Abstract
Using a photothrombotic mouse model of single stroke, we show that a single stroke onset increases the nuclear factor-κB (NF-κB), NLR family CARD domain containing protein 4 (NLRC4), and absent in melanoma 2 (AIM2) inflammasomes, as well as the mRNA levels of NLRP3. Next, using a photothrombotic mouse model of recurrent stroke, we found that recurrent strokes increased the activation of NLRP3, exacerbated the brain damage and the pro-inflammatory response in wild type (WT) mice, but not in NLRP3 knockout (NLRP3 KO) mice. Additionally, we found that apoptosis-associated speck-like protein containing a CARD (ASC) protein level surrounding the infarct area was comparatively increased, but that ASC specks outside of microglia in both the ipsilateral and contralateral of stroke site were decreased in NLRP3 KO mice relative to wild-type (WT) controls, and the number of ASC specks surrounding the second infarct area was positively correlated to the damage scores. Mechanistically, we found that recombinant ASC (RecASC) activated NLRP3 and induced pro-inflammatory responses, exacerbating the outcome of ischemic stroke, in WT mice, but not in NLRP3 KO mice. We therefore conclude that the NLRP3 inflammasome is activated by two attacks of stroke, which act together with ASC to exacerbate recurrent strokes.
Keywords: NLRP3, ASC, recurrent stroke, inflammasome, inflammation
Introduction
Stroke is the most common factor leading to death other than cardiovascular disease.1 Recurrent strokes account for almost 30% of all strokes. Importantly, recurrence significantly increases morbidity and mortality in stroke patients. Although the recurrence of stroke is multifactorial, inflammation is an important, common process in the pathophysiology of recurrent stroke. Recently, interleukin-1β (IL-1β), an inflammatory cytokine, has received much attention in the field of cardiovascular disease.2 Remarkably, inhibition of IL-1β reduces recurrent cardiovascular events including stroke,3 suggesting a prominent role for IL-1β in recurrent stroke. The activity of IL-1β is controlled by inflammasomes. Upon stimulation, inflammasomes undergo oligomerization, recruiting apoptosis-associated speck-like protein containing a CARD (ASC), triggering ASC assembly into fibrils and large ASC speck.4,5 This further facilitates the processing of caspase-1, as well as the secretion of pro-inflammatory cytokines including IL-1β.6 Inflammasome activity also results in the release of ASC specks, once released into the intercellular space, ASC specks can be taken up by neighbouring inflammatory cells to sustain the ongoing immune response.7 Inflammasomes are key elements in the innate immune system, which defends against infection. However, inappropriate activation of inflammasomes can be harmful to the human body under some conditions, due to the uncontrolled induction of inflammatory responses. NLRP3 (also known as cryopyrin or NLRP3) is the most extensively studied inflammasome protein,8 and NLRP3/ASC is reported to be involved in ischemic stroke. Activation of NLRP3/ASC has been shown to enlarge the infarct area; however, studies of the inflammasome in the pathogenesis of stroke have so far been controversial.
In the present study, we first examined ASC and NLRP3 activation in a photothrombotic model of single stroke. We found that after stroke, NLRP3 was primed, ASC was released from the affected cells, which was detected in the contralateral area, without affecting microglia and NLRP3 activation, and NLRP3 deficiency did not affect the outcome of single upset of stroke. Furthermore, brain damage and ASC aggregation were much more severe in the second stroke than in the first stroke in wild-type (WT) animals. Remarkably, this aggregation was abolished in NLRP3 KO mice relative to WT controls. In addition, intracerebroventricular injection of recombinant ASC (RecASC) exacerbated the injury and inflammasome activation in WT animals, but not in NLRP3 KO mice. These results demonstrate that ASC plays a predominant role in recurrent stroke, and that it acts in an NLRP3-dependent manner.
Methods
Animals
The study was conducted in accordance with National Institutes of Health guidelines and was approved by the Animal Research Committee of the First Affiliated Hospital of Sun Yat-sen University (Guangzhou, China; committee's reference number: [2013]97). All efforts were made to minimize the number and suffering of animals used in this study. This study used 84 wild type (WT) and 72 NLPR3 KO mice. NLRP3 KO mice were founded on a C57BL/6J background. WT mice were obtained from the Animal Center of Sun Yat-sen University, and NLPR3 KO mice were obtained from the Jackson laboratory (B6.129S6-Nlrp3tm1Bhk/J, Catalog number: 021302) and bred in the Laboratory Animal Monitoring Institute of Guangdong Province. All the animals were used at six to eight weeks of age and were housed under a 12:12 h light: dark cycle (light on from 07:00 to 19:00 h), with controlled temperature and humidity, and given food and water ad libitum. Animal experiments were carried out and reported in accordance with the ARRIVE guidelines.
Blood flow measurements
For in vivo imaging of blood flow dynamics, two-photon imaging was used. 9,10 Briefly, FITC-dextran (70,000 molecular weight [MW], Sigma-Aldrich, Darmstadt, Germany) was injected into mice through the tail, two-photon imaging was performed with a custom built two-photon laser scanning microscope (Leica) at a wavelength of 820 nm and a laser scanning system (Coherent, Santa Clara, CA,USA) equipped with a water immersion objective (10×, 25×). The pictures were firstly captured with 512 × 512 xyz stacks, then six arteries (diameter 15–20 μm) were selected and captured with xy slice, and the velocity was calculated by dX/dT of each RBC (Figure 1(a) and (b)).11
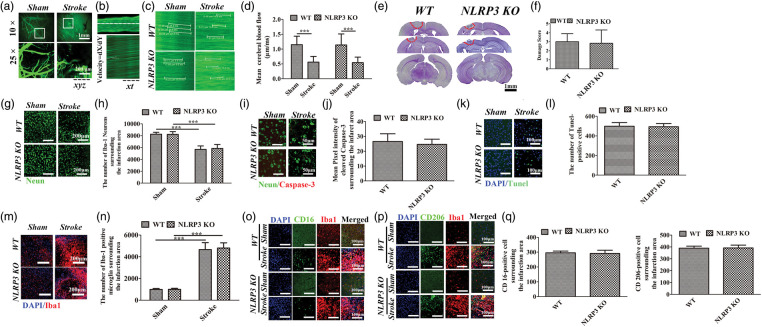
Figure 1.
