Abstract
Introduction
Adolescents experience enhanced social sensitivity and biopsychosocial changes that can be challenging. Much remains unknown about the effect of psychological characteristics and peer relationships on adolescents’ physiological responses to stress, due in part to methodological limitations.
Methods
To test how adolescents’ peer relationships and psychological characteristics are associated with their physiological and psychological response to stress, we administered the Group Public Speaking Task for Adolescents (GPST-A) to 54 adolescents (n = 40 girls; Mage = 16.6 years) from two schools in the United States. Salivary cortisol and alpha-amylase (sAA), and positive and negative affect were measured six times. Relationships among group members were measured, resulting in whole-network data. State and trait rumination, five factors of coping, and emotional reappraisal and suppression were measured along with symptoms of depression and anxiety.
Results
Greater levels of negative evaluation and victimization among group members were associated with a steeper increase and decline in the negative affect response, yet not associated with the physiological response to stress. Greater positive affect was associated with decreased cortisol reactivity, whereas negative affect was associated with steeper cortisol and sAA reactivity. Rumination, disengagement coping, and depression symptoms were related to the physiological response to stress.
Conclusions
The GPST-A is feasible to administer in a school context with adolescents to collect both physiological and psychological stress responses. Findings from the present study suggest peer relationships are important for understanding adolescents’ psychological response to stressors while psychological characteristics are important for adolescents’ physiological response to stress.
Introduction
Adolescence is characterized as a period of heightened psychological and physiological stress due to significant social, cognitive, and biological changes that occur during this developmental period (Spear, 2009; Steinberg & Lerner, 2004). Animal and human studies implicate the asymmetric development between the limbic and frontal regions of the brain and increased sensitivity to gonadal hormones in brain regions associated with emotion processing and threat detection, as contributing to the increased sensitivity to social evaluative threat and increased cortisol response to stress during this developmental period (Van den Bos, de Rooij, Miers, Bokhorst, & Westenberg, 2014; Nelson, Leibenluft, McClure, & Pine, 2005; Scherf, Smyth, & Delgado, 2013). Additionally, peer relationships also take on new importance for adolescents, referred to as social sensitivity, or a rise in the salience, emotional impact, and attention paid to social evaluation and social standing (Somerville, 2013). Thus, the peer context could be both an important source of support, but also stress during this period.
Given the neural reorganization, increased social and cognitive stress, and emergence of psychological disorders during adolescence (Dahl & Gunnar, 2009; McClure & Pine, 2007; Paus, Keshavan, & Giedd, 2008), adolescence may be a sensitive period of development where interventions focusing on competence building and wellbeing promotion may have the greatest impact on long term mental health. In turn, research examining adolescents’ physiological response in conjunction with psychological strategies for managing stress may offer new insights into how to intervene with this group to foster coping strategies to cultivate positive long-term mental health as well as physical wellbeing.
The Stress Response
Lazarus (1966) posited that the cognitive appraisal that one’s available resources are not sufficient to deal with the situational demands results in the subjective experience of stress and associated emotions. Neuroendocrine research supports this model, demonstrating that stimuli perceived as stressful – low in controllability or predictability and/or high in social evaluative threat – activate the HPA axis (Denson, Spanovic, & Miller, 2009; Hellhammer, Wüst, & Kudielka, 2009; Kirschbaum & Hellhammer, 1998). A typical response to stress triggers a cascade of coordinated biobehavioral responses beginning with a rapid release of catecholamines from the sympathetic branch of the autonomic nervous system (SNS) in the first minute of the response followed by the slower secretion of glucocorticoids from the hypothalamic-pituitary-adrenocortical (HPA) axis.
The SNS is fast-responding in its initiation of the fight-or-flight response (Cannon, 1932; Gunnar & Quevedo, 2007) and salivary alpha amylase (sAA) is considered a marker of SNS activation (Nater & Rohleder, 2009). Cortisol is a commonly used marker of HPA activity (Hellhammer et al., 2009), is a reliable indicator of emotional wellbeing (Juster et al., 2011) and has been studied extensively with samples of adolescents (e.g. Dahl & Gunnar, 2009). There is wide variation in people’s cortisol response profiles which are impacted by many factors including situational demands, life experiences, and an individual’s perception of the stressors (Kudielka et al., 2009).
