Abstract
Circulating levels of inter-alpha inhibitor proteins change dramatically in acute inflammatory disorders, which suggest an important contribution to the immunomodulatory system. Human blood-derived inter-alpha inhibitor proteins are neuroprotective and improve survival of neonatal mice exposed to lipopolysaccharide. Lipopolysaccharide augments inflammatory conditions and disrupts the blood–brain barrier. There is a paucity of therapeutic strategies to treat blood–brain barrier dysfunction, and the neuroprotective effects of human blood-derived inter-alpha inhibitor proteins are not fully understood. To examine the therapeutic potential of inter-alpha inhibitor proteins, we administered human blood-derived inter-alpha inhibitor proteins to male and female CD-1 mice after lipopolysaccharide exposure and quantified blood–brain barrier permeability of intravenously injected 14C-sucrose and 99mTc-albumin. We hypothesized that human blood-derived inter-alpha inhibitor protein treatment would attenuate lipopolysaccharide-induced blood–brain barrier disruption and associated inflammation. Lipopolysaccharide increased blood–brain barrier permeability to both 14C-sucrose and 99mTc-albumin, but human blood-derived inter-alpha inhibitor protein treatment only attenuated increases in 14C-sucrose blood–brain barrier permeability in male mice. Lipopolysaccharide stimulated a more robust elevation of male serum inter-alpha inhibitor protein concentration compared to the elevation measured in female serum. Lipopolysaccharide administration also increased multiple inflammatory factors in serum and brain tissue, including interleukin 6. Human blood-derived inter-alpha inhibitor protein treatment downregulated serum interleukin 6 levels, which were inversely correlated with serum inter-alpha inhibitor protein concentration. We conclude that inter-alpha inhibitor proteins may be neuroprotective through mechanisms of blood–brain barrier disruption associated with systemic inflammation.
Keywords: Inter-alpha inhibitor proteins, lipopolysaccharide, blood–brain barrier, interleukin 6, inflammation
Introduction
The blood–brain barrier (BBB) regulates the entry of blood-borne substances into the central nervous system (CNS). Lipopolysaccharide (LPS) is a gram-negative endotoxin that can disrupt the BBB under several experimental conditions.1–4 The use of LPS to induce BBB disruption was first pioneered by Eckman et al.5 and Allen.6 Subsequent studies have used LPS to study the CNS transport of chemotherapeutic agents,7 viruses,8 and peptides9 under conditions of systemic inflammation. The mechanisms of LPS-induced BBB disruption are complex and thought to be driven by inflammatory mediators including cytokines.3,10,11 Thus, models of LPS-induced BBB disruption are useful in the study of novel neuroprotective agents to attenuate systemic inflammation and related mechanism(s) of BBB disruption.
Inter-alpha inhibitor proteins (IAIPs) are a family of structurally related serine protease inhibitors. Two major forms are found in human blood: inter-alpha inhibitor (two heavy chains and a light chain called bikunin) and pre-alpha inhibitor (one heavy chain and bikunin).12 The light chain bikunin, also known as urinary trypsin inhibitor or ulinastatin, has two protease inhibitor domains facilitating its function as a serine protease inhibitor.13 The heavy chains are important in stabilizing and creating extracellular matrixes in tissues and synergizing with the activity of bikunin.14 IAIPs also inhibit destructive serine proteases and pro-inflammatory cytokines, increase anti-inflammatory cytokine levels, decrease complement activation during systemic inflammation, and enhance survival during inflammatory challenge in neonates and adults.15–17 Therefore, IAIPs have potentially critical roles as immunomodulatory proteins in many inflammatory disorders.18
The light chain is reported to have important neuroprotective effects.19–23 Bikunin pretreatment attenuates the severity of stroke-related infarcts in adult rats21 and protects oligodendrocytes and augments remyelination in experimental autoimmune encephalitis in adult rats.22 In addition, we have recently shown that intact human blood derived inter-alpha inhibitor proteins (hIAIPs) decreases neuronal cell death, enhances neuronal plasticity, reduces complex auditory processing deficits, and improves behavioral outcomes in male neonatal rodents after hypoxia/ischemia.24–26 Bikunin has recently been shown to reduce BBB disruption after global ischemia and to protect against cerebral ischemia and reperfusion by downregulating matrix metalloprotease expression and reducing the loss of tight junction proteins at the BBB.27,28 However, information is not available regarding the potential effects of the intact blood-derived form of hIAIPs on BBB function.
As outlined above, IAIPs and bikunin have important anti-inflammatory effects.15,29,30 Moreover, we have shown that attenuating inflammatory responses can reduce BBB disruption.1,31–33 Therefore, given the considerations summarized above, the objective of this study was to determine whether the hIAIPs attenuate LPS-induced BBB disruption to a small molecule (sucrose, MW 342 Da) and a large molecule (albumin, MW 66.5 kDa) in relation to associated inflammation in both brain and blood.
Materials and methods
Animals
Male CD-1 (N = 68) and female CD-1 (N = 68) mice (Charles River) aged 10–12 weeks (25–35 g) were used for this study. All mice had ad libitum access to food and water were maintained under a 12-h light/dark cycle and group housed. All animals were housed and handled in accordance with protocols approved by the Veterans Affairs Puget Sound Health Care System’s Institutional Animal Care and Use Committee, and all experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experiments reported are in compliance with the ARRIVE guidelines.
Human inter-alpha inhibitor proteins
As previously described,25 hIAIPs were extracted from fresh frozen human plasma (Rhode Island Blood Center, RI, USA). A scalable purification process to extract hIAIPs using a monolithic anion-exchange chromatographic media (CIMmultus™, BIA Separation, Ajdovščina, Slovenia) has been developed18,34,35 and an additional separation step using a proprietary synthetic chemical ligand affinity chromatographic media (Prometic Bioseparations, Cambridge, UK) was applied to obtain high yield, high purity (>90%), and biologically active hIAIPs.12,25
Eluted proteins were concentrated and buffer-exchanged using a tangential flow filtration device (Labscale, Millipore, Taunton, MA, USA). As part of the characterization of hIAIPs, endotoxin in the purified products was monitored using a limulus amebocyte lysate endotoxin-based chromogenic test (Pierce Biotechnology, ThermoFisher Scientific, Waltham, MA, USA). The chromatographic equipment and containers/tubing were treated with 1 M NaOH to reduce or eliminate potential endotoxin contamination during the purification process. Purified hIAIPs were frozen until use. The highly purified IAIP was reconstituted in sterile phosphate-buffered saline (PBS) then shipped frozen to the Veterans Affairs Puget Sound Health Care System to be administered to mice for subsequent experiments.
Experimental design
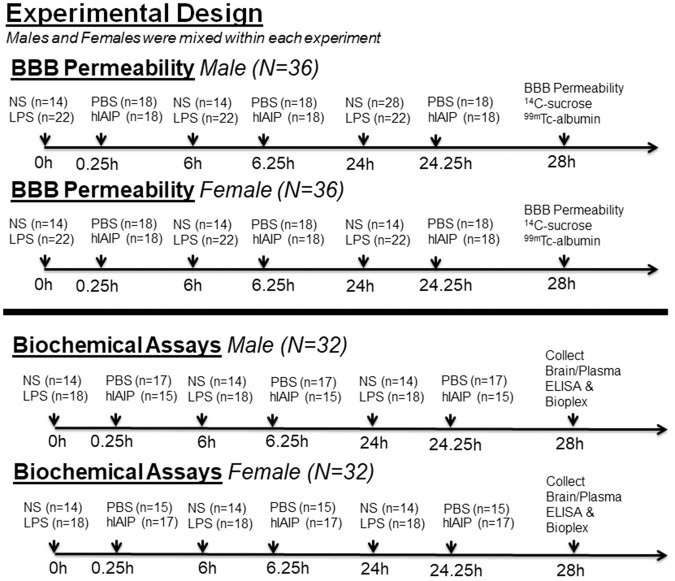
LPS (Sigma, St. Louis, MO) was prepared in sterile normal saline (NS). For BBB disruption experiments, equal numbers of male (N = 36) and female (N = 36) mice were randomly assigned to one of four groups: NS + PBS (n = 7), LPS + PBS (n = 11), NS + hIAIP (n = 7), or LPS + hIAIP (n = 11). For biochemical experiments, equal numbers of male (N = 32) and female (N = 32) mice were randomly assigned to one of four groups: NS + PBS (n = 7), LPS + PBS (n = 9), NS + hIAIP (n = 7), or LPS + hIAIP (n = 9). For both experiments, the effect size (α = 0.05; power = 0.95) was calculated using G*power statistical software.36
Mice in the LPS groups were given three intraperitoneal injections of LPS (3 mg/kg) at t = 0, 6, and 24 h. Experimental control mice were given three injections of sterile NS at the same time points. Mice in the IAIP groups were given three intraperitoneal injections of hIAIP (60 mg/kg), 15 min after each LPS or NS injection. Vehicle control mice were given three injections of sterile PBS. We selected a 60 mg/kg dose of hIAIPs based upon our previous work in neonatal rats.25 In our previous studies, we examined the effects of hypoxic ischemic injury in neonatal rats and found significant attenuation in brain injury with the hIAIP dose of 30 mg/kg.25 However, in this study, we administered three doses of LPS to adult mice, and, consequently, elected to test a larger dose of hIAIPs in this setting. Future work will also need to determine if smaller doses are also efficacious in reducing BBB permeability. No differences in BBB permeability or bioplex measurements were observed between hIAIP injected controls and NS injected controls (p > 0.05). Therefore, the two control groups were combined to form a collective NS group for subsequent analyses. The experimental design is shown in Figure 1.