Histological analysis of WT and NLRP3 KO mice following a single left photothrombotic stroke. (a) Imaging and XYZ scanning of cerebrovascular in cortex under two-photon microscope. (Above, 10 × , White box indicated the area zoomed in below, white dotted line indicated the occluded boundary; Below. 25 × , magnification field indicated in the above boxes). (b) xt scanning of target vessel using the two-photon imaging and equation to calculate the velocity. (c) Representative XT imaging in the sham and stroke animals. (d) Comparisons of blood flow between sham and stroke animals. (e) Nissl staining of coronal brain slices, red circle marks the damage area. (f) Comparisons of damage scores between WT and NLRP3 KO mice. (g–j) Immunofluorescence staining and comparison of Neurons and Cleaved caspase-3 between WT and NLRP3 KO mice surrounding the damage area. (k–l) TUNEL staining and comparison of Tunel-positive cells between WT and NLRP3 KO mice surrounding the damage area. (m–q) Immunofluorescence staining and comparisons of overall Iba-1-positive, CD-16 positive and CD-206 positive microglia in WT and NLRP3 KO mice surrounding the damage area. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 6.
Mouse model of single or recurrent stroke
We induced a single unilateral stroke on the left parietal cortex, according to methods used in a previous study.12 Briefly, mice were anesthetized with isoflurane and placed in a stereotaxic frame, with the left parietal cortex (2 mm lateral to the midline and 1.7 mm ahead of the lambdoid13) chosen as the area of interest. Rose Bengal (10 μl/g body weight) was injected into the tail vein so that the cerebral vessel could be seen clearly under the two-photon microscope. The cold light illuminator was switched on (fiber optic illuminator of 150 W intensity). After 15-min illumination, light exposure was stopped, and the wound was sutured. Mice were returned to their home cage after regaining consciousness. For mice undergoing a single stroke, animals were killed and histological analysis was performed one week later. For the mouse model of recurrent stroke, animals received the same surgery as described previously on the right parietal cortex one or two weeks later.
Purification of recombinant ASC protein from E. coli
We purified, refolded, and assembled ASC protein using recombinant ASC (RecASC) expressed in Escherichia coli as described. 14 Briefly pET-28-ASC plasmid was constructed and transformed in DH5a E. coli cells. Transformed E. coli cells were incubate overnight with shaking at 37℃, and expression was induced at an OD600 of 0.8 by 1 mM isopropyl β-D-1-thiogalactopyranoside. The cells were collected by centrifugation and sonicated in a buffer containing 20 mM Tris (pH 8.0), 500 mM NaCl and 5 mM imidazole. Cell lysate was centrifuged and resuspended in buffer A supplemented with 2 M guanidine-HCl centrifuged, and the supernatant was dialysed against buffer A . The sample was centrifuged and the supernatant was administered to a pre-equilibrated HisTrap column. The column was washed with Tris (pH 8.0), 500 mM NaCl and 20 mM imidazole, and protein was eluted in the same buffer containing 200 mM imidazole. The purified protein was dialysed against a buffer containing 20 mM Tris (pH 8.0) and 300 mM NaCl.
Intracerebroventricular injection of recombinant ASC
Mice were anesthetized with isoflurane and placed in a stereotaxic frame, and 5 μl recombinant ASC was injected intracerebroventricularly (1.5 mm behind bregma, 1 mm lateral to the midline, 2.5–3 mm from the dura).15
Histology
For histological analysis, 36 WT and 36 NLRP3 KO mice (n = 6/group) were used for histological analyses including Nissl staining, immunofluorescence staining or immunochemistry staining. Mice were perfused with 50 ml ice-cold saline, followed by 50 ml 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4). Brains were removed and incubated overnight in 4% paraformaldehyde, and dehydrated in 20–30% sucrose in PBS. Coronal brain slices (40 μm of thick) from the right parietal cortices were sectioned using a frozen microtome (Leica) at intervals of 100 μm to produce consecutive frozen sections.
For Nissl staining, sections from blocks proximal to the parietal cortex as well as more distal blocks containing hippocampus were stained with cresyl violet and inspected microscopically to detect the infarct. A cumulative score for brain injury at each level was given by an investigator blinded to animal identity using a scoring system similar to that described previously,16 with the cortical thickness divided into five areas. Without the effect of edema, which occurs at an early stage after photothrombotic cerebral infarction and returns to normal values by day 7,17 we obtained a cumulative score. The injury score was assigned to each region as follows: 0 for normal; 1 for <10% neuronal injury; 2 for 10–50% neuronal injury; 3 for >50% neuronal injury; 4 for confluent areas of pan-necrosis; and 5 for combined hippocampal injury.
For immunofluorescence staining, sections were boiled in citric acid buffer (pH 6.0) for 5 min in a microwave oven. After the sections were cooled, they were treated with 0.3% Triton X-100 and 10% goat serum for 1 h at room temperature. The sections were then incubated overnight at 4℃ with a primary antibody (1:100 Anti-Neun, Catalog number MAB377, Millipore, USA; 1:100 Anti-Cleaved caspase-3, Catalog number 9575S, Cell signaling technology, USA; 1:400 anti-ionized calcium binding adapter molecule 1 (IBA1) antibody, catalog number 019-19741, Wako, Japan; 1:200 anti-CD206, catalog number MA5-16868, Invitrogen, USA; 1:200 anti-CD16, catalog number ab25235, abcam, USA; 1:200 NLRP3, MA5-23919, ThermoFisher, USA; 1:100 anti-ASC, catalog number NBP1-78978, Novus Biologicals, USA), and then with a secondary antibody (1:300 AlexaFluor-488-conjugated goat anti-mouse IgG2a antibody, Life Technologies, catalog number A21131; 1:300 Alexa-Fluor-555-conjugated goat anti-rabbit IgG1 antibody, Life Technologies, catalog number A31572) in PBS containing 10% normal goat serum at room temperature for 1 h. Apoptosis was detected using a TUNEL Kit (TUNEL Apoptosis Detection Kit, catalog number 11684817910, Roche, China) according to the manufacturer's protocol. The sections were mounted onto slides, embedded with SlowFader Gold (Invitrogen), and covered with a coverslip.
Western blotting
For Western blotting, 36 WT and 36 NLRP3 KO mice (n = 6/group) were used; 20 μg protein in each lane was subjected to SDS-PAGE using 4–12% precast polyacrylamide gels (Novex, Invitrogen) at 200 V for 45 min. Proteins were transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA) at 100 V for 2 h. Membranes were incubated with 5% BSA for 1 h and incubated with primary antibodies overnight at 4℃, followed by anti-rabbit or anti-mouse immunoglobulin G secondary antibody (1:2000; Cell Signaling) incubation for 1 h in a dark room.
qPCR analysis
An additional 12 WT mice was used to detect the mRNA level of NLRP3. RNA extraction was performed using miRNeasy Extraction Kit (Qiagen) according to the manufacturer's instructions. Real-time qPCR analysis was performed using SYBR Green (BioRad). RT-qPCR primers for NLRP3 were provided by Beijing Genomics institute as follows, forward: 5′-ACTTGCAGAAGCTGGGGTTG-3′, reverse: 5′-AGTT TACAGTCCGGGTGCAG-3′.
