Abstract
Hemocytes, the immune cells in mosquitoes, participate in immune defenses against pathogens including malaria parasites. Mosquito hemocytes can also be infected by arthropod-borne viruses but the pro- or anti-viral nature of this interaction is unknown. Although there has been progress on hemocyte characterization during pathogen infection in mosquitoes, the specific contribution of hemocytes to immune responses and the hemocyte-specific functions of immune genes and pathways remain unresolved due to the lack of genetic tools to manipulate gene expression in these cells specifically. Here, we used the Gal4-UAS system to characterize the activity of the Drosophila hemocyte-specific hemolectin promoter in the adults of Anopheles gambiae, the malaria mosquito. We established an hml-Gal4 driver line that we further crossed to a fluorescent UAS responder line, and examined the expression pattern in the adult progeny driven by the hml promoter. We show that the hml regulatory region drives hemocyte-specific transgene expression in a subset of hemocytes, and that transgene expression is triggered after a blood meal. The hml promoter drives transgene expression in differentiating prohemocytes as well as in differentiated granulocytes. Analysis of different immune markers in hemocytes in which the hml promoter drives transgene expression revealed that this regulatory region could be used to study phagocytosis as well as melanization. Finally, the hml promoter drives transgene expression in hemocytes in which o'nyong-nyong virus replicates. Altogether, the Drosophila hml promoter constitutes a good tool to drive transgene expression in hemocyte only and to analyze the function of these cells and the genes they express during pathogen infection in Anopheles gambiae.
Keywords: Mosquito, Anopheles, Hemocytes, Immunity, Gal4-UAS system, Hemolectin, Granulocytes, Phagocytosis, Melanization, Arbovirus
Graphical abstract
Highlights
-
•
Characterization of the Drosophila hemolectin promoter in Anopheles gambiae.
-
•
A hemolectin-Gal4 line drives hemocyte-specific transgene expression in mosquitoes.
-
•
Hemocyte-specific transgene expression is induced after a blood meal.
-
•
Hemolectin-Gal4 can be used to analyze granulocytes, phagocytosis, melanization.
-
•
Hemolectin-Gal4 can be used to analyze arbovirus-hemocyte interactions.
1. Introduction
The mosquito Anopheles gambiae is the main African vector of Plasmodium falciparum, responsible for the severe forms of human malaria, which caused about 405 000 deaths in 2018 (WHO, 2019). In the absence of an efficient vaccine, control of mosquito vectors remains the most important tool to fight malaria. Indeed, malaria vector control interventions based on insecticide-treated bed nets and indoor residual spraying were responsible for 78% of the decline in malaria cases between 2000 and 2015 (Bhatt et al., 2015). However, these advances are hampered by the selection of insecticide resistance by mosquitoes (Churcher et al., 2016; Ranson and Lissenden, 2016) and changes in their behaviour with increased outdoor biting activity (Moiroux et al., 2012; Thomsen et al., 2017). The failure of existing methods for eradicating malaria as well as advances in the field of vector genomics and genetics including mosquito genome editing with notably the recent advent of CRISPR-Cas9 gene drive technology in mosquitoes have sparked interest in genetic vector control strategies, including replacing wild populations with malaria refractory mosquitoes (McLean and Jacobs-Lorena, 2016; Macias et al., 2017). This latter approach consists of introducing genes into the mosquito genome so the mosquitoes become resistant to parasite infection thereby blocking malaria transmission. To this aim, the identification of promoters for directing transgene expression is crucial as transgene expression should be limited to a specific tissue and time in the mosquito to achieve maximum effect during pathogen exposure while minimizing the fitness cost on the vector (Terenius et al., 2008; Macias et al., 2017). Importantly, effector genes conferring resistance to the parasite must also be identified. As mosquito immunity influences mosquito vector competence for malaria parasites (Clayton et al., 2014; Saraiva et al., 2016; Bartholomay and Michel, 2018; Kumar et al., 2018), i.e. the ability to become infected following an infectious blood meal and to subsequently transmit the parasite, some of the proposed antimalarial strategies could be based on amplifying mosquito immune defenses.
Mosquito innate immunity is principally composed of two arms: i/the humoral immune response including a complement-like system as well as the production of antimicrobial peptides (AMPs) and ii/a cellular immune response mediated by hemocytes, the immune cells of insects (Clayton et al., 2014; Saraiva et al., 2016; Bartholomay and Michel, 2018; Kumar et al., 2018). Mosquito hemocytes, which circulate in the hemolymph or are attached to tissues (sessile hemocytes), participate directly in immune defenses by phagocytizing pathogens in the hemolymph, or indirectly by secreting effector molecules mediating pathogen killing via lytic and melanization pathways (Blandin and Levashina, 2007; Hillyer, 2010; Hillyer and Strand, 2014; Bartholomay and Michel, 2018). In An. gambiae adults, three different types of hemocytes have been described based upon their morphology, granulocytes, oenocytoids and prohemocytes (Castillo et al., 2006). Granulocytes, which are the most abundant cell type, are large cells with numerous granules in the cytoplasm. They are capable of phagocytosis and they can bind and spread on solid surfaces, forming filopodia and focal adhesions. Oenocytoids measure approximatively 9 μm in diameter with a homogeneous cytoplasm. They show phenoloxidase activity (involved in melanization) and are non-phagocytic. Prohemocytes are round and small cells of approximatively 4–6 μm with a high nuclear to cytoplasm ratio and are thought to be the putative hemocyte progenitor cells with the capacity to differentiate into other cell types. In mosquitoes, little is known about the origin of adult hemocytes. In Drosophila, adult hemocytes consist of embryonic hemocytes and larval hemocytes, which ones are released from the lymph glands at the onset of metamorphosis (Holz et al., 2003). Whether adult flies do have or not a hematopoietic organ is an ongoing debate with a recent study showing that they possess active hematopoietic sites in the abdomen (Ghosh et al., 2015) while another study did not find an increase in hemocyte numbers during adult life (Bosch et al., 2019). Although no hematopoietic organ has yet been identified in mosquitoes, hemocyte numbers increase after a blood meal (Castillo et al., 2011; Bryant and Michel 2014, 2016) and upon infection with bacteria or parasites (King and Hillyer 2012, 2013; Ramirez et al., 2014). This increase in hemocyte number is thought to occur by mitosis of circulating hemocytes (King and Hillyer, 2013; Bryant and Michel, 2014).
In An. gambiae adult females, bacterial or parasite challenges also trigger hemocyte differentiation (King and Hillyer, 2012, 2013; Ramirez et al., 2014) and this is associated with differences in their gene and protein expression (Baton et al., 2009; Pinto et al., 2009; Smith et al., 2016). Moreover, hemocytes are involved in phagocytosis (Moita et al., 2005; King and Hillyer, 2012, 2013; Lombardo et al., 2013) and in immune responses against malaria parasites (Pinto et al., 2009; Ramirez et al., 2014; Smith et al., 2015; Lombardo and Christophides, 2016; Smith et al., 2016). Immune cell differentiation has also been implicated in innate immune memory (Rodrigues et al., 2010; Ramirez et al., 2014; Ramirez et al., 2015; Barletta et al., 2019). On the other hand, hemocytes can be infected by arthropod-borne virus (arbovirus) such as Sindbis virus in Aedes mosquitoes (Parikh et al., 2009) and o'nyong-nyong virus (ONNV) in An. gambiae (Carissimo et al., 2015) but the pro- or anti-viral nature of this interaction is largely unknown. Although there has been progress on hemocyte characterization during pathogen infections in mosquitoes thanks to the rise of omics approaches and RNA interference-based gene silencing, their specific contribution to immune responses remains unclear. Recently, the role of granulocytes in anti-bacterial and anti-plasmodial immune responses has been elegantly demonstrated by chemical depletion of phagocytic cells (Kwon and Smith, 2019). However, the hemocyte-specific functions of immune genes and pathways in mosquitoes are still unresolved due to the lack of genetic tools to manipulate gene expression in these cells specifically.
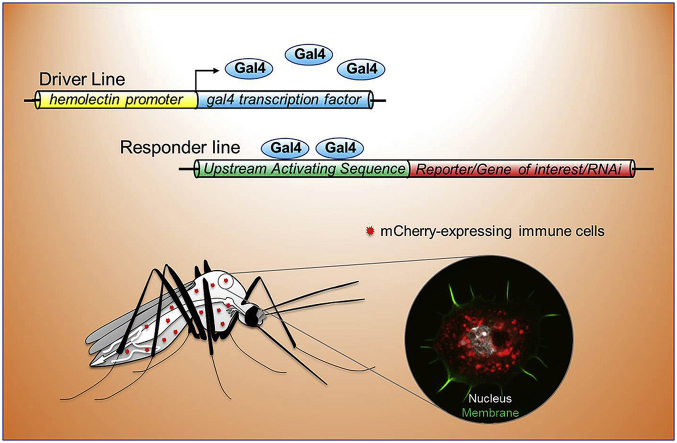
The Gal4-UAS system provides a perfect tool to characterize the expression pattern of promoters but also to study gene function at the tissue level. Indeed, this system (and its numerous extensions), established in the fly Drosophila melanogaster by Brand and Perrimon, (1993) and routinely used since, has proven to be one of the most powerful techniques for addressing gene function in vivo (Duffy, 2002; Southall et al., 2008). This system relies on two components: Gal4, a transcriptional activator from yeast, which is expressed in a spatio-temporal manner guided by the promoter placed upstream, and a transgene under the control of the upstream activating sequence (UAS) activated through Gal4 binding. The two components can be brought together using simple genetic crosses. This system is highly flexible, providing a versatile tool for controlling ectopic expression both spatially and temporally. Indeed, the expression of Gal4 can be controlled in a spatial and temporal manner using specific promoters, thereby dictating where and when the UAS-transgene is expressed. A key advantage of the system is the separation of the two components in different transgenic parental lines, which ensure that the transgene is silent until Gal4 is introduced in a genetic cross allowing transgenic lines encoding toxic or lethal proteins to be engineered. Moreover, a single UAS-transgene can be analyzed using different Gal4 drivers and different UAS-transgenes can be analyzed in the same tissue using a single Gal4 line, avoiding the creation of a new line for each promoter-gene combination. The Gal4-UAS system has since been developed in some model and pest species including Arabidopsis (Guyer et al., 1998), Zebrafish (Scheer and Campos-Ortega, 1999), Xenopus (Hartley et al., 2002), Bombyx (Imamura et al., 2003) and Tribolium (Schinko et al., 2010). The Gal4-UAS system has been shown to be also functional in mosquitoes. Gal4 lines allowing specific expression in the midgut and in the fat body have been established in the arbovirus vector Aedes aegypti (Kokoza and Raikhel, 2011; Zhao et al., 2014; Zhao et al., 2016). In An. gambiae, functional Gal4 lines have been established to drive transgene expression specifically in the midgut, in oenocytes or ubiquitously (Lynd and Lycett, 2012; Adolfi et al., 2018; Lynd et al., 2019). While hemocytes are important immune effectors, to date no hemocyte-specific Gal4 line has been established in mosquitoes. To develop such genetic tools, regulatory sequences need to be identified to drive gene expression in these cells only.
