Summary
Background
Urothelial carcinomas of the upper urinary tract (UTUCs) are rare, with poorer stage-for-stage prognosis than urothelial carcinomas of the urinary bladder. No international consensus exists on the benefit of adjuvant chemotherapy for patients with UTUCs after nephroureterectomy with curative intent. The POUT (Peri-Operative chemotherapy versus sUrveillance in upper Tract urothelial cancer) trial aimed to assess the efficacy of systemic platinum-based chemotherapy in patients with UTUCs.
Methods
We did a phase 3, open-label, randomised controlled trial at 71 hospitals in the UK. We recruited patients with UTUC after nephroureterectomy staged as either pT2–T4 pN0–N3 M0 or pTany N1–3 M0. We randomly allocated participants centrally (1:1) to either surveillance or four 21-day cycles of chemotherapy, using a minimisation algorithm with a random element. Chemotherapy was either cisplatin (70 mg/m2) or carboplatin (area under the curve [AUC]4·5/AUC5, for glomerular filtration rate <50 mL/min only) administered intravenously on day 1 and gemcitabine (1000 mg/m2) administered intravenously on days 1 and 8; chemotherapy was initiated within 90 days of surgery. Follow-up included standard cystoscopic, radiological, and clinical assessments. The primary endpoint was disease-free survival analysed by intention to treat with a Peto-Haybittle stopping rule for (in)efficacy. The trial is registered with ClinicalTrials.gov, NCT01993979. A preplanned interim analysis met the efficacy criterion for early closure after recruitment of 261 participants.
Findings
Between June 19, 2012, and Nov 8, 2017, we enrolled 261 participants from 57 of 71 open study sites. 132 patients were assigned chemotherapy and 129 surveillance. One participant allocated chemotherapy withdrew consent for data use after randomisation and was excluded from analyses. Adjuvant chemotherapy significantly improved disease-free survival (hazard ratio 0·45, 95% CI 0·30–0·68; p=0·0001) at a median follow-up of 30·3 months (IQR 18·0–47·5). 3-year event-free estimates were 71% (95% CI 61–78) and 46% (36–56) for chemotherapy and surveillance, respectively. 55 (44%) of 126 participants who started chemotherapy had acute grade 3 or worse treatment-emergent adverse events, which accorded with frequently reported events for the chemotherapy regimen. Five (4%) of 129 patients managed by surveillance had acute grade 3 or worse emergent adverse events. No treatment-related deaths were reported.
Interpretation
Gemcitabine–platinum combination chemotherapy initiated within 90 days after nephroureterectomy significantly improved disease-free survival in patients with locally advanced UTUC. Adjuvant platinum-based chemotherapy should be considered a new standard of care after nephroureterectomy for this patient population.
Funding
Cancer Research UK.
Introduction
Upper tract urothelial carcinoma (UTUC; transitional cell carcinoma of the ureter or renal pelvis) is rare, occurring in around two people per 100 000 population in high-income countries. Scant symptoms and delayed diagnosis mean that tumours are often muscle-invasive or locally advanced at presentation (56%), resulting in poorer survival figures than for urothelial carcinoma of the urinary bladder. More than 50% of patients diagnosed with UTUC die as a result of their disease, despite systemic platinum-based chemotherapy after local or metastatic recurrence.1 Improved management of early-stage disease, therefore, has the potential to save lives. At the inception of this study, systemic treatment had no proven role for locally advanced UTUC.2 Nephroureterectomy followed by surveillance has remained the routine treatment for localised UTUC.1
UTUC shares several clinicopathological features with muscle-invasive urothelial (transitional cell) carcinoma of the bladder. Robust survival improvements are seen with platinum-based chemotherapy in patients with urothelial bladder cancer, in both the neoadjuvant and metastatic settings.3, 4, 5 Similar benefits of platinum-based palliative chemotherapy have been seen for UTUC and urothelial bladder cancer at advanced stages.6 Thus, a clear rationale exists for investigating perioperative, platinum-based chemotherapy in patients with UTUC.
Research in context.
Evidence before this study
Before this study, findings of a literature review available online in 2010 (Audenet et al, 2013) showed that no level 1 randomised trial evidence was available assessing the efficacy of systemic chemotherapy for locally advanced upper tract urothelial carcinoma (UTUC). The paucity of research is partly because of the rarity of the disease. Undersized or retrospective studies had not shown a convincing survival benefit for chemotherapy. Guidelines from the European Association of Urology, therefore, recommended nephroureterectomy followed by surveillance as the standard of care. Most urothelial carcinomas (in both UTUC and bladder cancer) originate in the transitional epithelium (transitional cell carcinoma). It is logical, therefore, to consider data from trials of systemic bladder cancer treatment for signals to indicate whether chemotherapy could be efficacious in UTUC. Studies of perioperative chemotherapy for primary urothelial carcinoma of the bladder suggested localised urothelial carcinoma was chemosensitive, with (on meta-analysis) cisplatin-based neoadjuvant chemotherapy showing an absolute improvement of 5% in overall survival at 5 years (hazard ratio 0·86, 95% CI 0·77–0·95; p=0·003). Therefore, a comparable trial in UTUC was justified, particularly in view of the inferior stage-for-stage outcomes in UTUC when compared with bladder urothelial carcinoma. Challenges of obtaining definitive histology and accurate staging for UTUC before nephroureterectomy risk either undertreatment or overtreatment with neoadjuvant therapy. The POUT trial was, therefore, designed as a phase 3 randomised trial of adjuvant platinum-based chemotherapy, intended to provide, for the first time, robust evidence regarding its efficacy in UTUC.
Added value of this study
To the best of our knowledge, our study is the largest randomised controlled clinical trial done exclusively in patients with UTUC worldwide.
Implications of all the available evidence
We have shown that giving adjuvant platinum-based chemotherapy within 90 days after nephroureterectomy reduces subsequent rates of disease recurrence. Our data, therefore, suggest that adjuvant platinum-based chemotherapy should be recommended as a new standard of care after nephroureterectomy for all patients with locally advanced UTUC in whom there are no definitive contraindications to chemotherapy.
Because of the strength of evidence showing survival gain, neoadjuvant chemotherapy is the accepted standard of care for muscle-invasive bladder cancer. Although a neoadjuvant approach is attractive for patients with UTUC, particularly when the loss of renal function associated with nephrectomy is considered, the unreliability of preoperative UTUC staging and histopathology would probably result in overtreatment for some patients and undertreatment for others.7 Previous studies of adjuvant chemotherapy in UTUC are largely retrospective, with low statistical power and conflicting conclusions,8, 9, 10 providing insufficient evidence to recommend perioperative chemotherapy. Thus, for many patients with muscle-invasive UTUC, surgery alone is considered the standard approach.
Patient-reported outcome data for this rare population are also absent, with most available published work at the outset of this trial focusing on short-term outcomes after nephroureterectomy and no data obtained within the context of randomised controlled trials. We aimed to prospectively assess in a randomised controlled trial (the POUT trial) the effect of adjuvant platinum-based chemotherapy on disease-free survival, overall survival, safety, and quality of life after radical nephroureterectomy in patients with locally advanced UTUC.