Data and statistical analyses
ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to analyze the Nissl staining, immunohistochemical and Western blotting results. Independent t-tests were performed for comparisons between two samples. One-way or two-way repeated measures ANOVA with a Bonferroni's post hoc test for multiple comparisons were used to analyze all other data. A P value <0.05 was considered statistically significant, with SPSS or GraphPad used for statistical calculations (SPSS 19.0 software, Armonk, NY, USA; Prism 6, GraphPad, La Jolla, CA, USA). Data are expressed as the mean ± standard deviation of the means (SD).
Results
NLRP3 does not affect the damage score in a mouse model of single stroke
A photothrombotic model of brain ischemia was used to investigate the role of the NLRP3 inflammasome in a single-stroke model. We measured the cerebral blood flow (CBF) surrounding the occluded site (Figure 1(a) and (b)), which was comparably decreased in WT and NLRP3 KO mice compared with the sham animals (P < 0.001) (Figure 1(c) and (d)). Photo-activation of Rose Bengal induced a visible cortical lesion (Figure 1(e)). The damage score was comparable between WT and NLRP3 KO mice (Figure 1(f), P > 0.05). Furthermore, the numbers of neurons were comparably decreased surrounding the infarct area in WT and NLRP3 KO mice compared with sham groups without stroke (P < 0.001, Figure 1(g) and (h)). There was no visible cleaved caspase-3 expressed in sham animals, whereas which can be detected in WT and NLRP3 KO mice surrounding the infarct area, the mean pixel intensity showed no significant difference (Figure 1(i) and (j)). The numbers of TUNEL-positive cells (Figure 1(k) and (l), P > 0.05) and Iba-1 positive microglia around the infarction area were comparably increased in WT and NLRP3 KO mice compared with the sham controls (Figure 1(m) and (n), P > 0.05), as well as the CD-16 positive M1 microglia and CD-206 positive M2 microglia (Figure 1(o) to (q)).
NLRP3 knockout significantly reduces recurrent infarction
To explore the activation of the NLRP3/ASC inflammasome in recurrent ischemic stroke, we performed the second photothrombotic stroke one week or two weeks following the first stroke, which is termed a bilateral stroke model (B-Stroke). As shown in Figure 2(a) and (b), the CBF surrounding the occluded site was significantly decreased in WT and NLRP3 KO mice compared with the sham groups (P < 0.001), whereas there were no significantly differences between the second stroke (right) and first stroke (left) sites at interval of one or two weeks, both in WT and NLRP3 KO mice. The damage scores were significantly higher in the second stroke area (right side) than the first stroke area (left) at interval of one (P < 0.001) or two weeks (P < 0.01) in WT mice. In contrast, the damage score was not significantly different in the second stroke area (right) in NLRP3 KO mice (Figure 2(c) and (d)). Similarly, the numbers of neurons surrounding the infarct area were comparably decreased following the first stroke in WT (P < 0.001) and NLRP3 KO mice (P < 0.001), which showed further decreased during the second attack in WT mice without influence of interval time (P < 0.001) (Figure 2(e) and (f)). There was no visible cleaved caspase-3 expressed in sham animals, which can be detected surrounding the infarct area both in WT and NLRP3 KO mice, and the mean pixel intensities of cleaved caspase-3 were significantly increased surrounding the second stroke area at one (P < 0.01) or two weeks (P < 0.001) after the first attack in WT mice, which showed no significant difference in NLRP3 KO mice (Figure 2(g) and (h)). The numbers of Tunel-positive cells surrounding the second stroke area were significantly increased in WT mice (P < 0.001), which showed no significant difference in NLRP3 KO mice (Figure 2(i) and (j)). The numbers of Iba-1-positive microglia (Figure 2(k) and (l)) surrounding the first infarct area (left) were comparably increased in WT (P < 0.001) and NLRP3 KO (P < 0.001) mice compared with the sham controls, which showed further increased in the second stroke (right) at one (P < 0.001) or two weeks (P < 0.05) interval in WT mice. There was no visible activated microglia in the sham controls, whereas the polarized microglia was detected surrounding infarct area. Compared with the first stroke (left), the M1 microglia (Figure 2(m) and (n)) were significantly increased (P < 0.001, P < 0.01), whereas the M2 microglia surrounding the second stroke area. (Figure 2(o) and (p)) were decreased (P < 0.001, P < 0.001) in WT mice, but not in NLRP3 KO mice. Thus, our results indicate that the NLRP3 inflammasome contributes to microglial polarization towards the M1 phenotype after recurrent stroke.
Figure 2.
Histological analysis of WT and NLRP3 KO mice following bilateral photothrombotic stroke at interval of one or two weeks. (a) XY scanning of target vessels surrounding the infarct area. (b) Comparisons of the blood flows among the sham, first and recurrent stroke in WT and NLRP3 KO mice. (c) Nissl staining of coronal brain slices; red circle marks the damage area. (d) Comparisons of the damage scores between the first and recurrent strokes in WT and NLRP3 KO mice. (e–h) Immunofluorescence staining and comparisons of Neurons and Cleaved caspase-3 surrounding the damage area among the sham, first and recurrent stroke, in WT and NLRP3 KO mice. (k–l) TUNEL staining and comparison of Tunel-positive cells among the sham, first and recurrent stroke, in WT and NLRP3 KO mice (k–l). Immunofluorescence staining and comparisons of Iba-1-positive microglia surrounding the damage area among the sham, first and recurrent stroke in WT and NLRP3 KO mice. (m–P) Immunofluorescence staining and comparisons of the CD-16 positive and CD-206 positive microglia in WT and NLRP3 KO mice surrounding the damage area. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 6.