The HPA axis and the SNS have the potential to influence responses to one another (Boyce & Ellis, 2005; Sapolsky, Romero, & Munck, 2000), which is why multisystem approaches have become the focus of much neuroendocrine research (Laurent, Powers, & Granger, 2013). Bauer, Quas, and Boyce (2002) suggest an additive model in which the HPA axis and the SNS augment each other and may predict outcomes better when examined together than either system alone. This is important, as dysregulation of the stress response system, presenting as either hyper- or hypo-activation (McEwen, 1998), has been linked to depression, obesity, and externalizing behavior (Agorastos, Pervandidou, Chrousos, & Kolaitis, 2018).
Longitudinal research indicates that there is a change in stress reactivity across development (for a review see: Roberts & Lopez-Duran, 2019). Previous research indicates that basal levels of HPA axis hormones (e.g., adrenocorticotropic hormone [ACTH], cortisol) increase between childhood and adulthood (see for review, Gunnar & Vazquez, 2006) and that these basal levels increase for girls, but not consistently for boys, as they enter puberty (Scheifelbein & Susman, 2006). The magnitude of the physiological response to laboratory induced stressors increases over the course of development, peaking in mid-adolescence (14–15 years old) for both boys and girls with adult-like HPA axis responses continuing after that (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009b; Sumter, Bokhorst, Miers, Van Pelt, & Westenberg, 2010). The increase in cortisol reactivity to social-evaluative threat during adolescence may be explained by pubertal development (Van den Bos et al., 2014). Studies of rats found that males and females on the cusp of adolescence show longer glucocorticoid responses to stress (e.g., 45–60 minutes longer) than adults (Romeo et al., 2006). In human studies, boys and girls in later stages of adolescence (15–17 years old) display greater peak cortisol levels after stress compared to children or early adolescents (9–13 years old) that are similar to adults’ HPA reactivity patterns (Gunnar et al., 2009b; Stroud et al., 2009). Additionally, there are substantial shifts in stress reactivity from childhood to adolescence compared to stress reactivity in adulthood (Gunnar et al., 2009b). However, sAA reactivity patterns do not show the same age nor gender differences as cortisol (Nater & Rohleder, 2009; Vrijen, van Roekel, & Oldehinkel, 2018).
Psychological constructs and the stress response.
There is a large body of literature hypothesizing how a variety of psychological characteristics and coping response styles may explain differences in physiological responses to stress (Lazarus & Folkman, 1984; Carver & Scheier,1999; Dickerson & Kemeny, 2004). For example, rumination is a response style that involves the tendency to think about causes, situations, and consequences of one’s negative emotional experiences and has been associated with depression in adolescent populations (Nolen-Hoeksema, 1991). A meta-analysis of studies examining HPA axis activity to manipulated stress situations indicated that both emotions and appraisals were related to cortisol reactivity (Denson et al., 2009). There are some patterns that persist in the literature that will be tested in this study as well. For example, those who cope by suppressing their emotions generally show heightened physiological responses (Lam, Dickerson, Zoccola, & Zaldivar, 2009; Mauss, Cook, Cheng, & Gross, 2007; Egloff, Schmukle, Burns, & Schwedtfeger, 2006; Gross & Thompson, 2007; Harris, 2001) and rumination tends to be related to greater cortisol reactivity (Gianferante et al., 2014; Zoccola, Dickerson, & Zaldivar, 2008). The literature is less clear about how other coping styles, such as emotional reappraisal, may be related to the HPA axis. Many researchers hypothesize that increased reappraisal as a coping strategy could be associated with decreased physiological reactivity (e.g., Gross & John, 2003), although the results have been somewhat mixed with respect to the relationship between reappraisal strategies and stress reactivity (Egloff et al., 2006; Lam et al., 2009; Mauss et al., 2007).
