandić B., Novaković M., Vajs V., Milosavljević S., Trajković V. Isolation, Characterization, and In Vitro Cytotoxicity of New Sesquiterpenoids from Achillea clavennae. Planta Med. 2014;80:297–305. doi: 10.1055/s-0033-1360312. [DOI] [PubMed] [Google Scholar]
- 22.Haïdara K., Zamir L., Shi Q.-W., Batist G. The flavonoid Casticin has multiple mechanisms of tumor cytotoxicity action. Cancer Lett. 2006;242:180–190. doi: 10.1016/j.canlet.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Bader A., Abdallah Q., Abdelhady M., De Tommasi N., Malafronte N., Shaheen U., Bkhaitan M., Cotugno R. Cytotoxicity of Some Plants of the Asteraceae Family: Antiproliferative Activity of Psiadia punctulata Root Sesquiterpenes. Rec. Nat. Prod. 2019;13:307–315. doi: 10.25135/rnp.113.18.10.969. [DOI] [Google Scholar]
- 24.Abdallah Q., Al-Deeb I., Bader A., Hamam F., Saleh K., Abdulmajid A. Anti-angiogenic activity of Middle East medicinal plants of the Lamiaceae family. Mol. Med. Rep. 2018;18:2441–2448. doi: 10.3892/mmr.2018.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bader A., Flamini G., Cioni P.L., Morelli I. Essential oil composition of Achillea santolina L. and Achillea biebersteinii Afan. collected in Jordan. Flavour Fragr. J. 2003;18:36–38. doi: 10.1002/ffj.1147. [DOI] [Google Scholar]
- 26.Chizzola R. Composition of the Essential Oil from the Flower Heads of Achillea tomentosa L. J. Essent. Oil Bear. Plants. 2018;21:535–539. doi: 10.1080/0972060X.2017.1418682. [DOI] [Google Scholar]
- 27.Yordanova Z.P., Rogova M.A., Zhiponova M.K., Georgiev M.I., Kapchina-Toteva V.M. Comparative determination of the essential oil composition in Bulgarian endemic plant Achillea thracica Velen. during the process of ex situ conservation. Phytochem. Lett. 2017;20:456–461. doi: 10.1016/j.phytol.2017.03.011. [DOI] [Google Scholar]
- 28.Farajpour M., Ebrahimi M., Baghizadeh A., Aalifar M. Phytochemical and Yield Variation among Iranian Achillea millefolium Accessions. HortScience. 2017;52:827–830. doi: 10.21273/HORTSCI11654-16. [DOI] [Google Scholar]
- 29.Darmanin S., Wismayer P.S., Camilleri Podesta M.T., Micallef M.J., Buhagiar J.A. An extract from Ricinus communis L. leaves possesses cytotoxic properties and induces apoptosis in SK-MEL-28 human melanoma cells. Nat. Prod. Res. 2009;23:561–571. doi: 10.1080/14786410802228579. [DOI] [PubMed] [Google Scholar]
- 30.Murata S., Shiragami R., Kosugi C., Tezuka T., Yamazaki M., Hirano A., Yoshimura Y., Suzuki M., Shuto K., Ohkohchi N., et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013;30:2647–2652. doi: 10.3892/or.2013.2763. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Dahab R., Kasabri V., Afifi F.U. Evaluation of the volatile oil composition and antiproliferative activity of Laurus nobilis L. (Lauraceae) on breast cancer cell line models. Rec. Nat. Prod. 2014;8:136–147. [Google Scholar]
- 32.Duymuş H.G., Çiftçi G.A., Yıldırım Ş.U., Demirci B., Kırımer N. The cytotoxic activity of Vitex agnus castus L. essential oils and their biochemical mechanisms. Ind. Crops Prod. 2014;55:33–42. doi: 10.1016/j.indcrop.2014.01.041. [DOI] [Google Scholar]
- 33.Kumar D., Sukapaka M., Babu G.D.K., Padwad Y. Chemical Composition and In Vitro Cytotoxicity of Essential Oils from Leaves and Flowers of Callistemon citrinus from Western Himalayas. PLoS ONE. 2015;10:e0133823. doi: 10.1371/journal.pone.0133823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharifi-Rad J., Ayatollahi S.A., Varoni E.M., Salehi B., Kobarfard F., Sharifi-Rad M., Iriti M., Sharifi-Rad M. Chemical composition and functional properties of essential oils from Nepeta schiraziana Boiss. Farmacia. 2017;65:802–812. [Google Scholar]
