Abstract
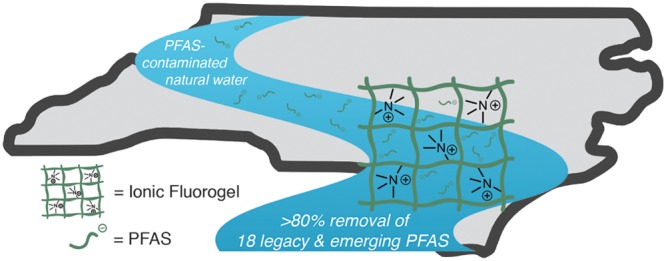
Per- and polyfluorinated alkyl substances (PFASs) contaminate groundwater, surface water, and finished drinking water internationally. Their ecological persistence and adverse human health effects demand effective remediation approaches. Motivated by the limitations in selectivity and performance of current PFAS removal technologies, we report a platform approach for the development of ionic fluorogel resins that effectively remove a chemically diverse mixture of PFAS from water. The synthesis of a material library with systematic variation in fluorous and ionic components led to the identification of a resin that demonstrated rapid removal of PFASs with high affinity and selectivity in the presence of nonfluorous contaminants commonly found in groundwater. The material can be regenerated and reused multiple times. We demonstrate ionic fluorogels as effective adsorbents for the removal of 21 legacy and emerging PFASs from settled water collected at the Sweeney Water Treatment Plant in Wilmington, North Carolina.
Short abstract
The ionic fluorogel resin developed herein removes a broad spectrum of PFAS compounds from water collected in the Cape Fear River Basin of North Carolina.
PFASs are a class of fluorinated compounds that are widely used as surfactants in the production of poly(tetrafluoroethylene) (Teflon), as water/stain repellent coatings in consumer products, and as components of fire retardants in aqueous film forming foams1−4. PFASs are distributed widely from contamination sites through waterways,5 and their long-term ecological persistence and adverse human health effects6−11 have resulted in increased regulatory attention to the concentration of PFASs in finished drinking water.12,13 The U.S. Environmental Protection Agency has set a lifetime health advisory level of 70 ng/L for the combined concentration of perfluorooctanoic acid (PFOA) and perfluoro-1-octanesulfonic acid (PFOS) in drinking water.14,15 Consequently, PFOA and PFOS were phased out in the United States in 2015 and were replaced with short-chain PFASs such as perfluoro-2-propoxypropanoic acid (GenX).16,17 While a complete toxicity assessment for GenX is ongoing, the US Environmental Protection Agency draft assessment concludes that it is less toxic than PFOA or PFOS,18 but there is suggestive evidence of carcinogenic potential for GenX.19 Currently, the state of North Carolina has set an upper limit of 140 ng/L for GenX as an emerging contaminant in drinking water due to widespread contamination of the Cape Fear River Watershed.20
A primary challenge for developing a resin for PFAS remediation is that nonfluorinated organic and inorganic species are present in natural waters at 3–8 orders of magnitude higher concentration than PFASs.21 Current and emerging PFAS remediation technologies typically remove waterborne contaminants nonspecifically, resulting in saturation by nonfluorinated species.22,23 For example, current PFAS remediation efforts using granular activated carbon (GAC) demonstrate substantial breakthrough at modest treatment volumes for short-chain PFASs.24 Furthermore, the binding affinities of organic contaminants to GAC are often higher than those of PFASs, which can result in PFASs leaching into filtered water over time.24,25 Emerging technologies include porous organic polymer adsorbents26−35 and ion exchange materials that contain a fluorinated component.36−38 While these materials show great promise for adsorbing long-chain PFASs, they are still at an early stage of development, have generally not been tested in real water, and/or display modest selectivity for short-chain PFASs.
We identified a materials design platform to remediate PFASs from water by combining two complementary strategies—fluorophilic sorption and targeted ion exchange. Our conceptual approach leverages the fluorophilicity of PFASs to selectively partition these micropollutants into a resin, similar to the method commonly used to separate desired fluorous-tagged products or catalysts from complex reaction mixtures.39−41 In addition, we reasoned that the incorporation of a tunable density of charged functional groups would enable ion exchange and sequestration of charged PFASs. We chose perfluoropolyethers (PFPEs) as the fluorophilic matrix material for resin development.42−44 PFPEs are amorphous, low-molecular-weight perfluorinated oligomers that are synthesized from the gas phase without the use of perfluorinated surfactants.45,46 PFPEs can be synthesized using supercritical CO2 as a solvent,47 and they are easier to oxidatively degrade than perfluorocarbons at the termination of their useful lifetime.48 The ionic fluorogels (IFs) described herein leverage a synergistic combination of fluorophilicity and ion exchange to generate high-performing and selective resins for PFAS remediation. The optimized materials demonstrate removal of a broad range of both legacy and emerging PFASs at environmentally relevant concentrations from settled water collected at the Sweeney Water Treatment Plant in Wilmington, NC.
