Short abstract
Lacunes on magnetic resonance imaging (MRI) are considered as a key hallmark for evaluating the progression and severity of cerebral small vessel diseases. We aimed to review the MRI diagnostic criteria, frequency, predictors and clinical impact of incident lacunes in the largest longitudinal studies. Analyses were restricted to cohort studies of more than 50 individuals that investigated incident lacunes over a duration of at least one year. We observed that: (1) MRI parameters and definition of lacunes are inconsistent across studies, (2) the frequency of incident lacunes is strongly related to the previous clinical and MRI status at individual level, (3) both age and hypertension diagnosed at onset predict incident lacunes but the exact impact of blood pressure level during follow-up remains undetermined, (4) the clinical correlates of these lesions on cognition are repeatedly observed but the exact consequences on motor or gait performances are not always evaluated. Homogenization of imaging techniques, the use of strict diagnostic criteria and a broader clinical assessment considering motor and gait performances should be recommended in future longitudinal studies of incident lacunes including clinical trials testing preventative treatments in cerebral small vessel diseases.
Keywords: Cerebral small vessel diseases, incidence of lacunes, lacunes, magnetic resonance imaging, stroke
Introduction
The word lacune (from the Latin lacuna(ae), a tiny hole, pit, or cavity) denotes a small, cystic cavity of the brain substance that usually results from an ischemic infarction in the territory of a penetrating arteriole, much more rarely from a small deep cerebral hemorrhage.1 The original description of lacunes in the brain was made at the end of the 19th century by Amedée Dechambre from La Salpétriêre Hospital in Paris and was confirmed 5 years later by Durand-Fardel.2 Durand-Fardel3,4 clearly distinguished lacunes from dilated perivascular spaces that lodged blood vessels. Decades later, Fisher5,6 reported the relationships between these small focal cerebral lesions and various segmental alterations in the wall of cerebral penetrating arterioles.
At the beginning of the 20th century, gait disturbances and cognitive decline in elderly individuals were related to the accumulation of multiple lacunes in the brain by Binswanger and Alzheimer.7,8 The various clinical consequences of single lacunes were described after in individual cases9 and detailed in a large number of stroke patients by Fisher in 1965 after post-mortem examination.5 From the 80s, lacunes became easily recognized in living individuals with the development of imaging techniques, particularly with the growing use of magnetic resonance imaging (MRI).10,11 They are frequently detected not only in stroke patients or in patients with dementia but also in the absence of any clinical event in large population-based studies. Thus, lacunes were found to be the main source of so-called “silent infarcts” detected in elderly individuals.12 Lacunes of presumed “vascular origin” are now clearly defined on MRI based on the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) criteria as a round or ovoid, subcortical, fluid-filled (similar signal as cerebral spinal fluid) cavity, of between 3 mm and about 15 mm in diameter, consistent with a previous acute small deep brain infarct or hemorrhage in the territory of a perforating arteriole (Figure 1).1 Recent MR data showed that only 50–80% of acute small deep infarcts become cavities, i.e. lacunes in the brain.13–15 Moreover, ultra-high field MRI techniques can reveal now microinfarcts resulting from small vessel disease which were previously invisible.16 Therefore, lacunes as defined by the STRIVE criteria may represent only the most severe focal lesions resulting from a small vessel disease that are visible using routine clinical MRI scans.
Figure 1.
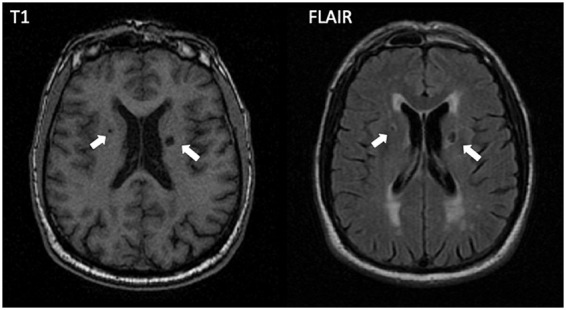
Incident lacunes on T1-weighted and FLAIR MRI sequences. Example of two incident lacunes (arrows) that appear in the paraventricular white matter.
Accumulating evidence shows that the cerebral location and number of these lesions mainly explain the extreme clinical variability related to lacunes observed at individual level on MRI. The location of a single lacune within a strategic cerebral area can have devastating clinical consequences, whereas in other areas, only subtle or no symptom is observed. On the other hand, their increasing number appears also clearly associated with the development of motor disability, gait disturbances, cognitive decline or dementia in individuals with sporadic or hereditary cerebral small vessel diseases (CSVDs).17–21 Recent data demonstrate that lacunes as defined on MRI are also strongly related to the development of cerebral atrophy22 and that even a single incident lesion can cause remote thinning of the cortex mantle.23 Thus, lacunes are now definitely considered as a key hallmark for evaluating the severity of CSVD.
Consequently, incident (newly developed) lacunes on MRI represent a key marker reflecting the progression of sporadic or hereditary CSVD and might become particularly useful in future clinical trials or longitudinal studies.24,25 The number of incident lacunes can be used not only after the occurrence of a stroke event or subcortical vascular cognitive impairment but also at the earliest stages of CSVD long before the occurrence of neurological manifestations26,27 for assessing the efficacy of various therapeutic strategies. In the present review, the frequency, predictors and clinical impact of incident lacunes on MRI were analyzed in the largest cohort studies in the literature.
Methods
Search strategy and selection criteria
A PubMed search has been conducted up to May, 2019 with the following terms (“Lacune*” OR “Lacuna” OR “Lacunar infarct*” OR “Lacunar infarction*” OR “Lacunar stroke*” OR “Lacunar lesion*” OR “Small deep infarct*” OR “Small deep infarction*” OR “Subcortical infarct*” OR “Subcortical infarction*” OR “Subcortical stroke*” OR “Small vascular disease stroke*” OR “Silent brain infarct*” OR “Silent brain infarction*” OR “Silent cerebral infarct*” OR “Silent cerebral infarct*” OR “Silent stroke*”) AND (“Magnetic Resonance Imaging” OR “MR*” OR “magnetic resonance”) AND (“incident” OR “Incidental” OR “Longitudinal” OR “Follow-up” OR “long term”). The searches were limited to original studies relating to human populations and written in English. The PubMed searches identified 831 articles. We also searched among all references in these 831 articles for further studies, resulting in another 15 additional articles.
We chose to analyze in details all longitudinal MRI studies that investigated incident lacunes over at least one year in only cohorts of more than 50 individuals. Cross-sectional studies, computer tomography (CT) studies, MRI studies with only diffusion-weighted images, case reports, and studies not reporting incident lacunes were not included. We also excluded studies on specific disorders such as performed in chronic kidney disease, heart failure, sickle cell disease, congenital protein C deficiency or cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).
Data analysis
A total of 37 articles covering 19 original studies were deemed eligible according to the previously mentioned criteria (Supplementary Figure 1 for the flow diagram).17–19,26,28–60 Data were reviewed descriptively and collated in Tables 1 to 3 and Supplementary Tables 1 to 3.
Table 1.