ASC oligomerization occurs in recurrent stroke in an NLRP3-dependent manner
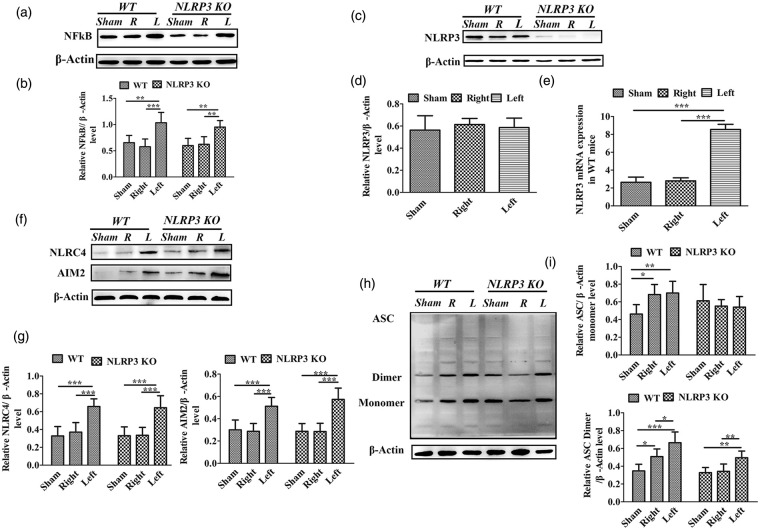
In order to define the different roles of NLRP3 in single versus recurrent strokes, we performed Western blot analysis in the peri-infacrt regions. Compared with the sham controls without stroke, the expressions of NF-κB, NLPR3,AIM2, NLRC4 proteins and the NLRP3 mRNA levels in right areas showed no significant differences in WT and NLRP3 KO mice with left stroke, but the ASC monomer and dimer levels were increased in WT mice (P < 0.05). Compared with the contralateral region (right), the expression of nuclear factor-κB (NF-κB) was the peri-infarct region (left) comparably increased in WT and NLRP3 KO mice (P < 0.001, P < 0.01; Figure 3(a) and (b)); the expression of NLRP3 was not significantly different in WT mice (Figure 3(c) and (d)), but the mRNA levels were significantly increased in stroke side (P < 0.001, Figure 3(e)), and the AIM2 and NLRC4 inflammasomes were significantly increased in the ischemic side in both WT and NLRP3 KO mice (P < 0.01, P < 0.001; Figure 3(f) and (g)). ASC bands corresponding to dimers were significantly increased in the ischemic side (left side) in both WT and NLRP3 KO mice (P < 0.05, P < 0.01; Figure 3(h) and (i)), suggesting an increase in ASC oligomerization. Our results indicate that NLRC4 and AIM2 inflammasomes, and not NLRP3, are responsible for the inflammation following single stroke.
Figure 3.
Western blot analysis of inflammasome proteins surrounding the infarct area in WT and NLRP3 KO mice following single photothrombotic stroke. (a) Chemiluminescence imaging of NF-κB and β-actin. (b) Comparison of the NF-κB/β-actin ratio among the sham control, unaffected (right, R) and the damaged (left, L) cortices in WT and NLRP3 KO mice. (c) Chemiluminescence imaging of NLRP3 and β-actin. (d) Comparisons of the NLRP3/β-actin ratio among the sham control, unaffected (right, R) and the damaged (left, L) cortices in WT mice. (e) Comparison of NLRP3 mRNA levels among the sham control, unaffected (right, R) and the damaged (left, L) cortices in WT mice. (f) Chemiluminescence imaging of NLRC4, AIM2 and β-actin. (g) Comparisons of the NLRC4 or AIM2/β-actin ratio among the sham control, unaffected (right, R) and the damaged (left, L) cortices in WT and NLRP3 KO mice. (h) Chemiluminescence imaging of ASC and β-actin I. Comparisons of ASC monomer/dimer abundance among the sham control, unaffected (right, R) and the damaged (left, L) cortices in WT and NLRP3 KO mice. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 6.
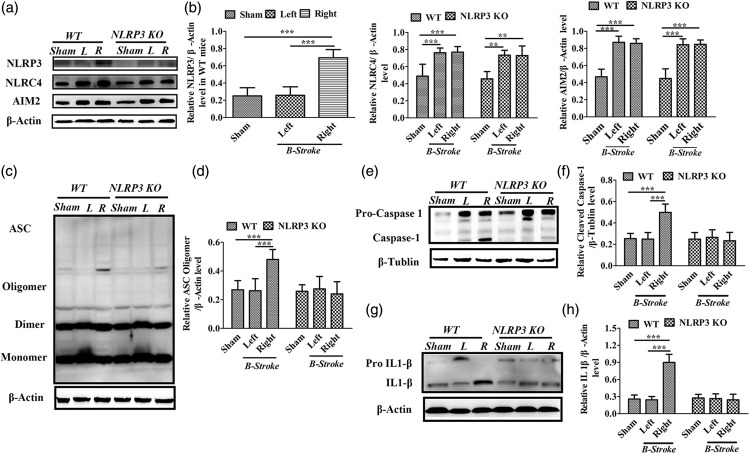
We then examined the involvement of the NLRP3 inflammasome in recurrent stroke after one and two weeks of the first upset of stroke, respectively (Figures 4 and 5). For WT and NLRP3 KO mice, Western blot analysis indicated the expressions of NLRP3, ASC, cleaved Caspase-1 and activated IL1- β in the first stroke area (right) were not significantly different from the sham controls without stroke, but the expressions of NLRC4 and AIM2 inflammasome were significantly increased (P < 0.001, P < 0.001). Compared with the first stroke, the expression of the NLRP3 inflammasome in the perinfarct region of the second stroke was significantly increased in WT mice (P < 0.001, Figure 4(a) and (b)), and there were no significant differences in the expression levels of the NLRC4, AIM2 inflammasomes (Figure 4(a) and (b)) and ASC monomers or dimers (Fig. 4C & D) in both in WT and NLRP3 KO mice. However, ASC oligomers were significantly increased in the second stroke (right), compared to the first stroke (left) in WT mice (P < 0.001, Figure 4(c) and (d)). In contrast, there was no significant difference in the levels of ASC oligomers between second (right side) and first stroke (left side) in NLRP3 KO mice. Our results therefore suggest that the NLRP3 inflammasome is essential for ASC oligomerization during recurrent stroke. Cleavaged caspase-1 and IL-1β were significantly increased in the second stroke (right) compared to the first stroke (left) in WT mice (P < 0.001, P < 0.001) (Figure 4(e) to (h)).
Figure 4.