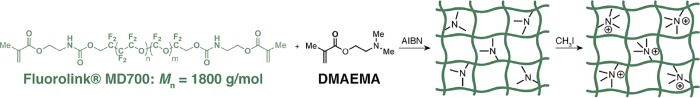
The synthesis of ionic fluorogels was achieved through thermally initiated radical copolymerization initiated by azobis(isobutyronitrile) (AIBN) of a commercially available PFPE with methacrylate chain-end functionality (Fluorolink MD 700) and an amine-containing monomer (2-dimethylaminoethyl methacrylate, DMAEMA) (Scheme 1). The composition of DMAEMA was varied from 10 to 60 wt % with respect to the total weight of the IF to generate a systematic library of materials that varies the ratio of fluorophilic and charged components. A portion of each formulation was subsequently treated with methyl iodide to access materials with quaternary ammonium groups that act as permanent charged species. Grinding and sieving the material provided a granular formulation with particle size between 75 and 125 μm for evaluation. This approach rapidly provides diverse IF formulations from the polymerization of commercially available components. A separate library of materials was prepared to act as negative controls in our structure–property studies. First, a PFPE elastomer with no electrostatic component (no DMAEMA) was polymerized. Second, nonfluorous ionic gels with charged groups but without a fluorous component were synthesized through the radical copolymerization of polyethylene glycol dimethacrylate (PEGMA Mn = 750 g/mol) and DMAEMA. This particular PEGMA was chosen to mimic a similar degree of polymerization between cross-links as Fluorolink MD 700. No significant difference was observed for the swelling ratio of these tightly cross-linked networks, indicating that it will not have a large influence on resin performance (Figure S16).
Scheme 1. Polymerization and Quaternization of Ionic Fluorogels.
Initial PFAS removal efficiency of each IF formulation was tested by conducting batch equilibrium adsorption experiments in simulated natural water, which was formulated by adding 200 mg/L NaCl and 20 mg/L humic acid to deionized water. Three substrates that represent long-chain (PFOA), short-chain (perfluorohexanoic acid, PFHxA), and branched (GenX) PFASs were spiked into the matrix each at 50 μg/L. This selection of PFASs targeted important legacy and emerging contaminants with various adsorption profiles. After exposing the contaminated water sample to 100 mg/L of ionic fluorogel for 21 h, PFAS removal efficiency was analyzed by liquid chromatography mass spectrometry (LC-MS). At this high PFAS concentration, PFAS removal by tertiary and quaternary IF was efficient, demonstrating >80% removal of PFASs in most cases (Figure S1). The exceptional adsorption of PFASs at these high concentrations led us to probe PFAS removal in a more challenging environmentally relevant scenario (1.0 μg/L of each PFAS, 10 mg/L ionic fluorogel, 21 h). The results of this systematic study revealed valuable structure–property information (illustrative results in Figure 1, complete results in Figure S2). Ionic fluorogels containing tertiary amines (IF-X, X = wt % amine comonomer incorporation) demonstrated lower affinity for PFASs than the respective materials that contained quaternary ammonium groups (IF-X+, X+ = wt % ammonium comonomer incorporation) across all formulations tested (Figure 1A), proving the importance of incorporating permanent charge. The highest performing formulations contained between 20 and 40 wt % ammonium comonomer incorporation (IF-20+ through IF-40+), demonstrating >80% removal of short-chain PFASs (PFHxA and GenX). We hypothesize that these formulations have enough ammonium content to enable efficient surface wetting while still containing enough fluorous content to provide selective PFAS adsorption.
Figure 1.