Overview of patient characteristics and frequency of incident lacunes in selected studies.
| Reference number | First author year | Study name or center | Country | Population | Terms used for lacunes | N | BL age, mean ± SD | BL prev (%) | FU, y | Freq (%) | Freq/y (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Population-based studies | |||||||||||
| 55 | Nylander et al. 2018 | PIVUS | Sweden | Age at 75 y, randomly selected | Lacunar infarcts | 406 | 75 | 21.9 | 5 | 10.3 | 2.1 |
| 17 | Sigurdsson et al. 2017 | AGES | Iceland | Age 66–97 y from Reykjavik | Infarcts | 2612 | 74.6 ± 4.8 | 30.7 | 5.2 | 20.9 | 4.0 |
| 31 | Knopman et al. 2011 | ARIC | USA | Age >55 y, stroke-free | Infarcts | 1112 | 61.7 ± 4.3 | 7.7 | 10.6 | 20.1 | 1.9 |
| 33 | Virtanen et al. 2013 | CHS | USA | Age over 65 y, stroke-free, randomly selected | Subclinical infarcts | 2293 | 75.1 | 23.3 | 5 | 16.1a | 3.2a |
| 34 | Satizabalet al. 2012 | 3C | France | Age over 65 y, randomly selected | Silent brain infarcts | 1841 | 72.5 ± 4.1 | 8.9 | 4 | 4.4 | 1.1 |
| 36 | Chen et al. 2009 | PTLS | Australia | Age 60–64 y, community residents | Lacunar infarcts | 477 | 62.6 ± 1.5 | 7.8 | 4.1 | 1.6 | 0.4 |
| 37 | van Dijk et al. 2008 | RSS | Netherlands | Age 60–90 y, no dementia | Lacunar infarcts | 668 | 71 ± 7 | 20 | 3.4 | 12 | 3.5 |
| 40 | Schmidt et al. 2006 | ASPS | Austria | Age 50–75 y, no neuropsychiatric disease, randomly selected | Lacunes | 505 | 63.9 ± 9.8 | 6.9 | 6 | 3.0 | 0.5 |
| Studies on patients with cerebrovascular disease | |||||||||||
| 59 | van Leijsen et al. 2018 | RUN DMC | Netherlands | Age 50–85 y, nondemented with CSVD | Lacunes | 258 | 62.4 ± 7.7 | 20.5 | 9 | 24.0 | 2.7 |
| 60 | Staszewsk et al. 2018 | SHEF-CSVD | Poland | Age >60 y, with CSVD | Lacunar infarcts | 123 | 72.2 ± 8 | 48.8 | 2 | 20.3 | 10.2 |
| 42 | Benjamin et al. 2016 | SCANS | UK | Patients with lacunar infarcts and confluent WMH on MRI | Lacunes | 121 | 70 ± 9.8 | 100 | 3 | 28.6 | 9.5 |
| 50 | Cavalieri et al. 2012 | VITATOPS | China | Patients with stroke or TIAs | Lacunes | 359 | 64.3 ± 12.8 | 36.5 | 2.1 | 7.0 | 3.4 |
| 35 | Gouw et al. 2008 | LADIS | Multiple centers in Europe | Age 65–84 y, no severe disability, with WMH on MRI | Lacunes | 396 | 73.6 ± 5.0 | 47.7 | 3.1 | 18.7 | 6.0 |
| 54 | Walters et al. 2003 | St Mary’s Hospital | UK | Patients with transient ischemic attacks, no cognitive impairment | Silent ischemic lesions | 60 | 71.7 | NS | 1 | 36.6 | 36.6 |
| Studies on patients with vascular risk factors | |||||||||||
| 41 | van Dalen et al. 2017 | preDIVA trial | Netherlands | Age 70–78 y, with hypertension | Lacunar infarcts | 126 | 77.2 ± 2.5 | 7.1 | 2.8 | 6.3 | 2.3 |
| 57 | Kloppenborg et al. 2017 | SMART-MR study | Netherlands | Patients with artery disease | Lacunar infarcts | 679 | 57.5 ± 9.6 | 16.5 | 3.9 | 10.3 | 2.6 |
| 58 | Uiterwijk et al. 2017 | HYBRiD | Netherlands | Uncomplicated hypertensive patients | Lacunes | 90 | 56.2 ± 11.9 | 23.3 | 4 | 5.6 | 1.4 |
| 51 | Saito et al. 2014 | Asahikawa Hospital | Japan | Age over 45 y, with non-valvular atrial fibrillation, stroke-free | Asymptomatic cerebral infarctions | 131 | 69.4 ± 9.2 | 28.6 | 2.7 | 10.4 | 3.9 |
| 52 | Umemura et al. 2011 | Chubu Rosai Hospital | Japan | Age over 45 y, with type 2 diabetes mellitus, stroke-free | Silent brain infarcts | 190 | 62.7 ± 8.1 | 24.2 | 6 | 24.3 | 4.1 |
aResults in patients without lacunes at baseline.
BL: baseline; Prev: prevalence; FU: follow-up; Freq: frequency; PIVUS: prospective investigation of the vasculature in uppsala seniors study; AGES: age gene/environment susceptibility-Reykjavik study; ARIC: atherosclerosis risk in communities study; CHS: cardio vascular health study; 3C: three-city-Dijon study; PTLS: PATH through life study; RSS: Rotterdam scan study; ASPS: Austrian stroke prevention study; RUN DMC: Radboud University Nijmegen diffusion tensor and magnetic resonance imaging cohort; SHEF-CSVD: significance of haemodynamic and haemostatic factors in the course of different manifestations of cerebral small vessel disease study; SCANS: St George’s Cognition and Neuroimaging in Stroke study; VITATOPS: VITAmins to prevent stroke MRI-substudy; LADIS : leukoaraiosis and disability study; preDIVA : prevention of dementia by intensive vascular care study; SMART-MR: second manifestations of arterial disease-magnetic resonance study; HYBRiD: hypertension and brain damage study; CSVD: cerebral small vessel diseases; MRI: magnetic resonance imaging; WMH: white matter hyperintensity; NS: not specified.
Table 2.
Predictors of incident lacunes in population based studies.
| BASELINE PREDICTORS | REFERENCES OF STUDIES WITH A SIGNIFICANT ASSOCIATION | REFERENCES OF STUDIES WITH NO SIGNIFICANT ASSOCIATION |
|---|---|---|
| Age | 19, 30, 31, 37 | |
| Sex | ||
| Male | 17 | |
| Female | 37 | |
| Blood pressure | ||
| Hypertension | 31 | |
| Systolic blood pressure | 31 | 19, 28, 37 |
| Diastolic blood pressure | 31, 37 | |
| Mean arterial blood pressure | 31 | |
| Dyslipidemia | ||
| Total cholesterol | 37 | |
| Triglycerides | 28 | |
| High-density lipoprotein | 28a | 37 |
| Diabetes | ||
| Diabetes | 31 | 37 |
| Glucose | 31 | |
| Metabolic syndrome and obesity | ||
| Metabolic syndrome | 28 | |
| Insulin resistance score | 28 | |
| Insulin | 28 | |
| Body mass index | 28 | |
| Waist circumference | 28 | |
| Waist/hip ratio | 28 | |
| Smoking | 37 | |
| Atrial fibrillation | 37 | |
| Previous stroke | 31 | |
| Vascular markers | ||
| Retinal microvascular abnormalities | 32 | 38 |
| Carotid atherosclerosis | 37 | |
| Ankle-arm index | 19a | |
| Creatinine | 19 | |
| MRI markers | ||
| White matter hyperintensities | 19 | |
| Others | ||
| APOE4 allele | 31, 37 | |
| IL-6 | 34 | |
| CRP | 34, 39, 40 | |
| Circulating omega‐3 polyunsaturated fatty acids | 33 | |
| Hyperhomocysteinemia | 37 | |
| Protein intake | 29 | |
| Daily activities | 19 | |
| Exercise | 19 | |
aPredictors with a significant adjusted OR <1.0.
