Abstract
Cortical spreading depolarization (CSD) induces pro-inflammatory gene expression in brain tissue. However, previous studies assessing the relationship between CSD and inflammation have used invasive methods that directly trigger inflammation. To eliminate the injury confounder, we induced CSDs non-invasively through intact skull using optogenetics in Thy1-channelrhodopsin-2 transgenic mice. We corroborated our findings by minimally invasive KCl-induced CSDs through thinned skull. Six CSDs induced over 1 h dramatically increased cortical interleukin-1β (IL-1β), chemokine (C-C motif) ligand 2 (CCL2), and tumor necrosis factor-α (TNF-α) mRNA expression peaking around 1, 2 and 4 h, respectively. Interleukin-6 (IL-6) and intercellular adhesion molecule-1 (ICAM-1) were only modestly elevated. A single CSD also increased IL-1β, CCL2, and TNF-α, and revealed an ultra-early IL-1β response within 10 min. The response was blunted in IL-1 receptor-1 knockout mice, implicating IL-1β as an upstream mediator, and suppressed by dexamethasone, but not ibuprofen. CSD did not alter systemic inflammatory indices. In summary, this is the first report of pro-inflammatory gene expression after non-invasively induced CSDs. Altogether, our data provide novel insights into the role of CSD-induced neuroinflammation in migraine headache pathogenesis and have implications for the inflammatory processes in acute brain injury where numerous CSDs occur for days.
Keywords: Cortical spreading depolarization, dexamethasone, inflammation, NSAIDs, optogenetics
Introduction
Cortical spreading depolarization (CSD) is a slowly propagating (2–5 mm/min) wave of near-complete depolarization of neurons and glia.1,2 A large body of evidence implicates CSD as the biological underpinning of migraine aura and a potential headache trigger.3 In experimental animals, CSD activates trigeminal nociception.4 Moreover, most drugs that are used for migraine prophylaxis inhibit CSD,5 and many drugs that inhibit CSD are clinically efficacious in migraine.6 Hence, CSD has been among the most commonly studied experimental models relevant to migraine pathophysiology. Moreover, electrophysiological studies both in animals and in humans have shown that numerous recurrent CSDs occur after ischemic, hemorrhagic, and traumatic brain injury,7,8 underscoring its clinical relevance.9
Several studies have shown that CSD induces pro-inflammatory gene expression in rodent cortex, in vivo, and in hippocampal slice cultures, in vitro.10–19 However, all of these studies employed highly invasive and potentially injurious approaches to trigger CSD such as craniotomy, durotomy, and pinprick. Therefore, both the magnitude and time course of the inflammatory response measured after CSD might have been confounded by the invasive CSD induction method itself,20 contributing to the conflicting and inconsistent data among the studies. To overcome this potential confounder, we adopted a novel and completely non-invasive method of inducing CSD through intact skull using optogenetics21 in transgenic mice expressing channelrhodopsin-2 in neurons (Thy1-ChR2 YFP transgenic mice).22,23 Optogenetic CSDs behave identical to CSDs induced by conventional means.21 We complemented this with a minimally invasive method of inducing CSDs by topical KCl applied briefly over thinned skull. Thus, eliminating the injury confounder, we determined the magnitude and time course of six different pro-inflammatory markers after CSD, and tested the effects of steroidal and non-steroidal anti-inflammatory drugs on this neuroinflammatory response. We also sought evidence for acute systemic inflammatory changes after CSD, including circulating pro-inflammatory markers and spleen size.
Materials and methods
Animals
Experiments were approved by the MGH Institutional Animal Care and Use Committee, and carried out in accordance with the Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996). Study design and reporting followed ARRIVE guidelines. We used 8- to 42-week-old, male and female Thy1-ChR2 YFP mice (n = 75; ChR2+; B6.Cg-Tg(Thy1-COP4/EYFP)18Gfng/J; Jackson Laboratories, Bar Harbor, ME),22,23 interleukin-1 receptor-1 knockout (IL-1R1 KO) mice (n = 5; B6.129S7-Il1r1tm1/mx/J; Jackson Laboratories), and wild-type mice (n = 206 ChR2−; C57BL/6; Charles River Laboratories, Wilmington, MA). Mice were housed under standard laboratory conditions with food and water available in 12-h light–dark cycle.
The experimenter was blinded for pharmacological experiments except for dexamethasone administered post-CSD. Animals were excluded when CSD induction procedure or assays failed. One Thy1-ChR2 YFP and 5 C57BL/6 and mice were excluded due to CSD induction failure. Six Thy1-ChR2 YFP and 18 C57BL/6 mice were excluded from reverse transcriptase polymerase chain reaction (RT-PCR) analysis due to technical failure. Two C57BL/6 mice and five data points were excluded from ELISA analyses due to technical failure.
General surgical preparation
Mice were anesthetized with isoflurane (4% for induction and 1–2% for maintenance) in 30% O2 and 70% N2O or N2. During the procedure, rectal temperature was maintained between 36° and 38° using a thermostatic heating pad. Mice were then placed into a stereotactic frame. After CSD induction, the scalp was sutured and mice allowed to recover until they were sacrificed.
Optogenetic induction of CSD
For unilateral CSD induction in transgenic Thy1-ChR2 YFP mice, the scalp was incised at midline and reflected for skull exposure. The intact skull was then soaked in mineral oil to prevent drying and maintain skull translucency. The optical fiber was placed over the skull at a position 2 mm lateral and 2 mm anterior to bregma. Illumination of intact skull surface with blue LED light (470 nm, 1–3 mW for 10 s) invariably induced a CSD. CSD was induced either once or six times (over 1 h) in one hemisphere, and detected using non-invasive optical intrinsic signal imaging through the intact skull (Figure S1).24 To test for possible direct effects of illumination, wild-type mice were also studied using the same protocols. For bilateral CSD induction, two small scalp incisions were made overlying the parietal bones, and the optical fiber was placed over the skull at a position 2 mm lateral and 2 mm posterior to bregma. Six CSDs were induced over 1 h in each hemisphere (total of 12 CSDs), and successful CSD induction was confirmed using a laser Doppler flowmeter probe placed near the optogenetic probe. In mice with unilateral CSD induction, contralateral hemisphere served as control. Mice with bilateral CSD induction were compared to sham controls that underwent the identical procedure but without illumination.
