Abstract
Computed tomography perfusion (CTP) allows the estimation of pretreatment ischemic core after acute ischemic stroke. However, CTP-derived ischemic core may overestimate final infarct volume. We aimed to evaluate the accuracy of CTP-derived ischemic core for the prediction of final infarct volume according to time from stroke onset to recanalization in 104 patients achieving complete recanalization after mechanical thrombectomy who had a pretreatment CTP and a 24-h follow-up MRI-DWI. A range of CTP thresholds was explored in perfusion maps at constant increments for ischemic core calculation. Time to recanalization modified significantly the association between ischemic core and DWI lesion in a non-linear fashion (p-interaction = 0.018). Patients with recanalization before 4.5 h had significantly lower intraclass correlation coefficient (ICC) values between CTP-predicted ischemic core and DWI lesion (n = 54; best threshold relative cerebral blood flow (rCBF) < 25%, ICC = 0.673, 95% CI = 0.495–0.797) than those with later recanalization (n = 50; best threshold rCBF < 30%, ICC = 0.887, 95% CI = 0.811–0.935, p = 0.013), as well as poorer spatial lesion agreement. The significance of the associations between CTP-derived ischemic core and clinical outcome at 90 days was lost in patients recanalized before 4.5 h. CTP-derived ischemic core must be interpreted with caution given its dependency on time to recanalization, primarily in patients with higher chances of early recanalization.
Keywords: Computed tomography perfusion, ischemic core, ischemic stroke, recanalization, thrombectomy
Introduction
Imaging has a central role for the evaluation of tissue viability, vessel permeability and collateral circulation in acute ischemic stroke.1,2 Diffusion-weighted imaging (DWI) on magnetic resonance imaging (MRI) has been established as the gold standard for the quantification of ischemic core, due to its high sensitivity and reproducibility.2,3 Computed tomography perfusion (CTP) imaging is also used to assess the hemodynamic state of brain parenchyma and may provide similar ischemic core estimations as those derived from MRI.4–7 However, the main limitation for the extended use of CTP is the imprecision of the optimal perfusion parameters used for the quantification of ischemic core, in part due to its poor signal-to-noise ratio and to the variability of pre- and post-processing platforms.2,7–10
Current data support the use of relative cerebral blood flow (CBF) with a threshold of 30% as the most accurate CTP estimator of ischemic core.4,6 However, a current concern is that several clinical, radiological and acute treatment variables may affect the predictive capacity of the CTP parameters and thresholds.2,7–12 Specifically, the impact of achieving an early and complete reperfusion on the capacity of a single CTP threshold to obtain a reliable prediction of final infarct volume or clinical outcomes is still uncertain.8,13–21
Under the hypothesis that early reperfusion may impair the predictive capacity of CTP, we aimed to explore the impact of the timing of complete reperfusion on the accuracy of CTP for predicting final infarct volume and clinical outcomes through a comprehensive analysis of perfusion parameters and thresholds.
Material and methods
Subjects
Patients were part of a prospectively collected clinical registry of acute stroke patients treated with reperfusion therapies between March 2010 and December 2017 in a referral comprehensive stroke center. The specific selection criteria for this analysis were: 1/ availability of a pretreatment whole-brain CTP scan, 2/ the presence of a proximal intracranial arterial occlusion in the terminal internal carotid artery or the M1 segment of the middle cerebral artery without tandem occlusions, 3/ complete reperfusion after mechanical thrombectomy (MT), and 4/ availability of a follow-up MRI imaging performed within the first 24–72 h after stroke onset. According to these criteria, a total of 104 consecutive patients were retrospectively analyzed, as shown in the flowchart (Supplementary Figure 1). Ethics approval was acquired from the local Clinical Research Ethics Committee from Hospital Clinic of Barcelona under the requirements of Spanish legislation in the field of biomedical research, the protection of personal data (15/1999) and the standards of Good Clinical Practice, as well as with the Helsinki Declaration of 1975/1983. Patient consent was not required due to the retrospective nature of the study design and the lack of patient interaction. We adhered to the STARD (Standards for Reporting of Diagnostic Accuracy) guidelines (http://www.stard-statement.org/).
Demographics, clinical course, baseline neurological status (monitored with the National Institutes of Health Stroke Scale score, NIHSS),22 stroke subtype (according to the Trial of Org 10 172 in Acute Stroke Treatment criteria, TOAST),23 functional outcome (scored with the modified Rankin Scale score, mRS) at 90 days, and reperfusion-therapy modality (primary or rescue MT) were prospectively recorded on all patients. In this cohort of patients, reperfusion therapies were administered following contemporary guideline recommendations. MT was performed within 8 h in eligible patients with proximal arterial occlusion on CT angiography. The presence of a CTP-defined infarct core volume higher than 70 mL, an ASPECTS score lower than 6 or the absence of mismatch in patients with symptoms lasting >4.5 h from stroke onset typo-redundant were considered as exclusion criteria for receiving MT. The mismatch profile was defined as the presence of a hypoperfusion lesion that was equal or higher than 120% of the infarct core. In patients treated with rescue MT after systemic thrombolysis, rtPA infusion was interrupted prematurely according to endovascular suite availability at the time of groin puncture.