Figure 1.
Experimental design for blood–brain barrier permeability and biochemical studies. LPS: lipopolysaccharide; NS: normal saline; PBS: phosphate-buffered saline; hIAIP: human inter-alpha inhibitor proteins; BBB: blood–brain barrier.
Competitive ELISA for endogenous mouse IAIP
Mouse serum concentrations of IAIPs were measured using a competitive ELISA with a purified biotinylated rat polyclonal antibody (R-21 pAb) as previously described.37 Purified rat IAIPs were coated on 96-well high-binding microplates (Microlon 600, Greiner Bio-One, Monroe, NC, USA) for 1 h at room temperature or overnight at 4℃. After blocking with 200 µL of 5% nonfat dried milk in PBS plus 0.05% Tween, 50 µL of mouse serum sample and the serially diluted rat serum-derived IAIP standards were added to the wells. Then, 50 µL of biotin-conjugated R-21 pAb (1:500 dilution in PBS) was also added to each well. Plates were incubated for 1 h at room temperature and washed with PBS plus 0.05% Tween. Streptavidin-poly-horseradish-peroxidase (Pierce Biotechnology, ThermoFisher) was added to each well to detect the bound biotinylated R-21 pAb, and the plate was incubated for 1 h at room temperature. After washing, 100 µL enhanced K-Blue TMB substrate (Neogen Corp, Lexington, KY, USA) was added to the wells and the reaction was stopped with 100 µL 1 N HCl solution. The absorbance at 450 nm was measured on SpectraMAX Plus microplate reader (Molecular Devices, Sunnyvale, CA, USA). Sample concentrations were calculated by comparison of the absorbance signal to the established standard curve. Each sample was measured in triplicate and assays were repeated at least twice on all samples.
Multiplex ELISA array for cytokines, chemokines, and growth factors
Cytokine, chemokine, and growth factor levels were measured using a mouse Bio-Plex Pro™ 23-plex Luminex assay (Bio-Rad Laboratories, Inc.; Hercules, CA, USA) in serum and brain tissue. Serum was obtained from the supernatant of coagulated blood from the descending aorta after centrifugation for 10 min at 2000g. Brain tissue was lysed in a 0.02% Triton-X homogenization buffer of 10 mM HEPES, 1.5 mM MgCl2, and 10 mM KCl, with fresh protease/phosphatase inhibitor cocktail (Sigma). All serum samples were diluted 1:4 in sample diluent provided with the Luminex assay; brain samples were diluted 1:3 in the homogenization buffer mentioned above. All prepared samples were then incubated with multiplex plate according to the manufacturer’s protocol. Plates were read on a Bio-Plex 200 (Bio-Rad). Serum cytokine values (pg/ml) were compared to Luminex assay standard curve. Brain cytokine values (pg/mg) were normalized to their respective bicinchoninic acid assay concentration (Pierce Biotechnology, Thermo Fisher).
Radiolabeled tracer preparation
Following established procedures,1 albumin (Sigma) was labeled with 99mTc (GE Healthcare, Piscataway, NJ, USA). A mixture of 240 mg/ml stannous tartrate and 1 mg/ml albumin was adjusted to pH 3.0 with HCl. One millicurie of 99mTc-NaOH4 was added to this mixture and allowed to incubate for 20 min. The 99mTc-albumin was purified on a column of G-10 Sephadex (GE Healthcare) in 0.1 ml fractions of phosphate buffer (0.25 M). Radioactivity in the purified 99mTc-albumin peak was more than 90% acid precipitable in an equal volume of 1% bovine serum albumin (BSA) and trichloroacetic acid (30%); 2 × 106 cpm/mouse of purified 99mTc-albumin fraction was combined with 2×106 dpm/mouse of 14C-sucrose (Perkin Elmer, Waltham, MA, USA) in a final volume (0.1 ml/mouse) of lactated Ringer’s solution containing 1% BSA.
Radiolabeled tracer intravenous injections
At 28 h after the first LPS or NS injection, mice were anesthetized with urethane (Sigma; 4 g/kg; 0.2 ml; ip). The mice were given an intravenous co-injection of 99mTc-albumin (2 × 106 cpm) and 14C-sucrose (2 × 106 dpm) in 0.1 ml of lactated Ringer’s solution in the jugular vein, followed by 10 min circulation time. Blood was collected from the descending abdominal aorta. The vascular space of the brain was then washed free of blood by opening the thorax, clamping the descending thoracic aorta, severing both jugular veins, and perfusing 20 ml of lactated Ringer’s solution through the left ventricle of the heart. After washout, the mouse was immediately decapitated, the brain was removed, and weighed. Brains with visible blood after washout were excluded from analysis (n = 2). Serum was obtained by centrifuging coagulated blood for 10 min at 2000g. Levels of 99mTc radioactivity in the serum and brain were determined in a gamma counter. The brain and sera were then solubilized, and the level of 14C radioactivity was determined by liquid scintillation counting. Brain tissue radioactivity was calculated by dividing the cpm (gamma) or dpm (beta) by brain weight (g). Serum radioactivity was calculated by dividing the cpm or dpm by serum volume (μl). The brain tissue radioactivity was then divided by the corresponding serum radioactivity and the results given in units of microliters per gram of brain tissue.
Statistical analysis
Statistical analyses were performed with GraphPad Prism® 8.0 (GraphPad Software, Inc., La Jolla, CA, USA) by at least two researchers blinded to experimental groups. Sample sizes are indicated in the figure legends and were estimated for this study according to our previous report of BBB disruption in this LPS model.1 Error bars represent the mean ± standard deviation (SD). Serum IAIPs were compared using a two-way analysis of variance (ANOVA) followed by a Sidak’s multiple comparisons test. BBB permeability and bioplex analyses were made using one-way ANOVA followed by a Tukey’s post hoc test. A Pearson linear regression analysis was used to find correlations between endogenous IAIP levels and cytokine levels.
Results
LPS effects on serum IAIP concentration in male and female mice
Blood levels of IAIPs change dramatically under inflammatory conditions.38–40 Therefore, we used a competitive ELISA to measure endogenous serum IAIP in male and female mice administered LPS. A two-way ANOVA revealed a significant treatment (p ≤ 0.001) and sex effect (p ≤ 0.001) for serum IAIP levels. A Sidak multiple comparisons test found that LPS treatment significantly increased serum IAIP levels in both male (Figure 2; t = 5.57; p ≤ 0.001) and female mice (Figure 2; t = 2.76; p ≤ 0.05) compared to NS controls. To compare the effects of sex, a Sidak test determined that males given LPS exhibited a significantly higher IAIP elevation compared to females given LPS (Figure 2; t = 5.15; p ≤ 0.001). No differences were observed between NS males and NS females (Figure 2; t = 2.13; p > 0.05). The neuroprotective effects of IAIPs in other inflammatory models20–22 allowed us to hypothesize that IAIPs may protect against LPS-induced BBB disruption. As such, we decided to administer exogenous hIAIP to male and female mice exposed to LPS and determine whether we could measure effects on BBB permeability.
Figure 2.
