Summary
Background
Buruli ulcer is a neglected tropical disease caused by Mycobacterium ulcerans infection that damages the skin and subcutis. It is most prevalent in western and central Africa and Australia. Standard antimicrobial treatment with oral rifampicin 10 mg/kg plus intramuscular streptomycin 15 mg/kg once daily for 8 weeks (RS8) is highly effective, but streptomycin injections are painful and potentially harmful. We aimed to compare the efficacy and tolerability of fully oral rifampicin 10 mg/kg plus clarithromycin 15 mg/kg extended release once daily for 8 weeks (RC8) with that of RS8 for treatment of early Buruli ulcer lesions.
Methods
We did an open-label, non-inferiority, randomised (1:1 with blocks of six), multicentre, phase 3 clinical trial comparing fully oral RC8 with RS8 in patients with early, limited Buruli ulcer lesions. There were four trial sites in hospitals in Ghana (Agogo, Tepa, Nkawie, Dunkwa) and one in Benin (Pobè). Participants were included if they were aged 5 years or older and had typical Buruli ulcer with no more than one lesion (caterories I and II) no larger than 10 cm in diameter. The trial was open label, and neither the investigators who took measurements of the lesions nor the attending doctors were masked to treatment assignment. The primary clinical endpoint was lesion healing (ie, full epithelialisation or stable scar) without recurrence at 52 weeks after start of antimicrobial therapy. The primary endpoint and safety were assessed in the intention-to-treat population. A sample size of 332 participants was calculated to detect inferiority of RC8 by a margin of 12%. This study was registered with ClinicalTrials.gov, NCT01659437.
Findings
Between Jan 1, 2013, and Dec 31, 2017, participants were recruited to the trial. We stopped recruitment after 310 participants. Median age of participants was 14 years (IQR 10–29) and 153 (52%) were female. 297 patients had PCR-confirmed Buruli ulcer; 151 (51%) were assigned to RS8 treatment, and 146 (49%) received oral RC8 treatment. In the RS8 group, lesions healed in 144 (95%, 95% CI 91 to 98) of 151 patients, whereas lesions healed in 140 (96%, 91 to 99) of 146 patients in the RC8 group. The difference in proportion, −0·5% (–5·2 to 4·2), was not significantly greater than zero (p=0·59), showing that RC8 treatment is non-inferior to RS8 treatment for lesion healing at 52 weeks. Treatment-related adverse events were recorded in 20 (13%) patients receiving RS8 and in nine (7%) patients receiving RC8. Most adverse events were grade 1–2, but one (1%) patient receiving RS8 developed serious ototoxicity and ended treatment after 6 weeks. No patients needed surgical resection. Four patients (two in each study group) had skin grafts.
Interpretation
Fully oral RC8 regimen was non-inferior to RS8 for treatment of early, limited Buruli ulcer and was associated with fewer adverse events. Therefore, we propose that fully oral RC8 should be the preferred therapy for early, limited lesions of Buruli ulcer.
Funding
WHO with additional support from MAP International, American Leprosy Missions, Fondation Raoul Follereau France, Buruli ulcer Groningen Foundation, Sanofi-Pasteur, and BuruliVac.
Introduction
Buruli ulcer, a necrotising skin disease caused by Mycobacterium ulcerans, is one of the 20 neglected tropical diseases.1 In Africa, the disease was first identified and described near the Nile River in the former Buruli county in Uganda. Buruli ulcer has been reported in at least 33 countries,2 with most cases occurring in west Africa. Sporadic cases occur in many locations in central America and South America (notably in French Guyana) and in Japan and the western Pacific region.3 Prevalence of the disease is highly variable, ranging from 3·1 to 30·7 cases per 100 000 population.4 Even in endemic areas, prevalence is highly focal and varies considerably in space and time.5 In sub-Saharan Africa, the median age of new cases is around 20 years,6, 7 whereas in the temperate climate of southeast Australia, the median age is around 60 years.8, 9
Research in context.
Evidence before this study
We searched PubMed from database inception until Dec 31, 2011, without language restrictions for clinical trials and randomised clinical trials using the search string: (“Buruli” OR “Mycobacterium” AND “ulcerans”) AND (“antimycobact*” OR “antimicrob*” OR “antibiotic*”) AND “treatment”. The standard treatment for Buruli ulcer is combination antibiotic therapy comprising intramuscular streptomycin and oral rifampicin daily for 8 weeks. Streptomycin injections are painful and can cause ototoxicity. A systematic review found case reports and observational cohort studies that showed the potential effect of fully oral antibiotic combinations for the treatment of Buruli ulcer. No previous trials in humans have tested the efficacy of a fully oral antibiotic regimen.
Added value of this study
This open-label, randomised, controlled, phase 3 trial evaluated the efficacy of a fully oral treatment with once daily rifampicin and clarithromycin 15 mg/kg extended release compared with standard of care using a non-inferiority design. Rates of healing of Buruli lesions were similar in both groups. Treatment-related adverse events, particularly otovestibular toxicity, were more common in patients receiving combined rifampicin and streptomycin than in patients receiving combined rifampicin and clarithromycin. In rural African regions endemic for Buruli ulcer, access to health care is difficult; hospital-based care and injections impose physical and financial barriers. Fully oral treatment provides the opportunity of decentralised care with optimal chances of healing, and an excellent safety profile.
Implications of all the available evidence
A fully oral treatment comprising rifampicin and clarithromycin is non-inferior to streptomycin–rifampicin in healing of early, limited Buruli ulcer lesions and is associated with a better safety profile. Fully oral rifampicin and clarithromycin can be provided as decentralised care, and should be considered the new standard of care for patients with Buruli ulcer disease; patients with limited lesions given the treatment have an excellent chance of healing without additional surgery.
Before 2005, surgery was the mainstay of treatment, which often required excision with a 1–2 cm rim of apparently healthy skin, followed by skin grafting. A proof-of-principle study in Ghana showed the efficacy of the combination of oral rifampicin 10 mg/kg plus streptomycin 15 mg/kg (intramuscularly) for 8 weeks (RS8) to kill M ulcerans in early, limited (lesions of 10 cm or less cross-sectional diameter) Buruli ulcer disease.10 WHO subsequently issued provisional guidelines for RS8 as the standard treatment in highly burdened regions in Africa. Early antimicrobial treatment gives excellent outcomes, but when treatment is delayed or disease is diagnosed late, severe morbidity, permanent disability, and social stigma with loss of productivity and school drop-out can ensue.11, 12, 13 RS8 has proven effective in healing all forms of Buruli ulcer disease and has led to a reduction of the recurrence rate from between 6% and 47% after surgery alone, to negligible after antimicrobial treatment.14, 15 However, daily streptomycin injections are painful and potentially toxic16 and this toxicity, in turn, jeopardises early reporting and compliance with therapy. Patients have to travel long distances to health facilities for daily treatment; hence, fully oral treatment is needed.