Methods
Study design
POUT is a phase 3, parallel group, open-label, randomised controlled trial done at 71 National Health Service (NHS) hospitals in the UK. An intervention was included to understand and then support recruitment to the trial.11 Eligible patients were aged at least 16 years, had received en-bloc radical nephroureterectomy for UTUC (including resection of all radiologically or macroscopically abnormal nodes), were postoperatively staged with either muscle-invasive (pT2–pT4, Nany) or lymph node-positive (pTany, N1–3) metastasis-free (M0) disease with predominantly transitional cell carcinoma histology, and were fit to receive adjuvant chemotherapy within 90 days after surgery.
Formal extended lymph-node dissection was not mandated. Participants with lymph-node involvement identified on preoperative imaging or during surgery had all grossly abnormal nodes resected. Postoperative imaging was mandated for these patients before randomisation; those with residual lymphadenopathy as ascertained by the local investigator were excluded. Participants had satisfactory haematological and biochemical blood profiles and a glomerular filtration rate (GFR) of 30 mL/min or higher.
Participants were recruited by their clinical care teams and provided written informed consent before enrolment. Regulatory approvals were obtained before trial activation from the Medicines and Healthcare products Regulatory Agency (MHRA) and the North West–Greater Manchester South research ethics committee (11/NW/0782). The POUT trial was undertaken according to the principles of Good Clinical Practice, and sponsored by The Institute of Cancer Research (ICR). The Clinical Trials and Statistics Unit at ICR (ICR-CTSU) coordinated the trial, did central statistical data monitoring, and undertook all analyses. The trial management group was overseen by independent data monitoring and trial steering committees (appendix p 12). The full study protocol is available in the appendix (pp 13–66).
Randomisation and masking
Treatment allocation was done centrally by ICR-CTSU using a minimisation algorithm incorporating a random element. Balancing factors were planned platinum agent (cisplatin vs carboplatin), preoperative radiologically or pathologically assessed nodal involvement (N0 vs N1 vs N2 vs N3), status of microscopic surgical margins (positive vs negative), and treating centre. Participants were randomly allocated (1:1) either surveillance or chemotherapy. Treatment allocation was not masked.
Procedures
Participants allocated chemotherapy received four 21-day cycles of platinum-based combination chemotherapy, to begin within 14 days after randomisation. Gemcitabine (1000 mg/m2) was given on days 1 and 8 of each cycle. Either cisplatin (70 mg/m2) or carboplatin (area under the curve [AUC]4·5 or AUC5, according to local practice, prespecified for each treatment centre) was given on day 1. Impaired renal function (GFR ≥30 mL/min and <50 mL/min) was the only permitted reason to give carboplatin rather than cisplatin. Protocol-specified recommendations were for chemotherapy to begin within 90 days of nephroureterectomy, for gemcitabine to be given as a 30-min intravenous infusion in 500 mL normal saline, cisplatin as a 4-h intravenous infusion in 1 L saline, and carboplatin as a 1-h intravenous infusion. Use of generic agents was allowed; no recommended manufacturer was specified. Hydration and infusion rates were in accordance with local practice. The protocol-recommended calculation of GFR was by the Cockcroft and Gault method; however, use of the Wright formula or estimation by radioisotope clearance were also permitted. Participating sites prespecified their intended assessment method before activation and were requested to use the same GFR assessment method for a participant throughout the study. Patients otherwise unsuitable to receive cisplatin were not permitted to join the trial to minimise the potential confounding effects of frailty and comorbidity. All participants receiving chemotherapy had haematology and serum biochemistry assessments and their GFR was estimated and body surface area calculated before every cycle of chemotherapy.
Adverse events during every chemotherapy cycle were assessed by the local investigator using National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Protocol-specified dose modifications were permitted for toxic effects of CTCAE grade 3 or worse. Patients intended to receive cisplatin were to switch to carboplatin if the estimated GFR fell to 30–49 mL/min. If the GFR fell from 70 mL/min or higher to 50–69 mL/min then the cisplatin dose was allowed to be split across 2 consecutive days. Participants allocated surveillance underwent adverse event assessment every 3 weeks after randomisation to mirror the assessment schedule of participants allocated chemotherapy.
Participants in both study groups were followed up at 3, 6, 9, and 12 months, then every 6 months to 36 months from randomisation, regardless of whether chemotherapy was complete, and annually thereafter. Assessment of disease recurrence included either plain film radiography or cross-sectional imaging (CT) of the thorax plus CT of the abdomen and pelvis at 3, 6, 9 (thorax only), 12, 18, 24, 30 (thorax only), and 36 months, then annually to 60 months. Cystoscopy was done every 6 months to 24 months, then annually to 60 months to detect recurrence in the lower urinary tract. Follow-up assessments were done in accordance with standard practice in the UK at the time. Assessment of adverse events was done at every follow-up visit to 24 months. Participants in both study groups who had disease recurrence were permitted to receive any appropriate further treatment as clinically indicated, including platinum–gemcitabine chemotherapy.
Participants in an optional patient-reported quality-of-life substudy were asked to complete on paper the European Organisation for Research and Treatment of Cancer (EORTC) quality-of-life of cancer patients questionnaire (QLQ-C30) and the EuroQol five dimensions five levels questionnaire (EQ-5D-5L) at baseline and before cycle three (week 7) and at 3 months, then at 6, 12, and 24 months post randomisation.
Outcomes
The primary endpoint was disease-free survival according to local assessment and was defined as time from randomisation to either first recurrence in the tumour bed, first metastasis, or death from any cause. Recurrence and metastasis could be established either radiologically or pathologically. Patients were censored at the date of diagnosis of a second primary cancer (including muscle-invasive bladder cancer and contralateral UTUC). New non-muscle-invasive bladder cancer was not regarded as an event or a reason to censor, although such events were recorded for future analysis. Secondary endpoints included metastasis-free survival, overall survival, treatment compliance, acute toxicity, late toxicity, and patient-reported quality of life.
Statistical analysis
The trial was designed to detect a hazard ratio (HR) of 0·65 in favour of chemotherapy, equivalent to a 15% absolute improvement in 3-year disease-free survival (from 40% to 55%, which was chosen to correspond with the magnitude of benefit noted for chemotherapy in muscle-invasive bladder cancer), with a two-sided significance of 5% and 80% power. On this basis, target recruitment was 345 participants (172 events), including a 2% inflation for loss to follow-up.
Time-to-event endpoints were analysed according to the intention-to-treat principle using the log-rank test and are presented using Kaplan-Meier plots. Estimates of treatment effect (with 95% CIs) were made using unadjusted and adjusted Cox regression models, with an HR less than 1 favouring chemotherapy. Adjusted models included planned chemotherapy type, nodal status and microscopic margin status (balancing factors) and pathological stage. A prespecified subgroup analysis was done of adjustment factors. The proportional hazards assumption of the Cox model held when tested with Schoenfeld residuals. Two-sided p values less than 0·05 were judged significant.