KCl induction of CSD
The scalp was incised at the midline and reflected for skull exposure. Skull was focally thinned (0.5 mm in diameter) at a position 2 mm lateral and 2 mm anterior to bregma for unilateral CSD induction, or at a position 2 mm lateral and 3.5 mm posterior to bregma for bilateral CSD induction. Six CSDs were induced over an hour in each hemisphere by briefly (<3 min) placing a <1 mm diameter cotton ball soaked in 1 M KCl over the thinned bone. Immediately after laser Doppler flowmetry confirmed a CSD, KCl was removed and the site washed with isotonic NaCl. In mice with unilateral CSD induction, the contralateral hemisphere served as control. Mice with bilateral CSD induction (i.e. six CSDs in each hemisphere) were compared to sham controls without KCl.
Drug administration
Dexamethasone 21-phosphate disodium (4 mg/kg i.p.; Sigma-Aldrich, St. Louis, MO) was administered 15 min prior to or 1, 2, or 3 h after the first CSD and pro-inflammatory markers were assessed at 1 or 4 h. Ibuprofen sodium (40 mg/kg i.p., Sigma-Aldrich) was given 15 min prior to CSDs and markers were studied at 1 or 4 h. These doses were chosen based on prior work.25–28 To prevent CSD, MK-801 hydrogen maleate (10 mg/kg i.p.; Sigma-Aldrich), non-competitive NMDA receptor antagonist,21 was administered 20 min prior to the CSD induction and marker was studied at 20 min. All of the drugs were dissolved in saline.
Quantitative RT-PCR
Ipsilateral and corresponding contralateral cortex was harvested 1, 2, 4, 12, or 24 h after the first CSD. In all KCl and in the majority of optogenetic CSD induction experiments, cortical tissue was harvested at least 1 mm away from the induction site. Tissues were then frozen in liquid nitrogen and kept in −80℃ freezer until RNA extraction. RNA was extracted using a commercial RNA extraction kit (Zymo Research, Irvine, CA) and converted to cDNA using SuperScript® III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). RT-PCR was performed using TaqMan® Gene Expression Assays and TaqMan® Fast Advanced Master Mix (Applied Biosystems, Foster City, CA) with the following primers: interleukin-1β (IL-1β), chemokine (C-C motif) ligand 2 (CCL2), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1). Cyclooxygenase-2 (Cox-2) expression was also measured as a known molecular response of brain tissue to CSD.29 18S was measured as the housekeeping gene. All primers were purchased from Applied Biosystems. RT-PCR was performed in a 7500 Fast Real-Time PCR system (Applied Biosystems) in triplicates. Relative mRNA expression levels were normalized to the housekeeping gene. Importantly, pilot experiments showed that arterial cannulation caused an overall increase in circulating levels of cytokines (Figure S2). Therefore, we avoided systemic procedures including cannulation in this study.
Plasma and spleen measurements
One, 4, or 6 h after the first CSD, mice were anesthetized with 3% isoflurane, and blood was collected from the heart using EDTA tube. Plasma IL-1β, CCL2, TNF-α, IL-6, ICAM-1, and VCAM-1 were measured in duplicates using commercial ELISA kits (R&D Systems, Minneapolis, MN). Plasma from mice injected with lipopolysaccharide (E.coli O111:B4; Sigma-Aldrich; 5 mg/kg i.p., 2 h before blood collection) served as a positive control (n = 2). To test whether CSD affects spleen size, mice were sacrificed 4 or 18–24 h after CSD, and spleens were removed and weighed. Spleens from mice with middle cerebral arterial occlusion served as positive controls (n = 6 ChR+ and 1 ChR−).
Statistical analysis
We first performed a general linear mixed model analysis with Bonferroni adjustment for post-hoc multiple comparisons in the entire dataset using independent variables of sex (male vs. female), CSD induction method (optogenetic vs. KCl), time after CSD (1–24 h), and hemisphere (ipsilateral vs. contralateral). We used gene expression for each pro-inflammatory marker as the dependent variable. We did not find a significant effect of sex or method of CSD induction on pro-inflammatory gene expression response to CSD. These data showed that the optogenetic method stimulated pro-inflammatory gene expression to the same extent as the minimally invasive KCl method in both male and female mice. Therefore, all optogenetic and KCl-induced CSD experiments in male and female mice were pooled for subsequent statistical analyses using two-way ANOVA for single or repeated measures followed by post-hoc Holm-Sidak's multiple comparisons test, and paired or unpaired t-test. All statistical tests are indicated in results and figure legends for each dataset. Statistical analyses were performed using Prism 7.02 (GraphPad Software, La Jolla, CA) or SPSS (IBM, Chicago, IL).
Results
Six CSDs induced over an hour (Figure 1(a)) increased Cox-2 expression as a known footprint of CSD,29,30 which peaked within 1 h, and remained elevated for at least 4 h (Figure 1(b)). Ipsilateral cortical IL-1β, CCL2, and TNF-α mRNA increased dramatically compared with contralateral hemisphere (Figure 1(c)). The peak increase in IL-1β occurred within 1 h after CSD, the earliest time point studied. However, this dramatic increase returned near baseline within 2 h. CCL2 expression was elevated at 1 h but peaked at 2–4 h, whereas TNF-α tended to rise at 1–2 h and peaked at 4 h. Both markers returned to normal levels by 24 h. Increases in IL-6 and ICAM-1 were much smaller compared to IL-1β, CCL2, and TNF-α, while VCAM-1 did not show a significant change after six CSDs. Altogether, these data showed a temporally orchestrated pro-inflammatory response (Figure S3). As a control experiment, 470 nm optogenetic light exposure in wild-type mice did not affect pro-inflammatory markers, ruling out a direct effect of illumination (n = 7; data not shown).
Figure 1.