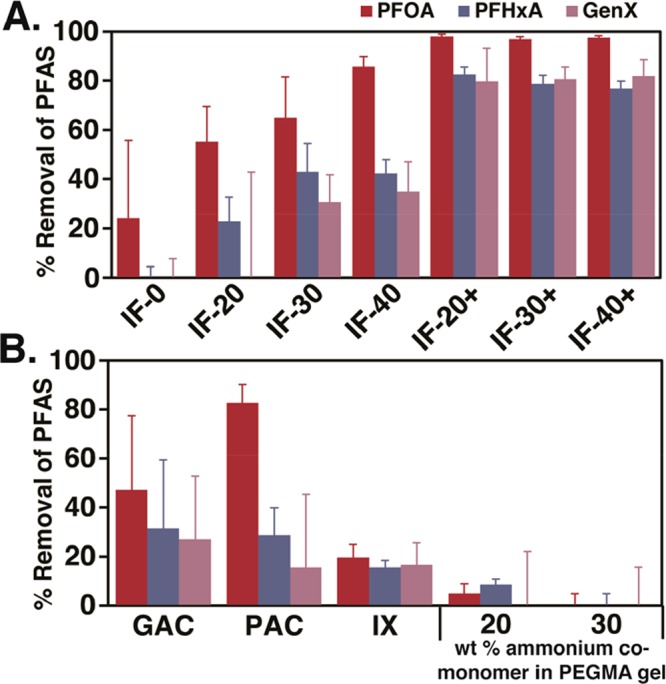
(A) Equilibrium PFAS removal by ionic fluorogels with amine (IF-X) or ammonium (IF-X+) groups where X = 0, 20, 30, or 40 wt %. (B) Equilibrium PFAS removal by GAC, powdered activated carbon (PAC), ion exchange resin (IX), and PEGMA gels with 20 or 30 wt % ammonium comonomer. Water constituents, 200 mg/L NaCl and 20 mg/L humic acid; pH = 6.4; [sorbent] = 10 mg/L; [PFAS]0 = 1 μg L–1 each; equilibrium time, 21 h. Error bars: standard deviation of 3 experiments.
Comparing ionic fluorogels against materials made to serve as controls illustrated the synergistic roles of fluorous interactions and ion exchange behavior. Removing ionic groups and exposing IF-0 (Figure 1A), an ionic fluorogel made solely of Fluorolink, to the equilibrium adsorption experiment led to no removal of PFHxA or GenX and modest PFOA removal. Furthermore, PEGMA gels made with a nonfluorous hydrocarbon equivalent of PFPEs demonstrated poor results for all formulations tested (<10% removal for all PFASs, Figure 1B).
Commercial materials representing the current state-of-the-art for PFAS removal were subsequently tested under these equilibrium adsorption conditions.49 Samples of GAC (Filtrasorb 400, Calgon Carbon), powdered activated carbon (PAC, PicaHydro MP23, Lenntech), and an anion exchange resin (IX, PFA 694E, Purolite) were exposed to simulated natural water for 21 h at a resin loading of 10 mg/L.12,21,50 Under these challenging conditions, the limitations of current technology are evident, especially for the adsorption of short-chain PFASs (Figure 1B). These head-to-head comparisons demonstrate the selectivity of ionic fluorogels for PFASs compared to conventional technologies, especially in a complex matrix that contains a 20 000 times higher concentration of organic contaminants (humic acid) compared to each PFAS.
GenX was chosen as an emerging short-chain contaminant to understand the kinetics of adsorption and capacity of ionic fluorogels. IF-20+ and IF-30+ were chosen as high-performing materials for further study. The adsorption kinetics of GenX at high concentration (200 μg/L) by IF-20+ (100 mg/L) were analyzed in deionized water (Figure 2). Rapid and quantitative removal of GenX was observed within 30 s. At 72 h, no desorption was observed, suggesting that the adsorption into the ionic fluorogel is irreversible under these conditions. Similarly, the adsorption kinetics at an environmentally relevant concentration of GenX (1 μg/L) by IF-20+ (10 mg/L) were also rapid, demonstrating 94% removal within 30 min and no desorption over time (Figure 2). This removal efficiency for GenX results in a final concentration (60 ng/L) is under the limit set by the state of North Carolina (140 ng/L).
Figure 2.
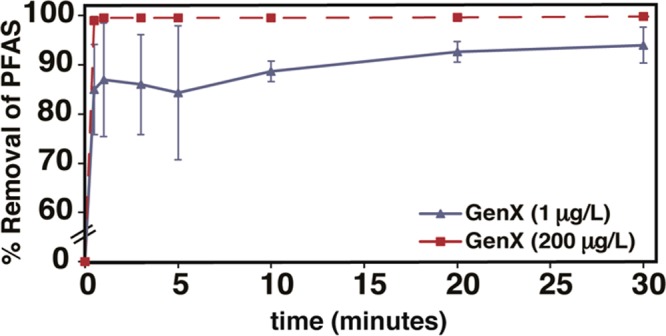
Time dependent GenX sorption by IF-20+ at high (red, dashed; GenX = 200 μg/L; sorbent = 100 mg/L) and low (blue, solid; GenX = 1 μg/L; sorbent = 10 mg/L) concentration. pH = 9.7. Error bars: standard deviation of 3 experiments.
A GenX binding isotherm was constructed to understand the binding capacity of IF-20+.51 The concentration of IF-20+ was fixed at 100 mg/L while the GenX concentration was varied from 0.20 to 50 mg/L. Data from triplicate experiments (Figure 3A) was fitted to the Langmuir adsorption model to yield an affinity coefficient (KL) of 5.9 × 106 M–1 and an estimated GenX capacity (Qm) of 278 mg/g. These represent the highest reported values in the literature for GenX at an environmentally relevant pH.31 The isotherm was also fitted to the Freundlich model, and Freundlich’s constant (KF) and the intensity of adsorption (n) were found to be 141 (mg/g)(L/mg)1/n and 2.2, respectively.31
Figure 3.