APOE4: apolipoprotein E4 ; IL-6: interleukin-6 ; CRP: C-reactive protein.
Table 3.
Clinical impacts of incident lacunes.
| Reference number | First author year | Study name or center | Population | N | FU, y | Lacunes freq/y (%) | Clinical evaluation | Testing | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Population-based studies | |||||||||
| 55 | Nylander et al. 2018 | PIVUS | Age at 75 y, randomly selected | 506 | 5 | 2.1 | Global cognitive | MMSE | >0.05 |
| Executive | The trail making tests A | 0.003 | |||||||
| Executive | The trail making tests B | 0.24 | |||||||
| 17 | Sigurdsson et al. 2017 | AGES | Age 66–97 y from Reykjavik | 2612 | 5.2 | 4.0 | Incident dementia | By consensus on diagnostic and statistical manual (DSM)-IV guidelines | >0.05 |
| 31 | Knopman et al. 2011 | ARIC | Age >55 y, stroke-free | 1112 | 10.6 | 1.9 | Incident stroke | Clinical examination | <0.05 |
| 18 | Longstreth et al. 2011 | CHS | Age over 65 y, stroke-free, randomly selected | 1446 | 5 and 9.6 | 3.5 | Incident heart failure | Clinical examination | 0.021 |
| Incident stroke | Clinical examination | <0.001 | |||||||
| Death | None | 0.002 | |||||||
| 37 | van Dijk et al. 2008 | RSS | Age 60–90 y, no dementia | 668 | 3.4 | 3.5 | Memory | 15-word verbal learning test | 0.15 |
| Psychomotor speed | Reading of Stroop test, one-letter subtask of paper-and-pencil memory scanning task, and letter–digit substitution task | <0.01 | |||||||
| Multiple tests | All tests above | <0.01 | |||||||
| Global cognition | Mini-mental state examination (MMSE) score | 0.44 | |||||||
| Studies in patients with cerebrovascular disease | |||||||||
| 26 | Jokinen et al. 2011 | LADIS | Age 65–84 y, no severe disability, with WMH on MRI | 387 | 3 | 6.2 | Global cognition | MMSE | 0.749 |
| Global cognition | Vascular dementia assessment scale–cognitive subscale (VADAS) | 0.146 | |||||||
| Speed and motor control | Trail-making test, part A (time), maze (time) and digit cancellation of VADAS. | 0.045 | |||||||
| Executive | Trail-making (B time-A time) Stroop test (Stroop III time-Stroop II time) symbol digit modalities test (correct responses) verbal fluency of VADAS | 0.021 | |||||||
| Memory | VADAS word recall, delayed recall, word recognition and digit span. | 0.973 | |||||||
| Studies in patients with vascular risk factors | |||||||||
| 53 | Imamine et al. 2011 | Chubu Rosai Hospital | Age over 45 y, with type 2 diabetes mellitus, stroke-free | 67 | 3 | 8.7 | Global cognition | Mini-mental state examination score | >0.05 |
| Verbal memory | Word recall-a subtest of Alzheimer’s disease assessment scale | >0.05 | |||||||
| Complex psychomotor skills | Digit symbol substitution (DSS) test | >0.05 | |||||||
| Attention/executive function | Modified Stroop color word (Stroop) test | >0.05 | |||||||
| Executive function | Visual elevator test, brixton spatial anticipation test, verbal fluency test | >0.05 | |||||||
FU: follow-up; RSS: Rotterdam scan study; ARIC: atherosclerosis risk in communities study; CHS: cardiovascular health study; AGES: age gene/environment susceptibility-Reykjavik study; LADIS: leukoaraiosis and disability study; PIVUS: prospective investigation of the vasculature in Uppsala seniors study.
The following aspects were analyzed systematically in all corresponding papers: MRI definition of lacunes, frequency, predictors and clinical impact of incident lacunes.
Results
Among the 37 articles selected, 17 (46.0%) were from 8 general population-based cohorts, 8 (21.6%) were from 6 cohorts of patients who presented with cerebrovascular disease, and 12 (32.4%) were from 5 cohorts of patients who only had vascular risk factors. A single study was an international cohort study obtained in different countries,26,35 5 studies were conducted in the Netherlands,37–39,41,43–48,57–59 2 in Japan,51–53 2 in United States18,19,28–33 and 2 in United Kingdom.42,54 A single study was performed in each of the following countries: Iceland,17 France,34 Australia,36 Austria,40 China,49,50 Sweden55,56 and Poland.60
MRI parameters
All information about MRI scanners, sequences, and imaging criteria used for diagnosis of lacunes are detailed in Supplementary Table 1. MRI magnet field strengths varied from 0.35 to 3.0 T. Two centers from two multi-center studies used a scanner with magnetic field equal or less than 0.5-T,33,35 and one study a 3-T scanner.48 In all other 16 studies, 1.5-T scanners were used.17,31,34,36,37,40,42,43,50–52,54–56,58–60 The section thickness was thinner than 4 mm in 7 studies,17,34,36,41,42,55,56,59 varied from 4 to 6 mm in 11 studies,31,33,35,37,40,43,50–52,57,58,60 and was not stated in 1 study.54 The in-plane resolution was described with the corresponding matrix in 11 studies31,34,36,37,40,42,43,50–52,54,57,58 but was not detailed in 7 studies.17,33,35,41,55,56,59,60
MRI definition of incident lacunes
The definition of lacunes varied among the different studies (details are given in Supplementary Table 1). In all studies, incident lacunes were defined as newly emerged cavities with cerebral spinal fluid intensity that were hyperintense on T2-weighted images and/or proton density images, and hypointense on T1-weighted images and/or T2 fluid-attenuated inversion recovery (FLAIR) images and analyzed as present or absent at individual level. In most studies (7 of 8 population-based studies17,28,33,34,36,37,55 and 7 of 11 other studies26,41–43,52,58,60), only focal lesions that were 3 mm or larger were considered as lacunes. In some studies,19,26,28,36,37,40–43,49,55,58–60 lacunes were considered for lesions with a maximum diameter of 10–20 mm after exclusion of larger or cortical infarcts. Only in few studies, the methods used to distinguish lacunes from dilated perivascular (Virchow–Robin) spaces were described. The definition criteria were based on the size of lesions (diameter >3 mm for lacunes to separate them from perivascular spaces),17,26,34,42,52,59 the presence of an hyperintense rim on FLAIR images,17,26,52,60 the hyperintense aspect on proton density,32,33 and according to the morphology and location of the lesion (round or ovoid shape for lacunes in contrast to linear shape along perforating arteries for perivascular spaces).34,43,55 After the STRIVE recommendations were reported in 2013, common diagnostic criteria of lacunes were used in all studies17,41,42,56–60 with the exception of two reports in 2014.49,51
Reliability and reproducibility of detection methods
The assessment of reliability and reproducibility both within and between-centers was sparsely performed. The intra-observer reliability (κ statistics) was reported only in two studies from 0.84 to 0.95.17,59 The inter-observer reliability was reported in four studies from 0.62 to 0.9517,52,58,59 (Supplementary Table 1). However, none of these studies specifically assessed the reproducibility of longitudinal measurements of lacunes (differences between the baseline and the follow-up scan).