Western blot analysis surrounding the infarct area in WT and NLRP3 KO mice following first and recurrent photothrombotic stroke at interval of 1 week. (a–b) Chemiluminescence imaging and comparisons of NLRP3, NLRC4, AIM2 and β-actin in the sham controls, first stroke area (left, L) and the second stroke area (right, R) in WT and NLRP3 KO mice. (c) Chemiluminescence imaging of ASC and β-actin. (d) Comparison of the relative levels of ASC oligomers among the sham controls, first (left, L) and second (right, R) stroke areas in WT and NLRP3 KO mice. (e–h) Chemiluminescence imaging and comparisons of caspase-1, IL-1β among the sham controls, first (left, L) and second (right, R) stroke areas in WT and NLRP3 KO mice. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 6.
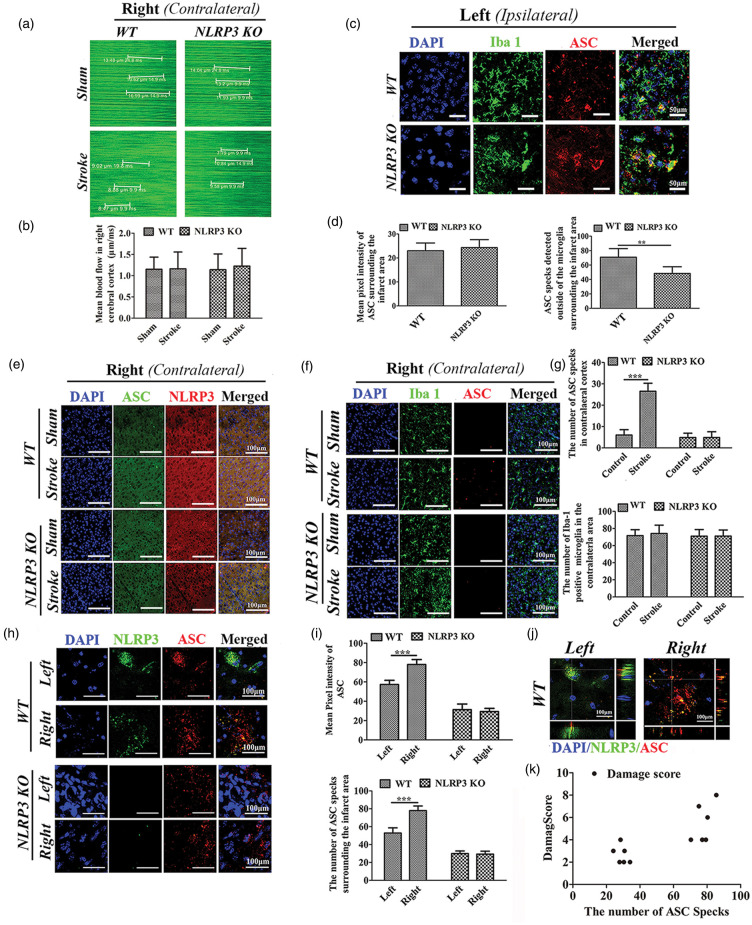
Figure 5.
Expression of NLRP3/ASC in the ipsilateral and contralateral cortices of WT and NLRP3 KO mice following single left photothrombotic stroke or bilateral stroke. (a) XY scanning of target vessels in the right cortex following single left photothrombotic stroke. (b) Comparisons of the blood flows in the right cortex. (c) Immunofluorescence staining of ASC and Iba-1-positive microglia. (d) Comparison of the ASC pixel intensity and the number of ASC specks surrounding the stroke area between WT and NLRP3 KO mice following single left photothrombotic stroke. (e–f) Immunofluorescence staining of ASC and NLRP3(E), ASC and Iba-1-positive microglia (f) in the right (contralateral) cortex following single left photothrombotic stroke. (g) Comparison of ASC specks and Iba-1-positive microglia in the right parietal cortex between WT and NLRP3 KO mice with or without single left photothrombotic stroke. (h) Immunofluorescence staining of ASC and NLRP3 surrounding the first (left) and recurrent (right) parietal infarct areas in WT and NLRP3 KO mice following bilateral stroke at interval of one week. (i) Comparisons of the pixel intensity and the number of ASC specks surrounding the infarct area following bilateral stroke. (j) Co-localization of ASC and NLRP3 in WT mice following bilateral stroke. (k) Pearson correlation analysis about the number of ASC specks surrounding the second infarct area and the damage score of the second stroke *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 6.
We also examined the activation of inflammasomes in the peri-infarct area during recurrence at two weeks after the first attack (Supplementary Figure 1). Similarly, the expression of the expressions of NLRP3, ASC oligomer, cleaved Caspase-1 and activated IL1- β during the first stroke (right) were not significantly different from the sham controls without stroke, but the NLRC4 and AIM2 levels were significantly increased (P < 0.001, P < 0.001). Compared with the first stroke, the expression of NLRP3 inflammasome was significantly increased in the recurrent stroke in WT mice (P < 0.001, Supplementary Figure 1(a) and (b)) but not in NLRP3 KO mice, and the expressions of the NLRC4 and AIM2 inflammasomes showed no significant differences in both in WT and NLRP3 KO mice (Supplementary Figure 1(a) and (b)); ASC oligomers were significantly increased in the second stroke (right) in WT mice (P < 0.001, Supplementary Figure 1(c) and (d)), but not in NLRP3 KO mice, and cleaved caspase-1 and IL-1β were also significantly increased in the second stroke (right) in WT mice (P < 0.001, P < 0.001), but not in NLRP3 KO mice (Supplementary Figure 1(e) to (h)). Our results indicate that NLPR3 contributes to the cleavage of caspase-1, as well as the activation of IL-1β.
It has been reported that ASC can form specks that contribute to the propagation of inflammation via its extracellular and prionoid activities. To investigate the possible role of ASC in recurrent stroke, we examined ASC specks in the brain following ischemia. As shown in Figure 5(a), the CBF of right cortex was not different from each other in animals with or without left stroke, both for WT and NLRP3 KO mice (Figure 5(b)).The number of ASC specks surrounding the infarct area was significantly increased in WT mice compared to NLRP3 KO mice (P < 0.01, Figure 5(c) and (d)). ASC specks were also visible in the contralateral side after stroke in WT mice, which was significantly increased compared to animals that were not subjected to stroke (P < 0.001). There was no significant difference in the number of Iba-1-positive microglia in the contralateral area compared with animals that were not subjected to stroke, for both WT and NLRP3 KO mice (Figure 5(e) and (f)). In addition, there was no detectable NLRP3 accumulation in the right parietal cortex following left parietal stroke both for WT and NLRP3 KO mice (Figure 5(e)). Our results indicate that ASC specks are formed in damaged cells, and are likely released and transported in an NLRP3-dependent manner.