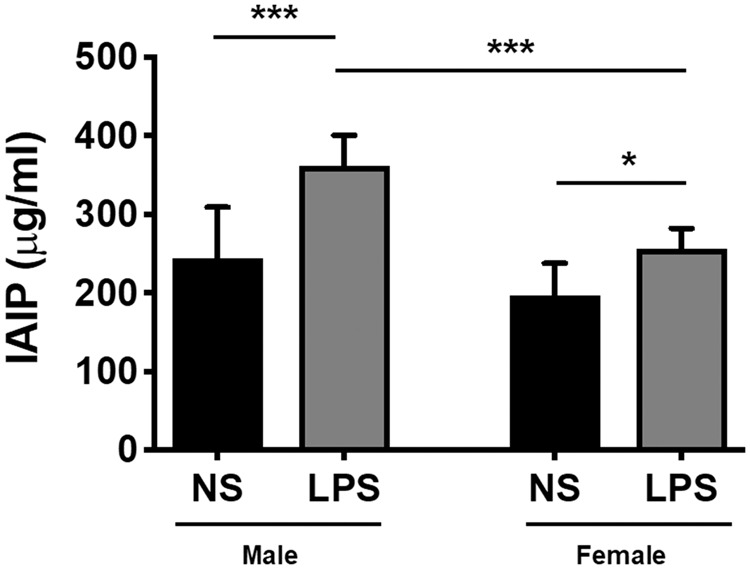
LPS increases endogenous serum IAIPs in male and female mice. IAIP concentration was measured in serum by competitive ELISA (µg/ml), and a significant effect of LPS was observed in male and female mice. Male LPS mice (n = 10) exhibited a more robust IAIP elevation compared to female LPS mice (n = 9). Differences were not observed between male and female NS control mice (p > 0.05; n = 8). Two-way ANOVA; Sidak’s multiple comparisons test. Values represent mean ± SD (***p ≤ 0.001; *p ≤ 0.05). NS: normal saline; LPS: lipopolysaccharide; IAIP: inter-alpha inhibitor proteins.
hIAIPs attenuate LPS-induced BBB disruption to 14C-sucrose in male mice
It is well established that LPS administration disrupts the BBB.1,41 As an initial examination, we tested whether hIAIP administration could mitigate LPS-induced 14C-sucrose permeability. Using well established methods,1 we quantified specific BBB permeability to peripherally co-administration of 14C-sucrose and 99mTc-albumin, both of which poorly cross the BBB under normal conditions.42–46
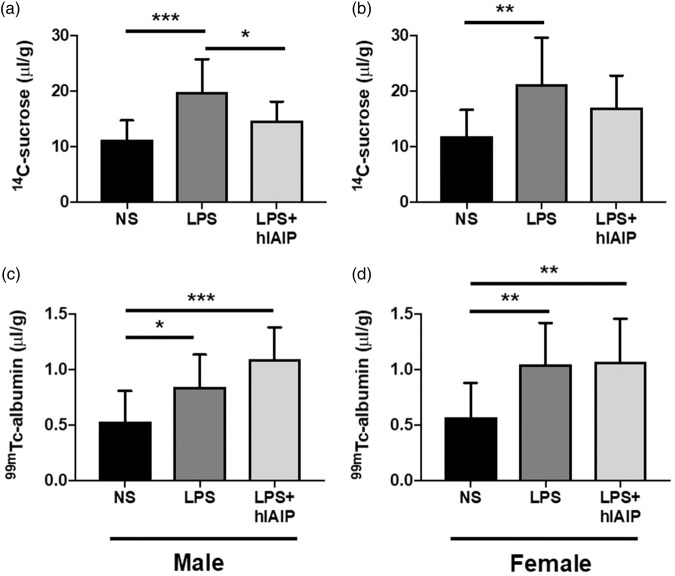
A significant LPS treatment effect of 14C-sucrose permeability was observed in both male (Figure 3(a); F(3,32) = 12.25; p ≤ 0.001) and female mice (Figure 3(b); F(3,32) = 6.95; p ≤ 0.01). Tukey’s post hoc analysis confirmed that LPS significantly increased 14C-sucrose permeability (µl/g) compared to NS injected male controls (Figure 3(a); p ≤ 0.001; q = 6.99) and NS female controls (Figure 3(b); p ≤ 0.01; q = 5.24). hIAIP administration after each LPS injection significantly attenuated LPS-induced BBB disruption to 14C-sucrose in males (Figure 3(a); p ≤ 0.05; q = 3.86) but not females (Figure 3(b); p > 0.05). These results suggest that under inflammatory conditions, hIAIPs may reduce BBB disruption to small molecules in a sex-specific manner.
Figure 3.
Exogenous hIAIPs attenuate LPS-induced BBB disruption to 14C-sucrose, but not 99mTc-albumin, in male mice. (a) Brain/serum ratios (μl/g) of 14C-sucrose were significantly increased in male LPS mice (n = 11) compared to male NS mice (n = 14), and hIAIP treatment (n = 11) significantly attenuated the effect. (b) 14C-sucrose was also significantly increased in female LPS mice (n = 11) compared to female NS mice (n = 14), but hIAIP treatment (n = 11) did not attenuate the effect (p > 0.05). 99mTc-albumin was significantly increased in the same male LPS mice (c) and female LPS mice (d), compared to NS control mice. No differences were observed between LPS mice and LPS mice treated with hIAIP (p > 0.05). One-way ANOVA; Tukey’s post hoc test. Values represent mean ± SD (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001). hIAIP: human inter-alpha inhibitor protein; NS: normal saline; LPS: lipopolysaccharide.
hIAIPs do not attenuate LPS-induced 99mTc-albumin permeability
The mice were co-injected with 14C-sucrose and 99mTc-albumin, so we could determine whether hIAIPs would also attenuate LPS-induced BBB disruption to larger molecules. A significant LPS treatment effect of 99mTc-albumin permeability was observed in both males (Figure 3(c); F(3,32) = 11.95; p ≤ 0.001) and females (Figure 3(d); F(3,32) = 7.91; p ≤ 0.01). Tukey’s post hoc analysis confirmed that LPS significantly increased 99mTc-albumin permeability compared to male NS controls (Figure 3(c); p ≤ 0.05; q = 3.85) and female NS controls (Figure 3(d); p ≤ 0.01; q = 4.71). hIAIP administration after each LPS injection was associated with increases in 99mTc-albumin permeability compared to male NS controls (Figure 3(c); p ≤ 0.001; q = 6.84) and female NS controls (Figure 3(d); p ≤ 0.01; q = 4.80), but not compared to LPS alone (p > 0.05). This further suggests that LPS is associated with increases in BBB disruption to albumin and that hIAIP administration does not alter this process.
Overall, the permeability studies suggest that hIAIPs have the ability to protect against LPS-induced BBB disruption to small, but not large molecules. In conjunction with our permeability studies, we investigated a panel of cytokines, chemokines, and growth factors to determine whether any changes were correlated with the BBB-protective effects of hIAIPs.
Brain cytokines upregulated by LPS were not affected by hIAIP treatment
LPS increases brain levels of inflammatory cytokines.1,3,41 Therefore, we employed a Bio-Rad Luminex assay to detect the concentrations of 23 distinct cytokines, chemokines, and growth factors in brain tissue from mice exposed to LPS and then compared these data with mice exposed to hIAIP after LPS. A significant increase in 10 of 23 brain analytes were measured in males given LPS (IL-1α, IL-1β, IL-6, IL-12, GCSF, CXCL1, CCL2, CCL3, CCL4, CCL5; see Table 1), and a significant increase in 11 of 23 brain analytes was measured in females given LPS (IL-1α, IL-1β, IL-6, IL-12, GCSF, GMCSF, CXCL1, CCL2, CCL3, CCL4, CCL5; see Table 1). The remaining 12 analytes were not significantly elevated by LPS (Table 2). hIAIP administration did not significantly affect brain analyte levels (Table 2). These findings suggest that peripherally administered hIAIPs have minimal influence on inflammatory factors in the brain. As such, we also measured serum analytes using the same Luminex assay after LPS and hIAIP treatment.
Table 1.
23-Plex analytes in mouse brain (pg/mg).