Time course of cortical pro-inflammatory gene expression after multiple CSDs. (a) CSDs were either induced by optogenetics (470 nm LED light) and monitored using full-field OIS imaging, or induced by brief topical KCl application through thinned skull and monitored by laser Doppler flowmetry. Six CSDs were induced in this manner over 1 h. Cortical tissue was harvested at 1–24 h after the first CSD. (b and c) Cox-2, IL-1β, CCL2, TNF-α, IL-6, ICAM-1, and VCAM-1 mRNA expression 1–24 h after the first CSD, expressed as % of average contralateral expression. Left (white) and right (gray) bars at each time point indicate contralateral and ipsilateral hemispheres, respectively (whiskers, full range; box, interquartile range; horizontal line, median; + , mean). Individual data points from each animal are also shown (circles, females; triangles, males; filled symbols, optogenetically induced CSDs; unfilled symbols, KCl-induced CSDs). Dashed lines show paired measurements (contralateral vs. ipsilateral) from the same brain. * – **** p < 0.05–0.0001 vs. contralateral hemisphere (two-way ANOVA for repeated measures followed by Holm-Sidak's multiple comparisons test). OIS: optical intrinsic signal; CSD: cortical spreading depolarization; Cox-2: Cyclooxygenase-2; IL-1β: interleukin-1β; CCL2: chemokine (C-C motif) ligand 2; TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; ICAM-1: intercellular adhesion molecule-1; VCAM-1: vascular cell adhesion molecule-1.
Because migraines are more likely to be associated with a single aura, rather than multiple auras, we also tested whether a single CSD is capable of triggering neuroinflammation, when measured at previously determined peak time points (Figure 2). A single optogenetic CSD indeed increased Cox-2 and IL-1β at 1 h, and CCL2 and TNF-α at 4 h. However, the magnitude of IL-1β, CCL2, and TNF-α responses after a single CSD was smaller than those induced by multiple CSDs (p <0.0001, p <0.05, p <0.0001, respectively; two-way ANOVA for followed by Holm-Sidak's multiple comparisons). In contrast, IL-6 (at 1 and 4 h) and ICAM-1 and VCAM-1 (at 4 h) did not show a change after a single CSD. When measured at 4 h after a single CSD, ipsilateral IL-1β and Cox-2 expressions have returned to normal.
Figure 2.
Cortical pro-inflammatory gene expression after a single CSD. (a) CSD was induced by optogenetics (470 nm LED light) and monitored using full-field OIS imaging. Cortical tissue was harvested at indicated times after the first CSD. All markers were studied 1 and/or 4 h after a single CSD. (b and c) Cox-2, IL-1β, CCL2, TNF-α, IL-6, ICAM-1, and VCAM-1 mRNA expression 1 and/or 4 h after CSD, expressed as % of average contralateral expression. Left (white) and right (gray) bars indicate contralateral and ipsilateral hemispheres, respectively (whiskers, full range; box, interquartile range; horizontal line, median; + , mean). Individual data points from each animal are also shown (circles, females; triangles, males). Dashed lines show paired measurements (contralateral vs. ipsilateral) from the same brain. * – **** p < 0.05–0.0001 vs. contralateral hemisphere (two-way ANOVA for repeated measures followed by Holm-Sidak's multiple comparisons test, or paired t-test). OIS: optical intrinsic signal; CSD: cortical spreading depolarization; Cox-2: Cyclooxygenase-2; IL-1β: interleukin-1β; CCL2: chemokine (C-C motif) ligand 2; TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; ICAM-1: intercellular adhesion molecule-1; VCAM-1: vascular cell adhesion molecule-1.
Given the substantial rise in IL-1β expression at 1 h after a single CSD, we set out to further characterize the pro-inflammatory gene expression at even earlier time points (Figure 3). When examined at 5, 10, or 20 min after a single CSD, we detected an ultra-early rise in tissue IL-1β expression that became statistically significant within 10 min. CCL2 and Cox-2, and to a lesser extent IL-6, increased between 10 and 20 min after a single CSD, once again showing that IL-1β expression precedes other pro-inflammatory markers. Increase of IL-1β at 20 min was completely blocked when CSD was prevented with MK-801, a well-known CSD inhibitor (Figure S4).
Figure 3.
Cortical pro-inflammatory gene expression after a single CSD at ultra-early time points (5, 10 or 20 min after). (a) CSD was induced by brief topical KCl application through thinned skull and monitored by laser Doppler flowmetry in male ChR2− mice. Cortical tissue was harvested at indicated times after the first CSD. Only markers which showed significant change at 1 h after multiple CSD inductions (see Figure 1) were studied. A sham group was also studied at 20 min. (b) IL-1β, CCL2, IL-6, and Cox-2 mRNA expression 5, 10, or 20 min after the first CSD, expressed as % of average contralateral expression. Left (white) and right (gray) at each time point indicate contralateral and ipsilateral hemispheres, respectively (whiskers, full range; box, interquartile range; horizontal line, median; + , mean). Individual data points from each animal are also shown. Dashed lines show paired measurements (contralateral vs. ipsilateral) from the same brain. ** – **** p < 0.01–0.0001 vs. contralateral hemisphere (two-way ANOVA for repeated measures followed by Holm-Sidak's multiple comparisons test). LDF: laser Doppler flowmetry; CSD: cortical spreading depolarization; Cox-2: Cyclooxygenase-2; IL-1β: interleukin-1β; CCL2: chemokine (C-C motif) ligand 2; IL-6: interleukin-6.
These data raised the possibility that IL-1β is an upstream mediator, driving the expression of the other pro-inflammatory markers. To test this, we used IL-1R1 KO mice and found a blunted CCL2 and TNF-α response 4 h after six CSDs, strongly suggesting that IL-1β augments the expression of other pro-inflammatory markers as an upstream mediator (Figure 4).
Figure 4.
Cortical pro-inflammatory gene expression 4 h after multiple CSDs in IL-1R1 KO mice. (a) CSDs were induced by brief topical KCl application through thinned skull and monitored by laser Doppler flowmetry in IL-1R1 KO or WT mice. Six CSDs were induced in this manner over 1 h. Cortical tissue was harvested at 4 h after the first CSD. (b) CCL2, TNF-α, and Cox-2 mRNA CSD expression 4 h after the first CSD, expressed as % of average contralateral expression. Left (white) and right (gray) bars at each time point indicate contralateral and ipsilateral hemispheres, respectively (whiskers, full range; box, interquartile range; horizontal line, median; + , mean). Individual data points from each animal are also shown. Dashed lines show paired measurements (contralateral vs. ipsilateral) from the same brain. * – **** p < 0.05–0.0001 vs. contralateral hemisphere, # – #### p < 0.05–0.0001 vs. ipsilateral hemisphere of WT mice (two-way ANOVA for repeated measures followed by Holm-Sidak's multiple comparisons test). LDF: laser Doppler flowmetry; CSD: cortical spreading depolarization; Cox-2: Cyclooxygenase-2; CCL2: chemokine (C-C motif) ligand 2; TNF-α: tumor necrosis factor-α; WT: wild-type; KO: knockout.