In mouse footpad studies comparing the standard RS8 therapy with rifamycins and other antimicrobials, clarithromycin (a macrolide drug) was deemed a suitable replacement for streptomycin, and treatment of these experimental animals with fully oral rifampicin 10 mg/kg plus clarithromycin 15 mg/kg for 8 weeks (RC8) was as effective as RS8.17, 18 Studies in humans have shown that switching from streptomycin to clarithromycin after 4 weeks15 or after 2 weeks19 yielded similar efficacy to RS8 therapy. In addition, a pilot study in Benin provided initial data suggesting the efficacy of fully oral RC8 treatment,20 but no direct comparison was made between RS8 and RC8, and some patients with larger lesions still needed surgical treatment. Here, we report results from a clinical study designed to compare the efficacy and tolerability of RC8 with that of RS8 for treatment of early Buruli ulcer lesions.
Methods
Study design and participants
We did an open-label, randomised, multicentre, phase 3 clinical trial with a non-inferiority design. The study adhered to the Consolidated Standards for the Reporting of Randomized Controlled Trials guidelines.21 The study was done in four hospitals in south Ghana (Agogo Presbyterian Hospital, Tepa Goverment Hospital, Nkawie Government Hospital, and Dunkwa Government Hospital) and in one hospital in south Benin (Pobè Health Centre).22
Trained community health workers referred patients clinically diagnosed with M ulcerans disease for possible enrolment, combining active and passive case finding approaches.23, 24 Patients were included in the study if they were aged 5 years or older and had clinically identified typical Buruli ulcer with no more than one lesion that was a maximum 10 cm in diameter. Exclusion criteria were children younger than 5 years, individuals with lesions bigger than 10 cm in diameter, pregnancy, drug intolerance, renal or hepatic impairment, HIV infection, or previous treatment with trial medication.
The protocol and consent forms were approved by the Ethics Review Committee of the Ghana Health Service (GHS-ERC01/03/11) and Benin (N°108/MS/DC/SGM/DFRS/CNPERS/SA). Ethics approval was also granted by the WHO Ethics Review Committee (RPC443), and the University Medical Centre Groningen Ethics Review Board reviewed the protocol (M11.097746). Written and verbal informed consent was obtained from all participants aged 12 years or older, and from parents, caretakers, or legal representatives of participants younger than 18 years. All staff involved in the study received formal training in good clinical practice.
Randomisation and masking
Eligible participants were randomly assigned (1:1) to receive RS8 or RC8; the dosing schedule is provided in the appendix (p 4). Randomisation was done with minimisation for study site and type of lesion (ulceration or non-ulceration). The randomisation sequence was computer-generated with blocks of six for each centre and concealed in opaque envelopes. Neither the investigators who took measurements of the lesions nor the attending doctors were masked to treatment assignment. As healing could be anticipated to occur at any timepoint in any of the study sites, and as the study teams in each centre not only assessed the image but also had the opportunity to palpate the lesion, and therefore had better assessment tools to verify healing (ie, full epithelialisation), we recorded the assessment by the study teams aware of treatment allocation as the primary endpoint. To evaluate whether this unmasked assessment of the primary endpoint (ie, healing) could be biased, we invited an independent technical expert panel masked to treatment assignment to review a sample of individual image sets. A study monitor (NFS) assessed data entries. Diagnostic uncertainties and adverse events were discussed with the (co-)principal investigators (TSvdW, ROP, JR) and the sponsor, using an electronic web-based platform. Annual auditing trips to the study sites were made. All data were entered on paper and uploaded into OpenClinica. The electronic data files were collected and managed by the Drugs for Neglected Diseases initiative, Africa Regional Office, Nairobi, Kenya.
Procedures
After participants had given informed consent, demographic details, clinical information, and blood samples were obtained. Pregnancy tests were done in female participants aged 10 years and older after pre-test counselling, and hearing tests were done for all participants (AS208 portable equipment, Interacoustics, Assens, Denmark) to obtain baseline audiometry data. HIV antibody tests (Alere Determine HIV 1/2 Kit, Alere, Waltham, MA, USA) were done after pre-test counselling.
The dimensions and aspect of lesions were documented by taking digital photographs to measure the surface area of lesions over time. An imaging system was used to compute the lesion size at baseline and during each follow-up visit (ARANZ, Wellington, New Zealand).
Diagnosis of Buruli ulcer was confirmed by quantitative PCR amplication of the IS2404 sequence from fine-needle aspirates or swab samples of lesions.25 In Ghana, samples were transported from study sites to the Kumasi Centre for Collaborative Research in Kumasi, Ghana, for analysis. Samples from Pobè Health Centre in Benin were initially all analysed in Centre de recherche en cancérologie et immunologie Nantes-Angers, Angers, France; subsequently, samples were also analysed in Pobè (with confirmation in Angers). All laboratories participated in an external quality assessment programme done by the WHO reference laboratory in the Institute of Tropical Medicine, Antwerp, Belgium.26
In the Ghanaian study sites, most patients were treated as outpatients. After assessment and initiation of treatment at the hospital, at the Pobè Health Centre in Benin, all patients recruited were treated as inpatients, in line with routine practice. Patients in Ghana who were treated as outpatients were given medication in weekly batches to take to the nearest health facility where they received directly observed therapy for the subsequent days, with daily wound care. Participants’ travel costs were reimbursed, and small meals were given to study participants. For ulcers with active discharge, absorptive dressing material (Beier Drawtex Healthcare, Pinetown, South Africa) was applied to maintain a moist environment for wound healing. When lesions were non-exuding, vaseline gauze was applied. Short stretch bandages were used to prevent lymphoedema. Only participants who had lesions involving joints or who resided in distant villages were admitted to hospital in Ghana. The health-care worker who observed the treatment recorded drug intake on the study form. The adherence to treatment according to treatment allocation was primarily assessed from the study file entries. In Ghana, a system to fully supervise treatment was implemented involving a network of village health workers and nurses, whereas the co-principal investigator and the study monitor in Ghana verified adherence personally on a regular basis. In the study centre in Benin, all patients were admitted for the time of treatment, allowing directly supervised treatment throughout. Each study site had dedicated trained staff for wound care and for prevention of disability.