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Analyte | NS | LPS | LPS + hIAIP | NS | LPS | LPS + hIAIP |
| IL-1α | 0.9 ± 0.6 | 79.7 ± 77.6*** | 62.0 ± 33.5** | 0.6 ± 0.3 | 191.3 ± 144.8*** | 118.2 ± 81.4** |
| IL-1β | 3.9 ± 1.6 | 7.7 ± 3.8** | 8.9 ± 1.9*** | 3.4 ± 1.2 | 11.0 ± 3.8*** | 10.2 ± 5.0*** |
| IL-2 | 5.9 ± 1.4 | 5.5 ± 1.5 | 5.7 ± 0.8 | 6.3 ± 1.1 | 5.2 ± 1.6 | 6.7 ± 3.0 |
| IL-3 | 6.1 ± 2.4 | 5.7 ± 2.1 | 5.9 ± 1.8 | 4.7 ± 1.7 | 5.6 ± 2.6 | 7.3 ± 3.0* |
| IL-4 | 9.7 ± 5.2 | 12.5 ± 9.1 | 12.1 ± 6.2 | 9.9 ± 6.0 | 10.8 ± 4.0 | 12.4 ± 5.2 |
| IL-5 | 2.7 ± 1.0 | 2.8 ± 1.2 | 2.8 ± 0.8 | 2.1 ± 0.8 | 2.9 ± 1.2 | 3.5 ± 1.2** |
| IL-6 | 28.9 ± 19.4 | 199.1 ± 188.8** | 159.7 ± 113.3* | 27.7 ± 23.1 | 437.9 ± 203.5** | 382.8 ± 417.6** |
| IL-9 | 174.8 ± 141.9 | 145.3 ± 159.9 | 127.9 ± 105.0 | 141.2 ± 99.9 | 102.8 ± 91.7 | 99.9 ± 100.5 |
| IL-10 | 47.80 ± 21.8 | 57.5 ± 35.0 | 49.0 ± 16.5 | 54.3 ± 32.4 | 52.1 ± 14.4 | 54.4 ± 13.1 |
| IL-12(p40) | 142.7 ± 46.6 | 10,442 ± 5490*** | 13,102 ± 8937*** | 144.7 ± 43.0 | 15,665 ± 9390*** | 13,170 ± 3653*** |
| IL-12(p70) | 980.9 ± 508.0 | 1037 ± 845.1 | 712.0 ± 264.2 | 1069 ± 572.5 | 675.2 ± 254.0 | 711.5 ± 380.3 |
| IL-13 | 99.1 ± 50.0 | 108.5 ± 72.0 | 88.7 ± 41.6 | 106.0 ± 63.8 | 88.7 ± 56.1 | 105.1 ± 52.6 |
| IL-17 | 31.1 ± 12.7 | 29.5 ± 10.2 | 31.6 ± 11.7 | 27.7 ± 9.1 | 27.8 ± 13.5 | 39.0 ± 20.2 |
| CCL11 | 277.1 ± 277.2 | 263.9 ± 304.1 | 212.7 ± 164.4 | 273.3 ± 264.4 | 158.2 ± 137.3 | 229.8 ± 200.1 |
| GCSF | 88.8 ± 169.7 | 5810 ± 5049*** | 3904 ± 2320** | 21.8 ± 18.5 | 13,239 ± 6166*** | 11,748 ± 9102*** |
| GMCSF | 7.9 ± 4.5 | 9.8 ± 4.9 | 10.5 ± 3.5 | 5.3 ± 2.7 | 12.5 ± 5.7** | 15.4 ± 6.8*** |
| IFNγ | 234.1 ± 209.5 | 213.9 ± 239.6 | 168.0 ± 158.4 | 219.1 ± 215.9 | 108.7 ± 111.7 | 126.3 ± 140.9 |
| CXCL1 | 46.5 ± 22.7 | 3845 ± 1711*** | 3592 ± 1055*** | 32.9 ± 12.3 | 7015 ± 1503*** | 5668 ± 2337*** |
| CCL2 | 38.4 ± 19.5 | 1495 ± 1344** | 2433 ± 1240*** | 25.6 ± 7.2 | 2276 ± 830.5*** | 1765 ± 1008*** |
| CCL3 | 6.3 ± 2.8 | 611.8 ± 681.1** | 536.5 ± 244.1** | 6.5 ± 2.4 | 1605 ± 1454*** | 1252 ± 1006** |
| CCL4 | 16.0 ± 5.3 | 101.1 ± 110.7* | 129.8 ± 80.5** | 14.7 ± 2.6 | 179.3 ± 133.7*** | 139.2 ± 116.7** |
| CCL5 | 13.0 ± 5.8 | 646.3 ± 258.5*** | 543.6 ± 170.6*** | 8.4 ± 4.8 | 1480 ± 1467*** | 1276 ± 472.6** |
| TNFα | 546.4 ± 353.0 | 539.9 ± 424.0 | 392.4 ± 92.3 | 506.9 ± 262.5 | 416.2 ± 312.8 | 501.7 ± 516.1 |
Note: Values represent mean difference ± SD; one-way ANOVA; Tukey’s post hoc test (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 vs. NS). Statistical differences occurred only relative to the NS group; there were no significant differences between the LPS vs. LPS + hIAIP groups. hIAIP: human inter-alpha inhibitor proteins; LPS: lipopolysaccharide; NS: normal saline; IL-1α: interleukin 1 alpha; IL-1β: interleukin 1 beta; IL-2: interleukin 2; IL-3: interleukin 3; IL-4: interleukin 4; IL-5: interleukin 5; IL-6: interleukin 6; IL-9: interleukin 9; IL-10: interleukin 10; IL-12(p40): interleukin 12 p40 homodimer; IL-12(p70): interleukin 12 p70 homodimer; IL-13: interleukin 13; IL-17: interleukin 17; CCL11: C-C motif chemokine ligand 11; GCSF: granulocyte colony-stimulating factor; GMCSF: granulocyte-macrophage colony-stimulating factor; IFNγ: interferon gamma; CXCL1: C-X-C motif chemokine ligand 1; CCL2: C-C motif chemokine ligand 2; CCL3: C-C motif chemokine ligand 3; CCL4: C-C motif chemokine ligand 4; CCL5: C-C motif chemokine ligand 5; TNFα: tumor necrosis factor alpha.
Table 2.
23-Plex analytes in mouse serum (pg/ml).
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Analyte | NS | LPS | LPS + hIAIP | NS | LPS | LPS + hIAIP |
| IL-1α | 15.8 ± 8.5 | 63.3 ± 19.9*** | 78.1 ± 36.7*** | 15.7 ± 10.3 | 92.9 ± 45.6*** | 78.3 ± 48.8*** |
| IL-1β | 61.9 ± 21.4 | 103.7 ± 29.7** | 115.5 ± 35.9*** | 50.9 ± 14.4 | 126.3 ± 36.2*** | 116.1 ± 51.1*** |
| IL-2 | 19.7 ± 7.4 | 34.6 ± 16.5* | 31.1 ± 11.5 | 17.2 ± 6.5 | 29.9 ± 9.7* | 29.7 ± 17.7 |
| IL-3 | 23.3 ± 7.8 | 25.8 ± 8.4 | 31.6 ± 10.3 | 21.2 ± 6.2 | 29.2 ± 12.3 | 30.3 ± 13.8 |
| IL-4 | 25.2 ± 11.9 | 14.2 ± 6.1 | 21.7 ± 10.0 | 18.7 ± 8.1 | 16.5 ± 11.1 | 19.6 ± 11.5 |
| IL-5 | 29.5 ± 8.3 | 27.9 ± 8.1 | 31.4 ± 9.6 | 25.1 ± 6.7 | 38.8 ± 17.3* | 41.1 ± 13.5* |
| IL-6 | 46.8 ± 39.8 | 25,024 ± 12,345*** | 14,080 ± 9216***,# | 21.4 ± 16.8 | 43,763 ± 20,895*** | 13,991 ± 9410*,### |
| IL-9 | 91.9 ± 35.6 | 72.2 ± 30.7 | 88.7 ± 36.7 | 83.3 ± 30.9 | 83.0 ± 39.8 | 87.0 ± 43.6 |
| IL-10 | 184.2 ± 59.5 | 666.1 ± 182.6*** | 546.9 ± 188.1*** | 161.3 ± 49.3 | 1152 ± 561.1*** | 1120 ± 695.6*** |
| IL-12(p40) | 1312 ± 423.8 | 5452 ± 1835*** | 6174 ± 2035*** | 1121 ± 817.6 | 6986 ± 3245*** | 5293 ± 2475*** |
| IL-12(p70) | 830.5 ± 417.5 | 1004 ± 505.7 | 1118 ± 551.7 | 734.1 ± 342.6 | 1168 ± 668.9 | 1095 ± 636.7 |
| IL-13 | 243.0 ± 134.3 | 308.7 ± 122.7 | 366.5 ± 177.1 | 253.0 ± 125.8 | 369.4 ± 175.3 | 392.8 ± 230.9 |
| IL-17 | 136.9 ± 38.8 | 298.0 ± 61.2*** | 237.6 ± 51.7***,# | 114.3 ± 23.0 | 277.3 ± 118.8*** | 216.7 ± 88.5*** |
| CCL11 | 861.7 ± 353.6 | 5368 ± 1884*** | 4686 ± 2000*** | 751.0 ± 318.9 | 5295 ± 2485*** | 2967 ± 2217*,# |