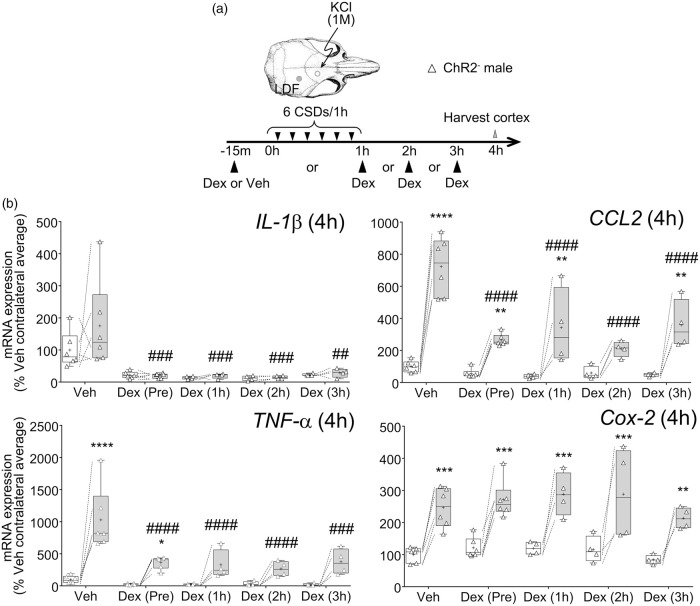
After characterizing the magnitude and time course of neuroinflammatory markers after CSD, we next tested whether the peak response can be suppressed by anti-inflammatory treatments (Figure 5). Dexamethasone, ibuprofen, or vehicle was administered 15 min before the first CSD. Compared to vehicle, dexamethasone significantly attenuated the increase in expression of cortical IL-1β, CCL2, and TNF-α after six CSDs, without affecting the Cox-2 response. In contrast, ibuprofen was ineffective at blocking the expression of the tested inflammatory mediators. Interestingly, ibuprofen significantly augmented the Cox-2 response at 1 h as well as at 4 h (p <0.05; not shown). To test whether dexamethasone could still suppress the neuroinflammatory response when administered after the CSDs have occurred, we treated animals with dexamethasone 1, 2, or 3 h after the first CSD and found this to be equally efficacious (Figure 6). Importantly, dexamethasone also reduced the expression of pro-inflammatory markers in the contralateral hemisphere.
Figure 5.
Effect of dexamethasone and ibuprofen pre-treatment on cortical pro-inflammatory gene expression. (a) CSDs were induced by brief topical KCl application through thinned skull and monitored by laser Doppler flowmetry. Six CSDs were induced in this manner over 1 h in male ChR2− mice. Dexamethasone, ibuprofen, or vehicle was administered 15 min prior to first CSD. Cortical tissue was harvested at indicated times after the first CSD, chosen based on peak responses (see Figure 1). (b) IL-1β, CCL2, TNF-α, and Cox-2 mRNA expression 1 or 4 h after the first CSD after vehicle, dexamethasone, or ibuprofen pre-treatment. Values are expressed as % of average contralateral expression of vehicle group. Left (white) and right (gray) bars at each drug indicate contralateral and ipsilateral hemispheres, respectively (whiskers, full range; box, interquartile range; horizontal line, median; + , mean). Individual data points from each animal are also shown. Dashed lines show paired measurements (contralateral vs. ipsilateral) from the same brain. * – **** p < 0.05–0.0001 vs. contralateral hemisphere, ### – #### p < 0.001–0.0001 vs. ipsilateral hemisphere of vehicle group (two-way ANOVA for repeated measures followed by Holm-Sidak's multiple comparisons test). LDF: laser Doppler flowmetry; CSD: cortical spreading depolarization; Cox-2: Cyclooxygenase-2; IL-1β: interleukin-1β; CCL2: chemokine (C-C motif) ligand 2; TNF-α: tumor necrosis factor-α.
Figure 6.
Effect of dexamethasone post-treatment on cortical pro-inflammatory gene expression. (a) CSDs were induced by brief topical KCl application through thinned skull and monitored by laser Doppler flowmetry in male ChR2− mice. Six CSDs were induced over 1 h. Dexamethasone was administered pre or post-CSD (1, 2, or 3 h) after first CSD. Cortical tissue was harvested at 4 h after the first CSD. (b) IL-1β, CCL2, TNF-α, and Cox-2 mRNA expression 4 h after CSD. Values are expressed as % of average contralateral expression of vehicle group. Left (white) and right (gray) bars at each time point indicate contralateral and ipsilateral hemispheres, respectively (whiskers, full range; box, interquartile range; horizontal line, median; + , mean). Individual data points from each animal are also shown. Dashed lines show paired measurements (contralateral vs. ipsilateral) from the same brain. ** – **** p < 0.01–0.0001 vs. contralateral hemisphere, ## – #### p < 0.01–0.0001 vs. ipsilateral hemisphere of vehicle group (two-way ANOVA for repeated measures followed by Holm-Sidak's multiple comparisons test). Vehicle and dexamethasone (pre-CSD treatment) data for CCL2 and TNF-α are re-plotted from Figure 5 for side-by-side comparisons. LDF: laser Doppler flowmetry; CSD: cortical spreading depolarization; Cox-2: Cyclooxygenase-2; IL-1β: interleukin-1β; CCL2: chemokine (C-C motif) ligand 2; TNF-α: tumor necrosis factor-α.
Because CSD-induced cortical pro-inflammatory gene expression was so profound, we hypothesized that the intense neuroinflammation may be detectable systemically. To maximize the impact, we induced six CSDs in each hemisphere, and measured the plasma levels of IL-1β, CCL2, TNF-α, ICAM-1, IL-6, and VCAM-1 6 h after the first CSD, and IL-1β, CCL2, and TNF-α when they peaked in the cortex (1 and 4 h after the first CSD, respectively) (Figure 7(a)). We did not detect a difference in plasma levels of any of the markers in CSD group compared with sham at any time point (Figure 7(b)). We used systemic lipopolysaccharide injection as a positive control and found markedly elevated plasma IL-1β (>40 pg/ml), CCL2 (>500 pg/ml), and TNF-α (>700 pg/ml) (n = 2). Furthermore, because spleen is known to contract after cerebral insults by releasing leukocytes into the systemic circulation,31,32 we also examined the spleen size 4 h and 18–24 h after the first CSD (Figure 7(a)), and did not find a difference between CSD and sham groups (Figure 7(c)). As a positive control, ischemic stroke induced by intravascular filament occlusion of the middle cerebral artery significantly decreased spleen size at 24 h (Figure 7(c)).