In Drosophila, hemocyte-specific transgene expression can be achieved using the hemolectin (hml) gene promoter (Goto et al., 2003). Hml is a large protein similar to von Willebrand factor (Goto et al., 2001) which is produced by differentiated hemocytes only and is involved in coagulation and immunity against bacteria (Goto et al., 2003; Lesch et al., 2007). Although different promoters can be used to drive gene expression in hemocytes in Drosophila, the hml promoter has the advantage that it drives expression only late during development (Goto et al., 2001; Goto et al., 2003; Shia et al., 2009) and genetic ablation of hemocytes driven by the hml promoter does not impede normal development (Shia et al., 2009). Therefore, this promoter can be used to study gene function in mature hemocytes without the interference of indirect developmental effects. Here, we used the Gal4-UAS system to characterize the activity of the Drosophila hml promoter in An. gambiae adults. We established an hml-Gal4 driver line that was further crossed to a fluorescent UAS responder line we established previously (Adolfi et al., 2018), and examined the expression pattern in the adult progeny driven by the hml promoter. We show that the hml regulatory region drives hemocyte-specific transgene expression in a subset of hemocytes, and that transgene expression is triggered after a blood meal. Hml drives transgene expression in differentiating prohemocytes as well as in differentiated granulocytes. Analysis of different immune markers in hemocytes in which the hml promoter drives transgene expression revealed that this regulatory region could be used to study phagocytosis as well as melanization. Finally, the hml promoter drives transgene expression in hemocytes in which ONNV replicates. Therefore, this regulatory region could also be used to study the function of immune cells during an arboviral infection. Altogether, the hml promoter constitutes a good promoter to drive transgene expression in hemocytes only in order to analyze the function of these cells and the genes they express.
2. Results
2.1. Characterization of the expression pattern driven by the Drosophila hml promoter
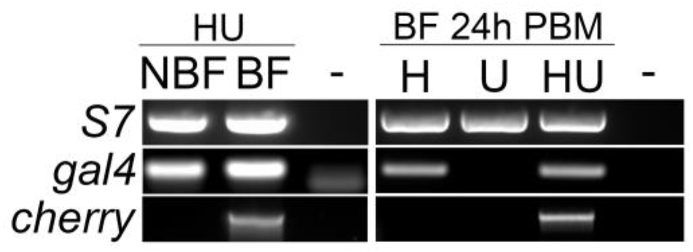
The hml-Gal4 line was crossed with a fluorescent responder line UAS-mCD8::Cherry line we established previously (Adolfi et al., 2018), to characterize the expression pattern driven by the hml regulatory sequence in the adult progeny, referred as hml > mCD8::Cherry. To collect hemocytes, hemolymph was perfused from non-blood fed (NBF) females and blood fed (BF) females 24 h post blood meal (PBM). Expression of cherry in hemocytes was analyzed by RT-PCR and by imaging. By RT-PCR, gal4 was detected in hemocytes from both NBF and BF females, however cherry could only be detected in hemocytes from BF females (Fig. 1, left panel). No cherry expression was detected in hemocytes collected from BF females from the control progeny of the crosses between the hml-Gal4 line or the UAS-mCD8::Cherry line to the E docking line (Fig. 1, right panel), showing no leakage of the UAS construct.
Fig. 1.
RT-PCR analysis of gal4 and cherry expression in perfused hemocytes. Left panel: Expression of gal4 and cherry in perfused hemocytes from non-blood fed (NBF) and blood fed (BF) females 24 h post blood meal (PBM) issued from the cross between hml-Gal4 and UAS-mCD8::Cherry lines (HU females). Right panel: Expression of gal4 and cherry in perfused hemocytes from BF females 24 h PBM issued from the cross between hml-Gal4 and UAS-mCD8::Cherry lines (HU females) and from the control progeny of the crosses between the hml-Gal4 line or the UAS-mCD8::Cherry line to the E docking line, referred as H and U females. S7 is used as a standard gene. -, water negative PCR control.
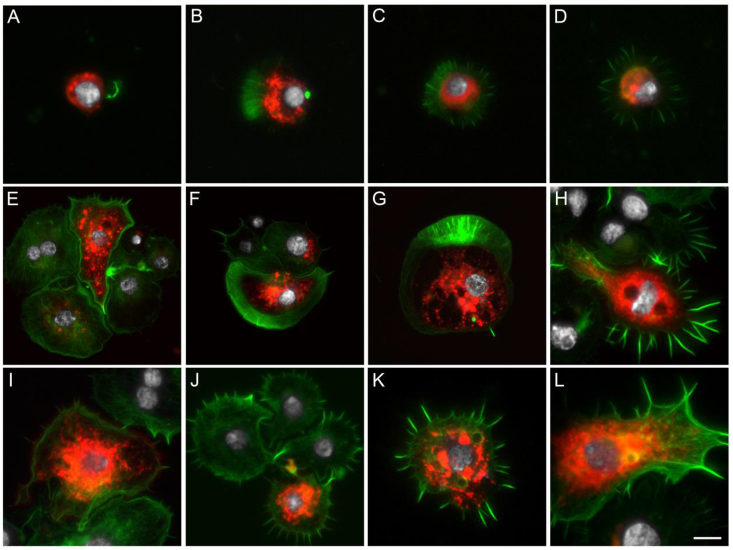
Microscopy analyses confirmed the RT-PCR results. While we observed endogenous Cherry fluorescence in hemocytes collected from hml > mCD8::Cherry females 24h PBM (Fig. 2), no Cherry fluorescence was detected in hemocytes perfused from the control progeny of the crosses between the hml-Gal4 line or the UAS-mCD8::Cherry line to the E docking line 24h PBM (data not shown). Perfused granulocytes can rapidly bind to the glass surface, start to enlarge and develop filopodia. Therefore, perfused hemocytes from BF females were fixed either 10 or 45 min after perfusion to analyze Cherry fluorescence at different hemocyte activation stages, e.g. small and round granulocytes as well as large granulocytes. The hemolymph fixed 10 min after perfusion contained a large proportion of small hemocytes (cell diameter of 5–6 μm) with a high nuclear to cytoplasm ratio and starting to produce filopodia (Fig. 2A) as well as relatively small granulocytes with filopodia (Fig. 2B–D). As no differentiation marker was used, it could not be determined whether the smaller hemocytes were prohemocytes starting to differentiate into granulocytes or already differentiated granulocytes. After 45 min on the slide, no small hemocytes could be observed and most hemocytes were large granulocytes, some of which measured up to 40 μm (Fig. 2E-L). Probably due to their lack of adhesion (Castillo et al., 2006), only few oenocytoids were observed and none of them displayed Cherry fluorescence. Cherry was present in both small and large granulocyte cell populations, although not in all cells from each population suggesting that different cell lineages exist with similar morphology (Fig. 2). Rarely, we also observed some giant rounded hemocytes expressing Cherry (Fig. 2F and G), which could correspond to the “rounded” hemocytes described by King and Hillyer, (2013). Interestingly, some large granulocytes contained two nuclei (e.g. Fig. 2E and H), which are likely hemocytes in mitosis as previously reported (King and Hillyer, 2013).
Fig. 2.
Microscopy analysis of hemocytes collected from hml > mCD8::Cherry blood fed females. Hemocytes expressing mCD8::Cherry under the control of hml promoter. (A–D) Hemocytes fixed 10 min post perfusion, (E–L) Hemocytes fixed 45 min post perfusion. Red: endogenous mCD8::Cherry; Green: Phalloidin; White: DAPI staining. Scale bar is 5 μm except for E, F and G where it is 10 μm.
In hemocytes expressing Cherry, the fluorescent protein was localized heterogeneously in the cytoplasm, most often in granules and vesicles. In the UAS responder line, Cherry is fused with mCD8, a transmembrane protein used as a cell membrane marker. Therefore, structures revealed by the membrane-bound Cherry are likely to be intracellular organelles such as endocytic vesicles. The cell surface staining around the filopodia of granulocytes was visible when using high levels of exposure for picture acquisition (Figure S1A and B). Anti-mCD8 antibody staining also confirmed that mCD8 and Cherry were exclusively expressed in the same hemocytes (Figure S1C and D). Cherry and mCD8 did not always colocalize within positive cells since we could observe Cherry but no mCD8 signal or mCD8 but no Cherry signal likely due to a cleavage of the fusion protein.
By imaging, very few hemocytes expressing Cherry were seen in perfusions either from NBF hml > mCD8::Cherry females, confirming the RT-PCR results (Fig. 1), or from hml > mCD8::Cherry males (data not shown) compared to BF hml > mCD8::Cherry females. In BF hml > mCD8::Cherry females, expression of the transgene under the hml promoter was activated after the blood meal, with a peak of positive hemocytes around 24–30h PBM and a decline to basal levels around 48–72h PBM. The proportion of perfused hemocytes which were positive varied greatly between experiments ranging roughly from 5 to 20% of total hemocytes from BF females. In rare cases and independently of the experimental context (pathogen or not), up to 60% of perfused hemocytes expressed the transgene. Some hemocytes also expressed low levels of Cherry and were only visible by microscopy when using high exposure levels. It is therefore possible that Cherry-positive hemocytes have been underestimated in some cases when observing the slides with the microscope objectives (e.g. Figure S2). As groups of 10 females were perfused per well, we wondered if the mix of Cherry-expressing and non-expressing hemocytes could be due to individual variation of transgene expression in hemocytes under the hml regulatory region. While there was variation in the proportion of Cherry-expressing hemocytes between perfusions from different individuals, every individual BF female contained both Cherry positive and negative hemocyte populations (data not shown).