| GCSF | 1265 ± 996.0 | 453,430 ± 132,765*** | 354,222 ± 96,063*** | 753.6 ± 543.4 | 524,700 ± 156,551*** | 586,491 ± 127,551*** |
| GMCSF | 86.8 ± 23.4 | 167.4 ± 39.0** | 170.4 ± 81.6** | 77.8 ± 17.9 | 315.6 ± 178.2* | 280.3 ± 347.7 |
| IFNγ | 99.7 ± 22.1 | 101.5 ± 25.7 | 118.0 ± 22.9 | 90.2 ± 20.7 | 141.1 ± 66.3* | 129.1 ± 53.9 |
| CXCL1 | 276.7 ± 101.0 | 14,239 ± 4258*** | 13,437 ± 6172*** | 255.6 ± 135.3 | 32,799 ± 21,151*** | 17,369 ± 14,547* |
| CCL2 | 645.4 ± 390.5 | 77,110 ± 39,764*** | 69,689 ± 46,305*** | 553.1 ± 389.3 | 88,821 ± 36,072*** | 62,675 ± 73,504** |
| CCL3 | 7.8 ± 2.9 | 92.1 ± 37.0*** | 99.0 ± 26.6*** | 6.8 ± 2.2 | 94.5 ± 35.4*** | 79.5 ± 41.1*** |
| CCL4 | 52.8 ± 31.0 | 677.6 ± 329.7*** | 722.9 ± 310.6*** | 45.4 ± 25.8 | 630.0 ± 207.6*** | 519.4 ± 430.9*** |
| CCL5 | 181.9 ± 101.3 | 9986 ± 2913*** | 8013 ± 4480*** | 140.8 ± 93.8 | 13,940 ± 4849*** | 7413 ± 3842***,### |
| TNFα | 150.2 ± 88.9 | 201.2 ± 110.0 | 243.7 ± 133.6 | 133.1 ± 70.1 | 225.8 ± 128.5 | 231.2 ± 148.1 |
Note: Asterisks represent statistical differences in comparison to NS; hashes represent statistical differences in comparison to LPS. Values represent mean difference ± SD; one-way ANOVA; Tukey’s post hoc test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 vs. NS; #p ≤ 0.05, ###p ≤ 0.001 vs. LPS). hIAIP: human inter-alpha inhibitor proteins; LPS: lipopolysaccharide; NS: normal saline; IL-1α: interleukin 1 alpha; IL-1β: interleukin 1 beta; IL-2: interleukin 2; IL-3: interleukin 3; IL-4: interleukin 4; IL-5: interleukin 5; IL-6: interleukin 6; IL-9: interleukin 9; IL-10: interleukin 10; IL-12(p40): interleukin 12 p40 homodimer; IL-12(p70): interleukin 12 p70 homodimer; IL-13: interleukin 13; IL-17: interleukin 17; CCL11: C-C motif chemokine ligand 11; GCSF: granulocyte colony-stimulating factor; GMCSF: granulocyte-macrophage colony-stimulating factor; IFNγ: interferon gamma; CXCL1: C-X-C motif chemokine ligand 1; CCL2: C-C motif chemokine ligand 2; CCL3: C-C motif chemokine ligand 3; CCL4: C-C motif chemokine ligand 4; CCL5: C-C motif chemokine ligand 5; TNFα: tumor necrosis factor alpha.
hIAIPs downregulated elevated serum IL-6 in both male and female mice exposed to LPS
LPS is known to induce a myriad of inflammatory cytokines that can be detected in serum of mice.1,3,10,41 We used the same Biorad Luminex assay mentioned above to detect the concentrations of 23 distinct cytokines, chemokines, and growth factors in serum from male and female mice exposed to LPS and then compared these data with mice exposed to hIAIP after LPS.
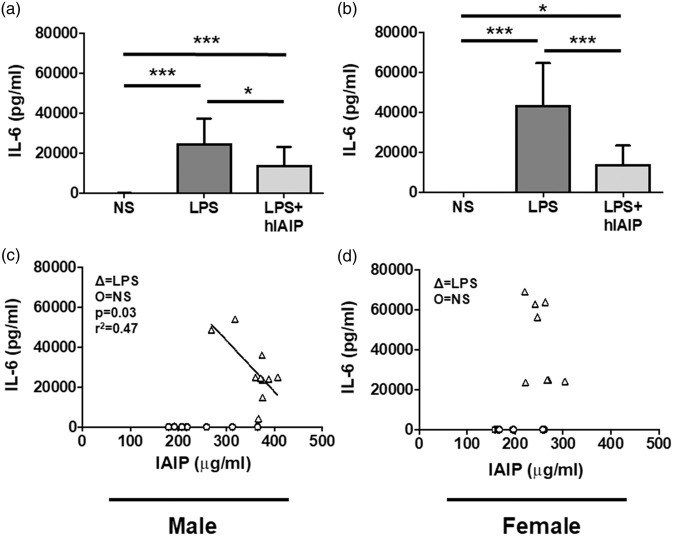
A significant increase in 15 of 23 serum analytes was measured in males given LPS (IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, IL-17, CCL11, GCSF, GMCSF, CXCL1, CCL2, CCL3, CCL4, CCL5; see Table 2). A significant increase in 16 of 23 serum analytes was measured in females given LPS (IL-1α, IL-1β, IL-5, IL-6, IL-10, IL-12, IL-17, CCL11, GCSF, GMCSF, IFNγ, CXCL1, CCL2, CCL3, CCL4, CCL5; see Table 2). hIAIPs downregulated serum IL-6, and IL-17 in male mice given LPS (Table 2), and hIAIPs downregulated serum IL-6, CCL11, and CCL5 in female mice given LPS (Table 2). Notably, hIAIP treatment downregulated serum IL-6 levels in both male (Figure 4(a); p ≤ 0.05; q = 4.06) and female mice (Figure 4(b); p ≤ 0.01; q = 7.70) exposed to LPS.
Figure 4.
hIAIPs downregulate LPS-induced serum IL-6 levels, which were inversely correlated to male serum IAIP concentration. (a) Serum IL-6 levels (pg/ml) measured by luminex assay were significantly increased in male LPS mice (n = 9) compared to male NS controls (n = 14), and hIAIP treatment (n = 9) significantly downregulates the LPS effect. (b) Serum IL-6 levels were significantly increased in female LPS mice (n = 8) compared to female NS controls (n = 14), and hIAIP treatment (n = 10) significantly downregulates the LPS effect. One-way ANOVA; Tukey’s post hoc test. Values represent mean ± SD. (*p ≤ 0.05; ***p ≤ 0.001). (c) LPS male serum IL-6 levels were inversely correlated to serum IAIP concentration (p = 0.03; r2 = 0.47). (d) LPS female serum IL-6 levels did not correlate to serum IAIP concentration (p > 0.05). Linear regression was performed on the LPS data points to determine statistical correlation. IL-6: interleukin 6; NS: normal saline; LPS: lipopolysaccharide; hIAIP: human inter-alpha inhibitor protein.
Serum IAIPs are inversely correlated to serum IL-6 in males, but not females, after LPS
Because we found that exogenous hIAIPs downregulated serum IL-6 after LPS (Table 2, Figure 4(a) and (b)), and LPS increased endogenous serum IAIP concentration (Figure 2), we determined whether serum IAIPs were correlated with serum IL-6. Male mice given LPS exhibited a significant inverse correlation between serum IAIPs and serum IL-6 (Figure 4(c); p ≤ 0.05; r2 = 0.47). Female mice did not show a significant correlation between IAIP and IL-6 after LPS exposure (Figure 4(d); p > 0.05; r2 = 0.26). Other serum analytes were downregulated by hIAIP treatment in both male and female mice given LPS (Table 2), and therefore, we determined whether those analytes were correlated to serum IAIP concentration as well.