Figure 7.
Plasma pro-inflammatory markers and spleen size after bilateral CSDs. (a) CSDs were induced either by optogenetics or by brief topical KCl application through thinned skull, and monitored by laser Doppler flowmetry. Six CSDs were induced in each hemisphere. In one subset (left panel), all six CSDs were induced in one hemisphere over an hour and then this was repeated in the other hemisphere. In the second subset (right panel), CSDs were induced bilaterally at the same time over an hour. Plasma was collected at 1, 4, or 6 h. Spleen was harvested at 4 or 18–24 h after the first CSD and weighed. Data from bilateral CSD animals were compared with sham controls. (b) Plasma inflammatory markers (IL-1β, CCL2, TNF-α, IL-6, ICAM-1, and VCAM-1) at 1, 4, or 6 h after first CSD or sham (whiskers, full range; box, interquartile range; horizontal line, median; + , mean). Individual data points from each animal are also shown. Dotted lines represent lower limit of reliable quantification based on the standard curves. There was no difference between sham and CSD group in any of the markers at any time point (unpaired t-test). (c) Spleen size in naïve animal, 4 or 18–24 h after first CSD or sham, or 24 h after middle cerebral artery occlusion (stroke). There was no significant difference between sham and CSD group within each time point. Stroke animal showed significant decrease compared to naïve animal. * – *** p < 0.05–0.001 vs. naïve (one-way ANOVA followed by Tukey's multiple comparisons test). LDF: laser Doppler flowmetry; CSD: cortical spreading depolarization; Cox-2: Cyclooxygenase-2; IL-1β: interleukin-1β; CCL2: chemokine (C-C motif) ligand 2; TNF-α: tumor necrosis factor-α; IL-6: interleukin-6; ICAM-1: intercellular adhesion molecule-1; VCAM-1: vascular cell adhesion molecule-1.
Discussion
Here, we show for the first time that non-invasive induction of CSD evokes a potent pro-inflammatory response in mice. Our approach avoids potential injury confounders associated with prior invasive approaches. This is also the first comprehensive study that examines six pro-inflammatory genes (cytokines IL-1β, TNF-α, IL-6; chemokine CCL2; cell adhesion molecules ICAM-1 ad VCAM-1) at several time points (5 min–24 h) after a single or multiple CSDs, in both male and female mice. These have been the most commonly studied markers consistently found elevated after CSD.11–19 We show that even a single CSD can trigger pro-inflammatory gene expression with a dose–response relationship. The response involves specific pro-inflammatory mediators with a precise temporal pattern led by IL-1β. Moreover, we show that dexamethasone but not ibuprofen acutely suppresses the pro-inflammatory response to CSD. Lastly, despite robust cortical neuroinflammation, we did not find any overt evidence of systemic inflammation.
We detected IL-1β mRNA expression as early as 10 min after a single CSD, which diminished within 2 h even after multiple CSDs. Prior studies have detected an increase in IL-1β expression in rat cortex12–14 or mouse CSF17 when measured 1–4 h after multiple CSDs, while others did not find an increase compared with sham.15,20 The increase in IL-1β appeared to last longer in prior invasive studies in rats compared with our non-invasive study in mice.12–14 CCL2 expression also increased after CSD in our study but with a more delayed peak at around 2 h. Only two other studies have acutely measured CCL2 expression within hours after CSD. One detected an increase in rats at 3 h, the earliest time point studied, which persisted for more than 24 h,15 whereas the other study did not find an increase in mice compared to a sham group.20 TNF-α expression reproducibly increased in our study, which appeared as early as 1 h after CSD, peaked at 4 h and resolved by 12 h. Previous work on TNF-α expression after CSD has been conflicting; one study in rats detected an increase at 4 h that lasted more than 16 h after multiple CSDs,12 while two others failed to detect an increase.15,20 We also detected an increase in IL-6 expression that appeared to have two separate peaks at 1 and 12 h after multiple CSDs, albeit much smaller in magnitude compared with IL-1β, CCL2, and TNF-α; IL-6 expression returned to baseline by 24 h. Previous results on IL-6 expression after CSD have also been mixed; one study in rats detected an increase at 2 h that persisted at 50 h after multiple CSDs,14 while two others failed to detect an increase compared with sham.15,20 ICAM-1 expression also showed a small increase around 4–12 h after CSD, peaking somewhat later than previous reports.11,15 Lastly, we did not detect an increase in VCAM-1 expression at any time point after CSD in mice, in contrast to two reports that showed increased expression in the rat.11,14 The discrepancies among studies (e.g. lack of difference from sham controls, or protracted inflammation) are likely related to assay methodology and invasive procedures (e.g. craniotomies, electrode insertions, CSD induction by pinprick), and perhaps to some extent species differences. It should be noted that we did not attempt to measure the translation of mRNA response into protein. Elevated protein levels of the pro-inflammatory mediators accompanying the upregulation of mRNA expression have been shown by others.11,12,19,33 Moreover, genetic ablation of IL-1R1 blunted the pro-inflammatory response, suggesting that IL-1β protein was indeed upregulated and this augmented the downstream response. Therefore, we are confident that the elevated mRNA signal in our studies is indeed translated into protein expression.