Frequency of incident lacunes at individual level
Population-based studies
Incident lacunes were detected at individual level with a frequency ranging from 1.6% to 20.9% over 3.4 to 10.6 years of follow-up in eight population-based cohort studies (Table1).17,31,33,34,36,37,40,55 The frequency of incident lacunes among community residents varied from 0.4% per year in the 477 individuals between 60 and 64 years included in the Austrian Stroke Prevention Study (ASPS)36 to 4.0% per year in the 2612 independently living elderly individuals aged from 66 to 97 years included in the Age Gene/Environment Susceptibility-Reykjavik Study (AGES-Reykjavik) study from Iceland.17 In three studies of individuals whose mean age was younger than 64 years, the estimated frequency ranged from 0.4% to 1.9% per year,31,36,40 in five studies of subjects whose mean age was older than 70 years, this frequency ranged from 1.1% to 4.0% per year.17,33,34,37,55
Cohorts of individuals with cerebrovascular disease
The incidence of lacunes in patients with stroke or transient ischemic attacks (TIA) largely varied among studies (Table1). In a longitudinal single-center investigation of 60 patients with TIA followed up over one year from United Kingdom, the frequency of incident lacunes was as high as 36.6% per year.54 Conversely, in the St George’s Cognition and Neuroimaging in Stroke (SCANS) study, a longitudinal study of 121 patients with lacunar infarcts and confluent white matter hyperintensities (WMH) on MRI also obtained in United Kingdom, the frequency of incident lacunes was found only 9.5% per year.42 A lower frequency of 3.4% per year was reported in 359 patients with stroke or TIA from a single center in China participating in the VITAmins TO Prevent Stroke (VITATOPS) MRI-Substudy.49 In patients presenting with CSVD on imaging, the frequency of incident lacunes was found 6.0% per year among 396 independently elderly subjects with WMH recruited from 11 European centers participating in the Leukoaraiosis and Disability (LADIS) study.35 Two studies of elder patients with any CSVD on MRI reported the frequency of incident lacunes as 2.7% and 10.2% per year, respectively.59,60
Cohorts of individuals with vascular risk factors
The frequency of incident lacunes among 190 Japanese patients with type 2 diabetes was 4.1% per year in a single study.52 A close frequency of incident lacunes (3.9% per year) was found among 131 Japanese patients with non-valvular atrial fibrillation followed 2.7 years.51 Two studies in Netherlands reported the frequency of incident lacunes in patients with hypertension. In the hypertension and brain damage (HYBRiD) study of 90 individuals, a frequency of 1.4% per year was found.58 Among 126 individuals in the Prevention of Dementia by Intensive Vascular Care (preDIVA) trial, the frequency of incident lacunes was 2.3%.41 The Second Manifestations of Arterial Disease-Magnetic Resonance (SMART-MR) study reported a similar frequency among 663 Dutch patients with manifest artery disease followed up four years (Table1).44
Predictors of incident lacunes
Population-based studies
In seven out of eight studies obtained in the general population17,19,28,29,31–34,37–40 information on predictors of incident lacunes were available. A summary of the main predictors and their association with incident lacunes is presented in Table 2.
Age and sex
Age is the most consistent predictor of incident lacunes. In the Cardiovascular Health Study (CHS) and the Rotterdam Scan Study (RSS), a significant association was detected between incident lacunes and age over a mean follow-up of 5 and 3.4 years with an odds ratio (OR) ranging from 1.04 (95% confidence interval (CI): 1.01, 1.07)19 to 1.07 (95% CI: 1.03, 1.10, per decade).37 Among 1112 individuals in the Atherosclerosis Risk in Communities (ARIC) study who were evaluated over a median follow-up of 10.6 years, age was also reported to be associated with incident lacunes.30 The number of subjects with incident lacunes on MRI was 16.1% between age 55 and 59 years, 20.9% between 60 and 64 years, and 25.4% between 65 and 72 years.31
For sex, the results appear inconsistent. An adjusted risk ratio for incident lacunes of 1.5 (95% CI: 1.1, 1.9) was related to the male sex in the 2612 individuals of the AGES-Reykjavik study.17 Conversely, an adjusted OR of 1.63 (95% CI: 1.01, 2.65) for female sex was detected among 668 individuals included in the RSS.37 The sex impact was not investigated in most other studies.19,28,29,31–34,40
Blood pressure
In cross-sectional studies, hypertension was strongly associated with the prevalence of lacunes.61,62 Consistently, in longitudinal studies, hypertension at baseline was also found independently associated with incident lacunes in the ARIC study (adjusted OR (95%CI): 1.58 (1.08, 2.30)).31 Systolic blood pressure (SBP) was investigated in 3/7 studies at population level. Only in the ARIC study,31 SBP at baseline was found to predict incident lacunes. The significant results obtained after adjustment for age, sex and race (OR: 1.54 (1.25, 1.90)) in 1113 individuals, were no longer significant after further adjustment for education, coronary artery disease, alcohol use and tobacco use (1.20 (0.97, 1.48)).28 In the RSS and CHS cohorts, no significant association between SBP and incident lacunes was detected.19,37 The association between diastolic blood pressure (DBP) and incident lacunes was not found significant either.31,37
Dyslipidemia, diabetes and metabolic syndrome
Vascular risk factor including dyslipidemia, diabetes and metabolic syndrome was found to raise the risk of incident lacunes, but less consistently.