Rifampicin tablets and extended release clarithromycin were shipped to the trial sites. The pharmacists at Agogo Presbyterian Hospital and Pobè Health Centre were responsible for storage of the trial medication.
Participants were followed up once every 2 weeks during the first 8 weeks and then monthly for 12 months. At review visits, clinical assessments included obtaining photographs of lesions. In addition, safety outcomes were assessed every 4 weeks by measuring liver, kidney, and auditory tests in all participants. Female participants aged 10 years and older received pregnancy tests at weeks 2, 4, and 6.
Lesions that did not heal were measured and photographed. Treatment failure was recorded if the lesion had not healed by week 52 or if the lesion had recurred within the year. Removal of necrosis and slough, along with skin grafting, were part of normal care. Requirements for these procedures were not considered evidence of treatment failure, as per protocol. Daily dressing changes were provided if wounds were discharging excessively.
Outcomes
The primary clinical endpoint was lesion healing (ie, full epithelialisation, or stable scar) without recurrence at 52 weeks after start of antimicrobial therapy. Secondary objectives were: (1) to compare the recurrence rates within 12 months of treatment initiation between the RC8 and RS8 groups; (2) to compare the treatment failure rates within 12 months of treatment initiation between the RC8 and RS8 groups; (3) to compare the incidences of paradoxical response, defined as increase of surface area of a lesion by more than 20% compared with a previous measurement, after initial reduction in lesion size,27 within 12 months of treatment initiation between the RC8 and RS8 groups; (4) to assess whether lesion healing depends on lesion category (I or II ≤10 cm in diameter) and lesion type (nodule, plaque, ulcer, or oedema) and treatment regimen, in terms of lesion sizes (surface area), time to complete lesion healing, healing without additional surgery or relapse, type of adjunctive surgical therapy, and time from treatment initiation to surgery; (5) to compare the rates of residual functional limitations at 12 months following treatment with the two antibiotic regimens (RC8 and RS8) and lesion category (I or II) and lesion type (nodule, plaque, oedema, or ulcer); (6) to compare the compliance rates in patients given RC8 and RS8 regimens; (7) to compare the incidences and relative risk of all adverse events, including treatment-related adverse events, serious adverse events, and grade 3–4 toxicity in patients given RC8 and RS8 regimens; and (8) to compare treatment discontinuation rates in patients given RC8 and RS8 regimens. If a serious adverse event was suspected or detected, we shared this information with all investigators and members of the data safety monitoring board, using the web-based platform. Safety outcomes were separately analysed and compared between study groups. For hearing loss, we used a threshold increase of more than 25 dB.
Statistical analysis
Assuming an efficacy of 96% for standard RS8 treatment,15 the sample size needed to detect inferiority of the experimental RC8 treatment by a margin of 12% for oral treatment was calculated to be 332 study participants with PCR-confirmed Buruli ulcer. Baseline comparisons of clinical and demographic characteristics according to study group allocation were done using Student's t test for parametric continuous data or the Mann-Whitney U-test for non-parametric data. Categorical data were compared using χ2 tests. The analysis was by intention to treat using a one-sided t test for the primary outcome. Time to healing was analysed by a Cox proportional hazards model to assess the cumulative probability of healing. Hazards ratios were reported with 95% CIs. We used Stata version 15 for analyses. A data safety monitoring board was used. This study was registered with clinicaltrials.gov, NCT01659437.
Role of the funding source
The sponsor and the donors had no role in study design, data collection, data analysis, data interpretation, or writing of the report. ROP, JR, RO, MOO, YS and TSvdW had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
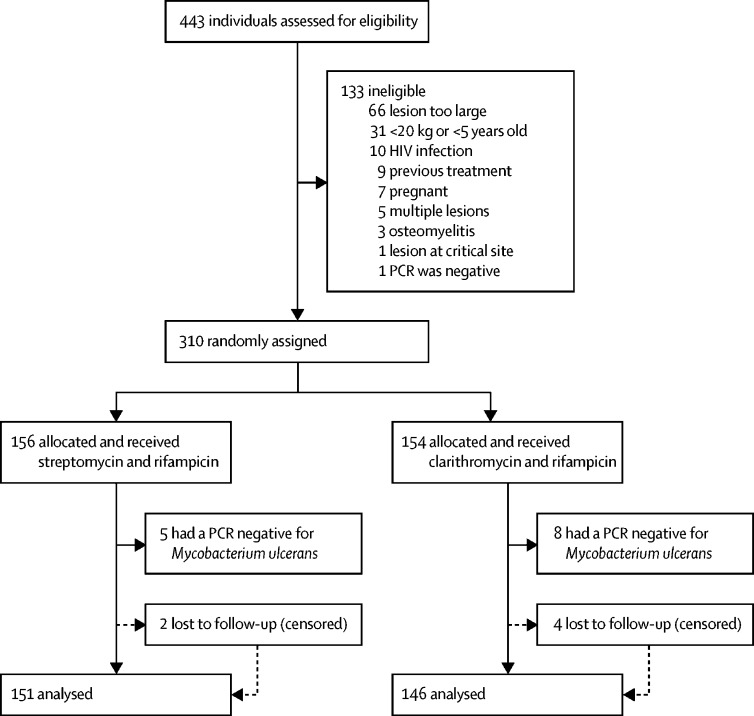
Participants were recruited to the trial between Jan 1, 2013, and Dec 31, 2017. We had initially planned a 2-year trial. Accrual was slow, despite active case finding and increased site visits. Accrual was extended twice (March 24, 2015, and March 21, 2017) following interim reports during biannual meetings with the study sponsor. All interim reports for this open-label study showed similarity of efficacy outcomes between treatment groups, so on March 21, 2017, the study sponsor and stakeholders decided to end further accrual at the end of 2017. 443 patients were assessed for eligibility, and accrual ended with 310 participants, 297 of whom had PCR-confirmed Buruli ulcer (figure 1).
Figure 1.
Trial profile
Table 1 shows that baseline characteristics of study participants were balanced; sex distribution was almost equal between groups (153 [52%] of 297 participants were female), and median age was 14 years (IQR 10–29). Most lesions were on the limbs; the lower limb was affected in 163 (55%) participants; the upper limb was affected in 108 (37%) participants. In 156 (53%) participants, lesions were ulcerated at presentation.