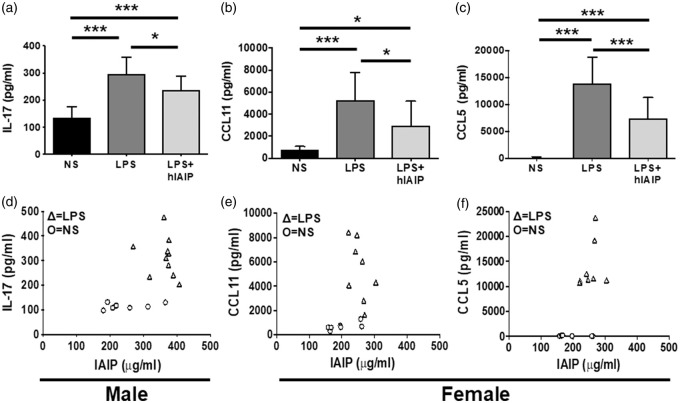
hIAIPs downregulated serum IL-17 in male mice exposed to LPS (Figure 5(a); p ≤ 0.05; q = 3.66), but the IL-17 levels did not correlate to serum IAIP concentration (Figure 5(d); p > 0.05; r2 = 0.05). In female mice exposed to LPS, hIAIP treatment downregulated CCL11 (Figure 5(b); p ≤ 0.05; q = 3.97) and CCL5 (Figure 5(c); p ≤ 0.05; q = 6.08). No significant correlation was observed between serum IAIP concentrations and CCL11 levels (Figure 5(e); p > 0.05; r2 = 0.12), or CCL5 levels (Figure 5(f); p > 0.05; r2 = 0.28). These data suggest that although IAIPs may have an effect on multiple inflammatory factors, IL-6 maintains the strongest association to hIAIP treatment.
Figure 5.
hIAIPs downregulate LPS-induced IL-17, CCL11, and CCL5; however, IL-17, CCL11, and CCL5 did not correlate with serum IAIP concentration. (a) LPS significantly increased serum IL-17 levels in male mice compared to male NS controls, and hIAIPs significantly downregulate the LPS effect. LPS significantly increased (b) serum CCL11 and (c) serum CCL5 levels in female mice compared to female NS controls, and hIAIPs significantly downregulate the LPS effect on both inflammatory factors. One-way ANOVA; Tukey’s post hoc test. Values represent mean ± SD. (*p ≤ 0.05; ***p ≤ 0.001). (d) LPS male serum IL-17 levels did not correlate to serum IAIP concentration (p > 0.05). LPS female serum (e) CCL11 and (f) CCL5 levels both did not correlate to serum IAIP concentration (p > 0.05). Linear regression was performed on the LPS data points to determine statistical correlation. IL-17: interleukin 17; CCL11: C-C motif chemokine 11; CCL5: C-C motif chemokine 5; IL-6: interleukin 6; NS: normal saline; LPS: lipopolysaccharide; hIAIP: human inter-alpha inhibitor protein.
Discussion
IAIPs play an important role in modulating inflammatory responses in several experimental models of newborn and adult systemic inflammation and in models of inflammation-related premature labor.15,16,47,48 hIAIPs are highly conserved in rodents (∼70%), and no adverse effects have been observed when multiple doses of hIAIPs had been administered to adult24 or neonatal rodents.25 In addition, we find that hIAIP alone did not provoke a significant difference in BBB permeability or cytokine production (see Experimental design). This suggests that hIAIP administration alone is not associated with an immunological reaction in mice. Due to the conservative nature of hIAIP, we were not concerned with any off-target effects, and therefore, we predicted hIAIP to primarily modulate effects associated with LPS administration.
A growing body of evidence suggests that LPS-induced systemic inflammation impairs BBB function.1,4,5,49–51 Thus, the primary objective of this study was to determine the effects of IAIPs on LPS-induced BBB disruption and its associated inflammatory mechanisms in male and female CD-1 mice. We measured the BBB permeability to 14C-sucrose and 99mTc-albumin in LPS-exposed mice with and without hIAIP treatment. We found that LPS administration was associated with increased BBB permeability to 14C-sucrose and 99mTc-albumin, which is consistent with our previous work.1 However, hIAIP treatment only attenuated LPS-induced BBB disruption to 14C-sucrose, but not to 99mTc-albumin, in the male mice. There was a nonsignificant decline in BBB disruption in the female mice, and so, the sex difference may be one of relative sensitivity.
Sucrose (MW 343 Da) is a small vascular tracer that does not readily cross the BBB and is often used as a marker for solute and ion permeability which predominantly leak through a disrupted BBB via a paracellular route.1,52 Albumin (MW 66.5 kDa) is a larger tracer that can be predominantly transported through the BBB by adsorptive-mediated transcellular endocytosis.1,52 Tight junction proteins (TJPs) play a critical role in BBB integrity by reducing the paracellular leakage of blood-borne molecules.45,46,53 Previously, we report TJP disorganization in our in vitro LPS model; however, we did not observe changes in TJP expression in mouse brain after LPS administration.1 It is currently unknown whether IAIPs attenuate BBB disruption via modulation of TJP function. Our findings suggest that the protective effects of IAIPs under inflammatory conditions are through paracellular, rather than transcellular mechanisms of BBB disruption. Sucrose is considered a sensitive paracellular marker of BBB disruption, as it has been shown to penetrate a BBB devoid of the TJP claudin-5.53 On the other hand, albumin has been implicated as a sensitive marker of transcellular BBB disruption due to a low baseline permeability measurement in vivo as well as a low signal-to-noise ratio compared to that of sucrose. The effects of hIAIPs on TJPs with LPS exposure will be investigated in future studies.
We and others have previously reported a decrease in endogenous IAIP concentration under inflammatory conditions,18,38,40,54 although in the context of human sepsis, which we are not modeling and often represents an immune suppressed state. In contrast, our three-dose LPS model invokes a hyperinflammatory state,1 and we measured an increase in endogenous serum IAIP concentration. Moreover, another LPS model reported decreased IAIPs15; however, that study was performed in neonatal Balb/c mice, which inherently evoke different immune responses to inflammatory stimuli.55 Alternatively, timing of our endogenous IAIP measurements could also explain the differences between our study and the study by Singh et al.15 Perhaps measuring IAIP levels shortly after a single LPS injection would have decreased serum IAIP levels; however, we would have dampened our BBB effect based on prior studies in our laboratory.3
A number of mechanisms have been described for the BBB disruption caused by LPS.50 Although LPS can cause BBB disruption through cell-autonomous mechanisms in brain endothelial cells in vitro, including modification of TJ protein architecture,1 increased blood concentration of inflammatory factors in response to LPS is also likely to contribute to paracellular pathways of BBB disruption in vivo.1,4,50,56,57 We found that mice given LPS exhibited an increase in multiple inflammatory factors in serum (Table 2), which is consistent with our previous studies using the same LPS model.3,41 Recent studies have identified the ability of IAIPs to inhibit the production of inflammatory factors and have suggested its potential as an effective therapeutic agent for systemic inflammation in neonates and adults.15,58–60 In our study, we found that exogenous hIAIP treatment downregulated four inflammatory factors (IL-6, IL-17, CCL5, and CCL11) in mice exposed to LPS. IL-6 was the only analyte downregulated by hIAIP in both male and female mice given LPS. IL-6 was also inversely correlated with endogenous IAIP concentrations in LPS-treated male mice. These data suggest that IL-6 may be a common target of IAIPs and may play a vital role in LPS-induced BBB disruption.
IL-6 has been demonstrated to increase BBB permeability in a mouse model of bacterial meningitis.61 This inflammatory cytokine has also been shown to reduce occludin and claudin-5 expression in microvessels isolated from sheep62 and can downregulate human brain microvascular endothelial barrier function through modification of TJPs.63 We have found that IL-6 inhibition modulates TJPs and attenuates BBB disruption in ovine fetuses with ischemic-reperfusion brain injury.33 Recent studies have shown that IAIPs inhibit systemic cytokines, including IL-6 in mice with histone challenge64 and granzyme K-induced increases of IL-6 production in vitro.65 Thus, our findings suggest that IAIPs ability to inhibit serum IL-6 production could contribute to the improvement of BBB disruption to small molecule permeability after LPS exposure. We also cannot rule out the possibility that IAIPs could have modulated LPS-mediated BBB permeability through other inflammatory mechanisms within the cerebral vascular endothelium, which could be tested as a future direction in an in vitro system.
We also found that the LPS injections increased the brain expression of multiple inflammatory factors (Table 1). This is consistent with our earlier studies in mice after LPS injections.3,66 LPS given peripherally can induce inflammatory responses in the brain, which may be caused by indirect effects.67 It is likely that LPS invokes neuroinflammation by activating LPS receptors located outside the BBB, stimulating afferent nerves, and/or acting at circumventricular organs.66 Serum inflammatory factors can also cross the BBB through a saturable transport mechanism and affect CNS function.68–70 Peripheral LPS injections can also activate brain microglia to elevate CNS inflammatory factors,67 including IL-6, which has the ability to disrupt the BBB.52 The lack of an hIAIP effect on inflammatory factors in brain tissue suggests that IAIP may specifically protect the BBB through luminal mechanisms associated with peripheral inflammatory factors.