CT perfusion imaging
Patients were scanned using a SIEMENS Somatom Definition Flash 128-section dual-source multidetector scanner (Siemens Healthineers, Erlangen, Germany), with a 98 mm z-coverage and 26 time points acquired each 1.5 s (total acquisition time, 39 s). Fifty milliliters of nonionic iodinated contrast were administered intravenously at 5 mL/s by using a power injector, followed by a saline flush of 20 mL at an injection rate of 2 mL/s. The imaging protocol included a baseline multimodal whole-brain CT scan, which included a plain CT (140 Kv, 127 mAs, FoV 225 mm, matrix 512 × 512, slice thickness 5 mm), a CT angiography (120 Kv, 663 mAs, FoV 261 mm, matrix 512 × 512, slice thickness 0.6 mm), and a CT perfusion (80 kV peak, 250 mAs, 1.5-s rotation, FoV 18 mm, matrix 512 × 512, and 49 2-mm thickness slices). CTP maps were then calculated by commercial software MIStar (Apollo Medical Imaging Technology, Melbourne, Australia) using a model-free singular value decomposition (SVD) algorithm with a delay and dispersion correction. The software automatically performs motion correction and selects an arterial input function (AIF) from an unaffected artery (usually the anterior cerebral artery) and a venous output function (VOF) from a large draining vein (the sagittal sinus). The software generates CBF, CBV, MTT and delay time (DT) maps. Of note, the delay corrected deconvolution method produces DT maps rather than the more extensively used Tmax maps. An image processing pipeline using in-house fully automated software running in Matlab (v.2017b, Mathworks, Natick, MA) was developed in order to implement a comprehensive analysis of the perfusion maps. An absolute threshold of 3 s was selected on the DT map to obtain the hypoperfused tissue (perfusion lesion). Inside this area, a range of relative and absolute thresholds was explored in the CBF and CBV maps at constant increments, as shown in Table 1. The relative thresholds were calculated as a percentage of mean perfusion values from the entire unaffected/contralateral hemisphere.
Table 1.
Range of thresholds explored in CT perfusion maps.
| CTP map | Parameter | Range of explored thresholds | Increments |
|---|---|---|---|
| CBF | aCBF | 0 to 36 mL/100 g/min | 2 mL/100 g/min |
| rCBF | 0 to 100% | 5% | |
| CBV | aCBV | 0 to 2.0 mL/100 g | 0.1 mL/100 g |
| rCBV | 0 to 100% | 5% |
Angiographic assessment
The occlusion was graded on dynamic subtraction angiography (DSA) according to the modified thrombolysis in cerebral infarction (mTICI) classification (Grade 0: no perfusion; Grade 1: minimal flow past the occlusion but no perfusion; Grade 2 a: partial reperfusion of less than half of the entire vascular territory; Grade 2 b: partial reperfusion of more than half of the entire vascular territory; Grade 3: complete reperfusion without any flow defects). Recanalization was defined as complete if a grade 3 was obtained at the end of the MT. The mTICI score was graded by the interventionist team at the end of the procedure.
MRI imaging
The follow-up MRI was performed within a median (IQR) 38 h (20–64 h) from treatment and included diffusion-weighted images (DWI, parameters: Repetition time (TR)/echo time (TE) 10,800/89 ms, matrix 192 × 192, field of view (FoV) 240 mm, slice thickness 3 mm, directions x,y,z, b-values: 0 and 1000 mm/s2) and gradient echo T2*-weighted (GRE: TR/TE 764/26 ms; matrix 384 × 512; FoV 240 mm; slice thickness 5 mm) sequences. DWI lesion was delineated using AMIRA software by means of a semi-automated thresholding method to identify regions of interest with high DWI signal intensity (selection of pixels exceeding the DWI signal intensity of the mean values in the contralateral hemisphere by more than three standard deviations), as shown in Supplementary Figure 2.24 In order to assure the comparability between pretreatment ischemic core CTP predictions and final DWI lesion, each CTP map was co-registered to the corresponding follow-up DWI using a rigid co-registration protocol (6-degrees of freedom) implemented with statistical parametric mapping (SPM12, Functional Imaging Laboratory, University College London, London, UK), as summarized in Supplementary Figure 2. CTP and DWI lesion overlap and spatial agreement were assessed using the Dice similarity coefficient that was calculated using the Imaging Processing Toolbox (MATLAB v.2017b, Mathworks, Natick, MA).