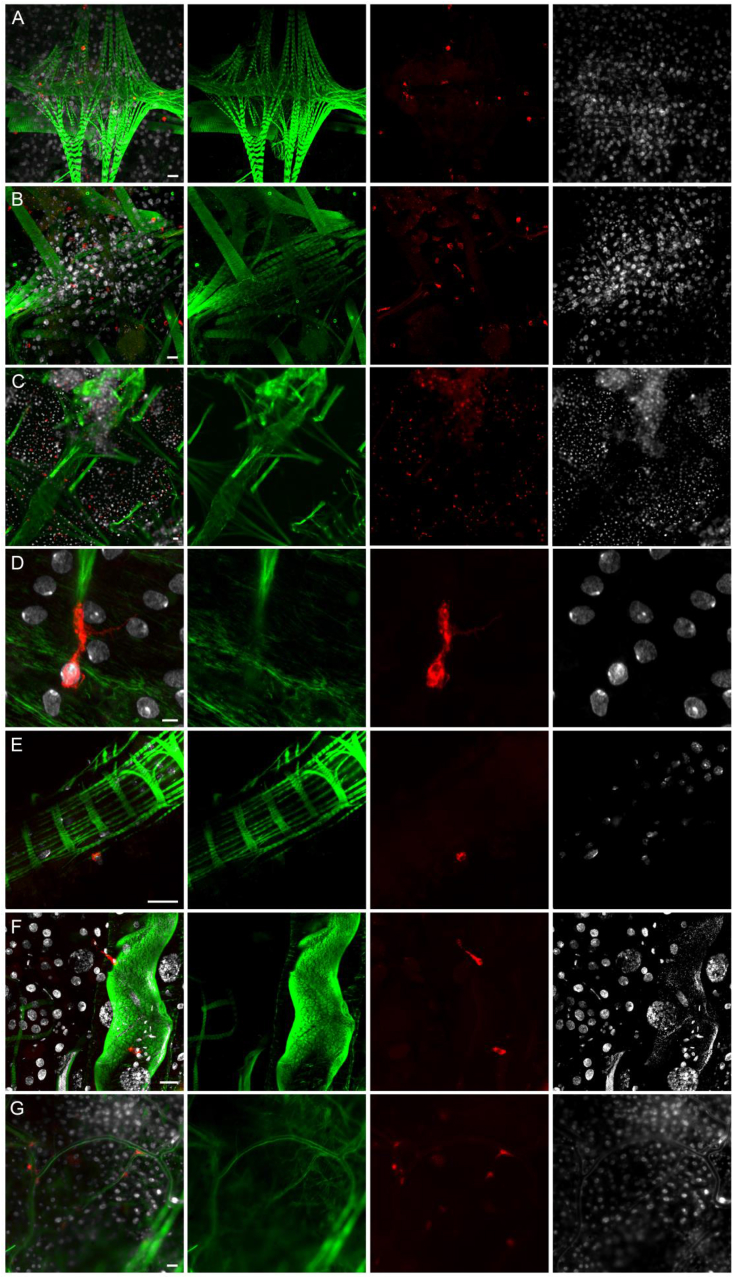
In BF hml > mCD8::Cherry females at 24h PBM, Cherry-expressing hemocytes were also found attached to tissues, mostly close to the heart, abdominal muscles and epidermis (Fig. 3A–C). These sessile hemocytes were differentiated with long filopodia which can be visualized with the mCD8::Cherry (Fig. 3D). A few Cherry-positive hemocytes were also found attached to the midgut and the Malpighian tubules (Fig. 3E and F). Cherry-expressing hemocytes were also found in close contact with tracheae (Fig. 3G). As observed with hemolymph samples, the number of hemocytes expressing Cherry was variable between individuals with some showing a large number of positive hemocytes in rare cases (Fig. 3C). Further analysis of the hml > mCD8::Cherry females tissues did not reveal other cells/tissues other than hemocytes expressing hml-driven Cherry fluorescence. Consistent with the perfusion results, very few hemocytes expressing Cherry were seen in the tissues from either NBF hml > mCD8::Cherry females or hml > mCD8::Cherry males compared to BF hml > mCD8::Cherry females and none in hml-Gal4 and UAS-mCD8::Cherry control females 24h PBM (data not shown).
Fig. 3.
Microscopy analysis of tissues from hml > mCD8::Cherry blood fed females. Hemocytes expressing the mCD8::Cherry under the control of the hml promoter. (A,B,C) Abdominal muscles and epidermis, including the heart, (D) Enlargement of a hemocyte attached to a muscle fiber, (E) Hemocyte attached to the anterior midgut, (F) Hemocytes attached to Malpighian tubule, (G) Hemocytes in close contact to tracheae. Red: endogenous mCD8::Cherry; Green: Phalloidin; White: DAPI staining. Scale bar is 20 μm except for D where it is 5 μm.
2.2. Characterization of hml-driven cherry-expressing hemocytes
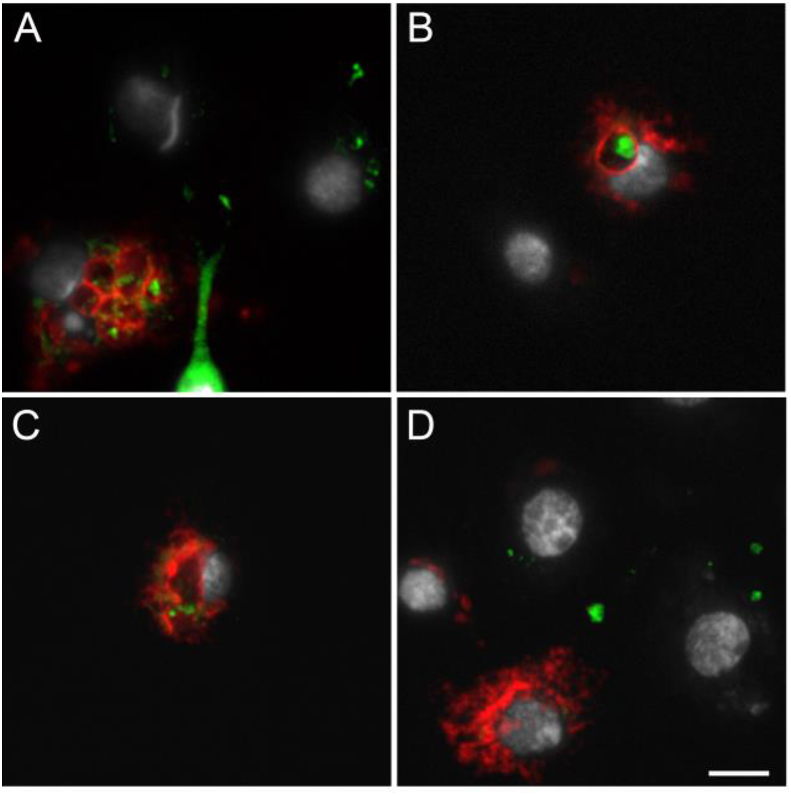
To better characterize the hemocytes in which the hml promoter drives transgene expression, we then analyzed different immune markers in hemocytes from hml > mCD8::Cherry females. Granulocytes can phagocytose bacteria but also microspheres (Hillyer et al., 2003; King and Hillyer, 2012). Therefore, hemocytes were perfused from hml > mCD8::Cherry females previously injected either with fluorescent microspheres (Fig. 4A and B) or with a GFP-expressing Escherichia coli strain. Microscopy analysis showed that granulocytes expressing Cherry, as well as granulocytes which do not, were able to phagocytose bacteria and microspheres (Fig. 4C and D). We also stained hemocytes perfused from BF hml > mCD8::Cherry females with an antibody against the thioester containing protein 1 (TEP1). TEP1 is a homolog of the mammalian C3 complement factor secreted in the hemolymph where it can bind to malaria parasites, leading to their elimination through lysis and melanization (Levashina et al., 2001; Blandin et al., 2004). In case of a bacterial challenge, TEP1 protein binds to bacteria and is taken up by hemocytes, most likely through phagocytosis of TEP1-tagged bacteria (Levashina et al., 2001; Moita et al., 2005; Volohonsky et al., 2017). We detected TEP1 in the cytoplasm of some granulocytes, expressing Cherry or not (Fig. 5). In granulocytes in which the hml promoter drives expression of the fluorescent protein, the mCD8 membrane marker revealed that TEP1 was localized in vesicles very similar to phagosomes described in Drosophila hemocytes (Shandala et al., 2013). Phagosomes are intracellular vesicles formed after internalization of extracellular material by invagination of the plasma membrane and they interact with endosomes and lysosomes to form acidic phagolysosomes, which degrade pathogens. These phagosome-like structures were also observed in other perfusion experiments (e.g. Fig. 6). Although we did not expose the females to a specific pathogen, mosquitoes were not reared in sterile conditions and the TEP1 staining in hemocytes could be due to phagocytosis of bacteria.
Fig. 4.
Hemocytes collected from hml > mCD8::Cherry females injected with fluorescent beads or bacteria. (A) hml > mCD8::Cherry control female showing eYFP expression in the eyes under the 3P3 promoter, (B) hml > mCD8::Cherry female injected with fluorescent beads, (C) Hemocytes collected from hml > mCD8::Cherry females injected with fluorescent beads, (D) Hemocytes collected from hml > mCD8::Cherry females injected with GFP-tagged Escherichia coli. Red: endogenous mCD8::Cherry; Green: fluospheres in B and C/Escherichia coli bacteria in D; White: DAPI staining. Scale bar is 5 μm.
Fig. 5.
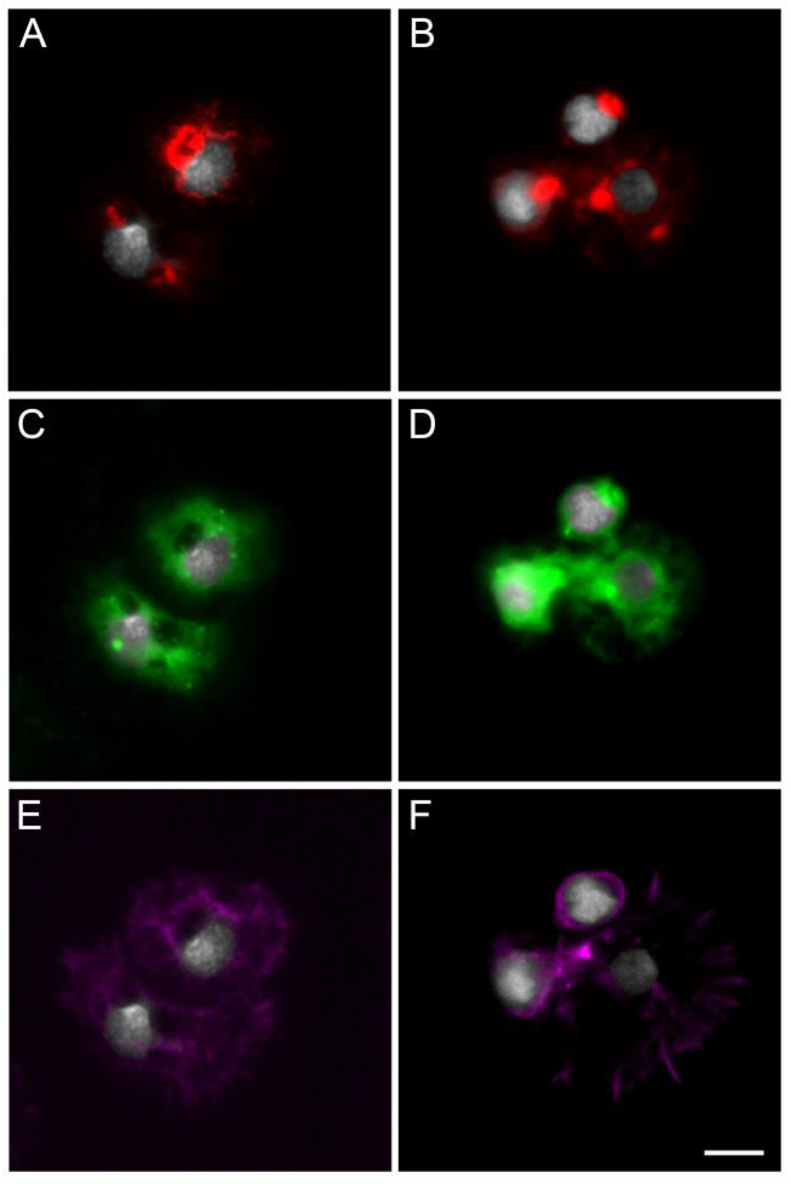
Anti-TEP1 immunostaining of hemocytes collected from hml > mCD8::Cherry blood fed females. Both the mCD8::Cherry under the control of hml promoter and TEP1 are detected in hemocytes (A, B, C), or only mCD8::Cherry (D), or only TEP1 (A, D), or none of the proteins (B). Red: endogenous mCD8::Cherry; Green: anti-TEP1 staining; White: DAPI staining. Scale bar is 5 μm.