The incidence of acute treatment-emergent adverse events, defined for both study groups as an increase in grade of any adverse event from baseline up to the 3-month timepoint, was compared by treatment received using the Wilcoxon rank-sum test (worst grade) and the χ2 test (proportion grade 3 or worse). Adverse events reported by more than 10% of participants in either group, or with significant differences between study groups using the Wilcoxon rank-sum test with a 1% significance level (to make some adjustment for multiple testing), were judged meaningful. Toxicity and treatment compliance data are reported in the safety population by treatment received at cycle one. Treatment compliance was assessed in all patients who received at least one dose of gemcitabine, cisplatin or carboplatin. When comparing the frequency of each adverse event type, we excluded participants who were not assessed for that adverse event type in the first 3 months of treatment (or equivalent timepoints for those allocated surveillance).
The global health score of the EORTC QLQ-C30 reported up to 12 months was summarised according to randomised allocation on an intention-to-treat basis. Data were analysed in accordance with the QLQ-C30 scoring manual. Change from baseline was compared between randomised groups using the ANCOVA model, adjusting for baseline score. Allowance for multiple testing was made by assessing scores at 3 months and 12 months only, with p values less than 0·01 judged significant; as a result, 99% CIs were used.
Accumulating safety and efficacy data were reviewed in confidence annually throughout the trial by an independent data monitoring committee. A Peto-Haybittle stopping rule (p<0·001) addressed both efficacy and inefficacy in disease-free survival.
Analyses are based on a snapshot of data taken on Nov 7, 2018, and include data from all follow-up visits up to and including May 31, 2018. This snapshot supersedes that used for the interim analysis, which led to the decision to close the trial early so that complete treatment and 3-month toxicity data could be reported. Analyses were done using Stata version 15.1.
This study is registered with the ISRCTN registry (ISRCTN98387754), ClinicalTrials.gov (NCT01993979), and Cancer Research UK (CRUK/11/027).
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
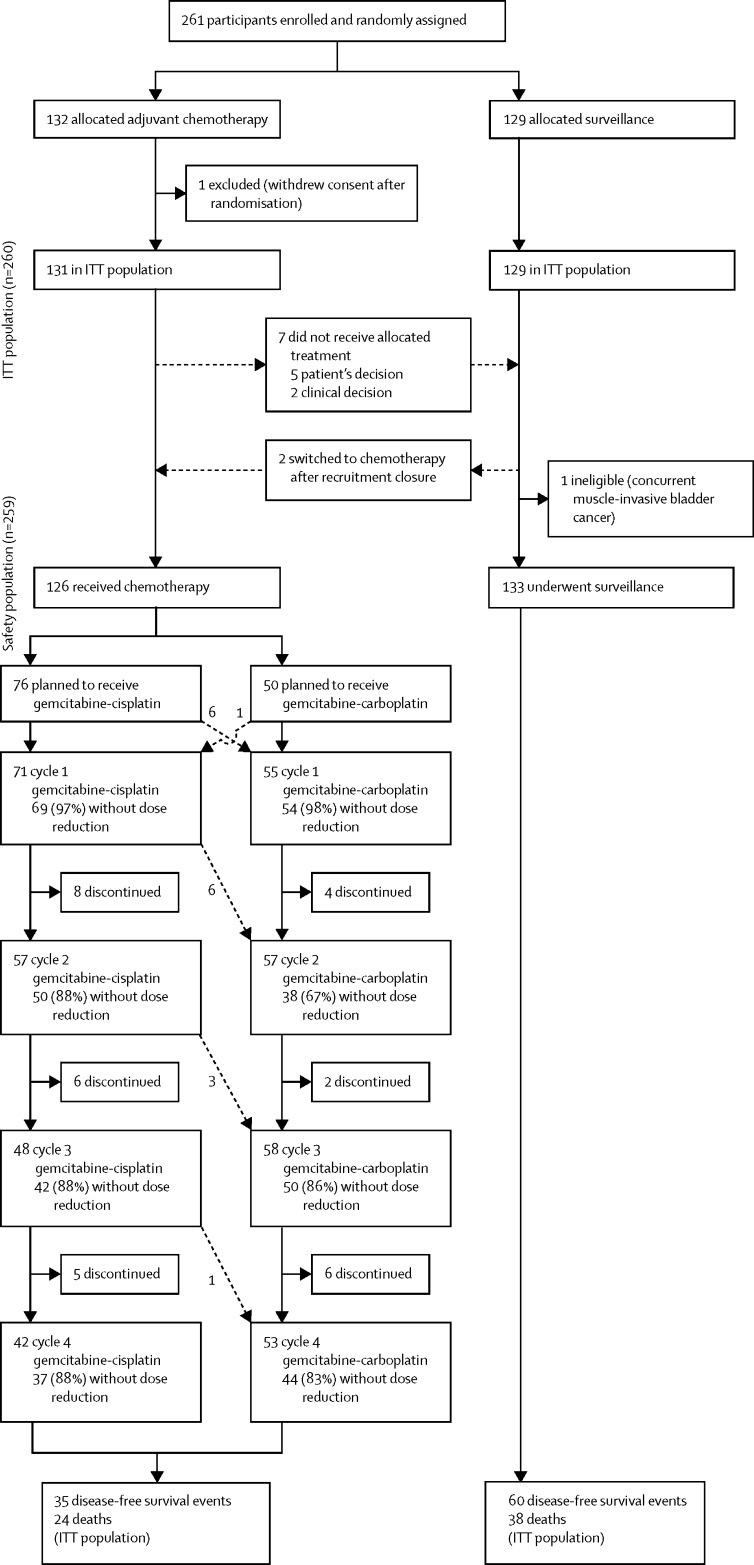
Between June 19, 2012, and Nov 8, 2017, 261 participants were recruited from 57 of 71 open sites (appendix pp 2–4), of whom 132 patients were allocated chemotherapy and 129 were allocated surveillance. 260 participants were included in the intention-to-treat population; one participant withdrew consent for data use after randomisation and is not included in any analyses (figure 1). Recruitment closed early on the recommendation of the independent data monitoring committee, having met the early stopping criterion for efficacy. At the point of trial closure, the independent data monitoring committee recommended that all participants who were still within the 90-day window from nephroureterectomy should be offered chemotherapy. Two participants allocated surveillance and still within this timeframe crossed over to receive chemotherapy but were included in the surveillance group for the intention-to-treat analysis (figure 1).
Figure 1.
Trial profile
Dotted lines indicate crossovers. ITT=intention to treat.
The median age of participants was 68·5 years (IQR 62·0–74·1; table). 245 (94%) of 260 participants were staged pT2–T3; of these, 223 (91%) were also staged N0 (appendix p 5). 166 (64%) participants had GFR of 50 mL/min or higher. Median follow-up was 30·3 months (IQR 18·0–47·5).
Table.