IL-1β was the earliest to peak among all the markers studied, and the pro-inflammatory response was partially blunted in IL-1R1 KO mice, suggesting that IL-1β stimulates the expression of other CSD-induced pro-inflammatory markers, as previously reported in other experimental models.34–36 We did not assess cell type(s) in which the pro-inflammatory response originated, but previous studies have implicated microglia, neurons, and astrocytes.12,17,19,37,38 For example, interleukin-1 converting enzyme activation has been detected in neurons using immunohistochemistry after CSD.17 In contrast, hippocampal cell cultures showed highest expression of IL-1β and TNF-α mRNA 1 h after CSD in microglial cells.38 Consistent with this, increased IL-1β staining has been detected in cortical microglia 8 h after CSD.12 In a model of chronic SD, increased levels of inflammatory markers has been demonstrated in astrocytes using immunohistochemistry.19 These data suggest that all three cell types, and possibly others in the brain can all contribute to the pro-inflammatory response after CSD. Regardless of the cell of origin, IL-1β has been reported to activate and sensitize meningeal nociceptors,39 and has been proposed as a key mediator in trigeminal activation after CSD.17 However, whether it plays a direct role in migraine headache pathogenesis is less clear. First, the earliest time point we detected a significant increase in IL-1β mRNA was 10 min after a single CSD. Therefore, it is unclear whether the typically short latency between aura onset and headache onset40–44 is long enough for the transcriptional signal to be mounted and translated into pro-IL-1β synthesis, for its conversion to mature IL-1β and release, and for the IL-1β released within the cortical tissue to reach its putative meningeal target(s) and activate nociception. Second, dexamethasone acutely suppressed the pro-inflammatory response in our study, including IL-1β (∼50%), even when given hours after CSD. However, clinical studies thus far failed to show an effect of dexamethasone in aborting acute migraine headache after its onset, although there are data suggesting that it may prevent recurrence.45,46 It is currently not recommended in practice.47–50 Conversely, ibuprofen was completely ineffective on the neuroinflammatory response after CSD in our study, but it is among the most effective and widely used acute migraine treatments. Consistent with our data, dexamethasone attenuated microglial activation, whereas indomethacin did not.51 Of course, non-steroidal anti-inflammatory drugs may still act at a downstream level after a pro-inflammatory response is triggered. Therefore, assuming that mouse brain biology is not diametric to human brain, a causal link between the pro-inflammatory response to CSD and migraine headache is not supported by our data. It is also worth noting that if the pro-inflammatory mediators induced by CSD indeed mediate the headache in migraineurs with aura, then patients with ischemic stroke and other forms of brain injury, who develop exponentially higher numbers of CSDs during the acute phase, should experience significantly more severe and lasting headaches. Yet, headaches after ischemic stroke are not as common (prevalence about 15%) and often not migrainous.52
Several studies have examined the systemic or jugular blood as well as CSF levels of pro-inflammatory markers ictally or interictally in migraineurs with mixed results. Under identical conditions, some detected elevated levels, whereas other did not.53–58 In addition, elevated circulating neutrophil to lymphocyte ratio has also been reported during migraine attacks.59 Experimentally, systemic inflammatory changes have been reported in juvenile rats when CSDs were induced once a week for a month by intracortical KCl injections through an implanted cannula.33 We measured plasma levels of all six protein markers but did not detect a change in any of the markers at 1, 4, or 6 h after CSD, despite maximizing the CSD burden by inducing six CSDs in each hemisphere. Moreover, we did not detect a change in spleen size as another potential indicator of systemic response. Overall, our data do not support a direct causal link between migraine aura and the systemic inflammatory biomarkers measured to date and suggest that any such relationship is likely to be indirect. Lack of a systemic response also suggests that the immunodepression observed after brain injury (e.g. ischemic stroke, subarachnoid hemorrhage60,61) is evoked by the actual injury rather than the tissue depolarizations accompanying it.
Our data have implications beyond migraine. First, we show that CSD alone (i.e. in the absence of any injury) can partially reproduce the characteristic neuroinflammatory response observed in brain injury,62,63 suggesting that intense but brief neuronal and glial depolarization is sufficient to trigger this programmed transcriptional response. Numerous CSDs occur for days after acute brain injury, such as ischemic stroke, subarachnoid hemorrhage, and trauma. Given the dose–response relationship we observed in this study, the pro-inflammatory response triggered by these recurrent CSDs is likely to be stronger and longer lasting than that triggered by a single CSD during migraine aura and may contribute to the injury progression, plasticity, and remodeling and perhaps even preconditioning in the injured brain.64 Indeed, numerous studies have examined the pro-inflammatory response to various forms of brain injury. When compared with the cytokine and chemokine expression after CSD, the response after ischemic or traumatic brain injury is somewhat late (several hours) but lasts significantly longer (days).65,66 The caveat of making side-by-side comparisons among studies from different labs notwithstanding, the magnitude of pro-inflammatory response after CSD appears smaller than that in brain injury.65,66 Second, the neuroinflammatory response triggered by chronic episodic attacks of migraine aura might alter the course of comorbid immune disorders of the central nervous system, such as multiple sclerosis.67,68 Indeed, migraineurs with multiple sclerosis suffer from increased relapse rates.68 Migraineurs are also known to have comorbidity with depression and anxiety disorders,69 where cytokines may play a role as well.70,71
In conclusion, we demonstrate for the first time an ultra-early pro-inflammatory gene expression response after CSD in mice using a non-invasive optogenetic approach. Detailed temporal analysis after a single or multiple CSDs reveals a concerted response led by IL-1β, which is suppressed by steroid but not by non-steroid anti-inflammatory drugs. Further studies are needed to examine whether this pro-inflammatory response is causally linked to headache, and whether it could exacerbate other neuroinflammatory diseases such as multiple sclerosis or brain injury.
Supplemental Material
Supplemental Material for Non-invasively triggered spreading depolarizations induce a rapid pro-inflammatory response in cerebral cortex by Tsubasa Takizawa, Tao Qin, Andreia Lopes de Morais, Kazutaka Sugimoto, Joon Yong Chung, Liza Morsett, Inge Mulder, Paul Fischer, Tomoaki Suzuki, Maryam Anzabi, Maximilian Böhm, Wen-sheng Qu, Takeshi Yanagisawa, Suzanne Hickman, Joseph El Khoury, Michael J Whalen, Andrea M Harriott, David Y Chung and Cenk Ayata in Journal of Cerebral Blood Flow & Metabolism
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided by National Institute of Neurological Disorders and Stroke at the National Institutes of Health (P01NS055104 and R01NS102969 to CA; and R25NS065743 and KL2TR002542 to DYC), Fondation Leducq and Ellison Foundation (CA), Heitman Foundation (CA and DYC), Lilly Scientific Fellowship Program, a grant from the Kanae Foundation for the Promotion of Medical Science, and a fellowship from the Uehara Memorial Foundation (TT), the Aneurysm and AVM Foundation and the Brain Aneurysm Foundation (DYC).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
TT, JEK, MJW and CA designed research; TT, TQ, AL, KS, JYC, LM, IM, PF, TS, MA, MB, WQ, TY, SH, AMH and DYC performed the experiments; TT and CA analyzed data; TT and CA wrote the manuscript; all authors critically reviewed the manuscript for intellectual content and approved the final manuscript.