Statistical analysis
Continuous variables were reported as mean and standard deviation (SD) or as median and interquartile range (IQR) and were compared with the Student t or Mann–Whitney tests. Categorical variables were reported as proportions and compared with the χ2 and Fisher exact test. To assess the correlation between CTP-predicted ischemic core and final DWI lesion volume on MRI, we conducted simple linear regression and intra-class correlation coefficient (ICC) and its 95% confidence intervals (95% CI). We used the ICC (two-way mixed model, absolute agreement for single measures) beyond other association tests to study the repeatability between the two repeated measurements of ischemic core, as the ICC estimation reflects both the degree of correlation and agreement between measurements of continuous data.25 Reliability was considered to be good when the ICC was 0.80 or higher. To assess the consistency of the optimal parameter thresholds for predicting ischemic core found in the whole population, a sensitivity analysis including only those patients in whom tissue time attenuation curves (TACs) were not truncated at the end of the CTP acquisition was performed. For this subset analysis, truncated curves were defined as those in which the TACs did not have return of the venous outflow function to at least 50% of peak and not truncated curves as those who were effectively completed. To analyze the agreement and association between the predicted ischemic core and final DWI infarct volume, we also conducted difference plots (Bland–Altman plots), spatial agreement analyses through the use of the Dice similarity coefficient and Spearman correlation analyses. The best perfusion threshold at an individual level was defined as the one that achieved the lowest volume difference between final DWI lesion and CTP-defined ischemic core. To evaluate the relationship between the individual best threshold and time to recanalization, a set of regression models were used: linear, logarithmic, quadratic and cubic. A general linear model adjusted for the HERMES collaboration confounding variables (age, sex, baseline NIHSS, baseline ASPECTS, previous rTPA administration and occlusion site defined as Internal Carotid Artery, segments M1 or M2 of the Middle Cerebral Artery) was applied to assess the association between CTP-derived ischemic core and functional outcome at 90 days (ordinal mRS shift) with exploratory purposes.26 For correlation and regression analysis, volume variables were transformed (cubic transformation) to meet normality criteria. The analysis was performed using SPSS Version 19.0 and R (v3.5.1), and the level of significance was established at a 0.05 level (2-sided).
Results
Baseline traits of the included population
Overall, 104 patients were included in the analysis from whom a total of 54 (52%) had complete recanalization within the first 4.5 h and 50 (48%) after 4.5 h from symptom onset. Descriptive data on demographics and baseline variables according to time to recanalization are shown in Table 2. Of note, the baseline clinical and radiological traits were similar across time groups except for a trend towards higher CTP-predicted ischemic core volumes in the earlier recanalization group.
Table 2.
Demographics, baseline and procedure-related variables according to time to recanalization.
| mTICI3 < 4.5 h (n = 54) | mTICI3 ≥ 4.5 h (n = 50) | p | |
|---|---|---|---|
| Age (years), median (IQR) | 73 (64–78) | 76 (64–82) | 0.172 |
| Prior mRS, median (IQR) | 0 (0–1) | 0 (0–1) | 0.646 |
| Females, n (%) | 26 (48) | 29 (58) | 0.315 |
| Smoking, n (%) | 5 (9) | 7 (14) | 0.450 |
| Hypertension, n (%) | 24 (44) | 31 (62) | 0.073 |
| Diabetes, n (%) | 4 (7) | 7 (14) | 0.275 |
| Dyslipidemia, n (%) | 25 (46) | 19 (38) | 0.392 |
| Atrial fibrillation, n (%) | 17 (32) | 21 (42) | 0.266 |
| Previous stroke, n (%) | 5 (9) | 4 (8) | 0.819 |
| Prior antithrombotic treatment, n (%) | 23 (43) | 25 (50) | 0.449 |
| Baseline SBP (mmHg), mean (SD) | 138 (21) | 143 (24) | 0.237 |
| Glucose (mg/dl), median (IQR) | 115 (105–136) | 119 (107–142) | 0.711 |
| Baseline NIHSS, median (IQR) | 16 (10–20) | 17 (8–19) | 0.604 |
| Pre-angio NIHSS, median (IQR) | 18 (11–21) | 17 (12–19) | 0.207 |
| Baseline ASPECTS, median (IQR) | 9 (9–10) | 9 (8–9) | 0.135 |
| Collateral score, median (IQR) | 2 (2–3) | 2 (1–3) | 0.472 |
| Time to CT perfusion (min), median (IQR) | 81 (60–119) | 269 (204–352) | <0.001 |
| Time to MT onset (min), median (IQR) | 156 (125–185) | 348 (290–425) | <0.001 |
| Duration of MT (min), median (IQR) | 26 (15–40) | 35 (16–45) | 0.151 |
| Time to mTICI 3 (min), median (IQR) | 195 (155–225) | 379 (318–477) | <0.001 |
| TOAST classification | 0.955 | ||
| Atherothrombotic origin, n (%) | 5 (9) | 4 (8) | |
| Cardioembolic origin, n (%) | 31 (58) | 30 (60) | |
| Other etiologies, n (%) | 18 (33) | 16 (32) | |
| Ischemic core (mL), median (IQR) | 23 (9–48) | 12 (6–28) | 0.055 |
| Hypoperfused tissue (mL), median (IQR) | 137 (103–168) | 135 (67–165) | 0.409 |
mTICI: modified thrombolysis in cerebral infarction; IQR: interquartile range; mRS: modified Rankin Scale; SBP: systolic blood pressure; NIHSS: National Institutes of Health Stroke Scale; ASPECTS: Alberta stroke program early CT score; CT: computed tomography; MT: mechanical thrombectomy; TOAST: trial of ORG 10172 in acute stroke treatment.