We double labeled tissue with NLRP3 and ASC, to investigate the extent of their co-localization and distribution. The mean pixel intensity of both NLRP3 and ASC was significantly increased after the second stroke (right) compared to the first stroke (left) in WT mice (P < 0.001, Figure 5(h) and (i)). In contrast, the mean pixel intensity of ASC was not significantly different between first and second stroke in NLRP3 KO mice (P > 0.05). We confirmed that there was no significant NLRP3 expression in NLRP3 KO mice. As shown in Figure 5(j), NLRP3 and ASC were co-localized in the first stroke (left) as well as in the second stroke (right)) in WT mice. However, the distribution of ASC differed; ASC was diffusely distributed around the nucleus in the first stroke, whereas it appeared in specks in the second stroke. Finally, Pearson correlation analysis indicated that the number of ASC specks surrounding the infarct area was positively correlated to the damage score of the recurrent stroke (P < 0.01, Figure 5(k)).
Recombinant ASC exacerbated the outcome of ischemic stroke in a NLRP3-dependent manner
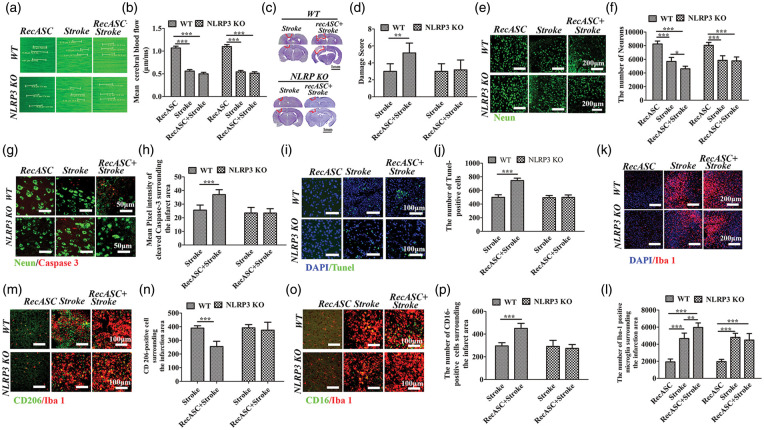
ASC specks were reported to aggregate the ischemic stroke, promote the spreading of inflammation. Also, we detected the ASC specks in the contralateral area after unilateral stroke, which might be involved in the recurrent stroke. To elucidate the role of ASC protein in the ischemic stroke, we intracerebroventricularly injected recombinant ASC specks (RecASC) one week before the upset of stroke in WT controls and NLRP3 KO mice. As shown in Figure 6(a) and (b), compared with the controls with injection of RecASC, the cerebral blood flows were reduced in stroke groups (P < 0.001), which were not affected by injection of RecASC (P > 0.05). Compared with the animals with single upset of stroke, injection of recombinant ASC increased the damage score in WT controls (P < 0.01), but not in NLRP3 KO mice (P > 0.05) (Figure 6(c) and (d)). Compared with sham controls with single RecASC injection, the numbers of neurons surrounding the infarct area were comparably decreased in WT and NLRP3 KO mice received stroke (P < 0.001, P < 0.001), which was further decreased in WT animals with RecASC injection plus stroke (P < 0.05), but not in NLRP3 KO mice (Figure 6(e) and (f)). There was no visible cleaved caspase-3 in animals received RecASC injection, which was detected surrounding the infarct area in WT and NLRP3 KO mice, with no significant difference. RecASC injection significantly increased the expression of cleaved caspase-3 surrounding the infarct area in WT mice (P < 0.001), but not NLRP3 KO mice (Figure 6(g) and (h)). There were no visible Tunel-positive cells in animals received RecASC injection, which was detected surrounding the infarct area in WT and NLRP3 KO mice, with no significant difference. RecASC injection significantly increased the number of tunel-positive cells surrounding the infarct area in WT mice (P < 0.001), but not NLRP3 KO mice (Figure 6(i) and (j)). The numbers of microglia surrounding the infarct area were comparably increased in stroke animals, which was further increased by injection of RecASC, in WT controls (P < 0.01), but not in NLRP3 KO mice (Figure 6(k) and (l)). Recominant ASC did not activate the mcircoglial polarization in animals without stroke, but decreased the CD-206-positive microglia (M2) (P < 0.001), whereas increased the number of CD-16-positive microglia (M1) (P < 0.001), in WT controls, but not in NLRP3 KO mice (Figure 6(m) to (p)).
Figure 6.
Histological analysis of WT and NLRP3 KO mice following photothrombotic stroke with or without RecASC injection. (a) XY scanning of target vessels in the left cortex with RecASC injection or photothrombotic stroke. (b) Comparison of the velocities among the groups with RecASC injection, stroke and RecASC plus stroke. (c) Nissl staining of coronal brain slices; red circle marks the damage area. (d) Comparison of the damage score between the animals with stroke and animals with stroke plus recASC injection in WT and NLRP3 KO mice. (e–h) Immunofluorescence staining and comparisons of Neurons and Cleaved caspase-3 surrounding the damage area among the groups with RecASC injection, stroke and RecASC plus stroke, in WT animals and NLRP3 KO mice. (i–j) TUNEL staining and comparison of Tunel-positive cells surrounding the damage area among the groups with RecASC injection, stroke and RecASC plus stroke, in WT animals and NLRP3 KO mice. (k–p) Immunofluorescence staining and comparisons of the overall Iba-1-positive, CD206-positive and CD-16 positive microglia surrounding the damage area of the number of Iba-1-positive microglia among the animals with RecASC injection, animals with stroke and animals with stroke plus recASC injection in WT and NLRP3 KO mice. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 6.