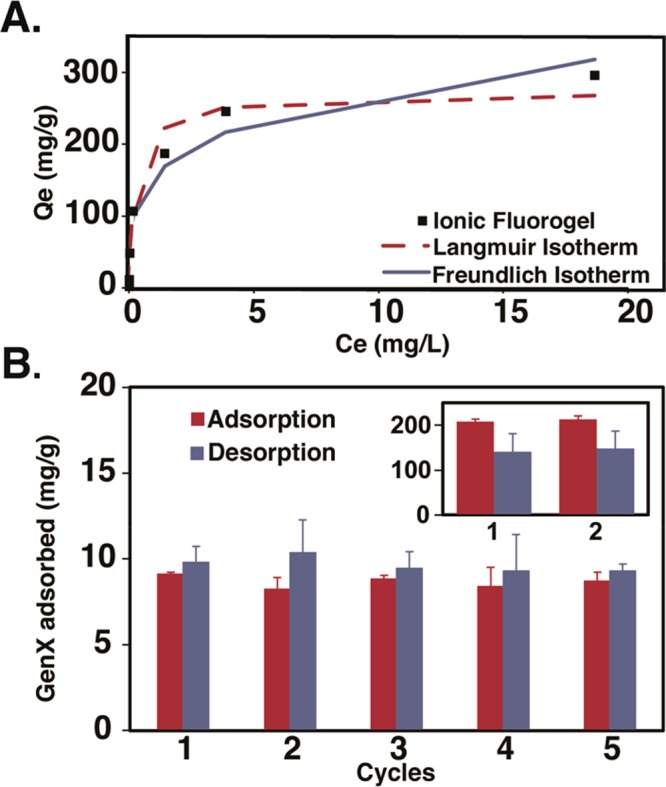
(A) GenX adsorption isotherm by IF-20+ (Adsorbent = 100 mg/L; GenX = 0.2–50 mg/L). Lines show fit to Langmuir (red, dashed) and Freundlich (blue, solid) models. (B) Regeneration and reuse of IF-20+. Sorbent = 20 mg, [GenX] = 10 mg/L (20 mL); extraction with 400 mM methanolic ammonium acetate (20 mL). Inset: sorbent = 5 mg, [GenX] = 200 mg/L (5 mL); extraction with 400 mM methanolic ammonium acetate (5 mL). Error bars: standard deviation of 3 experiments.
Subsequently, IF-20+ was tested for its ability to be regenerated for multiple reuse cycles (Figure 3B). Adsorption experiments were performed by loading IF-20+ (20 mg) onto a PTFE syringe filter (0.45 μm, 25 mm diameter). A GenX solution (10 mg/L, 20 mL) was passed through the filter over 2 min, and the residual GenX concentration in the filtrate was analyzed by LC-MS. The results showed >90% removal of GenX from the solution in such flow-through conditions, thus demonstrating the efficiency of adsorption even under short residence time conditions. Complete extraction of adsorbed GenX by IF-20+ was achieved by washing the material with a 400 mM methanolic ammonium acetate solution (20 mL, 2 min). This process was repeated 5 times without loss of efficiency in adsorption or reuse. Complementary to these rapid and low capacity regeneration investigations, we subjected IF-20+ (5 mg) to an analogous experiment under saturation conditions ([GenX] = 200 mg/L, 5 mL) similar to the scenario encountered during long-term water treatment. Exposure of IF-20+ nearly saturated with GenX to a 400 mM methanolic ammonium acetate solution led to efficient regeneration and was competent for an additional reuse, which represents an attractive attribute of these materials (Figure 3B, inset).
The ionic fluorogels provided rapid, efficient, and high capacity removal of a variety of PFASs under laboratory conditions. Real water matrices, however, contain an unpredictable cocktail of organic and inorganic contaminants that are difficult to model in a laboratory setting. To validate ionic fluorogels as a promising technology for PFAS removal from water, we obtained settled water collected at a site previously affected by PFAS contamination, the Sweeney Water Treatment Plant in Wilmington, NC.12 The water contained a total organic content (TOC) of 1.3 mg/L and had a pH of 6.2. In addition to PFASs found in the water upon collection (at levels of 20–50 ng/L), we spiked the matrix with 21 emerging and legacy PFASs. The real water matrix was exposed to IF-20+ and IF-30+ (100 mg/L), and PFAS removal was analyzed at 30 min and 2 h, with the data presented being the average of two experiments. After 2 h, short-chain PFASs that are traditionally challenging to adsorb, including PFHxA, GenX, and perfluorobutanesulfonate (PFBS) are removed from the water at >95% efficiency (representative data shown in Figure 4, complete analysis in Figure S11). We did not see evidence of long-chain PFASs such as PFOA and PFOS in the solution down to the detection limit of the LC-MS. Notably, IF-30+ removed all three perfluorinated sulfonic acids (PFBS, PFHxS, PFOS) at >95% efficiency, demonstrating the selectivity of the ionic fluorogel for PFASs beyond carboxylic acids. Lastly, IF-30+ performed impressively for removing the short-chain perfluorinated carboxylic acids PFBA (60%) and PFPeA (87%), which contain only 3 and 4 perfluorocarbons, respectively (Figure 4)30,31,34 and have demonstrated decreased sorption relative to the sulfonic acid analogues.52
Figure 4.