Table 1.
Baseline characteristics
| RS8 (n=151) | RC8 (n=146) | Total (n=297) | |
|---|---|---|---|
| Sex | |||
| Male | 74 (49%) | 70 (48%) | 144 (49%) |
| Female | 77 (51%) | 76 (52%) | 153 (52%) |
| Age, years | |||
| Mean | 20·4 (16·3) | 22·9 (17·7) | 21·6 (17·0) |
| Median | 14 (10–25) | 16 (10–32) | 14 (10–29) |
| Education level | |||
| None | 35 (24%) | 43 (30%) | 78 (27%) |
| Primary or middle school | 100 (67%) | 92 (64%) | 192 (66%) |
| Secondary school or above | 14 (9%) | 8 (6%) | 22 (8%) |
| Weight, kg | |||
| Mean | 41·5 (15·9) | 43·0 (17·5) | 42·3 (16·7) |
| Median | 41 (27–55) | 44 (26–58) | 42 (26–57) |
| Height, m | |||
| Mean | 1·5 (0·2) | 1·5 (0·2) | 1·5 (0·2) |
| Median | 1·5 (1·3–1·6) | 1·5 (1·3–1·6) | 1·5 (1·3–1·6) |
| Lesion location | |||
| Upper limb | 47 (31%) | 61 (42%) | 108 (37%) |
| Lower limb | 90 (60%) | 73 (50%) | 163 (55%) |
| Buttocks and perineum | 3 (2%) | 1 (1%) | 4 (1%) |
| Head and neck | 1 (1%) | 4 (3%) | 5 (2%) |
| Thorax | 4 (3%) | 3 (2%) | 7 (2%) |
| Abdomen | 2 (1%) | 3 (2%) | 5 (2%) |
| Back | 3 (2%) | 1 (1%) | 4 (1%) |
| Lesion type | |||
| Nodule | 28 (19%) | 34 (23%) | 62 (21%) |
| Plaque | 39 (26%) | 36 (25%) | 75 (25%) |
| Oedema | 1 (1%) | 3 (2%) | 4 (1%) |
| Ulcer | 83 (55%) | 73 (50%) | 156 (53%) |
Data are n (%), mean (SD), or median (IQR). RC8=oral rifampicin 10 mg/kg plus clarithromycin 15 mg/kg extended release once daily for 8 weeks. RS8=oral rifampicin 10 mg/kg plus intramuscular streptomycin 15 mg/kg once daily for 8 weeks.
Lesions had healed without relapse at week 52 after start of treatment in 144 (95%, 95% CI 91 to 98) of 151 patients receiving RS8 compared with 140 (96%, 91 to 99) of 146 patients receiving RC8. The difference in proportion of patients with healed lesions, −0·5 (95% CI −5·2 to 4·2), was not significantly greater than zero (p=0·59). The upper limit of the 95% CI of the difference of 4·2% is less than the non-inferiority margin of 12%, showing non-inferiority.
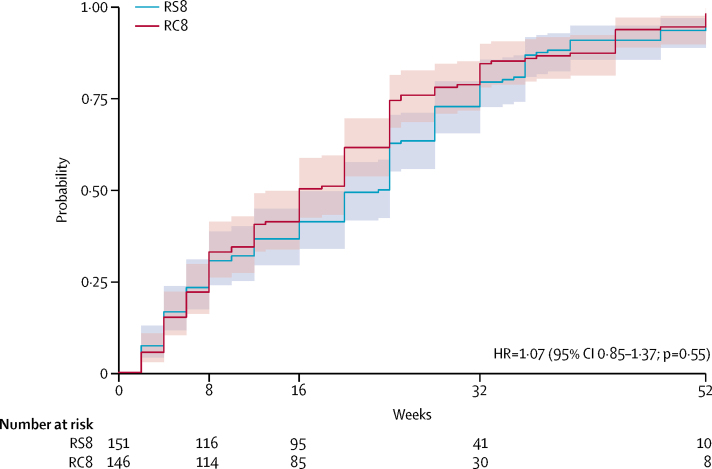
Time to healing was a secondary endpoint. Figure 2 shows the plot of cumulative probability of healing with 95% CIs and the results of Cox proportional hazard for time to healing. The median time to healing was 24 weeks (IQR 8–28) in the RS8 group, and 16 weeks (IQR 8–25) in the RC8 group. There were no recurrences and treatment failure rates were similar within 12 months of treatment. Treatment was unsuccessful for six patients receiving RC8 and for seven patients receiving RS8. The incidences of paradoxical response were not statistically different between study groups. If a paradoxical response was defined as an increase in lesion surface area by more than 20% compared with a previous measurement, after initial reduction in lesion size,27 the incidence was 34% in the RC8 group and 43% in the RS8 group (p=0·093). If other definitions were used, the difference remained non-significant. Further details are provided in the appendix (p 4). In the RS8 group, lesion healing in category I (<5 cm) lesions took a median of 16 weeks (IQR 6–28) compared with 28 weeks (16–38; p<0·0001). In the RC8 group, category I lesions healed at a median of 13 weeks (IQR 6–24) compared with 20 weeks (12–32) in category II (p=0·031; see appendix pp 5–6). Lesion type (ulcer, compared with non-ulcerated lesions at presentation: nodule, plaque, or oedema) did not influence time to healing. In the RS8 group, ulcers healed at a median of 20 weeks (IQR 8–28) versus 24 weeks (6–35) in non-ulcerated lesions (p=0·31). In the RC8 group, ulcers healed at a median of 12 weeks (IQR 8–24), while non-ulcerated lesions were healed at a median of 20 weeks (8–25; p=0·60; appendix p 8). Additional surgery consisted only of skin grafting, and this was necessary in four (1%) study participants (two in the RC8 group and two in the RS8 group), all of whom had category II lesions. All grafts were done after week 14. There were no residual functional limitations at week 52 following antimicrobial treatment. The compliance rates were 100% in all patients given RC8 and RS8 regimens. Using a threshold increase of more than 25 dB, three (2%) participants in the RS8 group had hearing loss compared with one (1%) in the RC8 group. In the RS8 group, five (3%) participants experienced dizziness with or without accompanying imbalance or tinnitus, versus none in the RC8 group. Taken together, otovestibular toxicity occurred in eight (5%) individuals in the RS8 group compared with one (1%) in the RC8 group. One (1%) participant in the RS8 group had to discontinue allocated treatment after week 6 (table 2). No renal damage was detected in blood chemistry tests. Few participants had electrocardiogram changes, which were similar in both study groups (four [3%] in the RS8 group and three [2%] in the RC8 group). Gastrointestinal and mucocutaneous adverse events were few and equally distributed. No neurological events were noted. Mild (grade 1–2) laboratory abnormalities were fairly common in both study groups; the proportion appeared largest at baseline.