Interestingly, sAA reactivity patterns are sensitive to a wider variety of experiences (social and cognitive) than cortisol (Adam, Hoyt, & Granger, 2011; Out, Granger, Sephton, Segerstrom, 2013; Susman et al., 2010a; Payne, Hibel, Granger, Tsao, & Zeltzer, 2014). sAA increases in response to both negative and positive emotional states suggesting that, unlike cortisol, it is more of a marker of general arousal and its release may be more sensitive to emotional states rather than social evaluative threat (Adam et al., 2011; Payne et al., 2014). However, not much is known about how psychological constructs such as coping and affect relate to the sAA response to stress. Thus, examining the response profile of sAA in a group of adolescents during a social-evaluative stressor remains exploratory. Diurnal sAA has been associated with momentary affect (Adam et al., 2011) but no studies to date include a comparison of sAA reactivity with momentary affect.
There is a high incidence of both depression and anxiety in adolescence (Andersen & Teicher, 2008; Walker, Sabuwalla, & Huot, 2004). A large body research shows that the HPA axis response to social-evaluative stress is altered in populations suffering from depression and anxiety. Overall, findings vary but there are some trends relating decreases in HPA reactivity with increased symptoms of depression (Cameron, McKay, Susman, Wynne-Edwards, Wright, & Weinberg, 2016; Burke, Davis, Otte, & Mohr, 2005; Schiefelbein, & Susman, 2006; Tsigos & Chrousos, 2002) and anxiety (Nelson, Leibenluft, McClure, & Pine, 2004; Walker et al., 2004). For example, a meta-analysis revealed that individuals with major depression show blunted cortisol responses to social-evaluative stressors (Burke et al., 2005). Additionally, rumination has been shown to impair cortisol recovery post stressor among adolescents with depression, but not in adolescents without depression (Stewart, Mazurka, Bond, Wynne-Edwards, & Harkness, 2013). In turn, this study includes symptoms of depression and anxiety to add to the body of research that explores the relationships between these symptoms and adolescents’ stress response systems.
While some researchers have explored the impact of coping styles as well as symptoms of anxiety and depression on HPA axis reactivity, simplistic statistical approaches that rely on assumed hormonal peaks between participants and that compare mean values across participants has limited what is known about the impact of coping styles on the reactivity, peak activation, and recovery phases of the stress response. Indeed, it is likely that heterogeneity in the research that attempts to link psychological factors with the stress response is due to inconsistencies in measurement and the use of analytic approaches that do not consider each phase of the stress response separately (Katz & Peckins, 2017; Lopez-Duran, Mayer, & Abelson, 2014; Dickerson & Zoccola, 2013). This study seeks to address the limitations in the field by modeling participants’ stress response profiles across time to elucidate how a variety of self-reported psychological constructs might impact specific phases of the participants’ stress response. In turn, we will explore the relationship between adolescents’ physiological response to stress along with their self-reported affect during the stressor, their coping styles as well as their symptoms of anxiety and depression with a sophisticated modeling approach that allows us to look more accurately at the phases of the response.
Measuring adolescents’ HPA axis and SNS response to stress.
In studies of stress reactivity, research teams have utilized a wide variety of stress paradigms to elicit a cortisol response in the laboratory setting (Gunnar, Talge, & Herrera, 2009a); however, most stress paradigms currently in use are resource intensive, lack ecological validity, or both. For example, the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) is one of the most widely used social-evaluative stress paradigms to study the stress response as it consistently elicits a mild to moderate HPA axis response and is validated for use with children, adolescents, and adults (e.g., Gunnar et al., 2009b). However, the TSST takes about 90 minutes for one participant to complete and is primarily administered in a laboratory setting. Group formats of the TSST (TSST-G) are also common in stress research with adults (Von Dawans, Kirschbaum, & Heinrichs, 2011). The TSST-G is more efficient than the individual version of the TSST as multiple participants can be assessed simultaneously and fewer resources are required including experimental time, staff hours, and research space (e.g., Häusser, Kattenstroth, & Mojzisch, 2012; Kumsta, Chen, Pape, & Heinrichs, 2013; Von Dawans et al., 2011). Like the TSST, the TSST-G reliably activates the HPA axis and elicits a cortisol response yet both are most commonly performed in a laboratory setting, limiting their ecological validity. Due to underutilization of the TSST-G in adolescent samples and in ecologically valid settings, less is known about adolescents’ physiological response to real-world challenges in a developmentally relevant setting.