Supplemental material
Supplemental material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Leao AA. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol 1947; 10: 409–414. [DOI] [PubMed] [Google Scholar]
- 2.Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev 2015; 95: 953–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayata C. Cortical spreading depression triggers migraine attack: pro. Headache 2010; 50: 725–730. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Levy D, Kainz V, et al. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol 2011; 69: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayata C, Jin H, Kudo C, et al. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol 2006; 59: 652–661. [DOI] [PubMed] [Google Scholar]
- 6.Ayata C. Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia 2009; 29: 1095–1114. [DOI] [PubMed] [Google Scholar]
- 7.Hartings JA, Shuttleworth CW, Kirov SA, et al. The continuum of spreading depolarizations in acute cortical lesion development: examining Leao's legacy. J Cereb Blood Flow Metab 2017; 37: 1571–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab 2017; 37: 1595–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lauritzen M, Dreier JP, Fabricius M, et al. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 2011; 31: 17–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eising E, Shyti R, Hoen PA, et al. Cortical spreading depression causes unique dysregulation of inflammatory pathways in a transgenic mouse model of migraine. Mol Neurobiol 2017; 54: 2986–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yisarakun W, Supornsilpchai W, Chantong C, et al. Chronic paracetamol treatment increases alterations in cerebral vessels in cortical spreading depression model. Microvasc Res 2014; 94: 36–46. [DOI] [PubMed] [Google Scholar]
- 12.Jander S, Schroeter M, Peters O, et al. Cortical spreading depression induces proinflammatory cytokine gene expression in the rat brain. J Cereb Blood Flow Metab 2001; 21: 218–225. [DOI] [PubMed] [Google Scholar]
- 13.Chen SP, Qin T, Seidel JL, et al. Inhibition of the P2X7-PANX1 complex suppresses spreading depolarization and neuroinflammation. Brain 2017; 140: 1643–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson CS, Hakim AM. Cortical spreading depression modifies components of the inflammatory cascade. Mol Neurobiol 2005; 32: 51–57. [DOI] [PubMed] [Google Scholar]
- 15.Urbach A, Bruehl C, Witte OW. Microarray-based long-term detection of genes differentially expressed after cortical spreading depression. Eur J Neurosci 2006; 24: 841–856. [DOI] [PubMed] [Google Scholar]
- 16.Pusic AD, Mitchell HM, Kunkler PE, et al. Spreading depression transiently disrupts myelin via interferon-gamma signaling. Exp Neurol 2015; 264: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karatas H, Erdener SE, Gursoy-Ozdemir Y, et al. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013; 339: 1092–1095. [DOI] [PubMed] [Google Scholar]
- 18.Kunkler PE, Hulse RE, Kraig RP. Multiplexed cytokine protein expression profiles from spreading depression in hippocampal organotypic cultures. J Cereb Blood Flow Metab 2004; 24: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaemi A, Alizadeh L, Babaei S, et al. Astrocyte-mediated inflammation in cortical spreading depression. Cephalalgia 2018; 38: 626–638. [DOI] [PubMed] [Google Scholar]
- 20.Choudhuri R, Cui L, Yong C, et al. Cortical spreading depression and gene regulation: relevance to migraine. Ann Neurol 2002; 51: 499–506. [DOI] [PubMed] [Google Scholar]
- 21.Chung DY, Sadeghian H, Qin T, et al. Determinants of optogenetic cortical spreading depolarizations. Cereb Cortex 2019; 29: 1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Peca J, Matsuzaki M, et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci U S A 2007; 104: 8143–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arenkiel BR, Peca J, Davison IG, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 2007; 54: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung DY, Sugimoto K, Fischer P, et al. Real-time non-invasive in vivo visible light detection of cortical spreading depolarizations in mice. J Neurosci Meth 2018; 309: 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertorelli R, Adami M, Di Santo E, et al. MK 801 and dexamethasone reduce both tumor necrosis factor levels and infarct volume after focal cerebral ischemia in the rat brain. Neurosci Lett 1998; 246: 41–44. [DOI] [PubMed] [Google Scholar]
- 26.Merkel SF, Andrews AM, Lutton EM, et al. Dexamethasone attenuates the enhanced rewarding effects of cocaine following experimental traumatic brain injury. Cell Transplant 2017; 26: 1178–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee B, Sur B, Yeom M, et al. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J Physiol Pharmacol 2016; 20: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teeling JL, Cunningham C, Newman TA, et al. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain Behav Immun 2010; 24: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yrjanheikki J, Koistinaho J, Copin JC, et al. Spreading depression-induced expression of c-fos and cyclooxygenase-2 in transgenic mice that overexpress human copper/zinc-superoxide dismutase. J Neurotrauma 2000; 17: 713–718. [DOI] [PubMed] [Google Scholar]
- 30.Yokota C, Inoue H, Kuge Y, et al. Cyclooxygenase-2 expression associated with spreading depression in a primate model. J Cereb Blood Flow Metab 2003; 23: 395–398. [DOI] [PubMed] [Google Scholar]
- 31.Kim E, Yang J, Beltran CD, et al. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab 2014; 34: 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol 2006; 176: 6523–6531. [DOI] [PubMed] [Google Scholar]
- 33.Ghaemi A, Sajadian A, Khodaie B, et al. Immunomodulatory effect of toll-like receptor-3 ligand poly I:C on cortical spreading depression. Mol Neurobiol 2016; 53: 143–154. [DOI] [PubMed] [Google Scholar]
- 34.Thompson WL, Van Eldik LJ. Inflammatory cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3 through NFkB and MAPK dependent pathways in rat astrocytes [corrected]. Brain Res 2009; 1287: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol 1990; 144: 2999–3007. [PubMed] [Google Scholar]
- 36.Crofford LJ, Wilder RL, Ristimaki AP, et al. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest 1994; 93: 1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothwell NJ. Functions and mechanisms of interleukin 1 in the brain. Trends Pharmacol Sci 1991; 12: 430–436. [DOI] [PubMed] [Google Scholar]