Best CTP definition of ischemic core in the whole population
According to the correlation coefficient analyses, the best pretreatment ischemic core perfusion definition for final DWI-lesion corresponded to the absolute CBF (aCBF) threshold of 6 mL/100 mg/min (ICC = 0.814, 95% CI = 0.758–0.890) and to the relative CBF (rCBF) threshold of 25% (ICC = 0.800, 95% CI = 0.743–0.881). Similar but slightly lower ICC values were obtained for the remainder perfusion parameters (rCBV < 45%, ICC = 0.787, 95% CI = 0.724–0.873; and aCBV < 1.1 mL/100 g ICC = 0.769, 95% CI = 0.701–0.861) (Figure 1). Volumetric agreement was tested with the Bland–Altman plots, and total volumetric bias (average difference between DWI–CTP-predicted ischemic core) was −1.39 mL (95% limits of agreement: −42.22–39.44) for the ischemic core definition of aCBF < 6 mL/100 mg/min and + 2.17 mL (95% limits of agreement: −36.34–40.68) for the ischemic core definition of rCBF < 25% (Supplementary Figure 3).
Figure 1.
Intraclass correlation coefficient (ICC) and 95% confidence intervals (95%CI) between volume of ischemic core defined on CT perfusion and final infarct volume defined on DWI for the set of relative (a) and absolute (b) thresholds analyzed on cerebral blood flow (CBF) and cerebral blood volume (CBV) maps.
Best CTP definition of ischemic core according to time from stroke onset to complete recanalization
A strong association was found between CTP lesion volume defined with each of the perfusion parameters and final DWI lesion. The strongest correlation was found for the rCBF threshold of 25% (Spearman rho = 0.522, p < 0.001). However, the time to complete recanalization modified significantly the association between the perfusion threshold and final DWI lesion (p-interaction = 0.018). The relationship between the best definition of CTP-predicted ischemic core for each patient (individual best threshold) and the time to recanalization was analyzed through the use of curve estimation regression analysis that included linear, logarithmic, quadratic and cubic models. Overall, the best R-squared values were obtained in cubic regression models for all the perfusion parameters (Supplementary Table 1). Specifically, the highest R-squared values were 0.235 for rCBF, 0.178 for rCBV, 0.193 for aCBF and 0.171 for aCBV. To determine a discrete time to recanalization cutoff point, we estimated the range of values where a plateau was reached in the cubic-fitted model for every perfusion parameter (Figure 2). Overall, the range of values fell between 240 and 420 min from symptom onset to recanalization. For practical purposes, a cutoff point of 4.5 h (270 min) was selected for further analyses.
Figure 2.
Individual best threshold (patient level) depending on the time from symptom onset to recanalization. The best fitting (highest r2) was observed in cubic regression models and the plateau was reached for every threshold in the range between 240 and 420 min: rCBF (a, 240–350 min), rCBV (b, 280–420 min), aCBF (c, 250–370 min) and aCBV (d, 260–380 min).