The potential impact of dyslipidemia was investigated in two studies.28,37 In the ARIC study, an increased risk of incident lacunes was observed with the level of triglycerides (adjusted OR (95%CI): 1.24 (1.04, 1.47)) and reduced risk with that of high-density lipoprotein (adjusted OR (95% CI): 0.77 (0.59, 0.99)).28 However, in the RSS, no significant association was observed with the level of total cholesterol or high-density lipoprotein was found.37
The diagnosis of diabetes was significantly associated with incident lacunes (adjusted OR (95%CI): 1.96 (1.23, 3.10)) in the ARIC study,31 whereas only a trend was observed in the RSS cohort after adjustment (adjusted OR (95%CI): 2.29 (0.98, 5.32)).37 A significant increase of incident lacune with the level of fasting glucose was also reported in the ARIC study (adjusted OR (95%CI): 1.27 (1.10, 1.46)). In the same cohort, a significant association between incident lacunes and the insulin resistance score (adjusted OR (95%CI): 1.33 (1.05, 1.68)) was also observed.28 More specifically, individuals in the highest tertile for both fasting blood glucose and systolic blood pressure at baseline had a risk of incident lacunes increased by 3.68 times (adjusted OR 95% CI: 1.89, 7.19) compared to subjects in the lowest tertile for both conditions.31
The metabolic syndrome (clustering of central obesity, hypertension, hyperglycemia, and dyslipidemia) (adjusted OR (95%CI): 1.98 (1.28, 3.05)) with central obesity measured by the waist/hip ratio (adjusted OR (95%CI): 1.30 (1.03, 1.65)) but not by the waist circumference alone (OR (95%CI): 1.05 (0.89, 1.25)) or body mass index (OR (95%CI): 1.01 (0.84, 1.21)) were also found associated with incident lacunes in the ARIC study.28
Smoking, atrial fibrillation and previous history of stroke
Studies selected in the review suggested that effects of vascular risk factors such as smoking, atrial fibrillation and previous history of stroke on incident lacunes might be less than anticipated. A previous history of stroke was not found associated with incident lacunes in the ARIC study (adjusted OR (95%CI): 1.02 (0.33, 3.11)).31 In the RSS, current smoking (OR (95%CI): 1.10 (0.56, 2.14)) or atrial fibrillation (OR (95%CI): 0.68 (0.15, 3.23))37 was not found associated with incident lacunes.
Vascular and MRI markers
The association between incident lacunes and markers of vascular alterations or MRI lesions was found relatively consistent.
A significant association between retinal hemorrhages and incident lacunes was observed in the ARIC study with OR as high as 3.25 (95%CI: 1.18, 8.92).32 In line, individuals with the largest venular diameters had a trend to develop more incident lacunes in the RSS cohort (OR (95%CI): 1.24 (0.72, 2.12))38 suggesting that some pathophysiological processes underlying diabetic and hypertensive retinopathy are shared by CSVD.32
In the RSS study, carotid atherosclerosis was also identified as a significant predictor of incident lacunes for both carotid intima media thickness (OR: 1.27 per SD (95%CI: 1.01, 1.61)), and the presence of carotid plaques (OR: 1.17 (95%CI: 1.00, 1.37)).37 Moreover, in the CHS, peripheral arterial alterations measured by the ankle-arm index was also found to be an independent risk factor of incident lacunes (adjusted OR (95%CI): 0.36 (0.14, 0.88)).19
At the cerebral level, tissue lesions related to the presence of CSVD as the severity of WMH observed at baseline was found to predict incident infarcts in the CHS cohort (per grade adjusted OR (95%CI): 2.09 (1.21, 3.60)).19 In line, at the kidney level, a significant association was reported between creatinine level presumably related to small vessel alterations and incident lacunes (adjusted OR (95%CI): 1.57 (1.40, 1.75)).19
Patients with cerebrovascular disease
In elderly subjects with MRI, white-matter hyperintensities from the LADIS study, systolic blood pressure, high-density lipoprotein, baseline lacunes and baseline white matter hyperintensities were found to be independent predictors of incident lacunes (adjusted OR (95%CI): 2.1 (1.4, 3.3), 0.5 (0.3, 0.7), 1.2 (1.0, 1.3) and 2.1 (1.2, 3.6), respectively) (Supplementary Table 2).35 However, an unexpected negative association was also detected with high values of diastolic blood pressure (adjusted OR (95% CI): 0.6 (0.4, 0.9)) and of low-density lipoprotein (adjusted OR (95%CI): 0.5 (0.3, 1.0)) in the same analysis.35 In contrast, age, female sex, previous stroke, hypertension, total cholesterol, triglycerides, diabetes, glucose, body mass index, smoking, atrial fibrillation and myocardial infarction were not found significant in multivariate analysis.35 In patients with CSVD, the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Imaging Cohort (RUN DMC) study found that plasma Aβ38 levels were independently associated with incident lacunes,59 and the Significance of Haemodynamic and Haemostatic Factors in the Course of Different Manifestations of Cerebral Small Vessel Disease (SHEF-CSVD) Study reported that homocysteine and Z-score for a set of markers related to systemic inflammation were associated with incident lacunes.60 In patients with stroke or TIA, the results of the VITATOPS study have not shown that the pre-stroke status49 or B-vitamin supplementation50 were associated with incident lacunes.
Patients with vascular risk factors
In patients with manifest arterial disease of the SMART-MR study, age, male sex, cerebrovascular disease46 and hyperhomocysteinemia47 were found to be independent predictors of incident lacunes (adjusted OR (95%CI): 4.61 (2.06, 10.33), 3.29(1.84, 5.86), 2.15 (1.01, 4.58) and 1.19 (1.10, 3.60), respectively) (Supplementary Table 3). Other risk factors such as the parenchymal cerebral blood flow,43 number of organ damage related to hypertension,44 stiffening of carotid arteries45 and serum angiotensin-converting-enzyme were not.48 The same study further found that hyperhomocysteinemia was only independently associated with incident lacunes in the basal ganglia. In contrast, hypertension and progression of WMH volume were independent risk factors for incident lacunes in the deep white matter.57 In a single study of type 2 diabetic patients, a significant association between the baseline serum level of soluble intercellular adhesion molecule-1 whose expression is induced by elevated blood glucose in the vascular endothelium (1.67 (95% CI 1.02 to 3.05)) and incident lacunes were found at six-year follow-up; however, the significant association between baseline high-sensitivity C-reactive protein levels (1.54 (95% CI 1.01 to 2.36)) and incident lacunes was only found at three-year follow-up.52 In a single study of patients with atrial fibrillation, the number of cerebral microbleeds at baseline was significantly associated with the occurrence of incident lacunes (hazard ratio (HR), 5.414; 95% CI 1.03–28.43).51 In the preDIVA trial performed in patients with hypertension, no significant effect of long-term multidomain intensive vascular care (OR (95%CI): 2.2 (0.4–12.1)) was detected on incident lacunes.41
Clinical impact
The clinical significance of incident lacunes was finally depicted in five population-based studies, in a single study of patients with cerebrovascular disease and in another one of patients with vascular risk factors17,18,26,31,37,53,55 (Table 3).