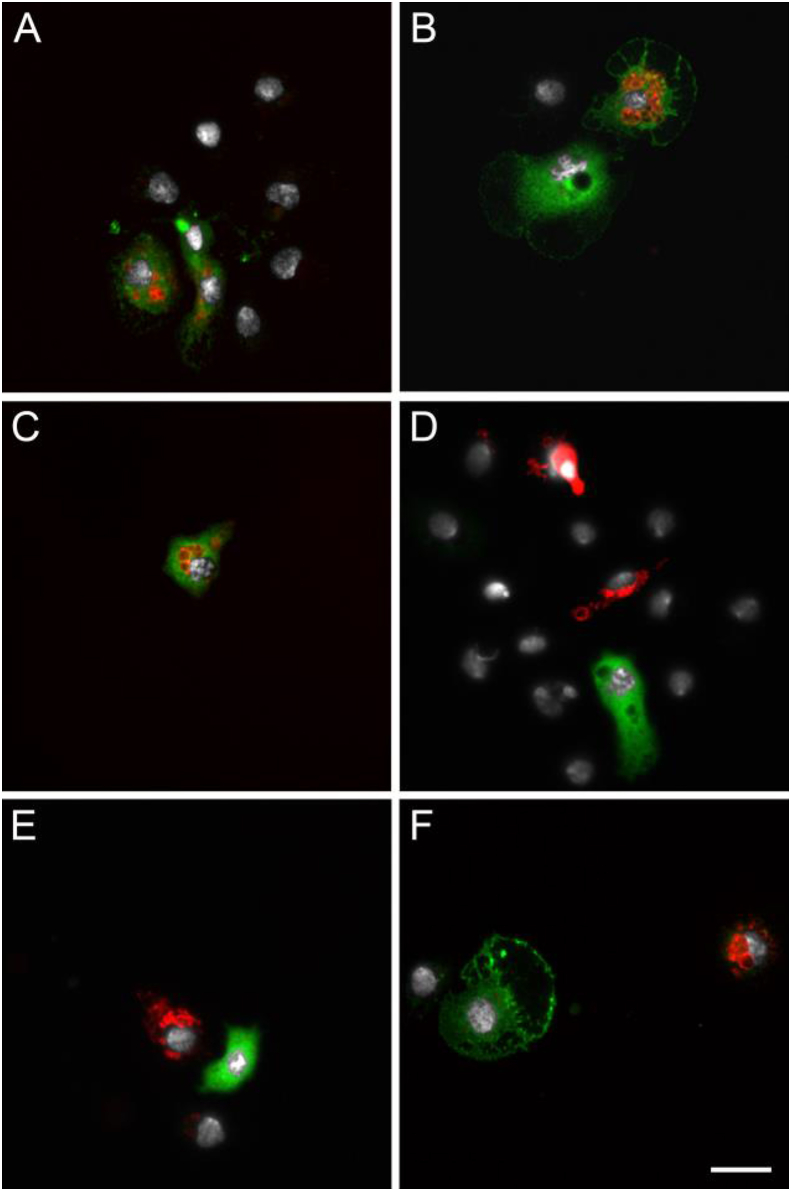
Fig. 6.
Anti-PPO2 immunostaining of hemocytes collected from hml > mCD8::Cherry blood fed females. Hemocytes express either both the mCD8::Cherry under the control of hml promoter and PPO2 (A, B, C), or only mCD8::Cherry (D, E, F), or only PPO2 (B, D, E, F), or none of the proteins (A,B,D,E,F). Red: endogenous mCD8::Cherry; Green: anti-PPO2 staining; White: DAPI staining. Scale bar is 10 μm.
In addition to phagocytosis, hemocytes also participate in melanization, a humoral immune response leading to the sequestration of invading pathogens into a thick proteinaceous layer. Hemocytes express phenoloxidases (POs), which are essential enzymes in the melanization cascade (Kumar et al., 2018). To determine if hemocytes in which the hml regulatory sequence drives transgene expression could be involved in the melanization process, we analyzed the expression of the prophenoloxidase 2 (PPO2) by immunostaining in perfused hemocytes from BF hml > mCD8::Cherry females. PPO2 is one of the ten An. gambiae PPOs and it is expressed in a hemocyte-like cell line (Muller et al., 1999) and in the hemolymph (Fraiture et al., 2009). PPO2 was found in granulocytes and only in a subset of Cherry-expressing granulocytes. Four different granulocyte populations were observed, some hemocytes expressing both PPO2 and Cherry (Fig. 6A–C), some expressing PPO2 or Cherry (Fig. 6D–F) and some hemocytes expressing neither PPO2 nor Cherry. We also perfused hemocytes from adult transgenic mosquitoes expressing the tdTomato fluorescent protein under the control of the An. gambiae promoter of the PPO6 gene (Volohonsky et al., 2015). As for PPO2 and the hml-driven transgene expression, PPO6-driven expression of tdTomato was found in only some granulocytes of BF females (Fig. 7). While the hml promoter drives transgene expression in few hemocytes in NBF females and in males compared to BF females, the expression pattern driven by PPO6 promoter in perfused hemocytes was similar between NBF females, BF females and males (NBF females and males not shown).
Fig. 7.
Hemocytes collected from PPO6-tdTomato mosquitoes. Red: endogenous tdTomato; Green: phalloidin; White: DAPI staining. Scale bar is 5 μm.
2.3. Characterization of hml-driven cherry-expressing hemocytes upon arbovirus infection
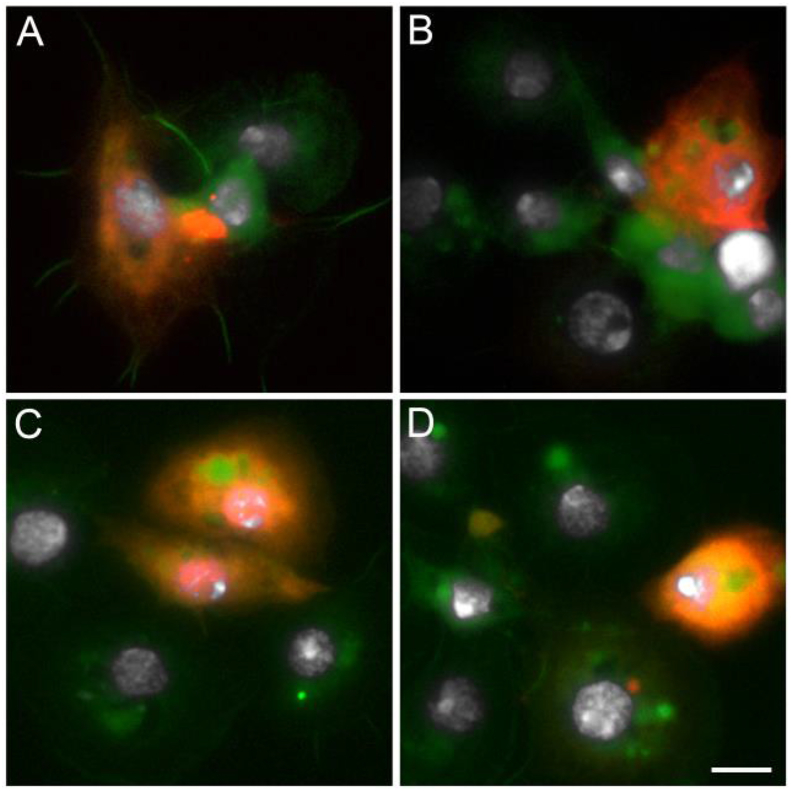
While most arthropod-borne viruses (arboviruses) transmitted by mosquitoes are transmitted by Aedes and Culex but not Anopheles mosquitoes, An. gambiae can be infected by, and transmit, ONNV (Williams et al., 1965; Vanlandingham et al., 2005). Although the roles of hemocytes in mosquito antiviral immunity have not been extensively studied due to the lack of genetic tools, it has been shown that arboviruses can infect hemocytes in Aedes aegypti (Sindbis virus, (Parikh et al., 2009)) and An. gambiae (ONNV, (Carissimo et al., 2015)). To assess if the hml promoter could drive transgene expression in hemocytes infected with ONNV, hml > mCD8::Cherry females were given either a non-infected blood meal or a blood meal infected with ONNV-eGFP (Brault et al., 2004). We perfused their hemocytes seven days later, a time at which ONNV has disseminated from the gut to other tissues and hemocytes (Carissimo et al., 2015). The GFP infection marker was expressed in hemocytes from infected females and some of the infected hemocytes expressed Cherry (Fig. 8), showing that the hml-Gal4 line constitutes a valuable tool for investigating further the contribution of hemocytes to arbovirus infection in mosquitoes.
Fig. 8.
Hemocytes collected from hml > mCD8::Cherry females infected with o'nyong-nyong virus (ONNV). (A,B) Red channel: endogenous mCD8::Cherry; (C,D) Green channel: ONNV-eGFP; (E,F) Far red channel: phalloidin; White: DAPI staining. A, C and E as well as B, D and F show the same field. Scale bar is 5 μm.
3. Discussion
Insect hemocytes play a fundamental role in insect immunity (Strand, 2008; Hillyer and Strand, 2014). However, their specific contributions in mosquitoes remain poorly understood due to the lack of genetic tools to study gene function in these specific cells. Here, we used the Gal4-UAS system to characterize the gene expression pattern driven by the Drosophila hml promoter in An. gambiae adult females. The Drosophila hml gene is homologous to the von Willebrand factor in vertebrates, and it is well conserved across animals including insects from different orders such as the lepidopteran Bombyx mori (Kotani et al., 1995; Arai et al., 2013) and in the hymenopteran Apis mellifera (Gabor et al., 2017). At the time of this study design, we could not identify an orthologue of hml in the genome of An. gambiae, suggesting a putative gene loss event or the failure to sequence and assemble the locus encoding the hml orthologue. Subsequent progress in genome sequencing efforts, including from the Anopheles 16 genomes project (Neafsey et al., 2015), has greatly improved genomic sampling of mosquitoes and other dipteran species. Intriguingly, no hml orthologues could be identified from any of the mosquito genomic resources at VectorBase (Giraldo-Calderon et al., 2015), while clear orthologues are present in tsetse flies and the housefly (Figure S3), thereby strongly supporting the loss of the hml gene from the ancestral mosquito genome. Despite this relatively ancient loss of hml, our study reveals that the Drosophila hml promoter can drive transgene expression in An. gambiae, demonstrating that some mosquito transcription factors can still bind to this regulatory region and activate transgene expression.
Although the hml promoter could drive gene expression in a few hemocytes in males and NBF females, we found that transgene expression was mostly activated by blood feeding. We observed that transgene expression under the hml promoter peaked around 24h PBM and returned to pre-blood meal levels 48 h after a blood meal. In An. gambiae, hemocytes respond to blood feeding by increasing their proliferation, size and granularity (Castillo et al., 2006; Bryant and Michel, 2014). Blood feeding also triggers a shift in proteins detected in hemocytes, including the immune factors TEP1, a complement-like molecule, and PPO6, which is involved in the melanization pathway (Bryant and Michel, 2014; Smith et al., 2016). Similarly to the hml-driven gene expression, this hemocyte immune activation following a blood meal is transient with a return to pre-blood meal state within 48 h (Bryant and Michel, 2016). This suggests that blood feeding itself could prime the female immune system for potential blood-borne pathogen challenge. As Hml is involved in immunity in Drosophila (Lesch et al., 2007) and is a marker of differentiated hemocytes (Goto et al., 2003; Evans et al., 2014), it is therefore not surprising that the hml promoter becomes active after blood feeding in a similar way to other mosquito immune genes expressed in granulocytes.