Participants' and tumour characteristics at trial entry
| Surveillance (n=129) | Chemotherapy (n=131) | Total (n=260) | |
|---|---|---|---|
| Sex | |||
| Male | 83 (64%) | 93 (71%) | 176 (68%) |
| Female | 46 (36%) | 38 (29%) | 84 (32%) |
| Age group (years) | |||
| <50 | 5 (4%) | 5 (4%) | 10 (4%) |
| 50–59 | 24 (19%) | 19 (15%) | 43 (17%) |
| 60–69 | 52 (40%) | 50 (38%) | 102 (39%) |
| 70–79 | 40 (31%) | 51 (39%) | 91 (35%) |
| ≥80 | 8 (6%) | 6 (5%) | 14 (5%) |
| Median (IQR) | 66·5 (61·5–73·3) | 69·2 (57·8–75·0) | 68·5 (62·0–74·1) |
| WHO performance status | |||
| 0 | 85 (66%) | 90 (69%) | 175 (67%) |
| 1 | 43 (33%) | 40 (31%) | 83 (32%) |
| Missing data | 1 (1%) | 1 (1%) | 2 (1%) |
| Smoking status | |||
| Current | 14 (11%) | 13 (10%) | 27 (10%) |
| Previous | 67 (52%) | 70 (53%) | 137 (53%) |
| Never | 47 (36%) | 48 (37%) | 95 (37%) |
| Missing data | 1 (1%) | 0 (0%) | 1 (<1%) |
| Concomitant drugs | |||
| No | 27 (21%) | 25 (19%) | 52 (20%) |
| Yes | 102 (79%) | 105 (80%) | 207 (80%) |
| Antihypertensive | 51 (40%) | 60 (46%) | 111 (43%) |
| Analgesic | 30 (23%) | 21 (16%) | 51 (20%) |
| Antidiabetes | 11 (9%) | 15 (11%) | 26 (10%) |
| Anticoagulant | 19 (15%) | 9 (7%) | 28 (11%) |
| Antiangina | 7 (5%) | 7 (5%) | 14 (5%) |
| Other | 80 (62%) | 77 (59%) | 157 (60%) |
| Missing data | 0 (0%) | 1 (1%) | 1 (<1%) |
| Pathological T stage | |||
| pT2 | 30 (23%) | 44 (34%) | 74 (28%) |
| pT3 | 88 (68%) | 83 (63%) | 171 (66%) |
| pT4 | 11 (9%) | 4 (3%) | 15 (6%) |
| Nodal stage* | |||
| N0 | 118 (91%) | 118 (90%) | 236 (91%) |
| N1 | 7 (5%) | 8 (6%) | 15 (6%) |
| N2 | 4 (3%) | 4 (3%) | 8 (3%) |
| N3 | 0 (0%) | 1 (1%) | 1 (<1%) |
| GFR (mL/min) | |||
| 30–49 | 45 (35%) | 49 (37%) | 94 (36%) |
| ≥50 | 84 (65%) | 82 (63%) | 166 (64%) |
| Site of tumour | |||
| Renal pelvis | 44 (34%) | 47 (36%) | 91 (35%) |
| Ureter | 42 (33%) | 47 (36%) | 89 (34%) |
| Both | 40 (31%) | 37 (28%) | 77 (30%) |
| Missing data | 3 (2%) | 0 (0%) | 3 (1%) |
| Type of surgery | |||
| Open | 17 (13%) | 21 (16%) | 38 (15%) |
| Laparoscopic | 104 (81%) | 109 (83%) | 213 (82%) |
| Robotic | 4 (3%) | 1 (1%) | 5 (2%) |
| Other† | 1 (1%) | 0 (0%) | 1 (<1%) |
| Missing data | 3 (2%) | 0 (0%) | 3 (1%) |
| Microscopic margin status | |||
| Positive | 14 (11%) | 17 (13%) | 31 (12%) |
| Negative | 115 (89%) | 114 (87%) | 229 (88%) |
| Number of lymph nodes dissected | |||
| 0 | 92 (71%) | 86 (66%) | 178 (68%) |
| 1–3 | 21 (16%) | 25 (19%) | 46 (18%) |
| 4–9 | 6 (5%) | 6 (5%) | 12 (5%) |
| ≥10 | 6 (5%) | 3 (2%) | 9 (3%) |
| Missing data | 4 (3%) | 11 (8%) | 15 (6%) |
Data are n (%), unless otherwise stated. GFR=glomerular filtration rate.
Ascertained radiologically when pathological staging was not available.
Kidney and ureter freed laparoscopically and removed through an open incision at the iliac fossa.
Seven of 131 participants allocated chemotherapy did not start treatment. 95 (75%) of 126 participants who started chemotherapy (including two patients who switched from surveillance after closure to recruitment) received four cycles of treatment. The proportion of participants starting chemotherapy who received four cycles of treatment did not differ by planned platinum agent (57 [75%] of 76 were planned to receive gemcitabine–cisplatin and 38 [76%] of 50 were planned to received gemcitabine–carboplatin; p=0·90).
31 participants discontinued chemotherapy early (clinician's decision [n=11], toxicity [n=10], patient's choice [n=8], or another unspecified reason [n=2]). Of the 95 participants receiving four cycles of chemotherapy, 52 started with gemcitabine–cisplatin and 43 started with gemcitabine–carboplatin. 41 (58%) of 71 participants who started cisplatin completed four cycles of cisplatin (a further patient received only gemcitabine on cycle four). 198 (91%) of 218 cycles of gemcitabine–cisplatin and 186 (83%) of 223 cycles of gemcitabine–carboplatin were delivered without a dose reduction. 16 (21%) of 76 participants intended for cisplatin switched to carboplatin because of a post-randomisation drop in GFR (figure 1). Six participants switched before the start of treatment and a further ten changed chemotherapy regimen from gemcitabine–cisplatin to gemcitabine–carboplatin at cycle two or later; of these, six switches were because of a reduction in GFR, as per protocol, two were attributable to suspected renal impairment, and two were because of grade 3 toxicity (joint pain or tinnitus). One (2%) of 50 participants planned to receive carboplatin switched to cisplatin because of a post-randomisation increase in GFR before treatment began.