The Group Public Speaking Task for Adolescents (GPST-A) was developed to be as effective as the single subject TSST at eliciting a physiological stress response but more efficient (Hostinar, McQuillan, Mirous, Grant, & Adam, 2014). The GPST-A is ideally carried out in groups of 5 and no larger than 7, for a uniform HPA axis response across participants, with participants in the same room but separated by opaque dividers (Hostinar et al., 2014). Participants are called on randomly to present a speech about themselves to two confederate judges. Similar to the TSST, Participants are told that their performance will be evaluated and the judges are instructed to give no feedback and remain neutral during the task. In the GPST-A when participants stand to speak, they present to the confederate judges with their group members watching them from behind. The GPST-A was validated for use among adolescents between the ages of 11–18 who did not know one another in a laboratory setting as it elicited a significant increase in cortisol concentrations in participants (approximately 60% above baseline) and in self-reported negative affect with no gender differences in reactivity (Hostinar et al., 2014). In a previous methodology paper with data from the present study (Katz & Peckins, 2017), we showed that the GPST-A was both feasible and effective at eliciting a cortisol and sAA response from our adolescent participants similar to Hostinar and colleagues (2014). The magnitude of the reactivity responses of both cortisol and sAA were positively associated, indicating that participants’ SNS and HPA axis were similarly reactive to the GPST-A.
One critical gap in the field is that past research on group stressor paradigms (TSST-G or GPST-A) have not examined how the relationships between participants in the group affect the stress response. This is important, given peer relationships have the potential to impact participants’ physiological and psychological response to stressors, especially in situations where group-members know one another. Previous research suggests that both social support and negative relationships influence participants’ responses to the TSST (Kirschbaum, Klauer, Filipp, & Hellhammer, 1995; Knack, Jensen-Campbell, & Baum, 2011). Another study showed that negative peer relationships, particularly peer victimization experienced in childhood, are associated with a dysregulated stress response in adolescence (Knack et al., 2011). In a recent study with children, researchers correlated children’s perceptions of their current peer relationships with their cortisol and sAA levels and found that children with lower levels of these hormones reported the highest density of friendships (Ponzi, Muehlenbein, Geary, & Flinn, 2016). Given adolescents’ sensitivity to social evaluation, it is imperative that research on the stress response system in adolescence directly examine the influence peer relationships have on the stress response in social-evaluative paradigms.
There is substantial individual variability in the physiological response to acute stress including individual variation in baseline concentrations, reactivity and peak activation, and recovery following stressors (Hellhammer et al., 2009). Additionally, peer relationships impact adolescents’ psychological assessments (Albert, Chein, & Steinberg, 2013) and both the HPA and SNS are influenced by social dynamics during the stress response (Massey, Byrd-Craven, Auer, & Swearingen, 2015; Stroud et al., 2009). Understanding the body’s physiological response to stress is important for understanding the mechanisms involved in risk for and resilience against health and behavior problems. Limited research is available on the physiological and psychological response to ecologically-valid stressors in adolescents. Further, it is not known how peer relationships affect the physiological and psychological response to stress. The present study capitalizes on the ecological validity created by the setting – inside a school building among adolescents who were classmates – to offer a model for exploring both psychological characteristics and social connections in relation to stress reactivity.