While hemocyte proliferation after a blood meal is controlled at least by the insulin signaling pathway (Castillo et al., 2011), factors mediating hemocyte activation after a blood meal are still unknown. This transient activation occurs simultaneously to an increase in Ras-MAPK signaling, suggesting that this pathway could be involved in hemocyte proliferation and differentiation (Bryant and Michel 2014, 2016). This could also be triggered by 20-hydroxyecdysone (20E) as the titers of this vitellogenic hormone, produced by ovaries after a blood meal, follow the same temporal pattern in mosquitoes including An. gambiae (Clements, 1992; Pondeville et al., 2008). This latter hypothesis is further supported by the fact that blood feeding as well as 20E injection activates the expression of the Leucine-Rich repeat IMmune protein LRIM9, an antagonist of Plasmodium berghei development (Upton et al., 2015). The expression of PPO1, another prophenoloxydase involved in melanization, is also up-regulated by 20E in an. gambiae cell line (Ahmed et al., 1999). In D. melanogaster, steroid hormone signaling during metamorphosis in hemocytes is required for hemocyte immune functions and survival to bacterial infections (Regan et al., 2013). Altogether, these findings suggest that mosquito female evolution towards anautogeny (requirement of a blood meal to develop eggs) co-opted molecules with functions in reproduction and whose expression is triggered by the blood meal to anticipate the risk of exposure to blood-borne pathogens. Others have hypothesized that this immune activation following a blood meal could be due to the leak of gut-resident bacteria into the hemolymph caused by the midgut epithelium stretch occurring during blood feeding (Bartholomay and Michel, 2018). However, the peritrophic matrix, which is induced by the gut microbiota after a blood meal, normally prevents systemic dissemination of bacteria from the gut (Rodgers et al., 2017). Therefore, bacterial leak from the gut might be a rare event happening accidently. Even though hml-driven expression in hemocytes was always induced in blood fed females, the proportion of positive hemocytes was much higher in rare cases for unidentified reasons. Therefore, it is possible that this rare and strong induction could be due to bacteria leaking from the gut. This would be consistent with Hml being involved in clotting and responses against bacteria as well as hml expression being induced by injuries in adult flies (Goto et al., 2001; Lesch et al., 2007).
In Drosophila, hml is only expressed in differentiating and differentiated hemocytes, and more precisely in crystal cells and plasmatocytes (Goto et al., 2003; Lesch et al., 2007). Although they are named differently, the classification of hemocytes based on morphology, immune markers and function shows that crystal cells and plasmatocytes in flies correspond to oenocytoids and granulocytes respectively in mosquitoes (Castillo et al., 2006). In An. gambiae, we also found that the Drosophila hml promoter drives transgene expression in granulocytes. We did not detect oenocytoids in our experiments, probably because of their lack of adhesion. Therefore, we cannot exclude that the hml promoter could drive gene expression in these cell type as well. In addition, the hml promoter drove expression in a subset of granulocytes only, although they had the same morphology, either in collected hemolymph or attached to tissues. As previously described, sessile hemocytes expressing Cherry were found mostly attached to the epidermis and abdominal muscles, in close contact to the tracheae and in periostal regions (King and Hillyer, 2013). It was difficult to estimate the exact proportion of granulocytes expressing the transgene under the control of the hml promoter, due to variation between individuals and experiments, over the time after a blood meal, not only in the number but also in the intensity of the responder fluorescent protein. We did not identify the reason of this variation. However, as already observed and discussed for the An. gambiae PPO6-tdTomato line (Volohonsky et al., 2015), variation could be due to variegation effects. As mosquitoes were not reared in sterile conditions, it might also be possible that some microbes influence hml-driven expression.
Our data showing that gene expression driven by the hml promoter in a subset of granulocytes, either in circulation or attached to tissues, is consistent with what has been described in Drosophila (Goto et al., 2003; Shia et al., 2009) and in Apis mellifera (Gabor et al., 2017). Similarly, PPO2 was not expressed in all granulocytes, sometimes co-expressed with the transgene and sometimes not, highlighting at least four populations of granulocytes. Similar results were obtained with the PPO6-tdTomato line (Volohonsky et al., 2015; Severo et al., 2018) in which we also detected transgene expression in some granulocytes only. Our data are in agreement with previous studies in Anopheles mosquitoes. Indeed, different immune markers such as Sp22D, PPO4 and PPO6 were expressed only in a subset of granulocytes (Castillo et al., 2006; Bryant and Michel, 2014; Severo et al., 2018; Kwon and Smith, 2019). Therefore, different populations of granulocytes are likely to be present in Anopheles mosquitoes, even though they display the same morphology. Therefore, genetic analysis of immune cell functions as well as hemocyte-specific gene functions in mosquitoes might require the use of different regulatory regions to target the different subpopulations of hemocytes, as in Drosophila (Evans et al., 2014). Such regulatory regions could be identified by enhancer trapping, previously used in An. stephensi to identify salivary gland, midgut and fat body regulatory regions (O'Brochta et al., 2012). Transcriptomic and proteomic studies comparing gene and protein expression between hemocytes and carcass in An. gambiae identified some genes enriched in immune cells (Baton et al., 2009; Lombardo et al., 2013; Smith et al., 2016; Kwon and Smith, 2019). Promoters of these genes could therefore constitute other good candidates to drive expression in mosquito hemocytes. However, this does not exclude that these genes are expressed at low levels in other tissues, or that they can be expressed in other tissues under different experimental conditions or during mosquito development (e.g. embryonic and larval development). For instance, transgene expression in Drosophila hemocytes can be achieved using the hml promoter, but also using the peroxidasin or serpent promoters. These two other promoters drive expression from embryonic development, resulting in lethality during development when combined with some transgenes, and can sometimes be non-specific to hemocytes, as observed for peroxidasin, driving also expression in the fat body (Shia et al., 2009).
One extension of the Gal4-UAS system to overcome this limitation is the use of the Temporal And Regional Gene Expression Targeting (TARGET) system developed in Drosophila (McGuire et al., 2004) and further established in the Zebrafish (Faucherre and Lopez-Schier, 2011). In this system, flexible temporal and/or tissue control of Gal4 activity can be achieved by using the temperature-sensitive version of the Gal80 protein (Gal80ts) under the control of tissue- or time-specific promoters. At permissive temperatures (18 °C–20 °C), Gal80ts binds to Gal4 and inhibits Gal4 activity, while at restrictive temperatures (28–30 °C), it no longer binds to Gal4, allowing Gal4-dependent transgene expression. This system would also be suitable for studying Plasmodium falciparum development in transgenic mosquitoes as the human malaria parasite, contrary to the rodent parasite, Plasmodium berghei, can develop within temperatures ranging from 21 up to 34 °C, with most studies analyzing malaria infection in Anopheles at 24–28 °C (Shapiro et al., 2017). The hml-gal4 line presented here was initially designed to carry, in tandem with hml-gal4, the gal80ts sequence under the control of a second and identical hml promoter. Although we could detect by RT-PCR the expression of gal80ts in perfused hemocytes, we could not observe any obvious difference in Cherry expression between blood fed mosquitoes reared at 20 versus 30 °C (data not shown). Different hypotheses could explain this negative result. First, contrary to D. melanogaster, An. gambiae mosquitoes live and can cope with high temperatures. Therefore, the thermo-sensitivity of Gal80ts could be decreased in mosquitoes if chaperone proteins stabilize Gal80ts. Secondly, as the hml promoter does not drive expression in all hemocytes, with variation between individuals and experiments, and sometimes in a low proportion of granulocytes, it might be difficult to observe a significant decrease. Further studies will be required to assess the functionality of the Gal80ts system in mosquitoes. This could be undertaken by using an ubiquitous promoter such as the one of the An. gambiae Polyubiquitin-c (PUBc) gene (Adolfi et al., 2018).
In summary, the Drosophila hml promoter combined with the Gal4-UAS system is a promising tool to study gene function in hemocytes, more particularly in a subpopulation of granulocytes, and their specific contributions to immunity in the malaria mosquito. Our findings show that it could be used to study blood meal-induced activation of hemocytes, phagocytosis, melanization as well as arbovirus-hemocyte interactions. Moreover, genes enriched in hemocytes and whose functions are yet unknown could be analyzed using this system. As phagocytosis of Plasmodium occasionally occurs (Hillyer et al., 2003) but is not believed to be required for an efficient anti-parasitic response in mosquitoes, we did not determine if granulocytes expressing the transgene under the hml promoter control could also phagocytose sporozoites after Plasmodium infection. Nevertheless, granulocytes are definitely involved in the response against the parasite (King and Hillyer, 2012; Bartholomay and Michel, 2018; Kwon and Smith, 2019), suggesting that the hml promoter could also constitute a good driver for the analysis of hemocyte-mediated anti-malaria immunity. Some other fundamental questions, already raised by Hillyer and Strand, (2014), about hemocyte origin lineage, function of hemocytes during post-embryonic development as well as the function of sessile hemocytes in vivo, could also be addressed using such genetic tools. The gal4 sequence used in this study encodes the classic Gal4 form. Future studies could also assess whether transgene expression driven by the hml regulatory sequence can be increased by using Gal4 modified forms, for example Gal4Δ (Ma and Ptashne, 1987) or Gal4GFY, shown to be more active than the classic Gal4 in an An. gambiae cell line (Lynd and Lycett, 2011). Finally, it would be interesting to assess if the hml regulatory region from Drosophila could also be active in other mosquito species, including Ae. aegypti, the main mosquito vector of arboviruses.
4. Material and methods
4.1. Rearing of mosquito transgenic strains
The E phiC31 docking line (Meredith et al., 2011), the hml-Gal4 line, the UAS-mCD8::Cherry line (Adolfi et al., 2018) and the PPO6-tdTomato line (Volohonsky et al., 2015) were maintained at 27 °C, under 68% relative humidity and a 12/12h light/dark cycle. Mosquito larvae were reared in deionized water supplemented with minerals and fed on TetraMin Baby-E fish food from the day of hatching to the fourth larval instar supplemented with pieces of cat food. Male and female adults were provided free access to a 10% wt/vol sucrose solution for the first five days post-emergence (PE). Female mosquitoes were fed for 30 min on the blood of anesthetized mice. Adult progeny from Gal4-UAS crosses were maintained at 28 °C.
4.2. Plasmid construction
In a first step, the 3′ terminus of EYFP was removed from the plasmid pGEM-T[3xP3-eYFPafm-attB] (Adolfi et al., 2018) by BsrG1-XbaI digestion and replaced by a 3’ terminus of eYFPnls amplified by PCR from pSL[UAS-eYFPnls-g] (Lynd and Lycett, 2012) and digested by BsrG1 and XbaI to create the recipient plasmid pGEM-T[3xP3-eYFPnls-afm-attB]. The 1160 bp hml regulatory region from D. melanogaster was amplified by PCR from the D. melanogaster BAC genomic clone BACR02M20 (https://bacpacresources.org/). The reverse primer was designed to mutate an ATG located 79 bp before the hml start codon to AAG and therefore suppressing a putative upstream ORF. The NotI-hml-EcoRI PCR fragment was further cloned into psL[LRIM-gal4] (Lynd and Lycett, 2011) after removal of the LRIM promoter with the same enzymes to give psL[hml-gal4]. The hml-gal4-SV40 cassette was then digested using AscI and FseI to be sub-cloned into the recipient plasmid pGEM-T[3xP3-eYFPnls-afm-attB]. The hml promoter was then amplified from psL[hml-gal4] with FseI forward and PmeI reverse primers. The gal80ts-SV40 cassette was amplified from pCASPER[DEST-gal80ts] (gift of Jacques Montagne) with PmeI forward and FseI reverse primers. Both PCR fragments were digested by FseI and PmeI and further cloned into pGEM-T[3xP3-eYFPnls-hml-gal4-attB] to create pGEM-T[3xP3-eYFPnls-hml-gal4-hml-gal80ts-attB]. This plasmid was sequenced to verify sequence integrity.