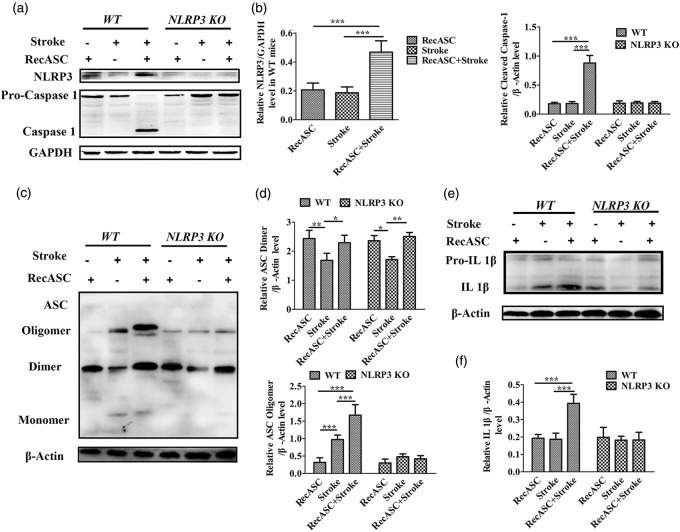
Western blot analysis showed that compared with the mice received single dose of RecASC specks injection, the expressions of NLRP3, cleaved caspase-1 (Figure 7(a) and (b)) and IL1-β proteins (Figure 7(e) and (f)) were not increased, both in WT and NLRP3 KO mice; the expression of ASC dimmers decreased (P < 0.01) whereas the ASC oligomer increased (P < 0.001) in WT mice (Figure 7(c) and (d)). But compared with the mice received single upset of stroke, recombinant ASC increased the expressions of NLRP3 (P < 0.001), cleaved caspase-1 (P < 0.001) and activated IL 1-β (P < 0.001) surrounding the infarct area in WT controls, not in NLRP3 KO mice. RecASC injection increased the expression of ASC dimmers and oligomers surrounding the infarct area in WT animal (P < 0.001), not in NLRP3 KO mice.
Figure 7.
Western blot analysis of WT and NLRP3 KO mice following photothrombotic stroke with or without recASC injection. (a) Chemiluminescence imaging of NLRP3 and Caspase-1 as well as GAPDH surrounding the stroke area. (b) Comparison of the relative level of NLRP3, cleaved Caspase-1 between the WT and NLRP3 KO mice following RecASC injection, stroke and RecASC injection plus stroke. (c) Chemiluminescence imaging of ASC and β-actin surrounding the stroke area. (d) Comparison of the relative level of ASC dimer and oligomer between the WT and NLRP3 KO mice following RecASC injection, stroke and RecASC injection plus stroke. (e–f) Chemiluminescence imaging and comparison of IL 1-β surrounding the stroke area between the WT and NLRP3 KO mice following RecASC injection, stroke and RecASC injection plus stroke. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. n = 6.
Discussion
In the present study, we have examined the role of the inflammasome in a mouse model of photothrombotic ischemia. We found that NLRP3 plays a key role in recurrent stroke, but not in primary stroke. We further demonstrated that ASC specks mediate recurrent stroke in a NLRP3-dependent manner. Given the critical role of ASC/NLRP3 in recurrent stroke, this study suggests that targeting ASC/NLRP3 may represent a novel therapeutic strategy for the treatment of recurrent stroke.
The involvement of multiple inflammasomes, including NLRC4 and AIM2, in ischemic stroke has been reported in the literature. However, consensus is lacking on the role of NLRP3 in ischemic brain injury. For example, NLRP3 deficiency has been shown to ameliorate neurovascular damage in an experimental model of ischemic stroke.18 In contrast, Denes et al.19 reported that NLRC4 and AIM2, but not NLRP3, contribute to ischemic injury. Consistent with Denes et al., we found here that NLRC4 and AIM2 inflammasomes, but not NLRP3, were increased in ischemic animals. Furthermore, NLRP3 deficiency did not have any remarkable influence on the outcome of ischemic stroke, indicating that NLRC4 and AIM2, but not NLRP3, contribute to the single onset of ischemic stroke. One explanation for this observation may be due to differing activation requirements between NLRP3 and NLRC4/AIM2. NLRP3 inflammasomes require a priming step prior to activation,19,20 whereas this priming step is not required for the activation of NLRC4/AIM2 inflammasomes. Following recurrent stroke, the expression levels of NLRP3 were noticeably increased, whereas those of NLRC4 and AIM2 remained unchanged. Furthermore, the loss of NLRP3, as modeled in NLRP3 KO animals, significantly attenuated the extent of recurrent ischemic brain injury. Therefore, NLRP3 is a major contributor to brain ischemic injury following recurrent stroke. A two-signal model, including a priming step (signal 1) and an activation step (signal 2) has been proposed for NLRP3 inflammasome activation. Consistent with the two-signal hypothesis, NLRP3 was distributed around the nucleus in the first stroke attack, yet diffusely distributed and co-localized with ASC specks in the recurrent stroke, indicating that NLRP3 is activated in recurrent stroke. It is possible that the first stroke serves as the signal 1 event, leading to the upregulation of molecules which can in turn prime NLRP3 during the second stroke. Because the levels of NLRP3 mRNA and NF-κB protein expression were increased after ischemic stroke, this indicates that NLRP3 was primed after the initial stroke.
ASC, a common component of most inflammasomes, mediates capase-1 activation and IL-1β production. In addition, ASC can be released into the extracellular space, propagating inflammation in a prion-like manner.21,22 In the present study, extracellular ASC specks could be detected on the contralateral side, yet it was not clear where these ASC specks originated. We believe it is less likely that ASC was produced locally, as microglia were not activated in the contralateral side. It is more likely that ASC specks originated from the ischemic side. In support of this, ASC has been reported in the cerebrospinal fluid following brain damage.22 Additional studies are warranted to investigate the exact source of ASC following nervous system injury. It has been reported that overexpression of ASC results in formation of small specks, without the presence of NRLP3, whereas NRLP3 is essential for the formation of large specks and the oligomerization of ASC. ASC oligomerization further activates caspase-1 and results in the production of IL-1β. Consistently, we found the ASC specks in the unaffected side after left parietal stroke did not induce NLRP3 or microglial activation. Additionally, injection of recombinant ASC before the upset of ischemic stroke increased the activation of NLRP3 inflammsome in the stroke and further increased the damage score, which could be abolished by NLRP3 deficiency. Our results further demonstrated that ASC plays an critical role in the spreading of the sustained inflammation, in a NLRP3 dependent manner, which might contribute to the recurrent stroke.
It should be noted that systemic inflammation is associated with poor outcomes in patients with acute ischemic stroke. Cerebral ischemia recruits the circulating leucocytes and damages the brain–blood barrier, infection and inflammatory disorders may trigger or prompt the ischemic stroke.23 Thus, systemic inflammation may play critical role on exacerbation in the second stroke. A future study is warranted to investigate the effect of systemic inflammation on second stroke in NLRP3 null animals.