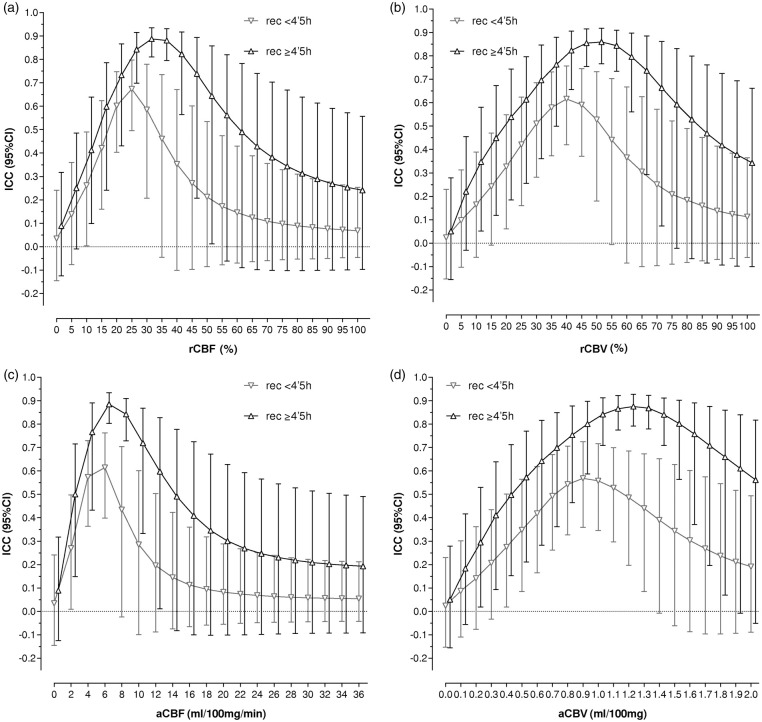
In stratified analyses, patients with recanalization after 4.5 h had significantly higher ICC values (n = 50; best threshold rCBF < 30% ICC = 0.887, 95% CI = 0.811–0.935) than those with earlier recanalization (n = 54; best threshold rCBF < 25% ICC = 0.673, 95% CI = 0.495–0.797), p = 0.013 (Figure 3). According to Bland–Altman plots, CTP-predicted ischemic core was overestimated in the group of patients with earlier complete recanalization (average difference of −4.54 mL, 95% limits of agreement: −36.78–32.24; rCBF < 25% threshold) in comparison with those with later recanalization (average difference of + 2.30 mL, 95% limits of agreement: −36.14–40.74; rCBF < 30% threshold) (Supplementary Figure 3). As shown in Figure 4, the volumetric difference analysis between CTP predicted ischemic core and final DWI lesion resulted in a significant overestimation of final DWI lesion in most of the explored perfusion thresholds in the group of patients with full recanalization within the first 4.5 h in comparison with those with later recanalization. The correlation between CTP-predicted ischemic core and final DWI lesion was also poorer in patients who recanalized earlier [(Spearman rho = 0.446 (95% confidence interval = 0.255–0.637); rCBF < 25% threshold)] in comparison with those with later recanalization [(Spearman rho = 0.679 (95% confidence interval = 0.553–0.805); rCBF < 30% threshold)], p = 0.049. According to spatial agreement analyses, the Dice similarity coefficient was significantly lower in the group of patients with earlier complete recanalization [median (IQR) 0.160 (0.118–0.202); rCBF < 25% threshold] in comparison with those with later recanalization [median (IQR) 0.228 (0.179–0.277); rCBF < 30% threshold)], p = 0.037.
Figure 3.
Intraclass correlation coefficient (ICC) and 95% confidence intervals (95% CI) between the volume of ischemic core defined on CT perfusion and final infarct volume defined on DWI depending on time from symptom onset to recanalization (complete recanalization before or after 4.5 h from symptom onset). Higher ICC values were found in patients with later recanalization for every analyzed threshold: rCBF (a), rCBV (b), aCBF (c) and aCBV (d).
Figure 4.
Volume difference between DWI final infarct and CTP ischemic core defined with a set of relative CBF thresholds. *p < 0.01 and **p < 0.005 for the comparison of volume differences according to recanalization groups (<4.5 h vs. ≥ 4.5 h).
To assess the consistency of the optimal parameter thresholds for predicting ischemic core that were found in the whole population, a sensitivity analysis including only those patients with not truncated TACs at the end of the CTP acquisition was performed. A total of 92 (88%) patients had non-truncated curves, and 50 (54%) of them had recanalization within the first 4.5 h from stroke onset. In this subset of patients, those with recanalization after 4.5 h had significantly higher ICC values (n = 42; best threshold rCBF < 30% ICC = 0.892, 95% CI = 0.809–0.940) than those with earlier recanalization (n = 50; best threshold rCBF < 25% ICC = 0.700, 95% CI = 0.583–0.819), p = 0.007 (Supplementary Figure 4), as in the whole study sample.
CTP definition of ischemic core and functional outcome
In the whole cohort of patients, a subset of thresholds of relative and absolute perfusion parameters was found to be significantly associated with functional outcome in exploratory analyses. These thresholds ranged from 30% to 40% of rCBF, 40% to 80% of rCBV and 1.2 to 2 mL/100 g of aCBV (Figure 5). However, the significance of these associations was influenced by the time from stroke onset to complete recanalization. In the subset of patients recanalized before 4.5 h of symptom onset, there were no significant associations between ischemic core and functional outcome, whereas some of these associations remained significant with wider confidence intervals in the subset of patients recanalized after 4.5 h (Figure 5).
Figure 5.