- 38.Hulse RE, Swenson WG, Kunkler PE, et al. Monomeric IgG is neuroprotective via enhancing microglial recycling endocytosis and TNF-alpha. J Neurosci 2008; 28: 12199–12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Burstein R, Levy D. Local action of the proinflammatory cytokines IL-1beta and IL-6 on intracranial meningeal nociceptors. Cephalalgia 2012; 32: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell MB, Iversen HK, Olesen J. Improved description of the migraine aura by a diagnostic aura diary. Cephalalgia 1994; 14: 107–117. [DOI] [PubMed] [Google Scholar]
- 41.Viana M, Linde M, Sances G, et al. Migraine aura symptoms: duration, succession and temporal relationship to headache. Cephalalgia 2016; 36: 413–421. [DOI] [PubMed] [Google Scholar]
- 42.Hansen JM, Lipton RB, Dodick DW, et al. Migraine headache is present in the aura phase: a prospective study. Neurology 2012; 79: 2044–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelman L. The aura: a tertiary care study of 952 migraine patients. Cephalalgia 2004; 24: 728–734. [DOI] [PubMed] [Google Scholar]
- 44.Russell MB, Olesen J. A nosographic analysis of the migraine aura in a general population. Brain 1996; 119(Pt 2): 355–361. [DOI] [PubMed] [Google Scholar]
- 45.Colman I, Friedman BW, Brown MD, et al. Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ 2008; 336: 1359–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelley NE, Tepper DE. Rescue therapy for acute migraine, part 3: opioids, NSAIDs, steroids, and post-discharge medications. Headache 2012; 52: 467–482. [DOI] [PubMed] [Google Scholar]
- 47.Friedman BW, Greenwald P, Bania TC, et al. Randomized trial of IV dexamethasone for acute migraine in the emergency department. Neurology 2007; 69: 2038–2044. [DOI] [PubMed] [Google Scholar]
- 48.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American Headache Society evidence assessment of migraine pharmacotherapies. Headache 2015; 55: 3–20. [DOI] [PubMed] [Google Scholar]
- 49.Evers S, Afra J, Frese A, et al. EFNS guideline on the drug treatment of migraine – revised report of an EFNS task force. Eur J Neurol 2009; 16: 968–981. [DOI] [PubMed] [Google Scholar]
- 50.Gelfand AA, Goadsby PJ. A neurologist's guide to acute migraine therapy in the emergency room. Neurohospitalist 2012; 2: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caggiano AO, Kraig RP. Eicosanoids and nitric oxide influence induction of reactive gliosis from spreading depression in microglia but not astrocytes. J Comp Neurol 1996; 369: 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumral E, Bogousslavsky J, Van Melle G, et al. Headache at stroke onset: the Lausanne Stroke Registry. J Neurol Neurosurg Psychiatry 1995; 58: 490–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Hilten JJ, Ferrari MD, Van der Meer JW, et al. Plasma interleukin-1, tumour necrosis factor and hypothalamic-pituitary-adrenal axis responses during migraine attacks. Cephalalgia 1991; 11: 65–67. [DOI] [PubMed] [Google Scholar]
- 54.Empl M, Sostak P, Riedel M, et al. Decreased sTNF-RI in migraine patients?. Cephalalgia 2003; 23: 55–58. [DOI] [PubMed] [Google Scholar]
- 55.Perini F, D'Andrea G, Galloni E, et al. Plasma cytokine levels in migraineurs and controls. Headache 2005; 45: 926–931. [DOI] [PubMed] [Google Scholar]
- 56.Sarchielli P, Alberti A, Baldi A, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache 2006; 46: 200–207. [DOI] [PubMed] [Google Scholar]
- 57.Bo SH, Davidsen EM, Gulbrandsen P, et al. Cerebrospinal fluid cytokine levels in migraine, tension-type headache and cervicogenic headache. Cephalalgia 2009; 29: 365–372. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, He Q, Ren Z, et al. Association of serum levels of intercellular adhesion molecule-1 and interleukin-6 with migraine. Neurol Sci 2015; 36: 535–540. [DOI] [PubMed] [Google Scholar]
- 59.Karabulut KU, Egercioglu TU, Uyar M, et al. The change of neutrophils/lymphocytes ratio in migraine attacks: a case-controlled study. Ann Med Surg 2016; 10: 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarrafzadeh A, Schlenk F, Meisel A, et al. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke 2011; 42: 53–58. [DOI] [PubMed] [Google Scholar]
- 61.Meisel C, Schwab JM, Prass K, et al. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci 2005; 6: 775–786. [DOI] [PubMed] [Google Scholar]
- 62.Taupin V, Toulmond S, Serrano A, et al. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion. Influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol 1993; 42: 177–185. [DOI] [PubMed] [Google Scholar]
- 63.Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann N Y Acad Sci 1997; 825: 179–193. [DOI] [PubMed] [Google Scholar]
- 64.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 2011; 17: 439–447. [DOI] [PubMed] [Google Scholar]
- 65.Chiu CC, Liao YE, Yang LY, et al. Neuroinflammation in animal models of traumatic brain injury. J Neurosci Meth 2016; 272: 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab 2012; 32: 1677–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moisset X, Ouchchane L, Guy N, et al. Migraine headaches and pain with neuropathic characteristics: comorbid conditions in patients with multiple sclerosis. Pain 2013; 154: 2691–2699. [DOI] [PubMed] [Google Scholar]
- 68.Kowalec K, McKay KA, Patten SB, et al. Comorbidity increases the risk of relapse in multiple sclerosis: a prospective study. Neurology 2017; 89: 2455–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buse DC, Silberstein SD, Manack AN, et al. Psychiatric comorbidities of episodic and chronic migraine. J Neurol 2013; 260: 1960–1969. [DOI] [PubMed] [Google Scholar]
- 70.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 2009; 65: 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neurosci Lett 2009; 456: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Non-invasively triggered spreading depolarizations induce a rapid pro-inflammatory response in cerebral cortex by Tsubasa Takizawa, Tao Qin, Andreia Lopes de Morais, Kazutaka Sugimoto, Joon Yong Chung, Liza Morsett, Inge Mulder, Paul Fischer, Tomoaki Suzuki, Maryam Anzabi, Maximilian Böhm, Wen-sheng Qu, Takeshi Yanagisawa, Suzanne Hickman, Joseph El Khoury, Michael J Whalen, Andrea M Harriott, David Y Chung and Cenk Ayata in Journal of Cerebral Blood Flow & Metabolism