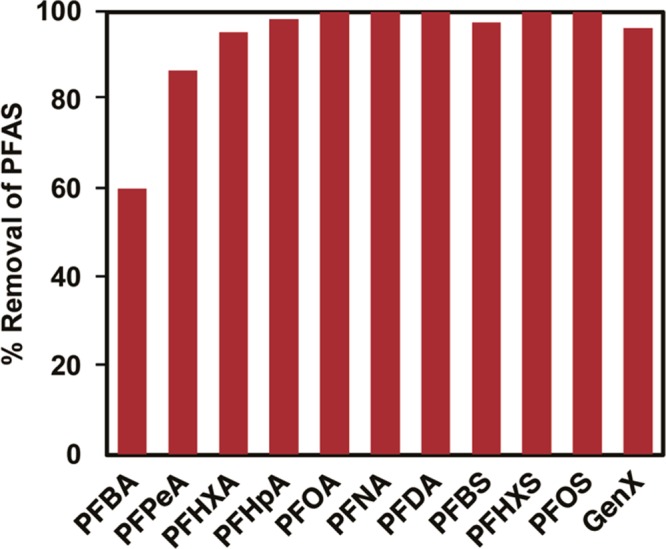
Removal of PFASs after 2 h by IF-30+ from settled water collected at the Sweeney Water Treatment Plant in Wilmington, NC. TOC = 1.3 mg/L; pH = 6.2; [sorbent] = 100 mg/L; [PFAS]0 = 1 μg/L each. Ten representative PFASs shown, complete analysis shown in Figure S11.
In conclusion, we introduce ionic fluorogels as a platform polymeric adsorbent to remove PFASs from water at environmentally relevant concentrations. The synergistic combination of fluorous and electrostatic interactions results in the high affinity, high capacity, and rapid sorption of a variety of PFASs from real water collected at an affected site in the Cape Fear River Watershed of North Carolina. The systematic material library and relationships identified between fluoropolymer and ionic content suggest general structure–property criteria to design improved sorbents for PFASs.
Acknowledgments
We acknowledge the members of the North Carolina PFAS Testing Network for insightful discussions and support surrounding PFAS in North Carolina.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.9b01224.
Materials and methods; experimental details; comprehensive data of all adsorption, regeneration, and kinetic studies; synthetic details; and additional characterization data (PDF)
Author Contributions
§ E.K. and I.M.M. contributed equally to this work
This research was supported by the North Carolina Policy Collaboratory from funding appropriated by the North Carolina General Assembly. Instrumentation used in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award P30ES010126. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare the following competing financial interest(s): The University of North Carolina at Chapel Hill has filed for intellectual property protection based on these findings.