Stroke and other vascular events
As expected, a strong association was observed between incident lacunes and incident stroke in the 1112 subjects without any history of stroke included in the ARIC study, incident lacunes were found associated with the occurrence of stroke during the follow-up (adjusted OR (95%CI): 19.4 (6.41–58.70)).31 More interestingly, in participants without adjudicated stroke or TIA but with a progression of covert MRI-defined brain vascular disease including the occurrence of incident lacunes, an increased risk of incident heart failure, stroke, and death was observed in the CHS study.18
Cognitive decline
Evidence from two population-based studies supports that incident lacunes may have an important role in the development of cognitive decline and decrease of motor performances.17,37 The results of AGES-Reykjavik study showed that after adjustment for age, sex, and time interval between MRI scans, the risk of incident dementia became 50% higher when incident lacunes are detected.17 Incident lacunes were also associated with the global cognitive and psychomotor speed decline but not with memory performances or incident dementia in the RSS cohort.37 The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study showed that incident lacunes in individuals aged 75–80 years were associated with impairment of executive function examined by the Trail Making Tests A.55 This impact was also confirmed in patients with WMH in the LADIS study where an association of incident lacunes with decline in speed motor control or with executive functions after three years.26 No association was detected between incident lacunes and cognitive tests in type 2 diabetic patients evaluated in a single Japanese study.53
Discussion
This review shows that the methods reported for assessing incident lacunes on MRI in the literature are largely heterogeneous. Three major sources of heterogeneity are identified in line with the results of a comparative analysis obtained at cross-sectional level by Zhu et al.63 First, variable sequence parameters were chosen for MRI examination in different centers and specific efforts of homogenization between centers or between repeated examinations were not systematically reported.64 Protocol harmonization is however crucial in multicenter and longitudinal studies to obtain sequences with acquisition parameters as close as possible for comparative assessment. Also, MRI data were not constantly acquired at high resolution and only a single study was performed at 3 Tesla which is better for detecting millimetric lesions in the clinical setting.65 A gap between slices was even present in 32% of studies. Second, variable diagnostic criteria were chosen for identifying lacunes which were also based on a variable number of sequences among studies. In the CHS study, the definition of lacunes even differs between the white and gray matter (Supplementary Table 1). Besides, the reproducibility of longitudinal measurements was sparsely evaluated and the assessment of incident lesions was never described in details. Third, variable strategies were used for separating small cavities secondary to infarctions from dilated perivascular (Virchow–Robin) spaces. Moreover, although the combination of both lesions is strongly increasing with age,37,42,66 their distinction was not even considered in 43% studies. This task is obviously needed for reducing misclassification of lacunes on imaging data but remains challenging and time-consuming.63 Automated and validated image-processing methods are not available yet for this purpose although promising techniques are emerging.64,67
Despite these limitations, data from the present review show that the occurrence of incident lacunes may widely vary among different populations. Variations related to the different MRI parameters and diagnostic criteria, alone, cannot explain the large differences detected between the frequency of incident lesions in population-based studies from 0.5 to 4%, in individuals with only vascular risk factors from 2.3 to 4.1% and in patients with cerebrovascular disease from 2.7 to 36.6%. This discrepancy might rather reflect different stages of the underlying CSVD in different samples. In the largest cohorts obtained from the general population, the effect of aging on incident lacunes was found highly significant.37,42 In the ARIC study, data support even an exponential increase of lacunes with aging after 60 years of age.31 This is in line with the strong association repeatedly observed at cross-sectional level between age and the prevalence of lacunes.61,62
In population-based studies, various risk factors were also found to predict incident lacunes on MRI independently of age such as hypertension, diabetes, metabolic syndrome or obesity detected at baseline.28,31 Some vascular markers were also found significantly related to incident lacunes. Thus, alterations of the retinal microvasculature32 as well as alterations in the wall of carotid37 or peripheral arteries19 were found associated with the occurrence of lacunes at the cerebral level. This further supports diffuse vascular changes related to common etiological factors in CSVD. The results observed for sex, blood pressure measures and dyslipidemia are inconsistent across studies17,19,28,31,37 but a real impact cannot be ruled out. The effects of such factors may largely vary according to the selected sample, duration of exposure and preventive treatments currently used during follow-up. The variable methods, diagnostic criteria, sample size and adjustments may also account for these discrepancies. Noteworthily, the potential relationships between the exact blood pressure level during follow-up and the risk of incident lacunes were not previously investigated. The lack of association with a previous history of stroke, smoking, atrial fibrillation or inflammatory biomarkers suggest that these factors might reflect other mechanisms, have changed during follow-up or have only small and undetectable effects due to the various limitations described above. On the other hand, the strong association between incident lacunes and prevalent WMH and lacunes indicates that at a certain stage of CSVD, the pathogenic mechanism leading to the accumulation of cerebral lesions may either accelerate or become self-reinforcing leading to an exponential progression of focal ischemic lesions.
The clinical impact of incident lacunes was in line with data obtained in previous cross-sectional studies. In the AGES-Reykjavik cohort from the general population, incident lacunes were found related to an increased risk of dementia.17 These results are similar to those obtained in two earlier cross-sectional studies in which the overall lacunes were shown to increase the risk of dementia.68,69 In the RSS, PIVUS and LADIS studies, incident lacunes participated in the reduction of mental and motor speed and in the alteration of executive functions, while memory was essentially unaffected. These findings are consistent with previous cross-sectional studies showing that the load of lacunes was associated with poorer executive abilities but not with memory performance in elderly populations.70,71 This pattern of cognitive changes is possibly related to the preponderance of lacunes in the anterior and central subcortical areas and to the lack of lesions in the hippocampus related to CSVD.12,72 Nevertheless, all these results support that accumulation of lacunes can play a causative role in cognitive decline.24 In line, accumulating evidence suggests that incident lacunes may also have a major impact on the cortex function and integrity.22,23 Interestingly, the association between incident lacunes and cognitive performances is not constantly detected. In the RSS cohort, incident lacunes were not found associated with global cognitive changes.37 Another study of elderly subjects with diabetes also failed to show a significant association between incident lacunes and cognitive decline.53 Multiple factors may explain this discrepancy as differences in study samples, risk factor distributions, imaging techniques and also the number of incident lacunes during follow-up and their exact location.
In conclusion, this review suggests that the frequency of incident lacunes is strongly related to the previous clinical and MRI status, and that both age and hypertension diagnosed at baseline are major predictors of such lesions. A potential influence of systolic or diastolic blood pressure level at onset remains uncertain and the impact of blood pressure during follow-up has not been investigated. Their clinical impact on cognition was repeatedly observed but their exact consequences on motor or gait performances or on other skill would need further investigations. Efforts of homogenization in imaging techniques and strict diagnostic criteria as following STRIVE standards are needed for evaluating the risk factors and natural history of incident lacunes on MRI. The assessment of incident lacunes should not present major technical difficulties and might become an important marker to follow objectively the progression of CSVDs and the effect of future preventative treatments of CSVD.
Supplemental Material
Supplemental material, JCB908361 Supplemental Material for Incident cerebral lacunes: A review by Yifeng Ling and Hugues Chabriat in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Association de Recherche en NEurologie Vasculaire (ARNEVA) and RHU (Recherche Hospitalo Universitaire) TRT_cSVD (Treat Cerebral Smal Vessel Diseases) (from Agence Nationale de la Recherche, France (Ministére de la Recherche, France).)
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
YLand HC designed the study. YL collected and analyzed the data. YL and HC wrote the initial draft of the paper. Both authors carefully revised the manuscript.
ORCID iD
Hugues Chabriat https://orcid.org/0000-0001-8436-6074
Supplemental material
Supplemental material for this article is available online.