In summary, first, we demonstrated that NLRC4 and AIM2 inflammasomes, together with ASC, contribute to injury following single ischemic stroke, but NLRP3, together with ASC, contributes to recurrent stroke. Second, extracellular ASC, likely released from damaged cells during the primary stroke event, plays a critical role in the aggravation of recurrent stroke in an NLRP3-dependent manner. Finally, extracellular ASC keeps its activity and activates the NLRP3/caspase-1/IL-1β cascade in ischemic stroke.
Supplemental Material
Supplemental Material for Extracellular ASC exacerbated the recurrent ischemic stroke in an NLRP3-dependent manner by Xiao-fei He, Yi-xuan Zeng, Ge Li, Yu-kun Feng, Cheng Wu, Feng-yin Liang, Yu Zhang, Yue Lan, Guang-qing Xu and Zhong Pei in Journal of Cerebral Blood Flow & Metabolism
Acknowledgement
We thank the Model Animal Research Center of Nanjing University for providing the NLRP3 knockout mice.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China (grant numbers: 81671102, 81572224, 81572230, 81371255 and 81772438), The National Key Research and Development Program of China, Stem Cell and Translational Research (2017YFA0105104), the National Key Clinical Department, National Key Discipline, Guangdong Provincial Key Laboratory For Diagnosis and Treatment of Major Neurological Disease (2014B030301035), the Science and Technology Planning Project of Guangdong Province, China (grant numbers: 2016A020213003, 2016B040404053, 2014B020212001, 2014A030304018, 2014B040404053, 2016B030230002 and 2017A040406007), the National Science and Technology Support Program (grant numbers: 2015BAI07B01), and the Science and Technology Planning Project of Guangzhou, China (grant numbers: 2016201604030036). Guangdong Provincial Key Laboratory for Diagnosis and Treatment of Major Neurological Diseases (2017B030314103); The Southern China International Cooperation Base for Early Intervention and Functional Rehabilitation of Neurological Diseases (2015B050501003); Guangzhou Clinical Research and Translational Center for Major Neurological Diseases (201604020010); Guangdong Provincial Engineering Center for Major Neurological Disease Treatment. The funding bodies had no involvement in the experimental design or the interpretation of the results.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
Xiao-fei He, Yi-xuan Zeng, Ge Li, Feng-yin Liang, Yu Zhang and Cheng Wu performed the experiments. Xiao-fei He, Yi-xuan Zeng and Yu-kun Feng drafted the manuscript. Yue Lan, Guang-qing Xu and Zhong Pei conceived and designed the research, and edited and revised the manuscript. Yue Lan, Guang-qing Xu and Zhong Pei approved the final version of the manuscript.
Supplementary material
Supplemental material for this article is available online.
References
- 1.World Health Organisation. Cardiovascular diseases fact sheet, www.who. Int/mediacentre/factsheets/fs317/en/ (accessed 14 December 2016).
- 2.Welsh P, Lowe GD, Chalmers J, et al. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke 2008; 39: 2226–2230. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1 moving upstream to identify novel targets for atheroprotection. Circ Res 2016; 118: 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu A, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014; 156: 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masumoto J, et al. ASC, a novel 22-kDa protein, aggregates during apoptosis of human promyelocytic leukemia HL-60 cells. J Biol Chem 1999; 274: 33835–33838. [DOI] [PubMed] [Google Scholar]
- 6.Nan Song, Zhao-Shan Liu, Wen Xue, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell 2017; 68: 1–13. [DOI] [PubMed] [Google Scholar]
- 7.Baroja-Mazo A, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol 2014; 15: 738–748. [DOI] [PubMed] [Google Scholar]
- 8.He Y, Hara H, Núñez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 2016; 41: 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih AY, Driscoll JD, Drew PJ, et al. Two-photon microscopy as a tool to study blood flow and neurovascular coupling in the rodent brain. J Cereb Blood Flow Metab 201; 32: 1277–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schrandt CJ, Kazmi SM, Jones TA, et al. Chronic monitoring of vascular progression after ischemic stroke using multiexposure speckle imaging and two-photon fluorescence microscopy. J Cereb Blood Flow Metab 2015; 35: 933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dan Wang, Jun Qian, Wei Qin, et al. Biocompatible and photostable AIE dots with red emission for in vivo two-photon bioimaging. Sci Rep 2014; 4: 4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Labat-gest V, Tomasi S. Photothrombotic ischemia: a minimally invasive and reproducible photochemical cortical lesion model for mouse stroke studies. J Visual Exp 2013; 76: e50370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature 2012; 484: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venegas C, Kumar S, Franklin BS, et al. Microglia-derived ASC specks cros-seed amyloid-β in Alzheimer's disease. Nature 2017; 552: 355–361. [DOI] [PubMed] [Google Scholar]
- 15.Koh EJ, Kim KJ, Song JH, et al. Spirulina maxima extract ameliorates learning and memory impairments via inhibiting GSK-3β phosphorylation induced by intracerebroventricular injection of amyloid-β 1-42 in mice. Int J Mol Sci 2017; 18: pii: E2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao M, Zhao Z, Barber PA, et al. Development of a model of recurrent stroke consisting of a mild transient stroke followed by a second moderate stroke in rats. J Neurosci Meth 2009; 184: 244–250. [DOI] [PubMed] [Google Scholar]
- 17.Hilger T, Blunk JA, Hoehn M. Characterization of a novel chronic photothrombotic ring stroke model in rats by magnetic resonance imaging, biochemical imaging, and histology. J Cereb Blood Flow Metab 2004; 24: 789–797. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Ziying W, Xinbing W, et al. NLRP3 deficiency ameliorates neurovascular damage in experimental ischemic stroke. J Cereb Blood Flow Metab 2014; 34: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adam D, Graham C, Nikolett L, et al. AIM2 and NLRC4 inflammasomes contribute with ASC to acute brain injury independently of NLRP3. Proc Natl Acad Sci U S A 2015; 112: 4050–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bauernfeind FG1, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 2009; 183: 787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamczak S, Dale G, de Rivero Vaccari JP, et al. Inflammasome proteins in cerebrospinal fluid of brain injured patients are biomarkers of functional outcome. J Neurosurg 2012; 117: 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin BS, Bossaller L, De Nardo D, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol 2014; 15: 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard M, Carine A, Olivier T, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 2011; 10: 471–480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Extracellular ASC exacerbated the recurrent ischemic stroke in an NLRP3-dependent manner by Xiao-fei He, Yi-xuan Zeng, Ge Li, Yu-kun Feng, Cheng Wu, Feng-yin Liang, Yu Zhang, Yue Lan, Guang-qing Xu and Zhong Pei in Journal of Cerebral Blood Flow & Metabolism