Supplementary Material
References
- Banks R. E.; Smart B. E.; Tatlow J. C.. Organofluorine Chemistry: Priciples and Comercial Applications; Springer US: Boston, MA, 1994. [Google Scholar]
- Knepper T. P.; Lange F. T.. Polyfluorinated Chemicals and Transformation Products; Springer Science & Business Media, 2011; Vol. 17. [Google Scholar]
- Kotthoff M.; Müller J.; Jürling H.; Schlummer M.; Fiedler D. Perfluoroalkyl and Polyfluoroalkyl Substances in Consumer Products. Environ. Sci. Pollut. Res. 2015, 22 (19), 14546–14559. 10.1007/s11356-015-4202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom A. B.; Strynar M. J.; Libelo E. L. Polyfluorinated Compounds: Past, Present, and Future. Environ. Sci. Technol. 2011, 45 (19), 7954–7961. 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Hu X. C.; Andrews D. Q.; Lindstrom A. B.; Bruton T. A.; Schaider L. A.; Grandjean P.; Lohmann R.; Carignan C. C.; Blum A.; Balan S. A.; et al. Detection of Poly- and Perfluoroalkyl Substances (PFASs) in U.S. Drinking Water Linked to Industrial Sites, Military Fire Training Areas, and Wastewater Treatment Plants. Environ. Sci. Technol. Lett. 2016, 3 (10), 344–350. 10.1021/acs.estlett.6b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow L. A.; Groth A. C.; Winquist A.; Shin H.-M.; Bartell S. M.; Steenland K. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environ. Health Perspect. 2016, 124 (8), 1227–1233. 10.1289/ehp.1510391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaak I.; de Cock M.; de Boer M.; Lamoree M.; Leonards P.; van de Bor M. Prenatal Exposure to Perfluoroalkyl Substances and Behavioral Development in Children. Int. J. Environ. Res. Public Health 2016, 13 (5), 511. 10.3390/ijerph13050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria A.; Trachtman H.; Malaga-Dieguez L.; Trasande L. Association between Perfluoroalkyl Acids and Kidney Function in a Cross-Sectional Study of Adolescents. Environ. Health 2015, 14 (1), 89. 10.1186/s12940-015-0077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry V.; Winquist A.; Steenland K. Perfluorooctanoic Acid (PFOA) Exposures and Incident Cancers among Adults Living near a Chemical Plant. Environ. Health Perspect. 2013, 121 (11–12), 1313–1318. 10.1289/ehp.1306615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D.; Rice N.; Depledge M. H.; Henley W. E.; Galloway T. S. Association between Serum Perfluorooctanoic Acid (PFOA) and Thyroid Disease in the US National Health and Nutrition Examination Survey. Environ. Health Perspect. 2010, 118 (5), 686–692. 10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. T.; Adami H.-O.; Boffetta P.; Wedner H. J.; Mandel J. S. A Critical Review of Perfluorooctanoate and Perfluorooctanesulfonate Exposure and Immunological Health Conditions in Humans. Crit. Rev. Toxicol. 2016, 46 (4), 279–331. 10.3109/10408444.2015.1122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.; Arevalo E.; Strynar M.; Lindstrom A.; Richardson M.; Kearns B.; Pickett A.; Smith C.; Knappe D. R. U. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 2016, 3 (12), 415–419. 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Cousins I. T.; Vestergren R.; Wang Z.; Scheringer M.; Mclachlan M. S. The Precautionary Principle and Chemicals Management : The Example of Perfluoroalkyl Acids in Groundwater. Environ. Int. 2016, 94, 331–340. 10.1016/j.envint.2016.04.044. [DOI] [PubMed] [Google Scholar]
- Boone J. S.; Vigo C.; Boone T.; Byrne C.; Ferrario J.; Benson R.; Donohue J.; Simmons J. E.; Kolpin D. W.; Furlong E. T. Per-and Polyfluoroalkyl Substances in Source and Treated Drinking Waters of the United States. Sci. Total Environ. 2019, 653, 359–369. 10.1016/j.scitotenv.2018.10.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA); EPA, 2016.
- Strynar M.; Dagnino S.; McMahen R.; Liang S.; Lindstrom A.; Andersen E.; McMillan L.; Thurman M.; Ferrer I.; Ball C. Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS). Environ. Sci. Technol. 2015, 49 (19), 11622–11630. 10.1021/acs.est.5b01215. [DOI] [PubMed] [Google Scholar]
- McCord J.; Strynar M. Identification of Per-and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening. Environ. Sci. Technol. 2019, 53 (9), 4717–4727. 10.1021/acs.est.8b06017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technical Fact Sheet: Draft Toxicity Assessments for GenX Chemicals and PFBS; EPA, 2019; pp 1–9.
- Human Health Toxicity Values for Hexafluoropropylene Oxide (HFPO) Dimer Acid and Its Ammonium Salt (CASRN 13252-13-6 and CASRN Also Known as “GenX Chemicals”; EPA, 2018.
- NC DHHS Preliminary Risk Assessment FAQ for GenX (Perfluoro2-Propoxypropanoic Acid); North Carolina Department of Health and Human Services, 2017.