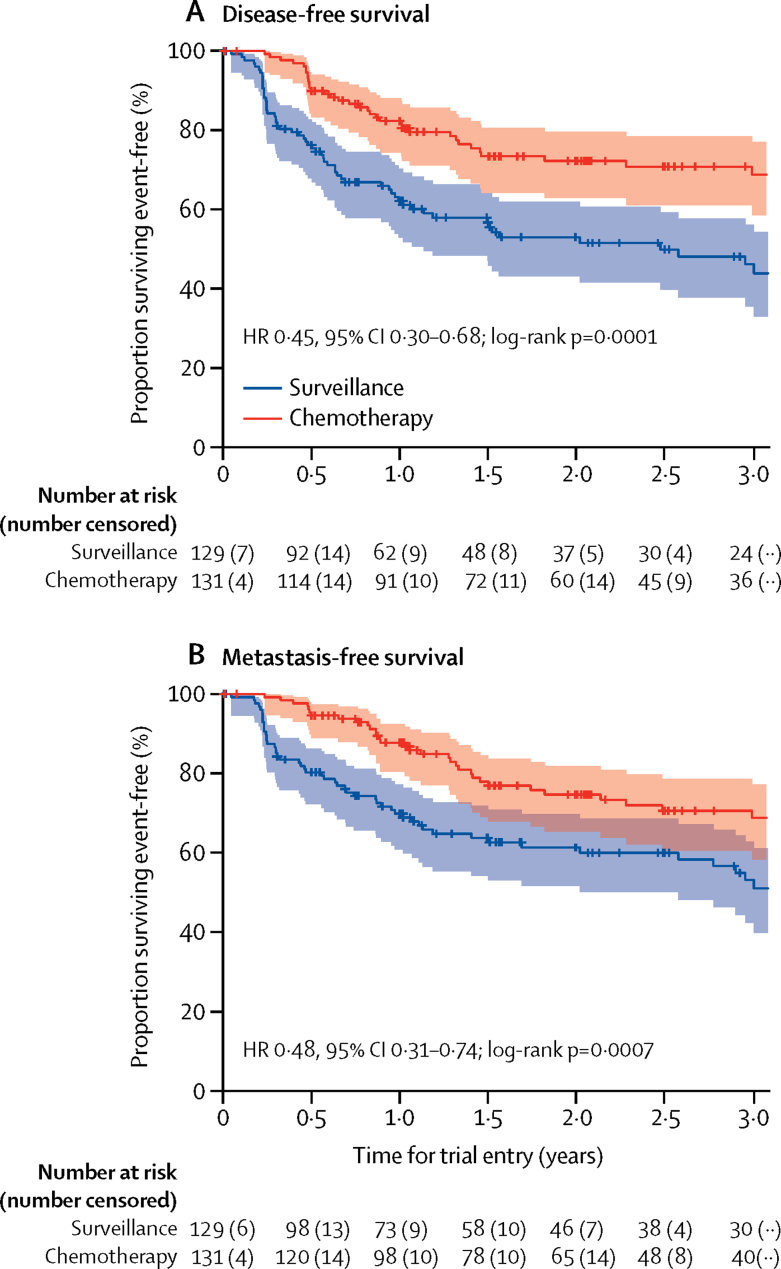
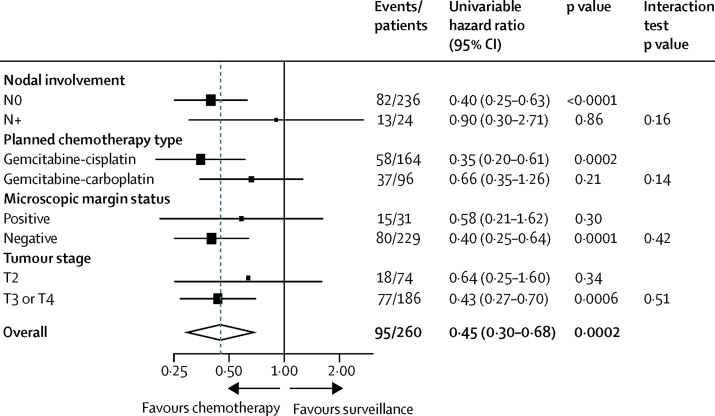
Fewer disease-related events contributing to the primary endpoint were reported in participants allocated chemotherapy (35 [27%] of 131) than in participants allocated surveillance (60 [47%] of 129). Chemotherapy conferred a 55% reduction in relative risk of disease recurrence or death (HR 0·45, 95% CI 0·30–0·68; log-rank p=0·0001; figure 2A). 3-year disease-free survival estimates were 71% (95% CI 61–78) in patients allocated chemotherapy and 46% (36–56) in those allocated surveillance, with an estimated absolute difference of 25% (95% CI 11–38). Median disease-free survival among patients allocated surveillance was 29·8 months (IQR 6·3–not reached; 95% CI 13·6–incalculable), and was not reached among those allocated chemotherapy. The benefit of chemotherapy was largely unchanged after adjustment for known prognostic factors (HR 0·46, 95% CI 0·30–0·71; p=0·0004; appendix p 6). Sensitivity analyses, including second primary muscle-invasive bladder cancers as recurrence events, gave similar results (appendix p 7). No heterogeneity of disease-free survival treatment effect was seen by prespecified balancing factors or tumour stage (figure 3).
Figure 2.
Disease-free survival and metastasis-free survival
Shaded areas denote 95% CIs. HR=hazard ratio.
Figure 3.
Subgroup analysis of disease-free survival
Participants allocated chemotherapy also had a lower risk of metastasis or death (HR 0·48, 95% CI 0·31–0·74; log-rank p=0·0007; figure 2B). 3-year event-free rates were 71% (95% CI 60–79) among patients allocated chemotherapy and 53% (42–63) among those allocated surveillance, with an estimated absolute difference of 17% (95% CI 4–31). Results were similar in multivariable analyses (appendix p 6).
Analysis of overall survival is planned once 88 deaths have occurred or all participants have at least 2 years of follow-up (whichever occurs first). 62 deaths have been recorded to date (24 assigned chemotherapy and 38 assigned surveillance). 49 deaths were attributed to UTUC, four to bladder cancer, one to other malignant disease, and eight to other causes. No treatment-related deaths were reported.
Grade 3 or worse acute treatment-emergent adverse events were reported for 55 (44%) of 126 participants who started chemotherapy (31 [44%] of 71 who started gemcitabine–cisplatin and 24 of 55 [44%] who started gemcitabine–carboplatin) compared with five (4%) of 129 managed by surveillance (p<0·0001). For each chemotherapy regimen, adverse events accorded with those frequently reported in routine clinical practice (appendix pp 8–11). Participants who received chemotherapy were more likely than those managed by surveillance to have grade 3 or worse decreases in neutrophils (45 [36%] of 126) and platelet count (13 [10%]), nausea (eight [6%]), febrile neutropenia (eight [6%]), and vomiting (seven [6%]). 54 serious adverse events were reported for 42 (32%) of 126 participants who received chemotherapy; 39 events were related to treatment. Analysis of late toxicity is planned once 2-year data are available for all participants.
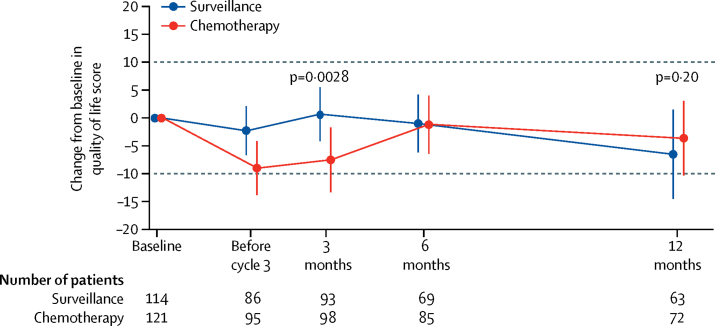
256 (98%) of 261 study participants consented to take part in the optional patient-reported quality-of-life study, including one participant who withdrew consent to use data after randomisation. Questionnaire return rates did not differ by allocated study group at any timepoint. Questionnaires were returned by 243 (95%) of 255 participants at baseline (119 [95%] of 125 allocated surveillance and 124 [95%] of 130 allocated chemotherapy), 208 (82%) of 255 at 3 months (101 [81%] of 125 and 107 [82%] of 130, respectively), and 166 (70%) of 237 at 12 months (78 [70%] of 112 and 88 [70%] of 125, respectively). Mean overall global health status score at baseline was 77% (SD 19) for the chemotherapy group and 76% (19) for the surveillance group. Overall global health status was lower during chemotherapy (before cycle 3) and immediately afterwards (at 3 months) in participants allocated chemotherapy versus surveillance. This difference had resolved by 6 months (figure 4). A full quality-of-life data analysis is planned once 2-year data are available for all participants.