The Present Study
In a previous study with these data, we demonstrated the effectiveness of the GPST-A at eliciting a cortisol and sAA response in adolescents in a school setting (Katz & Peckins, 2017). Furthermore, we used a novel, multisystem analytic approach that captured the intraindividual variability in responsivity of the HPA axis and the SNS following the GPST-A (Katz & Peckins, 2017). The present study seeks to expand on our previous research by exploring how connections with peers and a sampling of psychological constructs (affect during the visit, coping styles, symptoms of depression or anxiety) may be related to the response trajectories of cortisol and sAA during the GPST-A. Thus, the aims of the present study were two-fold. First, to demonstrate how whole-network data collected during each visit can be used to test the impact that relationships among group members may have on participants’ physiological and psychological responses to a stressful event. Second, to determine if any of the psychological constructs measured through self-report – momentary affect during the visit, coping styles, and symptoms of depression and anxiety – influenced their cortisol and sAA reactivity profiles in response to the GPST-A.
Methods
Recruitment and Participants
Students from two suburban high-schools (enrollment = ~1500 in each) were recruited to participate in this study. Researchers utilized established relationships with teachers in the schools to gain access to classes for recruitment and buy-in from the principals. Once researchers met with the principals and teachers, the superintendent was contacted to grant the final permission to recruit students and conduct the study at the schools. Authorization to recruit participants and conduct the study came from the district and school levels. Students from Grade 11 health classes were recruited because of enthusiasm from the teachers and it is a required course for all students. This recruitment strategy allowed us to target the largest number of participants who were potentially in later stages of puberty (Susman et al., 2010b) but were still in high school because pubertal development is known to impact physiological stress reactivity (Sumter et al., 2010). Researchers made a 10-minute presentation about the study and passed out fliers about the study with consent forms to students in their health class, reaching all juniors (~800 total students recruited). Teachers hung fliers about the study in their classrooms and the library as well as facilitated the collection of parental permission forms from students who wanted to participate. Researchers contacted students who returned consent forms via email to schedule an after-school meeting. The researchers worked with the principals and janitorial staff to find space at the schools where the study could take place every day after school for a number of weeks without disrupting extracurricular activities.
No students who returned consent forms were excluded from the study. Fifty-four adolescents (female=40, male=14, mean age=16.6 years) from two high schools participated in the study. Participants predominantly identified as Caucasian (n = 40) but also identified as Asian or Filipino (n = 4); Black or African American (n = 4); Hispanic, Latino/a, or Spanish (n = 1), or Biracial (n = 5). All participants spoke English fluently, had written consent from their parents, and provided assent to participate in the current study.
Procedure
All data collection procedures were conducted following a protocol approved by the Institutional Review Board at The Pennsylvania State University. Data were collected through two methods: self-report questionnaires and in-person physiological assessment. Participants attended one 90-minute appointment after-school in a classroom in the participant’s school and were part of groups made up of their classmates. Of the 12 total groups, 9 groups included 5 participants which was the target group size for this study based on findings from Hostinar and colleagues (2014). Due to last minute participant absences there were 3 groups with fewer than 5 participants; 1 group included 4, 1 group included 3, and 1 group included 2 participants. Data collection took place over a four-week period after school hours between 2:30 and 4:00 PM to control for the normative diurnal decline in cortisol.