- Ross I.; McDonough J.; Miles J.; Storch P.; Thelakkat Kochunarayanan P.; Kalve E.; Hurst J.; S. Dasgupta S.; Burdick J. A Review of Emerging Technologies for Remediation of PFASs. Remediat. J. 2018, 28 (2), 101–126. 10.1002/rem.21553. [DOI] [Google Scholar]
- Yu J.; Lv L.; Lan P.; Zhang S.; Pan B.; Zhang W. Effect of Effluent Organic Matter on the Adsorption of Perfluorinated Compounds onto Activated Carbon. J. Hazard. Mater. 2012, 226, 99–106. 10.1016/j.jhazmat.2012.04.073. [DOI] [PubMed] [Google Scholar]
- Dudley L. A.; Arevalo E. C.; Knappe D. R. U.. Removal of Perfluoroalkyl Substances by PAC Adsorption and Anion Exchange; Water Research Foundation: Denver, CO, 2015. [Google Scholar]
- Hopkins Z. R.; Sun M.; DeWitt J. C.; Knappe D. R. U. Recently Detected Drinking Water Contaminants: GenX and Other Per- and Polyfluoroalkyl Ether Acids. J. - Am. Water Works Assoc. 2018, 110 (7), 13–28. 10.1002/awwa.1073. [DOI] [Google Scholar]
- Schachtman B.CFPUA Filtered Water Still Has More Contaminants than Raw Water. Here’s Why, and What’s Being Done; Port City Daily: Wilmington, NC, 2019. [Google Scholar]
- Alsbaiee A.; Smith B. J.; Xiao L.; Ling Y.; Helbling D. E.; Dichtel W. R. Rapid Removal of Organic Micropollutants from Water by a Porous β-Cyclodextrin Polymer. Nature 2016, 529 (7585), 190–194. 10.1038/nature16185. [DOI] [PubMed] [Google Scholar]
- Xiao L.; Ling Y.; Alsbaiee A.; Li C.; Helbling D. E.; Dichtel W. R. β - Cyclodextrin Polymer Network Sequesters Perfluorooctanoic Acid at Environmentally Relevant Concentrations. J. Am. Chem. Soc. 2017, 139, 7689–7692. 10.1021/jacs.7b02381. [DOI] [PubMed] [Google Scholar]
- Ling Y.; Klemes M. J.; Xiao L.; Alsbaiee A.; Dichtel W. R.; Helbling D. E. Benchmarking Micropollutant Removal by Activated Carbon and Porous β - Cyclodextrin Polymers under Environmentally Relevant Scenarios. Environ. Sci. Technol. 2017, 51, 7590–7598. 10.1021/acs.est.7b00906. [DOI] [PubMed] [Google Scholar]
- Xiao L.; Ching C.; Ling Y.; Nasiri M.; Klemes M. J.; Reineke T. M.; Helbling D. E.; Dichtel W. R. Cross-Linker Chemistry Determines the Uptake Potential of Perfluorinated Alkyl Substances by β-Cyclodextrin Polymers. Macromolecules 2019, 52, 3747–3752. 10.1021/acs.macromol.9b00417. [DOI] [Google Scholar]
- Klemes A. M. J.; Ling Y.; Ching C.; Wu V.; Helbling E.; Dichtel W. R. Reduction of a Tetrafluoroterephthalonitrile-β-Cyclodextrin Polymer to Remove Anionic Micropollutants and Perfluorinated Alkyl Substances from Water. Angew. Chem., Int. Ed. 2019, 58, 12049–12053. 10.1002/anie.201905142. [DOI] [PubMed] [Google Scholar]
- Ji W.; Xiao L.; Ling Y.; Ching C.; Matsumoto M.; Bisbey R. P.; Helbling D. E.; Dichtel W. R. Removal of GenX and Perfluorinated Alkyl Substances from Water by Amine-Functionalized Covalent Organic Frameworks. J. Am. Chem. Soc. 2018, 140 (40), 12677–12681. 10.1021/jacs.8b06958. [DOI] [PubMed] [Google Scholar]
- Cao F.; Wang L.; Ren X.; Sun H. Synthesis of a Perfluorooctanoic Acid Molecularly Imprinted Polymer for the Selective Removal of Perfluorooctanoic Acid in an Aqueous Environment. J. Appl. Polym. Sci. 2016, 133 (15), 1–10. 10.1002/app.43192. [DOI] [Google Scholar]
- Yu Q.; Deng S.; Yu G. Selective Removal of Perfluorooctane Sulfonate from Aqueous Solution Using Chitosan-Based Molecularly Imprinted Polymer Adsorbents. Water Res. 2008, 42, 3089–3097. 10.1016/j.watres.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Ateia M.; Attia M. F.; Maroli A.; Tharayil N.; Alexis F.; Whitehead D. C.; Karanfil T. Rapid Removal of Poly- and Perfluorinated Alkyl Substances by Poly(Ethylenimine)-Functionalized Cellulose Microcrystals at Environmentally Relevant Conditions. Environ. Sci. Technol. Lett. 2018, 5 (12), 764–769. 10.1021/acs.estlett.8b00556. [DOI] [Google Scholar]
- Ateia M.; Pellizzeri S.; Attia M. F.; Tharayil N.; Anker J. N.; Karan T. Cationic Polymer for Selective Removal of GenX and Short-Chain PFAS from Surface Waters and Wastewaters at Ng/L Levels. Water Res. 2019, 163, 114874. 10.1016/j.watres.2019.114874. [DOI] [PubMed] [Google Scholar]