Odds ratios (OR) and 95% confidence intervals (95%CI) of the association between ischemic core defined with different absolute and relative thresholds and functional outcome. The significant associations are shown in bold (p < 0.05). A range of absolute and relative thresholds are significantly associated with functional outcome on the whole cohort of patients, fewer in the subset of patients recanalized after 4.5 h from symptom onset but no associations were found in those recanalized earlier.
Discussion
In this study, we implemented a comprehensive approach to evaluate the accuracy of pretreatment CTP hemodynamic parameters and thresholds for the prediction of final infarct volume and long-term clinical outcome in a cohort of acute ischemic stroke patients successfully reperfused after MT. We demonstrated that the association between CTP-derived ischemic core predictions and final DWI lesion volume was significantly modified by time from stroke onset to recanalization in a non-linear fashion. Thus, the reliability of CTP-predicted ischemic core was good in patients with complete recanalization after 4.5 h from stroke onset, whereas it was only moderate in those who achieved earlier and complete recanalization. We also found associations between CTP-derived ischemic core and functional outcome regardless of time from stroke onset to complete recanalization, although these associations were lost in patients recanalized before 4.5 h from symptom onset. Altogether, these results highlighted the relevance of time to recanalization in the accuracy of pretreatment CTP imaging prediction of final infarct and long-term clinical outcome.
Previous studies have shown strong correlations between CTP-derived ischemic core volume estimation with both final infarct volume and clinical outcome.4–8,11–16,20,21,26–29 In agreement with these data, we found significant associations between CTP-derived ischemic core values, final infarct volume and clinical outcome at follow-up in the full cohort of patients who achieved a complete recanalization after MT. However, a number of pretreatment variables, including baseline imaging features and acute reperfusion treatment modality have been extensively proved to modify the performance of CTP for final infarct prediction.7–12 Our results reinforce and expand previous observations supporting the dependency of optimal CTP thresholds on time to recanalization and the notion that CBF thresholds for tissue infarction may be more restrictive in patients with shorter time from stroke onset to reperfusion.8,13–19 Beyond the differences in CTP acquisition and post-processing protocols that were used in these studies, herein we included only a highly homogeneous stroke population consisting in patients that achieved full reperfusion at the end of MT thus minimizing the risk of infarct growth from the end of MT to follow-up MRI secondary to non-complete recanalization. Moreover, as a novel approach, we modeled the relationship between time to recanalization and CTP-DWI volumetric differences through curve estimation analyses across different perfusion parameters and thresholds and also explored their impact on long-term clinical outcome. Indeed, in contraposition with previous published data and according to ICC analyses,13,14,16 in patients with earlier recanalization, the reliability of CTP for the prediction of final infarct volume was only moderate; even using restrictive perfusion thresholds and ischemic core predicted values tended towards the overestimation of irreversible tissue injury. Reassuringly, Dice similarity coefficient and Spearman correlation analyses showed poorer spatial agreement and association between CTP-predicted ischemic core and final DWI lesion in patients with earlier recanalization compared to those who recanalized later than 4.5 h. Moreover, the significance of the association of CTP-predicted ischemic core values with clinical outcome in multivariate models adjusted for the effect of confounders was lost in the subgroup of patients with earlier and complete recanalization regardless of the perfusion parameters and thresholds used for the prediction of infarct core. Overall, these data support current guidelines arguing against the use of CTP-predicted ischemic core as selection criteria for MT in patients with short evolution from stroke onset to expected complete recanalization (e.g. if complete recanalization is expected within the first 4.5 h from stroke onset).30
The main strength of the study was the comprehensive analysis of absolute and relative CTP parameters and the exhaustive scanning of multiple different thresholds in whole-brain CTP acquisitions, a methodology that allowed obtaining perfusion measures of most of the affected brain tissue. Moreover, the imaging modality used for final infarct definition was CTP co-registered DWI in all the study population thus avoiding volumetric biases related to the use of different modalities (CT or MRI).31,32 In addition, patients were collected consecutively and managed following homogeneous therapeutic protocols according to contemporary clinical guidelines. Nonetheless, the study has several limitations. First, there should be acknowledged that the CTP methods used for the definition of ischemic core are a pragmatic approximation to a complex, dynamic and heterogeneous pathological process.33 Furthermore, a