Sexually dimorphic immune responses could influence the brain’s response to a systemic inflammatory insult such as LPS exposure.41 Our results did show a sexually dimorphic cytokine response of after LPS, which is consistent with our previous publication.41 Our results also show a sex difference in both the endogenous IAIP response to LPS, and the exogenous hIAIP effects on LPS-induced BBB permeability. Although we observed a sexually dimorphic inflammatory response to hIAIP treatment following LPS, we did observe that serum levels of IL-6 were downregulated by hIAIPs in both males and females. However, a significant correlation between the serum IL-6 levels and IAIP levels were only observed in males. These findings may suggest that attenuation of LPS-induced BBB disruption by hIAIP treatment could be achieved in part by a common IL-6-mediated pathway. Nevertheless, we cannot rule out the possibility that other signal transduction pathways could also have contributed to the hIAIP-mediated reduction of sucrose permeability in male mice exposed to LPS.
There are several limitations to our study along with potential opportunities for future study. First, the molecular/cellular mechanisms of IAIP in relation to TJPs at the BBB under inflammatory conditions have not yet been examined, but are likely candidates for future mechanistic studies. Second, sexually dimorphic responses to LPS and effects of LPS on the BBB may vary by mouse strain,41,71 and future studies along these lines could identify important genetic components of BBB responses to inflammatory stimuli. Furthermore, mechanistic studies could also be explored to determine whether the IAIP/IL-6 axis is mediating BBB disruption with LPS. Finally, we only studied a single time point following LPS administration, and therefore, future studies should consider measuring the inflammatory factors known to peak at earlier time points prior to BBB disruption.3
In conclusion, LPS increased endogenous serum levels of IAIPs compared to saline-injected controls. IAIP treatment attenuated LPS-induced BBB disruption to 14C-sucrose, but not 99mTc-albumin in CD-1 male mice. IAIP treatment also downregulated serum IL-6, IL-17, CCL5, and CCL11 in mice exposed to LPS. IAIP treatment did not affect brain cytokines after LPS exposure. Finally, endogenous serum IAIPs were inversely correlated to serum IL-6 levels in male mice given LPS. This study provides important insights into the effects of hIAIPs on BBB disruption and related inflammatory mechanisms, providing new potential targets for inflammatory disorders.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by T32AG052354 (AFL), 5R01AG046619 (WAB), 1R21NS095130 (BSS), 1R21NS096525 (BSS), 2R01HD057100 (BSS), and 2R44 NS084575 (YPL/BSS).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Yow-Pin Lim and Joseph Qiu are employed by ProThera Biologics. Yow-Pin Lim has a significant financial stake in the company. All other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Study design (AFL, MAE, XC, YPL, BSS, and WAB); conducted experiments (AFL, MAE, JQ, and YPL); analyzed data (AFL, MAE, XC, JQ, YPL, BSS, and WAB); wrote manuscript (AFL, MAE, XC, YPL, BSS, and WAB); and provided critical scientific input in manuscript preparation and interpreting results (AFL, MAE, XC, YPL, BSS, and WAB).
References
- 1.Banks WA, Gray AM, Erickson MA, et al. Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J Neuroinflamm 2015; 12: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Vries HE, Blom-Roosemalen MC, de Boer AG, et al. Effect of endotoxin on permeability of bovine cerebral endothelial cell layers in vitro. J Pharmacol Exp Ther 1996; 277: 1418–1423. [PubMed] [Google Scholar]
- 3.Erickson MA, Banks WA. Cytokine and chemokine responses in serum and brain after single and repeated injections of lipopolysaccharide: multiplex quantification with path analysis. Brain Behav Immun 2011; 25: 1637–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin L, Nation RL, Li J, et al. Species-dependent blood-brain barrier disruption of lipopolysaccharide: amelioration by colistin in vitro and in vivo. Antimicrob Agents Chemother 2013; 57: 4336–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckman PL, King WM, Brunson JG. Studies on the blood brain barrier. I. Effects produced by a single injection of gramnegative endotoxin on the permeability of the cerebral vessels. Am J Pathol 1958; 34: 631–643. [PMC free article] [PubMed] [Google Scholar]
- 6.Allen IV. The effect of bacterial pyrogen on the blood-brain barrier for Trypan Blue. J Pathol Bacteriol 1965; 89: 481–494. [PubMed] [Google Scholar]
- 7.Minami T, Okazaki J, Kawabata A, et al. Penetration of cisplatin into mouse brain by lipopolysaccharide. Toxicology 1998; 130: 107–113. [DOI] [PubMed] [Google Scholar]
- 8.Banks WA, Kastin AJ, Brennan JM, et al. Adsorptive endocytosis of HIV-1gp120 by blood-brain barrier is enhanced by lipopolysaccharide. Exp Neurol 1999; 156: 165–171. [DOI] [PubMed] [Google Scholar]
- 9.Xaio H, Banks WA, Niehoff ML, et al. Effect of LPS on the permeability of the blood-brain barrier to insulin. Brain Res 2001; 896: 36–42. [DOI] [PubMed] [Google Scholar]
- 10.Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation 2011; 8: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis 2010; 37: 26–32. [DOI] [PubMed] [Google Scholar]
- 12.Josic D, Brown MK, Huang F, et al. Proteomic characterization of inter-alpha inhibitor proteins from human plasma. Proteomics 2006; 6: 2874–2885. [DOI] [PubMed] [Google Scholar]
- 13.Potempa J, Kwon K, Chawla R, et al. Inter-alpha-trypsin inhibitor. Inhibition spectrum of native and derived forms. J Biol Chem 1989; 264: 15109–15114. [PubMed] [Google Scholar]
- 14.Zhu L, Zhuo L, Watanabe H, et al. Equivalent involvement of inter-alpha-trypsin inhibitor heavy chain isoforms in forming covalent complexes with hyaluronan. Connect Tissue Res 2008; 49: 48–55. [DOI] [PubMed] [Google Scholar]
- 15.Singh K, Zhang LX, Bendelja K, et al. Inter-alpha inhibitor protein administration improves survival from neonatal sepsis in mice. Pediatr Res 2010; 68: 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S, Lim YP, Zhou M, et al. Administration of human inter-alpha-inhibitors maintains hemodynamic stability and improves survival during sepsis. Crit Care Med 2002; 30: 617–622. [DOI] [PubMed] [Google Scholar]
- 17.Garantziotis S, Hollingsworth JW, Ghanayem RB, et al. Inter-alpha-trypsin inhibitor attenuates complement activation and complement-induced lung injury. J Immunol 2007; 179: 4187–4192. [DOI] [PubMed] [Google Scholar]
- 18.Lim YP. ProThera Biologics, Inc.: a novel immunomodulator and biomarker for life-threatening diseases. R I Med J 2013; 96: 16–18. [PubMed] [Google Scholar]
- 19.Abe H, Sugino N, Matsuda T, et al. Effect of ulinastatin on delayed neuronal death in the gerbil hippocampus. Masui 1996; 45: 38–43. [PubMed] [Google Scholar]
- 20.Feng M, Shu Y, Yang Y, et al. Ulinastatin attenuates experimental autoimmune encephalomyelitis by enhancing anti-inflammatory responses. Neurochem Int 2014; 64: 64–72. [DOI] [PubMed] [Google Scholar]
- 21.Yano T, Anraku S, Nakayama R, et al. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology 2003; 98: 465–473. [DOI] [PubMed] [Google Scholar]
- 22.Shu Y, Yang Y, Qiu W, et al. Neuroprotection by ulinastatin in experimental autoimmune encephalomyelitis. Neurochem Res 2011; 36: 1969–1977. [DOI] [PubMed] [Google Scholar]
- 23.Jiang XM, Hu JH, Wang LL, et al. Effects of ulinastatin on global ischemia via brain pro-inflammation signal. Transl Neurosci 2016; 7: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudet CM, Lim YP, Stonestreet BS, et al. Effects of age, experience and inter-alpha inhibitor proteins on working memory and neuronal plasticity after neonatal hypoxia-ischemia. Behav Brain Res 2016; 302: 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Threlkeld SW, Gaudet CM, La Rue ME, et al. Effects of inter-alpha inhibitor proteins on neonatal brain injury: age, task and treatment dependent neurobehavioral outcomes. Exp Neurol 2014; 261: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Threlkeld SW, Lim YP, La Rue M, et al. Immuno-modulator inter-alpha inhibitor proteins ameliorate complex auditory processing deficits in rats with neonatal hypoxic-ischemic brain injury. Brain Behav Immun 2017; 64: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XF, Zhang XJ, Zhang C, et al. Ulinastatin protects brain against cerebral ischemia/reperfusion injury through inhibiting MMP-9 and alleviating loss of ZO-1 and occludin proteins in mice. Exp Neurol 2018; 302: 68–74. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, Shen J, Zou F, et al. Effect of ulinastatin on the permeability of the blood-brain barrier on rats with global cerebral ischemia/reperfusion injury as assessed by MRI. Biomed Pharmacother 2017; 85: 412–417. [DOI] [PubMed] [Google Scholar]