4.3. hml-Gal4 line establishment
The transgenic line was created by injecting 2387 embryos of the E phiC31 docking line (Meredith et al., 2011) with 250 ng/μl of hml-gal4 plasmid and 800 ng/μl of mRNA encoding an insect codon optimized mutant phiC31 integrase (Franz et al., 2011). Out of the 510 G0 larvae that survived (21.4%), 175 showing transient fluorescence (34.3%) were kept for further rearing. Recovered G0 adults (72 females and 71 males) were backcrossed to mosquitoes of the E line (pools of 10 G0 males and 50 females; all G0 females with 5x males) and all females blood fed. Among the progeny of these backcrosses, five transgenic G1 larvae were obtained (one from the G0 female pool of and four from the G0 male pools) and used to establish a homozygous transgenic hml-Gal4 driver line. Considering half of G0 individuals were sterile, the transformation efficiency is estimated ranging from 2.8% (if only one out of the four G0 males produced transgenic gametes) to 7% (if the four G0 males produced transgenic gametes). Preparation of the injection mix, microinjections, screening and stable homozygous line generation were carried out as previously described (Pondeville et al., 2014). Correct site-specific integration was confirmed by PCR across the resulting attL and attR using specific primers (Meredith et al., 2011). Backcross to docking strain confirmed normal Mendelian inheritance of the eYFPnls marker.
4.4. Bacteria and beads injection
To obtain bacteria expressing GFP, E. coli bacteria (Sure 2, Agilent Technologies) were transformed with pFPV25.1 (Valdivia and Falkow, 1996) (Addgene plasmid # 20668) and grown overnight in a shaking incubator at 37 °C in Luria-Bertani's rich nutrient medium (LB broth) supplemented with ampicillin. After centrifugation, bacteria were resuspended in LB broth and cultures were normalized to OD600 = 0.5 using a spectrophotometer prior to injection. Yellow-green fluorescent FluoSpheres beads (1 μm diameter, Molecular Probes) were mixed with PBS to a final concentration of 0.08% solids per volume prior to injection. Cold-anesthetized 5-6 day-old females were injected into their thorax using a nanoinjector (Nanoject II, Drummond Scientific) with 69 nL of bacteria or 138 nL of fluorescent beads. Injected females were maintained with 10% (wt/vol) sucrose at 27 °C until hemocyte perfusions three days after bacteria injection or one day after beads injection.
4.5. Infection of mosquitoes with ONNV
The ONN-eGFP virus stocks were produced as described previously (Carissimo et al., 2015) from an infectious clone tagged with GFP in a duplicated subgenomic promoter (Keene et al., 2004). Female mosquitoes were allowed to feed for 15 min through a Hemotek membrane (Hemotek) covering a glass feeder containing the blood/virus mixture maintained at 37 °C. The infectious blood meal was composed of a virus suspension diluted (1:3) in washed rabbit blood and resuspended at 50% (vol/vol) in dialyzed rabbit serum (R4505; Sigma). ATP was added to a final concentration of 5 μM. The final blood-meal titer fed to mosquitoes was between 1 and 3 × 107 pfu/mL. Non-infected females were treated the same way except that media used to produce the virus in cell culture was used instead of the virus suspension. Fully engorged females were transferred to small cardboard containers and maintained with 10% (wt/vol) sucrose at 28 ± 1 °C until hemocyte perfusions.
4.6. Hemolymph perfusions
To collect circulating hemocytes, the last segment of the abdomen was cut, and mosquitoes were then injected into the thorax with phosphate buffered saline (PBS, pH 7.0) using a glass capillary mounted on a syringe. For RT-PCR experiments, PBS diluted hemocytes were collected in tubes on ice and centrifuged at 2000 rpm for 15 min at 4 °C. Supernatant was gently removed before adding TRI Reagent (Molecular Research Center). For immunostaining, hemocytes were perfused on slides (ibidi; 10 females per well). Slides were left in the dark for 10 or 45 min at 28 °C before removal of PBS-T and fixation.
4.7. RNA extraction and RT-PCR
RNA from perfused hemocytes in TRI Reagent was extracted according to the manufacturer's instructions, except that 1-Bromo-3-chloropropane was used instead of chloroform and DNAse (TURBO DNase, Ambion) treatment was carried out. Reverse-transcription (RT) was performed using the MMLV retro-transcriptase (Promega) from 500 ng of total RNA in a final volume of 60 μl cDNA was aliquoted and further stored at −20 °C until PCR using the DreamTaq Green DNA polymerase (ThermoFisher) according to manufacturer's instructions (40 cycles). Sequence of specific primers for S7, gal4, gal80ts and mCD8::cherry are given in Table S1.
4.8. Immunostainings of tissues and hemocytes
Abdomen were dissected and carefully cut along the cuticle. Perfused hemocytes and abdomen tissues were fixed at room temperature (RT) for 20 min in 4% (w/vol) paraformaldehyde (PFA) diluted in PBS. Hemocytes were washed (three times, 15 min each at 4 °C) in PBS before overnight staining with DAPI 1X (405 nm, Sigma) and Phalloidin 1X (488 nm, Sigma or 647 nm, Cell Signaling) diluted in PBS at 4 °C. After PBS washes (three times, 15 min each at 4 °C), mounting medium (ibidi) was used to replace PBS in wells. Tissues were treated the same way but PBS-Tween 0.05% was used instead of PBS. Tissues were mounted between slide and coverslip (24 mm × 24 mm) with an imaging spacer (1 well, diameter x thickness: 13 mm × 0.12 mm Grace Bio-Labs SecureSeal imaging spacer, Sigma-Aldrich) using mounting medium (ibidi). For mCD8 and PPO antibodies staining, fixed and washed hemocytes were blocked for at least 30 min in blocking solution (PBS-T 0.05%, 5% FCS [vol/vol], 5% BSA [w/vol], 0.05% Triton X-100 [vol/vol]) at 4 °C. Hemocytes were incubated at 4 °C overnight with a rat anti-mCD8 antibody (Ancell) diluted 1:100 or a rabbit anti-PPO2 (Fraiture et al., 2009) at 1:1000, or a rabbit anti-TEP1 (Levashina et al., 2001) at 1:300 in blocking solution. Samples were washed (five times, 15 min each at 4 °C) in PBS-T 0.05% and incubated with either an Alexa Fluor 488 goat anti-rat IgG (Thermo Fisher Scientific) or an Alexa Fluor 488 goat anti-rabbit IgG (Thermo Fisher Scientific) diluted 1:1000, DAPI 1X (405 nm, Sigma) and Phalloidin 1X (647 nm, Cell Signaling) in blocking solution for 2 h at RT. Three washes in PBS were carried out. Mounting medium (ibidi) was used to replace PBS in hemocyte wells. All steps were carried in the dark to preserve the endogenous mCD8::Cherry fluorescence. Images were acquired on an inverted Zeiss Observer Z1 (Axio vision software) for hemocytes, on a Leica (SP8) confocal microscope for tissues and processed with ImageJ and Adobe Photoshop.
Ethical compliance
This study complied with all relevant ethical guidelines and regulations. Project (n° 2013–0132) approved by the Ministère de l’Enseignement Supérieur et de la Recherche – Direction Générale pour la Recherche et l’Innovation – Secrétariat « Autorisation de projet » - 1, rue Descartes, 75231 PARIS cedex 5.
Data availability
All data are available upon request.
Funding
Support to E.P. was from an ANR-07-MIME-O25-01 award from the French Agence Nationale de la Recherche to C.B., from Fondation Roux (Institut Pasteur) fellowship and by the UK Medical Research Council to E.P. (MC_UU_12014/8), to C.B. from ANR-07-MIME-O25-01 award from the French Agence Nationale de la Recherche to C.B. and ANR-10-LABX-62-IBEID. R.M.W. was supported by Swiss National Science Foundation grant PP00P3_170664.
CRediT authorship contribution statement
Emilie Pondeville: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Nicolas Puchot: Formal analysis, Investigation, Visualization. Jean-Philippe Parvy: Formal analysis, Investigation, Writing - review & editing, Visualization. Guillaume Carissimo: Methodology, Formal analysis, Investigation, Writing - review & editing. Robert M. Waterhouse: Formal analysis, Writing - review & editing. Eric Marois: Resources, Writing - review & editing. Catherine Bourgouin: Conceptualization, Formal analysis, Writing - review & editing, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
None.
Acknowledgments
We thank Frank Schnorrer for supply of pUAS-mCD8::Cherry plasmid, Amy Lind and Gareth Lycett for pSL[UAS-eYFPnls-g] and pSL[UAS-eYFPnls-g] and useful discussions, Ernst Wimmer for pBac[3xP3] and pslfa1180, Michele Calos pour pattB, Francois-Xavier Camval for pFPV 25.1 and Anthony James for phiC31 integrase optimized mutant plasmid template. We are also grateful to Jacques Montagne for confocal access.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ibmb.2020.103339.
Contributor Information
Emilie Pondeville, Email: emilie.pondeville@glasgow.ac.uk.