Figure 2.
Cumulative probability of a Buruli ulcer lesion healing over time
95% CIs are shown. Lesion healing was defined by full epithelialisation or stable scar. HR=hazard ratio. RC8=oral rifampicin 10 mg/kg plus clarithromycin 15 mg/kg extended release once daily for 8 weeks. RS8=oral rifampicin 10 mg/kg plus intramuscular streptomycin 15 mg/kg once daily for 8 weeks.
Table 2.
Summary of adverse drug reactions
| RS8 (n=151) | RC8 (n=146) | Total (n=297) | ||
|---|---|---|---|---|
| Any adverse event | 20 (13%) | 9 (6%) | 29 (10%) | |
| Serious adverse event* | 1 (1%) | 0 | 1 (<1%) | |
| Adverse event, not serious | 19 (13%) | 9 (6%) | 29 (10%) | |
| Otovestibular toxicity | 8 (5%) | 1 (1%) | 9 (3%) | |
| Ototoxicity, >25 dB hearing loss | 3 (2%) | 1 (1%) | 4 (1%) | |
| Vestibular toxicity | 5 (3%) | 0 | 5 (2%) | |
| Mucocutaneous manifestations | 3 (2%) | 0 | 3 (1%) | |
| Skin rash (grades 1–2 only) | 2 (1%) | 0 | 2 (1%) | |
| Conjunctivitis | 1 (1%) | 0 | 1 (<1%) | |
| Constitutional symptoms | 1 (1%) | 0 | 1 (<1%) | |
| Fever | 1 (1%) | 0 | 1 (<1%) | |
| Headache | 1 (1%) | 2 (1%) | 3 (1%) | |
| Malaise | 0 | 1 (1%) | 1 (<1%) | |
| Lymphadenopathy | 1 (1%) | 1 (1%) | 2 (1%) | |
| Cough | 0 | 1 (1%) | 1 (<1%) | |
| Gastrointestinal | 2 (1%) | 0 | 2 (1%) | |
| Nausea and vomiting | 1 (1%) | 0 | 1 (<1%) | |
| Abdominal pain | 1 (1%) | 0 | 1 (<1%) | |
| Confusion | 0 | 0 | 0 | |
| ECG changes | 4 (3%) | 3 (2%) | 7 (2%) | |
| QTc prolongation (but <490 msec) | 3 (2%) | 2 (1%) | 5 (2%) | |
| Other (down-slope ST, P wave increase) | 1 (1%) | 1 (1%) | 2 (1%) | |
Data are n (%). RC8=oral rifampicin 10 mg/kg plus clarithromycin 15 mg/kg extended release once daily for 8 weeks. RS8=oral rifampicin 10 mg/kg plus intramuscular streptomycin 15 mg/kg once daily for 8 weeks. ECG=electrocardiogram.
Older patient with hearing loss, dizziness, and imbalance leading to discontinuation at week 6.
The 13 study participants whose treatment was unsuccessful (seven [5%] in the RC8 group and six [4%] in the RS8 group) are summarised in the appendix (p 8). Ten (3%) participants with lesions that did not heal by week 52 had category II lesions. Seven of the patients whose treatment was unsuccessful were actually lost to follow-up: two went on to heal after 52 weeks, one had osteomyelitis, two had non-healed lesions at the lateral malleolus where recurrent trauma was suspected as a cause of non-healing, malignancy was suspected in one participant, and none were suspected to have ongoing M ulcerans infection.
Just one participant dropped out of the study. One participant in the RC8 group had detected pregnancy at week 6, but she continued in the allocated treatment. Six participants were lost to follow-up at some point in time after completion of drug treatment. The censored data could still be used for analysis of these participants, and adherence to drug treatment was high in both study groups. No recurrences were noted during follow-up.
Discussion
With these findings, we provide evidence that a fully oral antimicrobial treatment for early, limited Buruli ulcer with rifampicin and an extended release formulation of clarithromycin (RC8) is non-inferior to the treatment with rifampicin and injected streptomycin (RS8). Although both treatments were generally well tolerated, safety outcomes were in favour of fully oral treatment with RC8. Eight individuals receiving streptomycin had side-effects attributed to the drug. Our results support the use of fully oral treatment with RC8 as first-line treatment for early, limited Buruli ulcer lesions. Oral treatment is far more convenient for patients and also prevents the toxicity (predominantly acoustic and vestibular) that can occur with the use of the injectable aminoglycoside streptomycin.16 This study addressed a question that was also deemed important by former patients who prioritised research into treatment that avoided painful injections and had few side-effects.28 Age and sex distribution, as well as socioeconomic status and educational level in this study population were all typical for the population affected by Buruli ulcer in west Africa.
Our study had limitations, the most important being the open-label design. Concealment of active study medication was not considered appropriate as it would not have been appropriate to give children a placebo injection. We were unable to fully rule out bias in the judgments by the investigators of the primary endpoint (ie, healing at week 52) and the secondary endpoint (time to healing). Assessments were, however, made by attending team members, and all staff involved in the study had formal training in good clinical practice. Monitoring and digital photographic documentation at follow-up helped reach consensus in determining the primary endpoint of healing. We explored any potential bias by assessing healing by a panel of wound experts masked to treatment allocation, and we were unable to detect bias in the reading of the imaging. Occasional shortages in dressing materials were infrequent, and we believe that these randomly occurring events did not influence the data, with block randomisation applied in each study site. Most materials could still be purchased on the local market if stock-outs occurred, with the exception of short-stretched bandage.
Although this study is the largest treatment trial for Buruli ulcer disease to date, our study did not reach the initially intended sample size of 332 participants with PCR-confirmed Buruli ulcer. The duration of the study had already been extended twice, and it was decided to stop accrual based on an ad-hoc interim analysis suggesting that extending the study any further would not result in different study outcomes. Since the start of the study, with declining numbers of recorded patients with new Buruli ulcer, the number of patients steadily declined at the study sites, a phenomenon that has been observed in most west African endemic regions.6, 29, 30 The incidence of Buruli ulcer has fluctuated and has recently emerged, unprecedentedly, in Victoria, Australia.4, 5, 8, 9 Another limitation was that we are unable to address the efficacy of RC8 on larger Buruli ulcers (ie, larger than 10 cm), which were excluded.