number of additional variables beyond time to recanalization such as collateral status, age, pretreatment hyperglycemia or leukoaraiosis might hamper CTP-derived measures of ischemic core and penumbra.18 Further studies with larger numbers of patients to allow the comparison of CTP performance in subgroups of patients defined by more than one characteristic are warranted. Second, we analyzed the best perfusion parameters in two acquisitions that were not concurrent as pretreatment CTP-derived ischemic core estimates were compared with post-treatment DWI lesions. Although we restricted our analysis to patients who achieved a complete recanalization at the end of MT to minimize the odds of lesion change overtime between CTP acquisition and recanalization, individual imbalances in time from CTP acquisition to recanalization or post-recanalization lesion changes could have derived in some uncontrolled volume differences. Of note, CT perfusion acquisition parameters and post-processing platforms are an essential source of variability regarding the values obtained from perfusion maps and therefore our results may apply only to the protocol of acquisition and analysis that were employed in these series and may not be generalizable to other methodologies.7,9,11 Specifically, in this study CTP maps were calculated using a deconvolution algorithm with delay and dispersion correction and therefore the obtained results may not be applicable to delay-sensitive algorithms and software. Regarding the acquisition protocols, a limited acquisition time (<60 s) may result in a delayed arrival of contrast agent with a consequent incomplete capture of the tissue TACs during acquisition. This delay may derive in truncation of tissue TACs that may preclude an accurate calculation of CTP parameters with a shift towards overestimation of perfusion deficits. Reassuringly, in this study, the consistency of the thresholds found in the whole population was confirmed in a sensitivity subgroup analysis that included only those patients without truncated typo-redundant TACs. In relation with the spatial agreement analysis, in this cohort of patients, the Dice values were generally poor in agreement with previous reports. As previously described, the low values of the Dice coefficient might be related to limitations of co-registering different imaging modalities (pretreatment CTP and post-treatment DWI) that include non-isotropic data, different slice angulations and inherent distortions in DWI images.7,18 Finally, the analyses of the association between perfusion thresholds and clinical outcome were not corrected for multiple comparisons for their exploratory nature and might have been underpowered because of the limited size of the study.
In summary, this comprehensive threshold-finding study showed that time from symptom onset to recanalization modifies the association of CTP-derived ischemic core with final infarct DWI volumes and with long-term clinical outcome measures in patients who achieve complete recanalization after MT. We found accurate predictions of final infarct volume and long-term clinical outcome from CTP-derived ischemic core definitions, especially in those patients that achieved recanalization after 4.5 h from symptom onset. Conversely, in the subset of patients who achieved complete and early recanalization within the first 4.5 h from stroke onset, we did not find a single CTP typo-redundant parameter and threshold for the definition of pretreatment ischemic core accurate enough for the prediction of final infarct volume or long-term clinical outcome. Overall, these findings suggest that the reliability of CTP for the prediction of tissue fate or long-term clinical outcome must be interpreted with caution in the setting of acute stroke patients eligible for MT, primarily in those with short time of infarct evolution and with higher chances of early recanalization.
Supplemental Material
Supplemental Material for The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: A comprehensive whole-brain computed tomography perfusion study by Carlos Laredo, Arturo Renú, Raúl Tudela, Antonio Lopez-Rueda, Xabier Urra, Laura Llull, Napoleón G Macías, Salvatore Rudilosso, Víctor Obach, Sergio Amaro and Ángel Chamorro in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We thank the support of the Spanish Ministry of Economy and Competitiveness for grant to AC (project PI15/00430 funded by Instituto de Salud Carlos III and co-funded by European Regional Development Fund ERDF). CL receives funding from Instituto de Salud Carlos III, with a Predoctoral Grant for Health Research (PFIS, FI16/00231). This work was partially developed at the building Centro Esther Koplowitz, Barcelona, CERCA Programme/Generalitat de Catalunya.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
CL obtained the imaging data, implemented the post-processing of the perfusion maps, performed the statistical analysis and wrote the first draft of the manuscript. AR, XU, LLl and SR obtained the clinical data. AR, RT, ALR and NGM supervised the neuroimage post-processing and analysis. AR, RT, XU, LLl, SR, VO, SA and AC revised the article critically for intellectual content. SA and AC designed the study, interpreted the data and wrote the final draft of the manuscript.
Supplementary material
Supplemental material for this article is available online.