Catherine Bourgouin, Email: catherine.bourgouin@pasteur.fr.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adolfi A., Pondeville E., Lynd A., Bourgouin C., Lycett G.J. Multi-tissue GAL4-mediated gene expression in all Anopheles gambiae life stages using an endogenous polyubiquitin promoter. Insect Biochem. Mol. Biol. 2018;96:1–9. doi: 10.1016/j.ibmb.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Ahmed A., Martin D., Manetti A.G., Han S.J., Lee W.J., Mathiopoulos K.D., Muller H.M., Kafatos F.C., Raikhel A., Brey P.T. Genomic structure and ecdysone regulation of the prophenoloxidase 1 gene in the malaria vector Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 1999;96(26):14795–14800. doi: 10.1073/pnas.96.26.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai I., Ohta M., Suzuki A., Tanaka S., Yoshizawa Y., Sato R. Immunohistochemical analysis of the role of hemocytin in nodule formation in the larvae of the silkworm, Bombyx mori. J. Insect Sci. 2013;13:125. doi: 10.1673/031.013.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barletta A.B.F., Trisnadi N., Ramirez J.L., Barillas-Mury C. Mosquito midgut prostaglandin release establishes systemic immune priming. iScience. 2019;19:54–62. doi: 10.1016/j.isci.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay L.C., Michel K. Mosquito immunobiology: the intersection of vector health and vector competence. Annu. Rev. Entomol. 2018;63:145–167. doi: 10.1146/annurev-ento-010715-023530. [DOI] [PubMed] [Google Scholar]
- Baton L.A., Robertson A., Warr E., Strand M.R., Dimopoulos G. Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genom. 2009;10:257. doi: 10.1186/1471-2164-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U., Battle K., Moyes C.L., Henry A., Eckhoff P.A., Wenger E.A., Briet O., Penny M.A., Smith T.A., Bennett A., Yukich J., Eisele T.P., Griffin J.T., Fergus C.A., Lynch M., Lindgren F., Cohen J.M., Murray C.L.J., Smith D.L., Hay S.I., Cibulskis R.E., Gething P.W. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S., Shiao S.H., Moita L.F., Janse C.J., Waters A.P., Kafatos F.C., Levashina E.A. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116(5):661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Blandin S.A., Levashina E.A. Phagocytosis in mosquito immune responses. Immunol. Rev. 2007;219:8–16. doi: 10.1111/j.1600-065X.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Bosch P.S., Makhijani K., Herboso L., Gold K.S., Baginsky R., Woodcock K.J., Alexander B., Kukar K., Corcoran S., Ouyang D., Wong C., Ramond E.J., Rhiner C., Moreno E., Lemaitre B., Geissmann F., Brückner K. Blood cells of adult Drosophila do not expand, but control survival after bacterial infection by induction of Drosocin around their reservoir at the respiratory epithelia. bioRxiv. 2019:578864. [Google Scholar]
- Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brault A.C., Foy B.D., Myles K.M., Kelly C.L., Higgs S., Weaver S.C., Olson K.E., Miller B.R., Powers A.M. Infection patterns of o'nyong nyong virus in the malaria-transmitting mosquito, Anopheles gambiae. Insect Mol. Biol. 2004;13(6):625–635. doi: 10.1111/j.0962-1075.2004.00521.x. [DOI] [PubMed] [Google Scholar]
- Bryant W.B., Michel K. Blood feeding induces hemocyte proliferation and activation in the African malaria mosquito, Anopheles gambiae Giles. J. Exp. Biol. 2014;217(Pt 8):1238–1245. doi: 10.1242/jeb.094573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant W.B., Michel K. Anopheles gambiae hemocytes exhibit transient states of activation. Dev. Comp. Immunol. 2016;55:119–129. doi: 10.1016/j.dci.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carissimo G., Pondeville E., McFarlane M., Dietrich I., Mitri C., Bischoff E., Antoniewski C., Bourgouin C., Failloux A.B., Kohl A., Vernick K.D. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl. Acad. Sci. U. S. A. 2015;112(2):E176–E185. doi: 10.1073/pnas.1412984112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J., Brown M.R., Strand M.R. Blood feeding and insulin-like peptide 3 stimulate proliferation of hemocytes in the mosquito Aedes aegypti. PLoS Pathog. 2011;7(10) doi: 10.1371/journal.ppat.1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J.C., Robertson A.E., Strand M.R. Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochem. Mol. Biol. 2006;36(12):891–903. doi: 10.1016/j.ibmb.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churcher T.S., Lissenden N., Griffin J.T., Worrall E., Ranson H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. Elife. 2016;5 doi: 10.7554/eLife.16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A.M., Dong Y., Dimopoulos G. The Anopheles innate immune system in the defense against malaria infection. J. Innate. Immun. 2014;6(2):169–181. doi: 10.1159/000353602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A.N. vol. 1. Chapman & Hall; 1992. p. 509. (The Biology of Mosquitoes). [Google Scholar]
- Duffy J.B. GAL4 system in Drosophila: a fly geneticist's Swiss army knife. Genesis. 2002;34(1–2):1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- Evans C.J., Liu T., Banerjee U. Drosophila hematopoiesis: markers and methods for molecular genetic analysis. Methods. 2014;68(1):242–251. doi: 10.1016/j.ymeth.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucherre A., Lopez-Schier H. Delaying Gal4-driven gene expression in the zebrafish with morpholinos and Gal80. PloS One. 2011;6(1) doi: 10.1371/journal.pone.0016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiture M., Baxter R.H., Steinert S., Chelliah Y., Frolet C., Quispe-Tintaya W., Hoffmann J.A., Blandin S.A., Levashina E.A. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5(3):273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Franz A.W., Jasinskiene N., Sanchez-Vargas I., Isaacs A.T., Smith M.R., Khoo C.C., Heersink M.S., James A.A., Olson K.E. Comparison of transgene expression in Aedes aegypti generated by mariner Mos1 transposition and PhiC31 site-directed recombination. Insect Mol. Biol. 2011;20(5):587–598. doi: 10.1111/j.1365-2583.2011.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor E., Cinege G., Csordas G., Torok T., Folkl-Medzihradszky K., Darula Z., Ando I., Kurucz E. Hemolectin expression reveals functional heterogeneity in honey bee (Apis mellifera) hemocytes. Dev. Comp. Immunol. 2017;76:403–411. doi: 10.1016/j.dci.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Singh A., Mandal S., Mandal L. Active hematopoietic hubs in Drosophila adults generate hemocytes and contribute to immune response. Dev. Cell. 2015;33(4):478–488. doi: 10.1016/j.devcel.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Calderon G.I., Emrich S.J., MacCallum R.M., Maslen G., Dialynas E., Topalis P., Ho N., Gesing S., VectorBase C., Madey G., Collins F.H., Lawson D. VectorBase: an updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015;43(Database issue):D707–D713. doi: 10.1093/nar/gku1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A., Kadowaki T., Kitagawa Y. Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Dev. Biol. 2003;264(2):582–591. doi: 10.1016/j.ydbio.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Goto A., Kumagai T., Kumagai C., Hirose J., Narita H., Mori H., Kadowaki T., Beck K., Kitagawa Y. A Drosophila haemocyte-specific protein, hemolectin, similar to human von Willebrand factor. Biochem. J. 2001;359(Pt 1):99–108. doi: 10.1042/0264-6021:3590099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer D., Tuttle A., Rouse S., Volrath S., Johnson M., Potter S., Gorlach J., Goff S., Crossland L., Ward E. Activation of latent transgenes in Arabidopsis using a hybrid transcription factor. Genetics. 1998;149(2):633–639. doi: 10.1093/genetics/149.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley K.O., Nutt S.L., Amaya E. Targeted gene expression in transgenic Xenopus using the binary Gal4-UAS system. Proc. Natl. Acad. Sci. U. S. A. 2002;99(3):1377–1382. doi: 10.1073/pnas.022646899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer J.F. Mosquito immunity. Adv. Exp. Med. Biol. 2010;708:218–238. doi: 10.1007/978-1-4419-8059-5_12. [DOI] [PubMed] [Google Scholar]
- Hillyer J.F., Schmidt S.L., Christensen B.M. Rapid phagocytosis and melanization of bacteria and Plasmodium sporozoites by hemocytes of the mosquito Aedes aegypti. J. Parasitol. 2003;89(1):62–69. doi: 10.1645/0022-3395(2003)089[0062:RPAMOB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hillyer J.F., Strand M.R. Mosquito hemocyte-mediated immune responses. Curr. Opin. Insect Sci. 2014;3:14–21. doi: 10.1016/j.cois.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz A., Bossinger B., Strasser T., Janning W., Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130(20):4955–4962. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- Imamura M., Nakai J., Inoue S., Quan G.X., Kanda T., Tamura T. Targeted gene expression using the GAL4/UAS system in the silkworm Bombyx mori. Genetics. 2003;165(3):1329–1340. doi: 10.1093/genetics/165.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene K.M., Foy B.D., Sanchez-Vargas I., Beaty B.J., Blair C.D., Olson K.E. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 2004;101(49):17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.G., Hillyer J.F. Infection-induced interaction between the mosquito circulatory and immune systems. PLoS Pathog. 2012;8(11) doi: 10.1371/journal.ppat.1003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J.G., Hillyer J.F. Spatial and temporal in vivo analysis of circulating and sessile immune cells in mosquitoes: hemocyte mitosis following infection. BMC Biol. 2013;11:55. doi: 10.1186/1741-7007-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V.A., Raikhel A.S. Targeted gene expression in the transgenic Aedes aegypti using the binary Gal4-UAS system. Insect Biochem. Mol. Biol. 2011;41(8):637–644. doi: 10.1016/j.ibmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani E., Yamakawa M., Iwamoto S., Tashiro M., Mori H., Sumida M., Matsubara F., Taniai K., Kadono-Okuda K., Kato Y. Cloning and expression of the gene of hemocytin, an insect humoral lectin which is homologous with the mammalian von Willebrand factor. Biochim. Biophys. Acta. 1995;1260(3):245–258. doi: 10.1016/0167-4781(94)00202-e. [DOI] [PubMed] [Google Scholar]
- Kumar A., Srivastava P., Sirisena P., Dubey S.K., Kumar R., Shrinet J., Sunil S. Mosquito innate immunity. Insects. 2018;9(3) doi: 10.3390/insects9030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Smith R.C. Chemical depletion of phagocytic immune cells in Anopheles gambiae reveals dual roles of mosquito hemocytes in anti-Plasmodium immunity. Proc. Natl. Acad. Sci. U. S. A. 2019;116(28):14119–14128. doi: 10.1073/pnas.1900147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch C., Goto A., Lindgren M., Bidla G., Dushay M.S., Theopold U. A role for Hemolectin in coagulation and immunity in Drosophila melanogaster. Dev. Comp. Immunol. 2007;31(12):1255–1263. doi: 10.1016/j.dci.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Levashina E.A., Moita L.F., Blandin S., Vriend G., Lagueux M., Kafatos F.C. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104(5):709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Lombardo F., Christophides G.K. Novel factors of Anopheles gambiae haemocyte immune response to Plasmodium berghei infection. Parasites Vectors. 2016;9:78. doi: 10.1186/s13071-016-1359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo F., Ghani Y., Kafatos F.C., Christophides G.K. Comprehensive genetic dissection of the hemocyte immune response in the malaria mosquito Anopheles gambiae. PLoS Pathog. 2013;9(1) doi: 10.1371/journal.ppat.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd A., Balabanidou V., Grosman R., Maas J., Lian L.-Y., Vontas J., Lycett G.J. bioRxiv; 2019. Development of a Functional Genetic Tool for Anopheles gambiae Oenocyte Characterisation: Application to Cuticular Hydrocarbon Synthesis; p. 742619. [Google Scholar]
- Lynd A., Lycett G.J. Optimization of the Gal4-UAS system in an Anopheles gambiae cell line. Insect Mol. Biol. 2011;20(5):599–608. doi: 10.1111/j.1365-2583.2011.01090.x. [DOI] [PubMed] [Google Scholar]