Figure 4.
Patient-reported quality of life
Error bars represent 99% CIs. Dotted lines represent the minimum important clinical difference. Negative numbers denote a decrease in quality of life and positive numbers an increase.
Discussion
To our knowledge, the POUT trial is the largest reported in this patient population. Our findings show that gemcitabine–platinum combination chemotherapy initiated within 90 days after nephroureterectomy significantly improves disease-free survival in patients with locally advanced UTUC. Chemotherapy was also associated with improved metastasis-free survival, with acceptable acute toxic effects consistent with existing data,12 and with no more than a transient effect on patient-reported quality of life.
The relative effect on survival of carboplatin and cisplatin remains unclear in urothelial carcinoma without sufficient data from clinical trials incorporating a direct randomised comparison between the two agents. Findings of a meta-analysis of outcomes of patients with advanced urothelial carcinoma treated with platinum-based chemotherapy showed superior tumour response rates in trials of cisplatin compared with those of carboplatin.13 In the POUT trial, a GFR greater than 50 mL/min was deliberately selected as the criterion for cisplatin delivery. Appropriate selection of the cisplatin-eligible population was an important consideration during development of the POUT trial, with input sought from potential investigators. Although we acknowledge that a GFR lower than 60 mL/min forms part of the Galsky definition of being cisplatin unfit, routine practice in the UK for treatment of patients with non-UTUC tumours is to use a cutoff point for GFR of greater than 50 mL/min. In view of UK oncologists' experience and familiarity with use of cisplatin in patients without UTUC, and our wish not to exclude patients in the rare UTUC setting for whom cisplatin could be a feasible treatment, we judged that the criterion for switching to carboplatin at a GFR less than 50 mL/min was appropriate.
Acknowledging low power for formal statistical testing, our analysis showed no apparent heterogeneity of treatment effect and results were consistent across prespecified subgroups, including planned platinum agent. POUT trial data, therefore, support the use of adjuvant platinum-based chemotherapy in all patients who have undergone nephroureterectomy with curative intent. Although cisplatin should be the preferred agent when possible, our results suggest that patients for whom cisplatin is contraindicated because of poor renal function could still derive benefit from the alternative gemcitabine–carboplatin regimen. Individuals with resected nodal disease and those with microscopically positive margins at surgery should also be offered adjuvant platinum-based chemotherapy, subject to their fitness for systemic treatment.
The limitations of our study largely relate to pragmatic decisions taken during study development to enable successful recruitment to this trial in a rare patient population while preserving our ability to address the primary endpoint.
At the time of study development, a feasibility survey across all UK sites confirmed that formal nodal dissection was not part of standard care, nor were data to support this procedure strong. Therefore, nodal dissection was not mandated in the protocol because it was deemed inappropriate to do so. Ongoing debate around the survival benefits of extended abdominal lymph node dissection (ELND) in UTUC14 meant that this procedure was only needed for patients with observable lymphadenopathy on baseline imaging. Since most participants had limited lymph node dissection (ie, removal of all macroscopic visible nodes), occult metastases might have been overlooked in some patients categorised as N0, because a proportion were likely to have been microscopically node-positive. A clear benefit from adjuvant chemotherapy was seen in the N0 group of patients; therefore, whether standard use of nodal dissection would offer additional benefit is uncertain. The role of ELND in N0 disease remains a subject for future studies.
We acknowledge that disease-free survival is not regarded as a fully validated surrogate of overall survival after nephroureterectomy for UTUC.14 However, in a rare disease such as UTUC, a suitably powered trial with overall survival as the primary endpoint was not judged feasible. A placebo control group was judged inappropriate; use of identical follow-up procedures in both study groups aimed to minimise the risk of assessment bias. Although mature survival data (as a secondary endpoint) are not yet available, the large improvement in disease-free survival we noted for the primary endpoint, together with improved metastasis-free survival recorded as a secondary endpoint, strongly suggest that patients have better outcomes with chemotherapy than without. In view of the rarity of UTUC and the urgent need to improve outcomes, we believe that evidence is now sufficient to advocate use of gemcitabine–platinum combination chemotherapy as a standard of care.
Whether perioperative systemic therapy would be most effective for UTUC in the neoadjuvant or adjuvant setting remains contentious. Meaningful pathological complete response rates15 and, in retrospective case series, survival benefits16 suggest similar potential advantages with neoadjuvant therapy in UTUC to those seen in bladder cancer. Furthermore, potentially nephrotoxic cisplatin-based chemotherapy might be safer and more feasible for UTUC if given before nephroureterectomy, when patients retain maximum renal function. Some patients were probably excluded from the POUT trial (and might be similarly excluded from adjuvant chemotherapy in real-life practice) because of insufficient recovery after surgery. These patients might be better served with neoadjuvant chemotherapy, albeit with the risk that the toxic effects of chemotherapy could prevent some individuals from proceeding with curative surgery. We had considered a trial of neoadjuvant chemotherapy when developing the POUT trial concept; however, we had concerns about the reliability of preoperative staging and histology in muscle-invasive UTUC.7 Before starting the POUT trial, we did a feasibility survey across all potential UK investigators, which strongly supported an adjuvant rather than a neoadjuvant study for the reasons we have outlined. Two patient focus groups undertaken during study development explored the different approaches, and their feedback favoured an adjuvant trial. Investigation of the relative feasibility of adjuvant and neoadjuvant cytotoxic chemotherapy in UTUC is underway (NCT02969083). Although the POUT trial has shown superiority of adjuvant chemotherapy over surgery alone, it is not clear that patients previously planned for neoadjuvant chemotherapy should now defer treatment until surgery is complete. However, until further robust evidence becomes available, we propose that adjuvant treatment should be considered the preferred setting for future trials of perioperative chemotherapy in UTUC.