- 35.Tundis R., Iacopetta D., Sinicropi M.S., Bonesi M., Leporini M., Passalacqua N.G., Ceramella J., Menichini F., Loizzo M.R. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae) Food Chem. Toxicol. 2017;106:155–164. doi: 10.1016/j.fct.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 36.Sampath S., Veeramani V., Krishnakumar G.S., Sivalingam U., Madurai S.L., Chellan R. Evaluation of in vitro anticancer activity of 1,8-Cineole–containing n-hexane extract of Callistemon citrinus (Curtis) Skeels plant and its apoptotic potential. Biomed. Pharmacother. 2017;93:296–307. doi: 10.1016/j.biopha.2017.06.056. [DOI] [PubMed] [Google Scholar]
- 37.Harassi Y., Tilaoui M., Idir A., Frédéric J., Baudino S., Ajouaoi S., Mouse H.A., Zyad A. Phytochemical analysis, cytotoxic and antioxidant activities of Myrtus communis essential oil from Morocco. J. Complement. Integr. Med. 2019;16:20180100. doi: 10.1515/jcim-2018-0100. [DOI] [PubMed] [Google Scholar]
- 38.Lee C.C., Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J. Ethnopharmacol. 2005;100:237–243. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 39.Pereira J.M., Peixoto V., Teixeira A., Sousa D., Barros L., Ferreira I.C.F.R., Vasconcelos M.H. Achillea millefolium L. hydroethanolic extract inhibits growth of human tumor cell lines by interfering with cell cycle and inducing apoptosis. Food Chem. Toxicol. 2018;118:635–644. doi: 10.1016/j.fct.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Giuliani C., Ascrizzi R., Tani C., Bottoni M., Maleci Bini L., Flamini G., Fico G. Salvia uliginosa Benth.: Glandular trichomes as bio-factories of volatiles and essential oil. Flora. 2017;233:12–21. doi: 10.1016/j.flora.2017.05.002. [DOI] [Google Scholar]
- 41.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing Corporation; Carol Stream, IL, USA: 1995. [Google Scholar]
- 42.Davies N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20M phases. J. Chromatogr. A. 1990;503:1–24. doi: 10.1016/S0021-9673(01)81487-4. [DOI] [Google Scholar]
- 43.Chahrour O., Abdalla A., Lam F., Midgley C., Wang S. Synthesis and biological evaluation of benzyl styrylsulfonyl derivatives as potent anticancer mitotic inhibitors. Bioorg. Med. Chem. Lett. 2011;21:3066–3069. doi: 10.1016/j.bmcl.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 44.Gouda A.M., Abdelazeem A.H., Abdalla A.N., Ahmed M. Pyrrolizine-5-carboxamides: Exploring the impact of various substituents on anti-inflammatory and anticancer activities. Acta Pharm. 2019;68:251–273. doi: 10.2478/acph-2018-0026. [DOI] [PubMed] [Google Scholar]
- 45.Shaheen U., Ragab E.A., Abdalla A.N., Bader A. Triterpenoidal saponins from the fruits of Gleditsia caspica with proapoptotic properties. Phytochemistry. 2018;145:168–178. doi: 10.1016/j.phytochem.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Attalah K.M., Abdalla A.N., Aslam A., Ahmed M., Abourehab M.A.S., ElSawy N.A., Gouda A.M. Ethyl benzoate bearing pyrrolizine/indolizine moieties: Design, synthesis and biological evaluation of anti-inflammatory and cytotoxic activities. Bioorg. Chem. 2020;94:103371. doi: 10.1016/j.bioorg.2019.103371. [DOI] [PubMed] [Google Scholar]