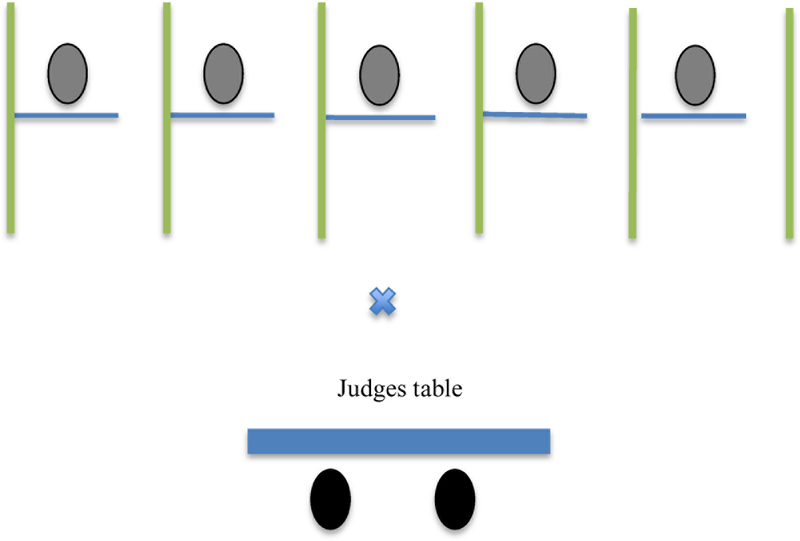
Each of the group members sat at a desk with opaque dividers between them (Figure 1). Dividers were made of PVC frame and opaque material that could be easily transported to and stored at the schools. After giving assent, participants were instructed on how to provide samples of saliva via passive drool and gave a practice sample (Time 0). Then participants completed computer-based questionnaires consisting of demographic information as well as cognitive and emotion measures during a 35-minute relaxation period. At the end of this period, the baseline saliva sample (Time 1) was collected. Participants then heard the task instructions for the GPST-A.
Figure 1.
GPST-A room layout Adapted from Hostinar et al., 2014.
The Group Public Speaking Task for Adolescents (GPST-A).
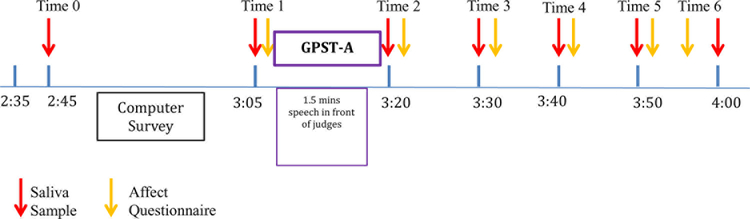
This study utilized the GPST-A (Hostinar et al., 2014). Participants were asked to give a 1.5 minute speech and to imagine they were introducing themselves to new classmates. Participants were told that they would be called on randomly to present their speech to two judges who would be assessing their speech and video recording them. Research assistants acting as judges were trained to have serious looks and to provide no feedback during the speech task. When participants stepped forward to give their speeches to the judges, they stepped in front of their desks and beyond the opaque dividers where they could be seen by, but not see, their peers (see Figure 1). Immediately after all speeches were completed, participants provided a saliva sample (Time 2) and then completed self-report measures assessing emotional responses to the stressors (i.e., current emotion ratings). These measures took approximately two minutes to complete. Once finished participants were instructed to sit quietly to wait for the judges’ feedback. After ten minutes participants provided another saliva sample and filled out the same self-report measures and then were told the judges were still deliberating (Time 3). Participants provided saliva and completed self-report measures of current emotion ratings on three additional occasions (Time 4 – Time 6; see Figure 2 for experimental timeline). After the final saliva sample and self-report (Time 6), a trained research assistant told the participants that the judges were fake and that their speeches were not being scored and compared to their peers. After participants were debriefed, participants were allowed to ask questions about the study and were given $20 gift certificates and pizza at the end of the appointment. Participants were also entered in a drawing for an iPad mini.
Figure 2.
Experimental timeline. Times are +/− 10 minutes depending on arrival time.
Salivary Cortisol and Alpha-Amylase (sAA).
Saliva samples were collected at six points via passive drool before and after the GPST-A (Time 1 – Time 6). Sampling procedure included one baseline sample prior to GPST-A, and five additional samples at approximately 10-minute increments starting at 0 minutes post-stressor to capture peak reactivity concentrations and recovery (Figure 2). Participants were given one minute to complete their saliva samples with a goal of 1ml of saliva.