Several aspects in the experimental design aided in producing a high-quality study in terms of level of care provided for other aspects than the drug treatment alone. These included a focus on wound care, rehabilitation, and pain management during dressing changes. However, despite these measures, the median time to wound healing was similar to that reported in previous studies.14, 15, 19
The strengths of the study were the large sample size, the diagnostic confirmation by validated PCR technology, extended monitoring and auditing, the high healing rate at week 52, the very low drop-out rate, and minimal loss to follow-up. We used internet-based data storage and data collection, and we used a web-based platform for intensive interactions and consultations within the study team. Very few study participants needed any form of surgery. No debridement surgery was done in any of the study participants, and only four patients had skin grafts. This is consistent with findings from a study in Benin addressing the role of surgery in Buruli ulcer disease.31
The RC8 treatment had excellent tolerability and efficacy, and our findings support data from Benin20 and Australia.32, 33 During screening, seven patients were excluded because of pregnancy. Although we counselled study participants to prevent pregnancy during the first 8 weeks of the study, one participant in the RC8 group had a positive pregnancy test at week 6. Although clarithromycin is not listed as safe for use during pregancy, we considered this drug combination relatively safe as it has been used frequently without reported ill effects, and she decided to complete her allocated treatment. Under service conditions, pregnancy tests are not routinely done, and we suspect a considerable risk of intrauterine streptomycin exposure, which would be prevented if oral RC8 treatment becomes the standard of treatment.
In real-world settings, daily drug treatment for 8 weeks in combination with regular wound dressing changes remains challenging in the rural endemic setting in west and central Africa. Not all patients necessarily need a full course of 8 weeks of antimicrobial treatment,34, 35 but how much treatment would suffice is unknown, and individualised treatment duration for Buruli ulcer has not been evaluated in a randomised trial. Drug–drug interactions between rifampicin and clarithromycin result in reduced exposure to clarithromycin within 2–3 weeks, as a result of hepatic enzyme induction by rifampicin,36 and perhaps the most important action of clarithromycin at onset of treatment is the prevention of clonal expansion of M ulcerans with reduced susceptibility to rifampicin.37 This phenomenon, however, appears uncommon,38 but surveillance for rpoB mutations in clinical isolates of M ulcerans would be helpful. Considering that the role of clarithromycin is primarily a companion drug, we suspect that providing this drug as a once-daily extended release preparation had no marked effect on overall efficacy. At the time of designing the study, and based on the limited exposure of the immediate release preparation of clarithromycin 7·5 mg/kg,36 we decided to double the dose in this extended release form.
Experimental treatments with shorter duration are under investigation,39, 40, 41 but novel drugs usually take a considerable time to reach the clinical arena. Our study provides evidence that this combination of oral generic rifampicin and extended release clarithromycin provides excellent tolerability and efficacy.
On the basis of its non-inferiority compared with streptomycin plus rifampicin (RS8), we recommend the combination of oral rifampicin and clarithromycin (RC8) for patients with Buruli ulcer lesions. We only provide evidence for this recommendation for lesions of limited size, in individuals aged 5 years and older. This oral antimicrobial treatment might also be beneficial for patients with larger lesions, and for younger patients.
Data sharing
Individual participant data that underlie the results reported in this Article, after de-identification and study protocol, statistical analysis plan, and analytic code will be made available 1 month after publication of this Article, with no end date, to researchers who provide a methodologically sound proposal (eg, an individual participant data meta-analysis). Proposals should be directed to Tjip S van der Werf (t.s.van.der.werf@umcg.nl) or Ymkje Stienstra (y.stienstra@umcg.nl). Data requestors will need to sign a data access agreement.
Acknowledgments
Acknowledgments
WHO sponsored the study with additional support in cash or kind provided by MAP International, American Leprosy Missions, Fondation Raoul Follereau France, Buruli ulcer Groningen Foundation, Sanofi-Pasteur France, and BuruliVac (EU FP7-241500). Sanofi-Pasteur supplied one of the three study drugs. Buruli ulcer Foundation is a legally recognised foundation situated in the Netherlands, supporting Buruli ulcer research, with volunteers contributing to support individual African researchers, and contributing in cash for supplies and travel expenses. We thank the members of the data safety monitoring board: David Ofori-Adjei, Sarah Eyangoh, Alan Knell, and William Faber. We thank Femke M Homan and Jose C Duipmans for their contribution in scoring and assessing digital images; Sandor Klis, Kristien Velding, and Till F Omansen for their assistance; Ohene Adjei and Joseph Akpaloo, Komfo Anokye Teaching Hospital, for their support; Collins Asare for safe driving; and all study participants for their patience and endurance.
Contributors
ROP, JR, KMA, FSS, GS, AC, EOA, MW-J, JG, JMMcD, TT, PS, AP, LL, MF, AT, S-AO, YS, KBA, and TSvdW contributed to study design. ROP, JR, KMA, WT, TW, GS, TG, AC, EOA, NFS, RS, and TSvdW contributed to GCP training. ROP, JR, KMA, WT, TW, GS, TG, AC, EOA, DA, EM, LG, JMMcD, TT, PS, LL, NFS, RS, AT, S-AO, KBA, and TSvdW contributed to management. FSS, EM, LG, and NFS contributed to laboratory examinations. KMA, TW, GS, TG, AC, RO, MOO, TWE, and MF contributed to data entry. KMA, WT, TW, GS, TG, AC, JMMcD, TT, LL, and MF contributed to wound care. WT, PS, AP, LL, and MF contributed to rehabilitation. NFS and TSvdW contributed to study monitoring. ROP, JR, KMA, WT, FSS, TW, GS, TG, AC, RO, MOO, EOA, DA, JMMcD, TT, PS, AP, LL, MF, NFS, RS, AT, S-AO, KBA, and TSvdW contributed to auditing. ROP, JR, RO, MOO, TWE, YS, and TSvdW contributed to data analysis. ROP, JR, KBA, YS, and TSvdW contributed to writing. All authors read and approved the final version of the manuscript.