Previous studies adding a third agent to gemcitabine–platinum combinations have met with little success in advanced disease,17, 18, 19 partly because of the high burden of toxicity. However, data suggest potential benefits from two new classes of agents, fibroblast growth factor receptor (FGFR) inhibitors and immune checkpoint therapeutics. Increased understanding of the biology of UTUC suggests that distinct molecular differences exist between UTUC and bladder urothelial carcinomas.20 Higher proportions of FGFR alterations and luminal-like urothelial cancer signatures have been noted in UTUC21 than in bladder cancer.22 Because FGFR alterations are associated with high response rates to FGFR inhibitors and luminal-like urothelial cancer signatures with low response rates to chemotherapy in advanced urothelial cancers, investigation of orally bioavailable FGFR inhibitors (eg, erdafitinib alone or in combination with gemcitabine–platinum regimens) in molecularly selected patient cohorts might have particular value.23, 24, 25, 26 Efficacy of checkpoint inhibitors (eg, pembrolizumab and atezolizumab) in advanced urothelial carcinoma27, 28, 29 has prompted trials of immunotherapy in the perioperative setting as monotherapy and in combination with cytotoxic chemotherapy for urothelial carcinomas of the bladder (eg, NCT02365766 and NCT03661320). Although the adjuvant trials have included preplanned cohorts of patients with UTUC, no phase 3 trials are underway to address the role of immunotherapy in the adjuvant treatment of UTUC alone. Both FGFR inhibitors and immune checkpoint inhibitors might, therefore, be suitable additions to chemotherapy in future phase 3 trials that specifically address optimisation of perioperative treatment in UTUC.
We conclude that adjuvant platinum-based chemotherapy should be adopted as a new standard of care for patients with locally advanced UTUC for whom systemic chemotherapy is not contraindicated. This regimen should be routinely considered for all patients in this population, and future studies should focus on combinations with novel agents in the adjuvant setting, which might further improve the prognosis for locally advanced UTUC.
Data sharing
Deidentified individual participant data, together with a data dictionary defining each field in the set, will be made available to other researchers on request. The Institute of Cancer Research (ICR) Clinical Trials and Statistics Unit (CTSU) supports wider dissemination of information from the research it conducts and increased cooperation between investigators. Trial data are obtained, managed, stored, shared, and archived according to ICR-CTSU standard operating procedures to ensure the enduring quality, integrity, and utility of the data. Formal requests for data sharing are considered in line with ICR-CTSU procedures, with due regard given to funder and sponsor guidelines. Requests are via a standard proforma describing the nature of the proposed research and extent of data requirements. Data recipients are required to enter a formal data sharing agreement, which describes the conditions for release and requirements for data transfer, storage, archiving, publication, and intellectual property. Requests are reviewed by the Trial Management Group (TMG) in terms of scientific merit and ethical considerations, including patients' consent. Data sharing is undertaken if proposed projects have a sound scientific or patients' benefit rationale, as agreed by the TMG and approved by the independent data monitoring and steering committee, as required. Restrictions relating to patients' confidentiality and consent will be limited by aggregating and anonymising identifiable patients' data. Additionally, all indirect identifiers that could lead to deductive disclosures will be removed in line with Cancer Research UK data sharing guidelines.
Acknowledgments
Acknowledgments
We thank the patients who participated in this trial and staff at the participating centres and at the Institute of Cancer Research (ICR) Clinical Trials and Statistics Unit (CTSU), including Michelle Newton, Lauren Maynard, and Michaela Hill; we also thank POUT Trial Management Group members past and present, and the independent data monitoring committee and trial steering committee for overseeing the trial. POUT was supported by Cancer Research UK (CRUK/11/027) with programme grants to support the work of ICR-CTSU (C1491/A15955; C1491/A25351). This study represents independent research supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and the ICR (London, UK). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Contributors
ABi is the POUT trial Chief Investigator and EH is the methodological lead; ABi and EH led study design and acquired funding for the trial. ABi, MJ, JC, RJ, RTB, CH, AW, JWFC, JLD, AF, FXK, RK, TP, CW, RL, and EH are members of the POUT Trial Management Group, which contributed to study design, was responsible for oversight throughout the trial and contributed to data interpretation and manuscript preparation. ABl, PC, PAE, SJ, and JW were involved in recruitment and treatment of participants and contributed to data collection and manuscript preparation. EH oversaw statistical analyses and was responsible for central management of the trial at the Institute of Cancer Research (ICR) Clinical Trials and Statistics Unit (CTSU), with RL's support. RT did central study management at ICR-CTSU and contributed to data acquisition, interpretation, and manuscript writing. DD and BJ did statistical analyses at ICR-CTSU and contributed to data interpretation and manuscript writing. All authors reviewed and approved the manuscript.
Declaration of interests
ABi reports personal fees from Bayer, Janssen, Astellas, Roche, and Sanofi-Genzyme, outside of the submitted work; and research support from Sanofi-Genzyme, outside of the submitted work. JC reports personal fees and non-financial support from MSD, outside of the submitted work. RJ reports non-financial support from NHS Greater Glasgow and Clyde Health Board, during the conduct of the study; grants from the Chief Scientist Office, Scotland, during the conduct of the study; grants and personal fees from Roche, AstraZeneca, Astellas, Exilixis, and Clovis, outside of the submitted work; personal fees and non-financial support from MSD, Bristol-Myers Squibb, Janssen, and Ipsen, outside of the submitted work; personal fees from Merck Serono, outside of the submitted work; and grants, personal fees, and non-financial support from Bayer, outside of the submitted work. DD reports grants from Cancer Research UK and Prostate Cancer UK, during the conduct of the study. RTB reports a patent issued on bladder cancer prognosis (WO/2016/083832) and has previously contributed to advisory boards for Olympus Medical Systems and Janssen, outside of the submitted work. JWFC reports personal fees from AstraZeneca, Janssen, Roche, Ferring, MSD, and Bristol-Myers Squibb, during the conduct of the study. JLD reports grants from Cancer Research UK, during the conduct of the study. SJ reports personal fees from Janssen and Novartis, outside of the submitted work; and grants from Ipsen and Astellas, outside of the submitted work. TP reports personal fees from AstraZeneca, Bristol-Myers Squibb, Exelexis, Incyte, Ipsen, MSD, Pfizer, Roche, and Seattle Genetics, outside of the submitted work; and grants from AstraZeneca, Roche, and Pfizer, outside of the submitted work. EH reports grants from Cancer Research UK, during the conduct of the study; grants from MSD, Janssen-Cilag, Aventis Pharma (Sanofi), Accuray, Varian, and Roche, outside of the submitted work; and grants and non-financial support from AstraZeneca and Bayer, outside of the submitted work. MJ, CH, AW, ABl, PC, PAE, AF, BJ, FXK, RK, JW, CW, RT, and RL declare no competing interests.