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Román GC.On the history of lacunes, etat criblé, and the white matter lesions of vascular dementia. Cerebrovasc Dis 2002; 13(Suppl 2): 1–6. [DOI] [PubMed] [Google Scholar]
- 3.Durand-Fardel M.Mémoire sur une altération particulière de la substance cérébrale. Gaz Méd Paris 1842; 10: 23–26, 33–38. [Google Scholar]
- 4.Durand-Fardel M.Traité clinique et pratique des maladies des Vieillards. Paris: JB Baillière, 1854. [Google Scholar]
- 5.Fisher CM.Lacunes: small, deep cerebral infarcts. Neurology 1965; 15: 774–784. [DOI] [PubMed] [Google Scholar]
- 6.Fisher CM.The arterial lesions underlying lacunes. Acta Neuropathol 1968; 12: 1–15. [DOI] [PubMed] [Google Scholar]
- 7.Binswanger O Ludwig.Die Abgrenzung der allgemeinen progressiven paralyse. Berl Klin Wochenschr 1894; 31: 1103–1105, 1137–1139, 1180–1186. [Google Scholar]
- 8.Alzheimer A.Die Seelenstörungen auf arteriosklerotischer Grundlage. Allg Z Psychiatr Psych Med 1902; 59: 695–711. [Google Scholar]
- 9.Marie P.Des foyers lacunaires de désintégration et des differents autres états cavitaires du cerveau. Rev Méd 1901; 21: 281–298. [Google Scholar]
- 10.Román GC.The identity of lacunar dementia and Binswanger disease. Med Hypotheses 1985; 16: 389–391. [DOI] [PubMed] [Google Scholar]
- 11.Lopes MA, Firbank MJ, Widdrington M, et al. Post-stroke dementia: the contribution of thalamus and basal ganglia changes. Int Psychogeriatr 2012; 24: 568–576. [DOI] [PubMed] [Google Scholar]
- 12.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003; 348: 1215–1222. [DOI] [PubMed] [Google Scholar]
- 13.Loos CMJ, Staals J, Wardlaw JM, et al. Cavitation of deep lacunar infarcts in patients with first-ever lacunar stroke a 2-year follow-up study with MR. Stroke 2012; 43: 2245–2247. [DOI] [PubMed] [Google Scholar]
- 14.Loos Caroline MJ, Makin Stephen DJ, Staals Julie, et al. Long-term morphological changes of symptomatic lacunar infarcts and surrounding white matter on structural magnetic resonance imaging. Stroke 2018; 49: 1183–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinter D, Gattringer T, Enzinger C, et al. Longitudinal MRI dynamics of recent small subcortical infarcts and possible predictors. J Cereb Blood Flow Metab 2018; 39(9): 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith EE, Schneider JA, Wardlaw JM, et al. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 2012; 11: 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigurdsson S, Aspelund T, Kjartansson O, et al. Incidence of brain infarcts, cognitive change, and risk of dementia in the general population: the AGES-Reykjavik study (age gene/environment susceptibility-Reykjavik study). Stroke 2017; 48: 2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Longstreth WT, Arnold AM, Kuller LH, et al. Progression of magnetic resonance imaging-defined brain vascular disease predicts vascular events in elderly. Stroke 2011; 42: 2970–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longstreth WT, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly the cardiovascular health study. Stroke 2002; 33: 2376–2382. [DOI] [PubMed] [Google Scholar]
- 20.Chabriat H, Pappata S, Poupon C, et al. Clinical severity in CADASIL related to ultrastructural damage in white matter in vivo study with diffusion tensor MRI. Stroke 1999; 30: 2637–2643. [DOI] [PubMed] [Google Scholar]
- 21.Chabriat H, Hervé D, Duering M, et al. Predictors of clinical worsening in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy prospective cohort study. Stroke 2016; 47: 4–11. [DOI] [PubMed] [Google Scholar]
- 22.Jouvent E, Viswanathan A, Mangin J-F, et al. Brain atrophy is related to lacunar lesions and tissue microstructural changes in CADASIL. Stroke 2007; 38: 1786–1790. [DOI] [PubMed] [Google Scholar]
- 23.Duering M, Righart R, Csanadi E, et al. Incident subcortical infarcts induce focal thinning in connected cortical regions. Neurology 2012; 79: 2025–2028. [DOI] [PubMed] [Google Scholar]
- 24.Ling Y, De Guio F, Duering M, et al. Predictors and clinical impact of incident lacunes in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke 2017; 48: 283–289. [DOI] [PubMed] [Google Scholar]
- 25.Ling Y, De Guio F, Jouvent E, et al. Clinical correlates of longitudinal MRI changes in CADASIL. J Cereb Blood Flow Metab 2019; 39: 1299–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jokinen H, Gouw AA, Madureira S, et al. Incident lacunes influence cognitive decline: the LADIS study. Neurology 2011; 76: 1872–1878. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt R, Seiler S, Loitfelder M.Longitudinal change of small-vessel disease-related brain abnormalities. J Cereb Blood Flow Metab 2016; 36: 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dearborn JL, Schneider ALC, Sharrett AR, et al. Obesity, insulin resistance, and incident small vessel disease on magnetic resonance imaging atherosclerosis risk in communities study. Stroke 2015; 46: 3131–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haring B, Misialek JR, Rebholz CM, et al. Association of dietary protein consumption with incident silent cerebral infarcts and stroke: the atherosclerosis risk in communities (ARIC) study. Stroke 2015; 46: 3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Windham BG, Griswold ME, Shibata D, et al. Covert neurological symptoms associated with silent infarcts from midlife to older age the atherosclerosis risk in communities study. Stroke 2012; 43: 1218–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopman DS, Penman AD, Catellier DJ, et al. Vascular risk factors and longitudinal changes on brain MRI. Neurology 2011; 76: 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung N, Mosley T, Islam A, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain 2010; 133: 1987–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virtanen JK, Siscovick DS, Lemaitre RN, et al. Circulating omega‐3 polyunsaturated fatty acids and subclinical brain abnormalities on MRI in older adults: the cardiovascular health study. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2013; 2: e000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satizabal CL, Zhu YC, Mazoyer B, et al. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon study. Neurology 2012; 78: 720–727. [DOI] [PubMed] [Google Scholar]
- 35.Gouw AA, Flier WM van der, Fazekas F, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period the leukoaraiosis and disability study. Stroke 2008; 39: 1414–1420. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Wen W, Anstey KJ, et al. Prevalence, incidence, and risk factors of lacunar infarcts in a community sample. Neurology 2009; 73: 266–272. [DOI] [PubMed] [Google Scholar]
- 37.van Dijk EJ, Prins ND, Vrooman HA, et al. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences Rotterdam scan study. Stroke 2008; 39: 2712–2719. [DOI] [PubMed] [Google Scholar]
- 38.Ikram MK, Jong FJD, Dijk EJV, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam scan study. Brain 2006; 129: 182–188. [DOI] [PubMed] [Google Scholar]
- 39.van Dijk EJ, Prins ND, Vermeer SE, et al. C-reactive protein and cerebral small-vessel disease the Rotterdam scan study. Circulation 2005; 112: 900–905. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt R, Schmidt H, Pichler M, et al. C-reactive protein, carotid atherosclerosis, and cerebral small-vessel disease results of the Austrian stroke prevention study. Stroke 2006; 37: 2910–2916. [DOI] [PubMed] [Google Scholar]