- Lynd A., Lycett G.J. Development of the bi-partite Gal4-UAS system in the African malaria mosquito, Anopheles gambiae. PloS One. 2012;7(2) doi: 10.1371/journal.pone.0031552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- Macias V.M., Ohm J.R., Rasgon J.L. Gene drive for mosquito control: where did it come from and where are we headed? Int. J. Environ. Res. Publ. Health. 2017;14(9) doi: 10.3390/ijerph14091006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S.E., Mao Z., Davis R.L. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE. 2004;2004(220):pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- McLean K.J., Jacobs-Lorena M. Genetic control of malaria mosquitoes. Trends Parasitol. 2016;32(3):174–176. doi: 10.1016/j.pt.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith J.M., Basu S., Nimmo D.D., Larget-Thiery I., Warr E.L., Underhill A., McArthur C.C., Carter V., Hurd H., Bourgouin C., Eggleston P. Site-specific integration and expression of an anti-malarial gene in transgenic Anopheles gambiae significantly reduces Plasmodium infections. PloS One. 2011;6(1) doi: 10.1371/journal.pone.0014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiroux N., Gomez M.B., Pennetier C., Elanga E., Djenontin A., Chandre F., Djegbe I., Guis H., Corbel V. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J. Infect. Dis. 2012;206(10):1622–1629. doi: 10.1093/infdis/jis565. [DOI] [PubMed] [Google Scholar]
- Moita L.F., Wang-Sattler R., Michel K., Zimmermann T., Blandin S., Levashina E.A., Kafatos F.C. In vivo identification of novel regulators and conserved pathways of phagocytosis in A. gambiae. Immunity. 2005;23(1):65–73. doi: 10.1016/j.immuni.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Muller H.M., Dimopoulos G., Blass C., Kafatos F.C. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J. Biol. Chem. 1999;274(17):11727–11735. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- Neafsey D.E., Waterhouse R.M., Abai M.R., Aganezov S.S., Alekseyev M.A., Allen J.E., Amon J., Arca B., Arensburger P., Artemov G., Assour L.A., Basseri H., Berlin A., Birren B.W., Blandin S.A., Brockman A.I., Burkot T.R., Burt A., Chan C.S., Chauve C., Chiu J.C., Christensen M., Costantini C., Davidson V.L., Deligianni E., Dottorini T., Dritsou V., Gabriel S.B., Guelbeogo W.M., Hall A.B., Han M.V., Hlaing T., Hughes D.S., Jenkins A.M., Jiang X., Jungreis I., Kakani E.G., Kamali M., Kemppainen P., Kennedy R.C., Kirmitzoglou I.K., Koekemoer L.L., Laban N., Langridge N., Lawniczak M.K., Lirakis M., Lobo N.F., Lowy E., MacCallum R.M., Mao C., Maslen G., Mbogo C., McCarthy J., Michel K., Mitchell S.N., Moore W., Murphy K.A., Naumenko A.N., Nolan T., Novoa E.M., O'Loughlin S., Oringanje C., Oshaghi M.A., Pakpour N., Papathanos P.A., Peery A.N., Povelones M., Prakash A., Price D.P., Rajaraman A., Reimer L.J., Rinker D.C., Rokas A., Russell T.L., Sagnon N., Sharakhova M.V., Shea T., Simao F.A., Simard F., Slotman M.A., Somboon P., Stegniy V., Struchiner C.J., Thomas G.W., Tojo M., Topalis P., Tubio J.M., Unger M.F., Vontas J., Walton C., Wilding C.S., Willis J.H., Wu Y.C., Yan G., Zdobnov E.M., Zhou X., Catteruccia F., Christophides G.K., Collins F.H., Cornman R.S., Crisanti A., Donnelly M.J., Emrich S.J., Fontaine M.C., Gelbart W., Hahn M.W., Hansen I.A., Howell P.I., Kafatos F.C., Kellis M., Lawson D., Louis C., Luckhart S., Muskavitch M.A., Ribeiro J.M., Riehle M.A., Sharakhov I.V., Tu Z., Zwiebel L.J., Besansky N.J. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science. 2015;347(6217):1258522. doi: 10.1126/science.1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brochta D.A., Pilitt K.L., Harrell R.A., 2nd, Aluvihare C., Alford R.T. Gal4-based enhancer-trapping in the malaria mosquito Anopheles stephensi. G3 (Bethesda) 2012;2(11):1305–1315. doi: 10.1534/g3.112.003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh G.R., Oliver J.D., Bartholomay L.C. A haemocyte tropism for an arbovirus. J. Gen. Virol. 2009;90(Pt 2):292–296. doi: 10.1099/vir.0.005116-0. [DOI] [PubMed] [Google Scholar]
- Pinto S.B., Lombardo F., Koutsos A.C., Waterhouse R.M., McKay K., An C., Ramakrishnan C., Kafatos F.C., Michel K. Discovery of Plasmodium modulators by genome-wide analysis of circulating hemocytes in Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 2009;106(50):21270–21275. doi: 10.1073/pnas.0909463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondeville E., Maria A., Jacques J.C., Bourgouin C., Dauphin-Villemant C. Anopheles gambiae males produce and transfer the vitellogenic steroid hormone 20-hydroxyecdysone to females during mating. Proc. Natl. Acad. Sci. U. S. A. 2008;105(50):19631–19636. doi: 10.1073/pnas.0809264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondeville E., Puchot N., Meredith J.M., Lynd A., Vernick K.D., Lycett G.J., Eggleston P., Bourgouin C. Efficient ΦC31 integrase–mediated site-specific germline transformation of Anopheles gambiae. Nat. Protoc. 2014;9(7):1698–1712. doi: 10.1038/nprot.2014.117. [DOI] [PubMed] [Google Scholar]
- Ramirez J.L., de Almeida Oliveira G., Calvo E., Dalli J., Colas R.A., Serhan C.N., Ribeiro J.M., Barillas-Mury C. A mosquito lipoxin/lipocalin complex mediates innate immune priming in Anopheles gambiae. Nat. Commun. 2015;6:7403. doi: 10.1038/ncomms8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J.L., Garver L.S., Brayner F.A., Alves L.C., Rodrigues J., Molina-Cruz A., Barillas-Mury C. The role of hemocytes in Anopheles gambiae antiplasmodial immunity. J. Innate. Immun. 2014;6(2):119–128. doi: 10.1159/000353765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson H., Lissenden N. Insecticide resistance in african Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32(3):187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Regan J.C., Brandao A.S., Leitao A.B., Mantas Dias A.R., Sucena E., Jacinto A., Zaidman-Remy A. Steroid hormone signaling is essential to regulate innate immune cells and fight bacterial infection in Drosophila. PLoS Pathog. 2013;9(10) doi: 10.1371/journal.ppat.1003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers F.H., Gendrin M., Wyer C.A.S., Christophides G.K. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog. 2017;13(5) doi: 10.1371/journal.ppat.1006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J., Brayner F.A., Alves L.C., Dixit R., Barillas-Mury C. Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science. 2010;329(5997):1353–1355. doi: 10.1126/science.1190689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva R.G., Kang S., Simoes M.L., Anglero-Rodriguez Y.I., Dimopoulos G. Mosquito gut antiparasitic and antiviral immunity. Dev. Comp. Immunol. 2016;64:53–64. doi: 10.1016/j.dci.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Scheer N., Campos-Ortega J.A. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 1999;80(2):153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Schinko J.B., Weber M., Viktorinova I., Kiupakis A., Averof M., Klingler M., Wimmer E.A., Bucher G. Functionality of the GAL4/UAS system in Tribolium requires the use of endogenous core promoters. BMC Dev. Biol. 2010;10:53. doi: 10.1186/1471-213X-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severo M.S., Landry J.J.M., Lindquist R.L., Goosmann C., Brinkmann V., Collier P., Hauser A.E., Benes V., Henriksson J., Teichmann S.A., Levashina E.A. Unbiased classification of mosquito blood cells by single-cell genomics and high-content imaging. Proc. Natl. Acad. Sci. U. S. A. 2018;115(32):E7568–E7577. doi: 10.1073/pnas.1803062115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shandala T., Lim C., Sorvina A., Brooks D.A. A Drosophila model to image phagosome maturation. Cells. 2013;2(2):188–201. doi: 10.3390/cells2020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L.L.M., Whitehead S.A., Thomas M.B. Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria. PLoS Biol. 2017;15(10) doi: 10.1371/journal.pbio.2003489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shia A.K., Glittenberg M., Thompson G., Weber A.N., Reichhart J.M., Ligoxygakis P. Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. J. Cell Sci. 2009;122(Pt 24):4505–4515. doi: 10.1242/jcs.049155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.C., Barillas-Mury C., Jacobs-Lorena M. Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 2015;112(26):E3412–E3420. doi: 10.1073/pnas.1420078112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.C., King J.G., Tao D., Zeleznik O.A., Brando C., Thallinger G.G., Dinglasan R.R. Molecular profiling of phagocytic immune cells in Anopheles gambiae reveals integral roles for hemocytes in mosquito innate immunity. Mol. Cell. Proteomics. 2016;15(11):3373–3387. doi: 10.1074/mcp.M116.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall T.D., Elliott D.A., Brand A.H. The GAL4 system: a versatile toolkit for gene expression in Drosophila. CSH Protoc. 2008 doi: 10.1101/pdb.top49. 2008: doi:10.1101/pdb.top49. [DOI] [PubMed] [Google Scholar]
- Strand M.R. The insect cellular immune response. Insect Sci. 2008;15(1):1–14. [Google Scholar]
- Terenius O., Marinotti O., Sieglaff D., James A.A. Molecular genetic manipulation of vector mosquitoes. Cell Host Microbe. 2008;4(5):417–423. doi: 10.1016/j.chom.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen E.K., Koimbu G., Pulford J., Jamea-Maiasa S., Ura Y., Keven J.B., Siba P.M., Mueller I., Hetzel M.W., Reimer L.J. Mosquito behavior change after distribution of bednets results in decreased protection against malaria exposure. J. Infect. Dis. 2017;215(5):790–797. doi: 10.1093/infdis/jiw615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton L.M., Povelones M., Christophides G.K. Anopheles gambiae blood feeding initiates an anticipatory defense response to Plasmodium berghei. J. Innate. Immun. 2015;7(1):74–86. doi: 10.1159/000365331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia R.H., Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 1996;22(2):367–378. doi: 10.1046/j.1365-2958.1996.00120.x. [DOI] [PubMed] [Google Scholar]
- Vanlandingham D.L., Hong C., Klingler K., Tsetsarkin K., McElroy K.L., Powers A.M., Lehane M.J., Higgs S. Differential infectivities of o'nyong-nyong and chikungunya virus isolates in Anopheles gambiae and Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 2005;72(5):616–621. [PubMed] [Google Scholar]
- Volohonsky G., Hopp A.K., Saenger M., Soichot J., Scholze H., Boch J., Blandin S.A., Marois E. Transgenic expression of the anti-parasitic factor TEP1 in the malaria mosquito Anopheles gambiae. PLoS Pathog. 2017;13(1) doi: 10.1371/journal.ppat.1006113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volohonsky G., Terenzi O., Soichot J., Naujoks D.A., Nolan T., Windbichler N., Kapps D., Smidler A.L., Vittu A., Costa G., Steinert S., Levashina E.A., Blandin S.A., Marois E. Tools for Anopheles gambiae transgenesis. G3 (Bethesda) 2015;5(6):1151–1163. doi: 10.1534/g3.115.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2019. World Malaria Report 2019. [Google Scholar]
- Williams M.C., Woodall J.P., Corbet P.S., Gillett J.D. O'nyong-Nyong fever: an epidemic virus disease in east africa. 8. Virus isolations from Anopheles mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 1965;59:300–306. doi: 10.1016/0035-9203(65)90012-x. [DOI] [PubMed] [Google Scholar]
- Zhao B., Hou Y., Wang J., Kokoza V.A., Saha T.T., Wang X.L., Lin L., Zou Z., Raikhel A.S. Determination of juvenile hormone titers by means of LC-MS/MS/MS and a juvenile hormone-responsive Gal4/UAS system in Aedes aegypti mosquitoes. Insect Biochem. Mol. Biol. 2016;77:69–77. doi: 10.1016/j.ibmb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Kokoza V.A., Saha T.T., Wang S., Roy S., Raikhel A.S. Regulation of the gut-specific carboxypeptidase: a study using the binary Gal4/UAS system in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2014;54C:1–10. doi: 10.1016/j.ibmb.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon request.