Additional members of Buruli ulcer clinical trial study team
Samuel Osei Mireku, Justice Abotsi, Joseph Ken Adu Poku, Richard Asamoah-Frimpong, Bright Osei-Wusu, Edward Sarpong (Agogo Presbyterian Hospital, Agogo, Ghana); Beatrice Konadu, Ernest Opoku (Nkawie-Toase Hospital, Nkawie, Ghana); Mark Forson, Mathias Ndogyele, Elizabeth Ofori, Felicity Aboagye, Thomas Berko (Tepa Government Hospital, Tepa, Ghana); Godfred Kwabena Sarpong, George Amofa, Anastasia Nsiah, Joyce Mensah-Bonsu (Dunkwa Government Hospital, Dunkwa-On-Offin, Ghana); Joseph Ofori Nyarko, Yaw Ampem Amoako, Elliot Koranteng Tannor, Justice Boakye-Appiah (Komfo Anokye Teaching Hospital, Kumasi, Ghana); Aloysius Dzibordzi Loglo, Mabel Sarpong-Duah, Bernadette Agbavor (Kumasi Centre for Collaborative Research, Kumasi, Ghana); Marie Françoise Ardent, Arnaud Yamadjako, Naomi Adanmado Gersande, Ambroise Adeye, Martial Kindjinou, Akpolan, Maxime Kiki, Espoir Sodjinou, Clémence Guegnard (Centre de diagnostic et de traitement de la lèpre et de l’Ulcère de Buruli Madeleine et Raoul Follereau, Ouémé-Plateau, Pobè, Bénin).
Declaration of interests
We declare no competing interests.
Contributor Information
Tjip S van der Werf, Email: t.s.van.der.werf@umcg.nl.
study team:
Samuel Osei Mireku, Justice Abotsi, Joseph Ken Adu Poku, Richard Asamoah-Frimpong, Bright Osei-Wusu, Edward Sarpong, Beatrice Konadu, Ernest Opoku, Mark Forson, Mathias Ndogyele, Elizabeth Ofori, Felicity Aboagye, Thomas Berko, George Amofa, Anastasia Nsiah, Joyce Mensah-Bonsu, Joseph Ofori Nyarko, Yaw Ampem Amoako, Elliot Koranteng Tannor, Justice Boakye-Appiah, Aloysius Dzibordzi Loglo, Mabel Sarpong-Duah, Bernadette Agbavor, Marie Françoise Ardent, Arnaud Yamadjako, Naomi Adanmado Gersande, Ambroise Adeye, Martial Kindjinou, Akpolan, Maxime Kiki, Espoir Sodjinou, Clémence Guegnard, Sandor-Adrian Klis, Kristien Velding, Till Omansen, David Ofori-Adjei, Sarah Eyangoh, Alan Knell, and William Faber
Supplementary Material
References
- 1.WHO Neglected tropical diseases. 2020. https://www.who.int/neglected_diseases/diseases/en/ (accessed Sept 17, 2019).
- 2.WHO Buruli ulcer. 2020. https://www.who.int/health-topics/buruli-ulcer#tab=tab_1 (accessed Sept 17, 2019).
- 3.WHO . Fourth WHO report in neglected tropical disease; integrating NTD into global health and development. World Health Organization; Geneva: 2017. https://apps.who.int/iris/bitstream/handle/10665/255011/9789241565448-eng.pdf [Google Scholar]
- 4.Simpson H, Deribe K, Tabah EN. Mapping the global distribution of Buruli ulcer: a systematic review with evidence consensus. Lancet Glob Health. 2019;7:e912–e922. doi: 10.1016/S2214-109X(19)30171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson PDR. Buruli ulcer: here today but where tomorrow? Lancet Glob Health. 2019;7:e821–e822. doi: 10.1016/S2214-109X(19)30233-5. [DOI] [PubMed] [Google Scholar]
- 6.Yeboah-Manu D, Aboagye SY, Asare P. Laboratory confirmation of Buruli ulcer cases in Ghana, 2008–2016. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeres DT, Horstman J, van der Tak P. The paediatric participation scale measuring participation restrictions among former Buruli ulcer patients under the age of 15 in Ghana and Benin: development and first validation results. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tai AYC, Athan E, Friedman ND, Hughes A, Walton A, O’Brien DP. Increased severity and spread of Mycobacterium ulcerans, southeastern Australia. Emerg Infect Dis. 2018;24:58–64. doi: 10.3201/eid2401.171070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loftus MJ, Tay E-L, Globan M. Epidemiology of Buruli ulcer infections, Victoria, Australia, 2011–2016. Emerg Infect Dis. 2018;24:1988–1997. doi: 10.3201/eid2411.171593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etuaful S, Carbonnelle B, Grosset J. Efficacy of the combination rifampin-streptomycin in preventing growth of Mycobacterium ulcerans in early lesions of Buruli ulcer in humans. Antimicrob Agents Chemother. 2005;49:3182–3186. doi: 10.1128/AAC.49.8.3182-3186.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stienstra Y, van der Graaf WTA, Asamoa K, van der Werf TS. Beliefs and attitudes toward Buruli ulcer in Ghana. Am J Trop Med Hyg. 2002;67:207–213. doi: 10.4269/ajtmh.2002.67.207. [DOI] [PubMed] [Google Scholar]
- 12.Stienstra Y, van Roest MHG, van Wezel MJ. Factors associated with functional limitations and subsequent employment or schooling in Buruli ulcer patients. Trop Med Int Health. 2005;10:1251–1257. doi: 10.1111/j.1365-3156.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 13.Vincent QB, Ardant M-F, Adeye A. Clinical epidemiology of laboratory-confirmed Buruli ulcer in Benin: a cohort study. Lancet Glob Health. 2014;2:e422–e430. doi: 10.1016/S2214-109X(14)70223-2. [DOI] [PubMed] [Google Scholar]
- 14.Sarfo FS, Phillips R, Asiedu K. Clinical efficacy of combination of rifampin and streptomycin for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother. 2010;54:3678–3685. doi: 10.1128/AAC.00299-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nienhuis WA, Stienstra Y, Thompson WA. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet. 2010;375:664–672. doi: 10.1016/S0140-6736(09)61962-0. [DOI] [PubMed] [Google Scholar]
- 16.Klis S, Stienstra Y, Phillips RO, Abass KM, Tuah W, van der Werf TS. Long term streptomycin toxicity in the treatment of Buruli ulcer: follow-up of participants in the BURULICO drug trial. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji B, Chauffour A, Robert J, Lefrançois S, Jarlier V. Orally administered combined regimens for treatment of Mycobacterium ulcerans infection in mice. Antimicrob Agents Chemother. 2007;51:3737–3739. doi: 10.1128/AAC.00730-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almeida D, Converse PJ, Ahmad Z, Dooley KE, Nuermberger EL, Grosset JH. Activities of rifampin, rifapentine and clarithromycin alone and in combination against Mycobacterium ulcerans disease in mice. PLoS Negl Trop Dis. 2011;5 doi: 10.1371/journal.pntd.0000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips RO, Sarfo FS, Abass MK. Clinical and bacteriological efficacy of rifampin-streptomycin combination for two weeks followed by rifampin and clarithromycin for six weeks for treatment of Mycobacterium ulcerans disease. Antimicrob Agents Chemother. 2014;58:1161–1166. doi: 10.1128/AAC.02165-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauty A, Ardant M-F, Marsollier L. Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in Benin. Clin Infect Dis. 2011;52:94–96. doi: 10.1093/cid/ciq072. [DOI] [PubMed] [Google Scholar]