- 29.Wisniewski HG, Hua JC, Poppers DM, et al. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol 1996; 156: 1609–1615. [PubMed] [Google Scholar]
- 30.Htwe SS, Wake H, Liu K, et al. Inter-alpha inhibitor proteins maintain neutrophils in a resting state by regulating shape and reducing ROS production. Blood Adv 2018; 2: 1923–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen X, Hovanesian V, Naqvi S, et al. Systemic infusions of anti-interleukin-1beta neutralizing antibodies reduce short-term brain injury after cerebral ischemia in the ovine fetus. Brain Behav Immun 2018; 67: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patra A, Chen X, Sadowska GB, et al. Neutralizing anti-interleukin-1beta antibodies reduce ischemia-related interleukin-1beta transport across the blood-brain barrier in fetal sheep. Neuroscience 2017; 346: 113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Sadowska GB, Chen X, et al. Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J 2015; 29: 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opal SM, Lim YP, Cristofaro P, et al. Inter-alpha inhibitor proteins: a novel therapeutic strategy for experimental anthrax infection. Shock 2011; 35: 42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spasova MS, Sadowska GB, Threlkeld SW, et al. Ontogeny of inter-alpha inhibitor proteins in ovine brain and somatic tissues. Exp Biol Med (Maywood) 2014; 239: 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009; 41: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 37.Disdier C, Zhang J, Fukunaga Y, et al. Alterations in inter-alpha inhibitor protein expression after hypoxic-ischemic brain injury in neonatal rats. Int J Dev Neurosci 2018; 65: 54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaaban H, Singh K, Huang J, et al. The role of inter-alpha inhibitor proteins in the diagnosis of neonatal sepsis. J Pediatr 2009; 154: 620–622.e1. [DOI] [PubMed] [Google Scholar]
- 39.Baek YW, Brokat S, Padbury JF, et al. Inter-alpha inhibitor proteins in infants and decreased levels in neonatal sepsis. J Pediatr 2003; 143: 11–15. [DOI] [PubMed] [Google Scholar]
- 40.Lim YP, Bendelja K, Opal SM, et al. Correlation between mortality and the levels of inter-alpha inhibitors in the plasma of patients with severe sepsis. J Infect Dis 2003; 188: 919–926. [DOI] [PubMed] [Google Scholar]
- 41.Erickson MA, Liang WS, Fernandez EG, et al. Genetics and sex influence peripheral and central innate immune responses and blood-brain barrier integrity. PLoS One 2018; 13: e0205769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levin VA, Fenstermacher JD, Patlak CS. Sucrose and inulin space measurements of cerebral cortex in four mammalian species. Am J Physiol 1970; 219: 1528–1533. [DOI] [PubMed] [Google Scholar]
- 43.Blasberg RG, Fenstermacher JD, Patlak CS. Transport of alpha-aminoisobutyric acid across brain capillary and cellular membranes. J Cereb Blood Flow Metab 1983; 3: 8–32. [DOI] [PubMed] [Google Scholar]
- 44.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983; 3: 1–7. [DOI] [PubMed] [Google Scholar]
- 45.Ziylan YZ, Robinson PJ, Rapoport SI. Blood-brain barrier permeability to sucrose and dextran after osmotic opening. Am J Physiol 1984; 247: R634–R638. [DOI] [PubMed] [Google Scholar]
- 46.Mayhan WG, Heistad DD. Permeability of blood-brain barrier to various sized molecules. Am J Physiol 1985; 248: H712–H718. [DOI] [PubMed] [Google Scholar]
- 47.Futamura Y, Kajikawa S, Kaga N, et al. Protection against preterm delivery in mice by urinary trypsin inhibitor. Obstet Gynecol 1999; 93: 100–108. [DOI] [PubMed] [Google Scholar]
- 48.Kaga N, Katsuki Y, Futamura Y, et al. Role of urinary trypsin inhibitor in the maintenance of pregnancy in mice. Obstet Gynecol 1996; 88: 872–882. [DOI] [PubMed] [Google Scholar]
- 49.Wispelwey B, Lesse AJ, Hansen EJ, et al. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest 1988; 82: 1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun 2017; 60: 1–12. [DOI] [PubMed] [Google Scholar]
- 51.Elwood E, Lim Z, Naveed H, et al. The effect of systemic inflammation on human brain barrier function. Brain Behav Immun 2017; 62: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7: 41–53. [DOI] [PubMed] [Google Scholar]
- 53.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161: 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaaban H, Shin M, Sirya E, et al. Inter-alpha inhibitor protein level in neonates predicts necrotizing enterocolitis. J Pediatr 2010; 157: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mills CD, Kincaid K, Alt JM, et al. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164: 6166–6173. [DOI] [PubMed] [Google Scholar]
- 56.de Vries HE, Blom-Roosemalen MC, van Oosten M, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol 1996; 64: 37–43. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh A, Birngruber T, Sattler W, et al. Assessment of blood-brain barrier function and the neuroinflammatory response in the rat brain by using cerebral open flow microperfusion (cOFM). PLoS One 2014; 9: e98143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horiguchi T, Harada Y. The effect of protease inhibitor on reperfusion injury after unilateral pulmonary ischemia. Transplantation 1993; 55: 254–258. [DOI] [PubMed] [Google Scholar]
- 59.Li XK, Suzuki H, Kimura T, et al. Ulinastatin, a protease inhibitor, attenuates intestinal ischemia/reperfusion injury. Transplant Proc 1994; 26: 2423–2425. [PubMed] [Google Scholar]
- 60.Endo S, Inada K, Yamashita H, et al. The inhibitory actions of protease inhibitors on the production of polymorphonuclear leukocyte elastase and interleukin 8. Res Commun Chem Pathol Pharmacol 1993; 82: 27–34. [PubMed] [Google Scholar]
- 61.Paul R, Koedel U, Winkler F, et al. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain 2003; 126: 1873–1882. [DOI] [PubMed] [Google Scholar]
- 62.Cohen SS, Min M, Cummings EE, et al. Effects of interleukin-6 on the expression of tight junction proteins in isolated cerebral microvessels from yearling and adult sheep. Neuroimmunomodulation 2013; 20: 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rochfort KD, Collins LE, Murphy RP, et al. Downregulation of blood-brain barrier phenotype by proinflammatory cytokines involves NADPH oxidase-dependent ROS generation: consequences for interendothelial adherens and tight junctions. PLoS One 2014; 9: e101815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chaaban H, Keshari RS, Silasi-Mansat R, et al. Inter-alpha inhibitor protein and its associated glycosaminoglycans protect against histone-induced injury. Blood 2015; 125: 2286–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma M, Merkulova Y, Raithatha S, et al. Extracellular granzyme K mediates endothelial activation through the cleavage of protease-activated receptor-1. FEBS J 2016; 283: 1734–1747. [DOI] [PubMed] [Google Scholar]
- 66.Banks WA, Robinson SM. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav Immun 2010; 24: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007; 55: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995; 2: 241–248. [DOI] [PubMed] [Google Scholar]
- 69.Threlkeld SW, Lynch JL, Lynch KM, et al. Ovine proinflammatory cytokines cross the murine blood-brain barrier by a common saturable transport mechanism. Neuroimmunomodulation 2010; 17: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadowska GB, Chen X, Zhang J, et al. Interleukin-1beta transfer across the blood-brain barrier in the ovine fetus. J Cereb Blood Flow Metab 2015; 35: 1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Everhardt Queen A, Moerdyk-Schauwecker M, McKee LM, et al. Differential expression of inflammatory cytokines and stress genes in male and female mice in response to a lipopolysaccharide challenge. PLoS One 2016; 11: e0152289. [DOI] [PMC free article] [PubMed] [Google Scholar]