References
- 1.Warach SJ, Luby M, Albers GW, et al. Acute stroke imaging research roadmap III imaging selection and outcomes in acute stroke reperfusion clinical trials: consensus recommendations and further research priorities. Stroke 2016; 47: 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lev MH. Perfusion imaging of acute stroke: its role in current and future clinical practice. Radiology 2013; 266: 22–27. [DOI] [PubMed] [Google Scholar]
- 3.Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010; 75: 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell BCV, Christensen SS, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011; 42: 3435–3440. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BCV, Christensen SS, Levi CR, et al. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke 2012; 43: 2648–53. [DOI] [PubMed] [Google Scholar]
- 6.Lin L, Bivard A, Krishnamurthy V, et al. Whole-brain CT perfusion to quantify acute ischemic penumbra and core 1. 0. Radiology 2016; 279: 876–887. [DOI] [PubMed] [Google Scholar]
- 7.Cereda CW, Christensen S, Campbell BC, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab 2016; 36: 1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.d’Esterre CD, Boesen ME, Ahn SH, et al. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke 2015; 46: 3390–3397. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer PW, Souza L, Kamalian SS, et al. Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke 2015; 46: 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen S, Lansberg MG. CT perfusion in acute stroke: practical guidance for implementation in clinical practice. J Cereb Blood Flow Metab. Epub ahead of print 22 October 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamalian S, Kamalian S, Maas MB, et al. CT cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke 2011; 42: 1923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Bivard A, Lin L, et al. Thresholds for infarction vary between gray matter and white matter in acute ischemic stroke: a CT perfusion study. J Cereb Blood Flow Metab 2019; 39: 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao Y, Zhu G, Patrie J, et al. Optimal perfusion computed tomographic thresholds for ischemic core and penumbra are not time dependent in the clinically relevant time window. Stroke 2014; 45: 1355–1362. [DOI] [PubMed] [Google Scholar]
- 14.Mui K, Yoo AJ, Verduzco L, et al. Cerebral blood flow thresholds for tissue infarction in patients with acute ischemic stroke treated with intra-arterial revascularization therapy depend on timing of reperfusion. AJNR Am J Neuroradiol 2011; 32: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copen WA, Yoo AJ, Rost NS, et al. In patients with suspected acute stroke, CT perfusion-based cerebral blood flow maps cannot substitute for DWI in measuring the ischemic core. PLoS One 2017; 12: e0188891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bivard A, Kleinig T, Miteff F, et al. Ischemic core thresholds change with time to reperfusion: a case control study. Ann Neurol 2017; 82: 995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martins N, Aires A, Mendez B, et al. Ghost infarct core and admission computed tomography perfusion: redefining the role of neuroimaging in acute ischemic stroke. Interv Neurol 2018; 7: 513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoving JW, Marquering HA, Majoie CBLM, et al. Volumetric and spatial accuracy of computed tomography perfusion estimated ischemic core volume in patients with acute ischemic stroke. Stroke 2018; 49: 2368–2375. [DOI] [PubMed] [Google Scholar]
- 19.Najm M, Al-Ajlan FS, Boesen ME, et al. Defining CT perfusion thresholds for infarction in the golden hour and with ultra-early reperfusion. Can J Neurol Sci 2018; 45: 339–342. [DOI] [PubMed] [Google Scholar]
- 20.Parsons MW, Pepper EM, Chan V, et al. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol 2005; 58: 672–679. [DOI] [PubMed] [Google Scholar]
- 21.Xie Y, Oppenheim C, Guillemin F, et al. Pretreatment lesional volume impacts clinical outcome and thrombectomy efficacy. Ann Neurol 2018; 83: 178–185. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Neurological Disorders National, rt-PA Stroke Study Group Stroke. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1588. [DOI] [PubMed] [Google Scholar]
- 23.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 24.Kakuda W, Lansberg MG, Thijs VN, et al. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab 2008; 28: 887–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kottner J, Audige L, Brorson S, et al. Guidelines for reporting reliability and agreement studies (GRRAS) were proposed. Int J Nurs Stud 2011; 48: 661–671. [DOI] [PubMed] [Google Scholar]
- 26.Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279. [DOI] [PubMed] [Google Scholar]
- 27.Haussen DC, Dehkharghani S, Rangaraju S, et al. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta Stroke Program Early CT Score): correlation and clinical outcome prediction in large vessel stroke. Stroke 2016; 47: 2318–2322. [DOI] [PubMed] [Google Scholar]
- 28.Bivard A, Spratt N, Miteff F, et al. Tissue is more important than time in stroke patients being assessed for thrombolysis. Front Neurol 2018; 9: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bivard A, Levi C, Krishnamurthy V, et al. Perfusion computed tomography to assist decision making for stroke thrombolysis. Brain 2015; 138: 1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals From the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 31.Lansberg MG, Albers GW, Beaulieu C, et al. Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology 2000; 54: 1557–1561. [DOI] [PubMed] [Google Scholar]
- 32.Boers AMM, Jansen IGH, Beenen LFM, et al. Association of follow-up infarct volume with functional outcome in acute ischemic stroke: a pooled analysis of seven randomized trials. J Neurointerv Surg 2018; 10: 1137–1142. [DOI] [PubMed] [Google Scholar]
- 33.del Zoppo GJ, Sharp FR, Heiss W-D, et al. Heterogeneity in the penumbra. J Cereb Blood Flow Metab 2011; 31: 1836–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The accuracy of ischemic core perfusion thresholds varies according to time to recanalization in stroke patients treated with mechanical thrombectomy: A comprehensive whole-brain computed tomography perfusion study by Carlos Laredo, Arturo Renú, Raúl Tudela, Antonio Lopez-Rueda, Xabier Urra, Laura Llull, Napoleón G Macías, Salvatore Rudilosso, Víctor Obach, Sergio Amaro and Ángel Chamorro in Journal of Cerebral Blood Flow & Metabolism