Supplementary Material
References
- 1.Roupret M, Babjuk M, Comperat E. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73:111–122. doi: 10.1016/j.eururo.2017.07.036. [DOI] [PubMed] [Google Scholar]
- 2.Audenet F, Yates DR, Cussenot O, Rouprêt M. The role of chemotherapy in the treatment of urothelial cell carcinoma of the upper urinary tract (UUT-UCC) Urol Oncol. 2013;31:407–413. doi: 10.1016/j.urolonc.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 3.International Collaboration of Trialists International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loehrer PJ, Sr, Einhorn LH, Elson PJ. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10:1066–1073. doi: 10.1200/JCO.1992.10.7.1066. [DOI] [PubMed] [Google Scholar]
- 5.Sternberg CN, Skoneczna I, Kerst JM. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:76–86. doi: 10.1016/S1470-2045(14)71160-X. [DOI] [PubMed] [Google Scholar]
- 6.Moschini M, Shariat SF, Roupret M. Impact of primary tumor location on survival from the European Organization for the Research and Treatment of Cancer advanced urothelial cancer studies. J Urol. 2018;199:1149–1157. doi: 10.1016/j.juro.2017.11.068. [DOI] [PubMed] [Google Scholar]
- 7.Chitale S, Mbakada R, Irving S, Burgess N. Nephroureterectomy for transitional cell carcinoma: the value of pre-operative histology. Ann R Coll Surg Engl. 2008;90:45–50. doi: 10.1308/003588408X242268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leow JJ, Martin-Doyle W, Fay AP, Choueiri TK, Chang SL, Bellmunt J. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol. 2014;66:529–541. doi: 10.1016/j.eururo.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Hellenthal NJ, Shariat SF, Margulis V. Adjuvant chemotherapy for high risk upper tract urothelial carcinoma: results from the Upper Tract Urothelial Carcinoma Collaboration. J Urol. 2009;182:900–906. doi: 10.1016/j.juro.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Necchi A, Lo Vullo S, Mariani L. Adjuvant chemotherapy after radical nephroureterectomy does not improve survival in patients with upper tract urothelial carcinoma: a joint study by the European Association of Urology-Young Academic Urologists and the Upper Tract Urothelial Carcinoma Collaboration. BJU Int. 2018;121:252–259. doi: 10.1111/bju.14020. [DOI] [PubMed] [Google Scholar]
- 11.Donovan JL, Rooshenas L, Jepson M. Optimising recruitment and informed consent in randomised controlled trials: the development and implementation of the Quintet Recruitment Intervention (QRI) Trials. 2016;17:283. doi: 10.1186/s13063-016-1391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Santis M, Bellmunt J, Mead G. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galsky MD, Chen GJ, Oh WK. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2012;23:406–410. doi: 10.1093/annonc/mdr156. [DOI] [PubMed] [Google Scholar]
- 14.Guo R, Zhu Y, Xiong G, Li X, Zhang K, Zhou L. Role of lymph node dissection in the management of upper tract urothelial carcinomas: a meta-analysis. BMC Urol. 2018;18:24. doi: 10.1186/s12894-018-0336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman-Censits J, Puligandla M, Trabulsi E. Phase II trial of neoadjuvant chemotherapy followed by extirpative surgery for patients with high grade upper tract urothelial carcinoma (HG UTUC): results from ECOG-ACRIN 8141. J Urol. 2018;199(suppl 4):e1166–e1167. doi: 10.1097/JU.0000000000000644. (abstr LBA26). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porten S, Siefker-Radtke AO, Xiao L. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer. 2014;120:1794–1799. doi: 10.1002/cncr.28655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geldart T, Chester J, Casbard A. SUCCINCT: an open-label, single-arm, non-randomised, phase 2 trial of gemcitabine and cisplatin chemotherapy in combination with sunitinib as first-line treatment for patients with advanced urothelial carcinoma. Eur Urol. 2015;67:599–602. doi: 10.1016/j.eururo.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galsky MD, Hahn NM, Powles T. Gemcitabine, cisplatin, and sunitinib for metastatic urothelial carcinoma and as preoperative therapy for muscle-invasive bladder cancer. Clin Genitourin Cancer. 2013;11:175–181. doi: 10.1016/j.clgc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg JE, Ballman KV, Halabi S. CALGB 90601 (Alliance): randomized, double-blind, placebo-controlled phase III trial comparing gemcitabine and cisplatin with bevacizumab or placebo in patients with metastatic urothelial carcinoma. Proc Am Soc Clin Oncol. 2019;37(suppl 15) doi: 10.1200/JCO.21.00286. abstr 4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss TJ, Qi Y, Xi L. Comprehensive genomic characterization of upper tract urothelial carcinoma. Eur Urol. 2017;72:641–649. doi: 10.1016/j.eururo.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 21.van Oers JM, Zwarthoff EC, Rehman I. FGFR3 mutations indicate better survival in invasive upper urinary tract and bladder tumours. Eur Urol. 2009;55:650–657. doi: 10.1016/j.eururo.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 22.Robinson BD, Vlachostergios PJ, Bhinder B. Upper tract urothelial carcinoma has a luminal-papillary T-cell depleted contexture and activated FGFR3 signaling. Nat Commun. 2019;10 doi: 10.1038/s41467-019-10873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bahleda R, Italiano A, Hierro C. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res. 2019;25:4888–4897. doi: 10.1158/1078-0432.CCR-18-3334. [DOI] [PubMed] [Google Scholar]
- 24.Siefker-Radtke AO, Necchi A, Park SH. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt) Proc Am Sco Clin Oncol. 2018;36(suppl 15) abstr 4503. [Google Scholar]
- 25.Papadopoulos KP, El-Rayes BF, Tolcher AW. A phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br J Cancer. 2017;117:1592–1599. doi: 10.1038/bjc.2017.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nogova L, Sequist LV, Perez Garcia JM. Evaluation of BGJ398, a fibroblast growth factor receptor 1-3 kinase inhibitor, in patients with advanced solid tumors harboring genetic alterations in fibroblast growth factor receptors: results of a global phase I, dose-escalation and dose-expansion study. J Clin Oncol. 2017;35:157–165. doi: 10.1200/JCO.2016.67.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellmunt J, de Wit R, Vaughn DJ. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376:1015–1026. doi: 10.1056/NEJMoa1613683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powles T, Durán I, van der Heijden MS. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018;391:748–757. doi: 10.1016/S0140-6736(17)33297-X. [DOI] [PubMed] [Google Scholar]
- 29.Balar AV, Galsky MD, Rosenberg JE. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified individual participant data, together with a data dictionary defining each field in the set, will be made available to other researchers on request. The Institute of Cancer Research (ICR) Clinical Trials and Statistics Unit (CTSU) supports wider dissemination of information from the research it conducts and increased cooperation between investigators. Trial data are obtained, managed, stored, shared, and archived according to ICR-CTSU standard operating procedures to ensure the enduring quality, integrity, and utility of the data. Formal requests for data sharing are considered in line with ICR-CTSU procedures, with due regard given to funder and sponsor guidelines. Requests are via a standard proforma describing the nature of the proposed research and extent of data requirements. Data recipients are required to enter a formal data sharing agreement, which describes the conditions for release and requirements for data transfer, storage, archiving, publication, and intellectual property. Requests are reviewed by the Trial Management Group (TMG) in terms of scientific merit and ethical considerations, including patients' consent. Data sharing is undertaken if proposed projects have a sound scientific or patients' benefit rationale, as agreed by the TMG and approved by the independent data monitoring and steering committee, as required. Restrictions relating to patients' confidentiality and consent will be limited by aggregating and anonymising identifiable patients' data. Additionally, all indirect identifiers that could lead to deductive disclosures will be removed in line with Cancer Research UK data sharing guidelines.