Assays were completed by the Biomarker Core Lab at the Pennsylvania State University (University Park, PA). Cortisol and sAA concentrations in each of the six salivary samples (Time 1-Time 6) were determined using Salimetrics assay kits (State College, PA). The first sample (Time 0) was not assayed because it reflects cortisol and sAA response to a novel experience (coming into the appointment for the experiment) and not cortisol or sAA at rest. Saliva samples for cortisol values were assayed in duplicate, averaged, and converted to nmol/L to produce values for analysis. For salivary cortisol, inter-assay covariances were less than 10% and intra-assay covariances were less than 5%. Saliva samples for sAA were assayed in singlet and converted to U/mL. The inter-assay coefficient of variability was 5.05%. To correct for skew and kurtosis, a log transformation was applied to cortisol and sAA values, and those log-transformed variables were used in analyses.
Social Connectedness Survey.
The Social Connectedness Survey (SCS) was used to examine the relationships between group members and their possible impact on participants’ reaction to the GPST-A. The SCS was developed for the present study by adapting The Social Anxiety Scale for Children (La Greca & Stone, 1993) and victimization scales developed by Olweus (1991) and Schwartz, Farver, Chang, and Lee-Shin (2002). Prior to survey administration, all participants within a group were assigned a number based on their speech order. The SCS consisted of 8 items and the first item asked participants how well they knew each participant identified by their assigned number. Responses included ‘this is me’, ‘not at all’, ‘somewhat’ or ‘very well’. For each participant number, if participants indicated ‘this is me’ or ‘not at all’, they did not answer the remaining 7 items for that participant number. If participants indicated that they knew a group member ‘somewhat’ or ‘very well’, they completed the remaining seven items (below). Participants rated their feelings toward each participant that they knew ‘somewhat’ or ‘very well’ on a 3-point Likert scale: ‘Not at all’, ‘Somewhat’ and ‘Very well/much/a lot’.
How much do you like participant #X?
How much do you trust/feel comfortable with participant #X?
This person understands me.
I worry about what this person thinks of me.
I’m afraid this person won’t like me.
How often has this person been physically or verbally mean to you? (includes behaviors in-person, via text and online)
How often has this person gossiped about you, kept you out of a group, ignored you or said negative things about you behind your back? (includes via text and online)
The SCS consists of four scales: (1) Victimization captured the participant’s feeling of victimization from the group (item 1+item 2); (2) Negative Evaluation is indicative of the participant’s feeling that he or she would be negatively evaluated by his or her peers (item 5 + item 6); (3) Comfort reflects the participant’s level of comfort with the group (item 3 + item 4); and (4) Connectedness represents how positive the participant feels about the group while taking into account negative group feelings. The Connectedness scale was created by subtracting the sum of all negative items (items 5+6+7+8) from the sum of all positive items (items 2+3+4). Victimization, Negative Evaluation, and Comfort scales were created for each participant by summing the participant’s responses to scale items for each group member.
Psychological Measures
Positive and Negative Affect.
Each time a saliva sample was collected post-stressor, participants were asked to rate how nervous, excited, angry, happy, embarrassed, confused, and sad they were feeling at that moment on a scale from 1 (“not at all”) to 5 (“very”). This measure was used to verify that the stress paradigm elicited an emotional response from participants and to provide a general measure of participants’ affect during each phase of their response to the stressor. Negative (i.e., nervous, angry, embarrassed, confused, sad) and positive (i.e., excited, happy) items were averaged to create negative and positive affect scales at each time point. The internal consistency for the negative affect scale ranged from α = .59–.78 (M = .69) and the positive affect scale ranged from α = .64–.79 (M = .73) at each time point it was collected.
State Rumination.
The Thoughts Questionnaire (Zoccola et al., 2008) was used to measure state rumination at Time 6, approximately 40 minutes post stressor. This stressor specific measure has been used in previous studies to assess state rumination after a social evaluative stressor (Edwards, Rapee, & Franklin 2003, Zoccola et al., 2008). Participants indicated how often they had certain thoughts in the time since their speech ended, using a 5-point scale ranging from “never” (0) to “very often” (4). Examples of rated statements included: “How often did you think about how bad your speech was?” and “How often did you think that you must have looked stupid?”. The Thoughts Questionnaire contains both negative and positive thoughts subscales. The 14-item negative thoughts subscale was used for analyses in this study. A higher score indicates more state rumination and there was high internal consistency for this scale (α =0.93).