- Huang P. J.; Hwangbo M.; Chen Z.; Liu Y.; Kameoka J.; Chu K. H. Reusable Functionalized Hydrogel Sorbents for Removing Long- and Short-Chain Perfluoroalkyl Acids (PFAAs) and GenX from Aqueous Solution. ACS Omega 2018, 3 (12), 17447–17455. 10.1021/acsomega.8b02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda Y.; Terashima T.; Sawamoto M. Fluorous Microgel Star Polymers: Selective Recognition and Separation of Polyfluorinated Surfactants and Compounds in Water. J. Am. Chem. Soc. 2014, 136, 15742–15748. 10.1021/ja508818j. [DOI] [PubMed] [Google Scholar]
- Koda Y.; Terashima T.; Takenaka M.; Sawamoto M. Star Polymer Gels with Fluorinated Microgels via Star – Star Coupling and Cross-Linking for Water Purification. ACS Macro Lett. 2015, 4, 377–380. 10.1021/acsmacrolett.5b00127. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Curran D. P. Synthetic Applications of Fluorous Solid-Phase Extraction (F-SPE). Tetrahedron 2006, 62 (51), 11837–11865. 10.1016/j.tet.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z.; Zhang Q.; Oderaotoshi Y.; Curran D. P. Fluorous Mixture Synthesis: A Fluorous-Tagging Strategy for the Synthesis and Separation of Mixtures of Organic Compounds. Science (Washington, DC, U. S.) 2001, 291 (5509), 1766–1769. 10.1126/science.1057567. [DOI] [PubMed] [Google Scholar]
- Curran D. P. Strategy-Level Separations in Organic Synthesis: From Planning to Practice. Angew. Chem., Int. Ed. 1998, 37 (9), 1174–1196. . [DOI] [PubMed] [Google Scholar]
- Bell G. A.; Howell J.. Perfluoropolyethers. In Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; Rudnick L. R., Ed.; CRC PRess: Boca Raton, FL, 2005; pp 157–174. [Google Scholar]
- Lopez G.; Améduri B.; Habas J.-P. A Perfluoropolyether-Based Elastomers Library with on-Demand Thermorheological Features. Eur. Polym. J. 2017, 95, 207–215. 10.1016/j.eurpolymj.2017.08.007. [DOI] [Google Scholar]
- Lopez G.; Granado L.; Coquil G.; Lárez-Sosa A.; Louvain N.; Améduri B. Perfluoropolyether (PFPE)-Based Vitrimers with Ionic Conductivity. Macromolecules 2019, 52 (5), 2148–2155. 10.1021/acs.macromol.8b02493. [DOI] [Google Scholar]
- Howell J. L.; Perez E. W.; Waterfeld A.; Friesen C. M.; Thrasher J. S.. Thermally Stable Perfluoropolyethers and Processes Therefore and Therewith. US Patent US 6753301 B2, 2001.
- Faucitano A.; Buttafava A.; Commincioli V.; Marchionni G.; Pasquale R. J. D. E. Kinetic Modeling of the Low-Temperature Photo-Oxidation of Hexafluoropropene. J. Phys. Org. Chem. 1991, 4, 293–300. 10.1002/poc.610040506. [DOI] [Google Scholar]
- Bunyard W. C.; Romack T. J.; Desimone J. M. Perfluoropolyether Synthesis in Liquid Carbon Dioxide by Hexafluoropropylene Photooxidation. Macromolecules 1999, 32 (24), 8224–8226. 10.1021/ma981588l. [DOI] [Google Scholar]
- Kasai P. H. Perfluoropolyethers: Intramolecular Disproportionation. Macromolecules 1992, 25 (25), 6791–6799. 10.1021/ma00051a011. [DOI] [Google Scholar]
- Xiao X.; Ulrich B. A.; Chen B.; Higgins C. P. Sorption of Poly- and Perfluoroalkyl Substances (PFASs) Relevant to Aqueous Film-Forming Foam (AFFF)-Impacted Groundwater by Biochars and Activated Carbon. Environ. Sci. Technol. 2017, 51 (11), 6342–6351. 10.1021/acs.est.7b00970. [DOI] [PubMed] [Google Scholar]
- Zaggia A.; Conte L.; Falletti L.; Fant M.; Chiorboli A. Use of Strong Anion Exchange Resins for the Removal of Perfluoroalkylated Substances from Contaminated Drinking Water in Batch and Continuous Pilot Plants. Water Res. 2016, 91, 137–146. 10.1016/j.watres.2015.12.039. [DOI] [PubMed] [Google Scholar]
- Bolster C. H.; Green B.; Hornberger G. M. On the Use of Linearized Langmuir Equations. Soil Sci. Soc. Am. J. 2007, 71 (6), 1796–1806. 10.2136/sssaj2006.0304. [DOI] [Google Scholar]
- Higgins C. P.; Luthy R. G. Sorption of Perfluorinated Surfactants on Sediments. Environ. Sci. Technol. 2006, 40 (23), 7251–7256. 10.1021/es061000n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.