- 21.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7 doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO Facilities involved in Buruli ulcer clinical trial 2012–2015 in Ghana and Benin. 2012. http://gamapserver.who.int/mapLibrary/Files/Maps/Ghana_Benin_BuruliUlcer_clinical_2012_2015.png
- 23.Abass KM, van der Werf TS, Phillips RO. Buruli ulcer control in a highly endemic district in Ghana: role of community-based surveillance volunteers. Am J Trop Med Hyg. 2015;92:115–117. doi: 10.4269/ajtmh.14-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barogui YT, Sopoh GE, Johnson RC. Contribution of the community health volunteers in the control of Buruli ulcer in Bénin. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbinger K-H, Adjei O, Awua-Boateng N-Y. Comparative study of the sensitivity of different diagnostic methods for the laboratory diagnosis of Buruli ulcer disease. Clin Infect Dis. 2009;48:1055–1064. doi: 10.1086/597398. [DOI] [PubMed] [Google Scholar]
- 26.Eddyani M, Lavender C, de Rijk WB. Multicenter external quality assessment program for PCR detection of Mycobacterium ulcerans in clinical and environmental specimens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nienhuis WA, Stienstra Y, Abass KM. Paradoxical responses after start of antimicrobial treatment in Mycobacterium ulcerans infection. Clin Infect Dis. 2012;54:519–526. doi: 10.1093/cid/cir856. [DOI] [PubMed] [Google Scholar]
- 28.Velink A, Woolley RJ, Phillips RO. Former buruli ulcer patients’ experiences and wishes may serve as a guide to further improve buruli ulcer management. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ampah KA, Asare P, Binnah DD-G. Burden and historical trend of buruli ulcer prevalence in selected communities along the Offin River of Ghana. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anagonou EG, Johnson RC, Barogui YT. Decrease in Mycobacterium ulcerans disease (Buruli ulcer) in the Lalo District of Bénin (west Africa) BMC Infect Dis. 2019;19:247. doi: 10.1186/s12879-019-3845-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadagni AC, Barogui YT, Johnson RC. Delayed versus standard assessment for excision surgery in patients with Buruli ulcer in Benin: a randomised controlled trial. Lancet Infect Dis. 2018;18:650–656. doi: 10.1016/S1473-3099(18)30160-9. [DOI] [PubMed] [Google Scholar]
- 32.Friedman ND, Athan E, Walton AL, O’Brien DP. Increasing experience with primary oral medical therapy for Mycobacterium ulcerans disease in an Australian cohort. Antimicrob Agents Chemother. 2016;60:2692–2695. doi: 10.1128/AAC.02853-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien DP, Friedman D, Hughes A, Walton A, Athan E. Antibiotic complications during the treatment of Mycobacterium ulcerans disease in Australian patients. Intern Med J. 2017;47:1011–1019. doi: 10.1111/imj.13511. [DOI] [PubMed] [Google Scholar]
- 34.Klis S, Kingma RA, Tuah W, van der Werf TS, Stienstra Y. Clinical outcomes of Ghanaian Buruli ulcer patients who defaulted from antimicrobial therapy. Trop Med Int Health. 2016;21:1191–1196. doi: 10.1111/tmi.12745. [DOI] [PubMed] [Google Scholar]
- 35.O’Brien DP, Friedman ND, Cowan R, Walton A, Athan E. Six versus eight weeks of antibiotics for small Mycobacterium ulcerans lesions in Australian patients. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz532. published online June 20. [DOI] [PubMed] [Google Scholar]
- 36.Alffenaar JWC, Nienhuis WA, de Velde F. Pharmacokinetics of rifampin and clarithromycin in patients treated for Mycobacterium ulcerans infection. Antimicrob Agents Chemother. 2010;54:3878–3883. doi: 10.1128/AAC.00099-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsollier L, Honoré N, Legras P. Isolation of three Mycobacterium ulcerans strains resistant to rifampin after experimental chemotherapy of mice. Antimicrob Agents Chemother. 2003;47:1228–1232. doi: 10.1128/AAC.47.4.1228-1232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansson M, Beissner M, Phillips RO. Comparison of two assays for molecular determination of rifampin resistance in clinical samples from patients with Buruli ulcer disease. J Clin Microbiol. 2014;52:1246–1249. doi: 10.1128/JCM.03119-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Converse PJ, Almeida DV, Tasneen R. Shorter-course treatment for Mycobacterium ulcerans disease with high-dose rifamycins and clofazimine in a mouse model of Buruli ulcer. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Converse PJ, Almeida DV, Tyagi S, Xu J, Nuermberger EL. Shortening buruli ulcer treatment with combination therapy targeting the respiratory chain and exploiting Mycobacterium ulcerans gene decay. Antimicrob Agents Chemother. 2019;63:e00426–e00519. doi: 10.1128/AAC.00426-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scherr N, Bieri R, Thomas SS. Targeting the Mycobacterium ulcerans cytochrome bc1:aa3 for the treatment of Buruli ulcer. Nat Commun. 2018;9:5370–5379. doi: 10.1038/s41467-018-07804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual participant data that underlie the results reported in this Article, after de-identification and study protocol, statistical analysis plan, and analytic code will be made available 1 month after publication of this Article, with no end date, to researchers who provide a methodologically sound proposal (eg, an individual participant data meta-analysis). Proposals should be directed to Tjip S van der Werf (t.s.van.der.werf@umcg.nl) or Ymkje Stienstra (y.stienstra@umcg.nl). Data requestors will need to sign a data access agreement.