- 41.van Dalen JW, Moll van Charante EP, Caan MWA, et al. Effect of long-term vascular care on progression of cerebrovascular lesions: magnetic resonance imaging substudy of the PreDIVA trial (prevention of dementia by intensive vascular care). Stroke 2017; 48: 1842–1848. [DOI] [PubMed] [Google Scholar]
- 42.Benjamin P, Zeestraten E, Lambert C, et al. Progression of MRI markers in cerebral small vessel disease: sample size considerations for clinical trials. J Cereb Blood Flow Metab 2016; 36: 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Veen PH, Muller M, Vincken KL, et al. Longitudinal relationship between cerebral small-vessel disease and cerebral blood flow the second manifestations of arterial disease-magnetic resonance study. Stroke 2015; 46: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 44.van der Veen PH, Geerlings MI, Visseren FLJ, et al. Hypertensive target organ damage and longitudinal changes in brain structure and function: the second manifestations of arterial disease-magnetic resonance study. Hypertension 2015; 66: 1152–1158. [DOI] [PubMed] [Google Scholar]
- 45.Jochemsen HM, Muller M, Bots ML, et al. Arterial stiffness and progression of structural brain changes: the SMART-MR study. Neurology 2015; 84: 448–455. [DOI] [PubMed] [Google Scholar]
- 46.van der Veen PH, Muller M, Vincken KL, et al. Longitudinal changes in brain volumes and cerebrovascular lesions on MRI in patients with manifest arterial disease: the SMART-MR study. J Neurol Sci 2014; 337: 112–118. [DOI] [PubMed] [Google Scholar]
- 47.Kloppenborg RP, Geerlings MI, Visseren FL, et al. Homocysteine and progression of generalized small-vessel disease: the SMART-MR Study. Neurology 2014; 82: 777–783. [DOI] [PubMed] [Google Scholar]
- 48.Jochemsen HM, Geerlings MI, Grool AM, et al. Angiotensin-converting enzyme and progression of white matter lesions and brain atrophy – the SMART-MR study. J Alzheimers Dis JAD 2012; 29: 39–49. [DOI] [PubMed] [Google Scholar]
- 49.Xiong Y, Wong A, Cavalieri M, et al. Prestroke statins, progression of white matter hyperintensities, and cognitive decline in stroke patients with confluent white matter hyperintensities. Neurotherapeutics 2014; 11: 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cavalieri M, Schmidt R, Chen C, et al. B Vitamins and magnetic resonance imaging–detected ischemic brain lesions in patients with recent transient ischemic attack or stroke the VITAmins to prevent stroke (VITATOPS) MRI-substudy. Stroke 2012; 43: 3266–3270. [DOI] [PubMed] [Google Scholar]
- 51.Saito T, Kawamura Y, Tanabe Y, et al. Cerebral microbleeds and asymptomatic cerebral infarctions in patients with atrial fibrillation. J Stroke Cerebrovasc Dis 2014; 23: 1616–1622. [DOI] [PubMed] [Google Scholar]
- 52.Umemura T, Kawamura T, Umegaki H, et al. Endothelial and inflammatory markers in relation to progression of ischaemic cerebral small-vessel disease and cognitive impairment: a 6-year longitudinal study in patients with type 2 diabetes mellitus. J Neurol Neurosurg Psychiatry 2011; 82: 1186–1194. [DOI] [PubMed] [Google Scholar]
- 53.Imamine R, Kawamura T, Umemura T, et al. Does cerebral small vessel disease predict future decline of cognitive function in elderly people with type 2 diabetes? Diabetes Res Clin Pract 2011; 94: 91–99. [DOI] [PubMed] [Google Scholar]
- 54.Walters RJL, Fox NC, Schott JM, et al. Transient ischaemic attacks are associated with increased rates of global cerebral atrophy. J Neurol Neurosurg Psychiatry 2003; 74: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nylander R, Kilander L, Ahlström H, et al. Small vessel disease on neuroimaging in a 75-year-old cohort (PIVUS): comparison with cognitive and executive tests. Front Aging Neurosci 2018; 10: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nylander R, Fahlström M, Rostrup E, et al. Quantitative and qualitative MRI evaluation of cerebral small vessel disease in an elderly population: a longitudinal study. Acta Radiol 2018; 59: 612–618. [DOI] [PubMed] [Google Scholar]
- 57.Kloppenborg RP, Nederkoorn PJ, Grool AM, et al. Do lacunar infarcts have different aetiologies? Risk factor profiles of lacunar infarcts in deep white matter and basal ganglia: the second manifestations of arterial disease-magnetic resonance study. Cerebrovasc Dis 2017; 43: 161–168. [DOI] [PubMed] [Google Scholar]
- 58.Uiterwijk R, Staals J, Huijts M, et al. MRI progression of cerebral small vessel disease and cognitive decline in patients with hypertension. J Hypertens 2017; 35: 1263–1270. [DOI] [PubMed] [Google Scholar]
- 59.van Leijsen EMC, Kuiperij HB, Kersten I, et al. Plasma Aβ (Amyloid-β) levels and severity and progression of small vessel disease. Stroke 2018; 49: 884–890. [DOI] [PubMed] [Google Scholar]
- 60.Staszewski J, Piusińska-Macoch R, Brodacki B, et al. IL-6, PF-4, sCD40 L, and homocysteine are associated with the radiological progression of cerebral small-vessel disease: a 2-year follow-up study. Clin Interv Aging 2018; 13: 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ.Silent brain infarcts: a systematic review. Lancet Neurol 2007; 6: 611–619. [DOI] [PubMed] [Google Scholar]
- 62.Fanning JP, Wong AA, Fraser JF.The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med 2014; 12: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu Y-C, Dufouil C, Tzourio C, et al. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke 2011; 42: 1140–1145. [DOI] [PubMed] [Google Scholar]
- 64.De Guio F, Jouvent E, Biessels GJ, et al. Reproducibility and variability of quantitative magnetic resonance imaging markers in cerebral small vessel disease. J Cereb Blood Flow Metab 2016; 36: 1319–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Veluw SJ, Charidimou A, van der Kouwe AJ, et al. Microbleed and microinfarct detection in amyloid angiopathy: a high-resolution MRI-histopathology study. Brain 2016; 139: 3151–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu Y-C, Tzourio C, Soumare A, et al. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke 2010; 41: 2483–2490. [DOI] [PubMed] [Google Scholar]
- 67.Ghafoorian M, Karssemeijer N, Heskes T, et al. Deep multi-scale location-aware 3D convolutional neural networks for automated detection of lacunes of presumed vascular origin. NeuroImage Clin 2017; 14: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham offspring study. Stroke 2010; 41: 600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 2003; 22: 13–22. [DOI] [PubMed] [Google Scholar]
- 70.Geerlings MI, Appelman AP, Vincken KL, et al. Association of white matter lesions and lacunar infarcts with executive functioning: the SMART-MR study. Am J Epidemiol 2009; 170: 1147–55. [DOI] [PubMed] [Google Scholar]
- 71.Carey CL, Kramer JH, Josephson SA, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke 2008; 39: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duering M, Gonik M, Malik R, et al. Identification of a strategic brain network underlying processing speed deficits in vascular cognitive impairment. NeuroImage 2013; 66: 177–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB908361 Supplemental Material for Incident cerebral lacunes: A review by Yifeng Ling and Hugues Chabriat in Journal of Cerebral Blood Flow & Metabolism