Responses to Stress Questionnaire.
The Responses to Stress Questionnaire (RSQ; Compas, Connor-Smith, Saltzman, Thomsen, & Wadsworth, 2001) captures the different ways individuals cope with stress. The RSQ includes 55 items that measure coping styles, including “when problems with my peers come up, I can’t stop thinking about how I am feeling.” Participants rated how often they use each coping method on a scale of 1 (“Not at all”) to 4 (“A lot”). The RSQ consists of 19 subscales (e.g., trait rumination, problem solving, cognitive restructuring, overall feelings of stress), which were used to create 3 factors of coping as well as capture involuntary engagement, and involuntary disengagement. The RSQ assesses 10 categories of voluntary coping strategies across three broad coping constructs. Primary Control coping reflects categories of responses (problem-solving, emotional expression, emotional regulation) aimed at actively changing the situation or one’s emotional reaction to the situation (e.g., “I try to think of different ways to change the problem or fix the situation.”). Secondary Control coping captures categories of responses (cognitive restructuring, positive thinking, acceptance, distraction) focused on adapting to the situation (e.g., “I think about the things I am learning from the situation.”). Disengagement Coping assesses categories of responses (denial, avoidance, wishful thinking) that emphasize withdrawal or escape from the situation (e.g., “I try to stay away from people and things that make me feel upset or remind me of the problem.”). Proportion scores were created for each factor, thus controlling for individual differences in rates of endorsing items. The internal consistency for these subscales and factors ranged from α = .59–.89. Of the 19 subscales, only trait rumination was examined separately because of our interest in understanding the effects of state and trait rumination on the physiological response to stress.
Emotion regulation (ERQ-CA).
The Emotion Regulation Questionnaire for Children and Adolescents (ERQ-CA; Gullone & Taffe, 2012) is a revised version of the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003) which is comprised of 10 items forming two subscales that assess the habitual use of two emotion regulation strategies: cognitive reappraisal (6 items, e.g. “When I’m faced with a stressful situation, I make myself think about it in a way that helps me stay calm”) and expressive suppression (4 items, e.g. “I keep my emotions to myself”). Participants rated the extent to which they agree with each statement on a Likert scale ranging from 1 (strongly disagree) to 7 (strongly agree). Results indicate sound internal consistency (Cronbach’s α: suppression = 0.69, reappraisal = 0.65).
Symptoms of Anxiety and Depression.
The GAD-7 was administered to assess anxiety severity in the past two weeks (Löwe et al., 2008; Spitzer, Williams, & Lowe, & Kroenke, 2006). The GAD-7 is a screening and severity measure for generalized anxiety disorder, and participants were asked to indicate how often they have been bothered by seven different problems in the last two weeks. Items included ‘Not being able to stop or control worrying’ and ‘Trouble relaxing.’ Responses ranged from 0 (Not at all) to 3 (Nearly every day). Total anxiety severity was determined by summing items together, with greater scores representing greater severity. This scale has good reliability (α = .91) and participants’ scores could range from 0 to 21. Scores of 5, 10, and 15 represent mild, moderate, and severe anxiety, respectively. Sum scores of 10 or above are used as a cut point for identifying an anxiety disorder (Spitzer et al., 2006).
The Primary Health Questionnaire (PHQ-8) was used to assess depression severity over the past two weeks (Spitzer, Kroenke, & Williams, 1999). The PHQ-8 consists of 8 items on a scale from 0 (Not at all) to 3 (Nearly every day). Items include ‘Little interest or pleasure in doing things’ and ‘Feeling tired or having little energy.’ Total depression severity was determined by summing items together, such that greater scores indicated greater depression severity. This scale demonstrated good reliability (α = .82) and participants’ scores ranged from 0 to 20. Scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively.
Covariates.
Participants self-reported their gender (boy or girl) and race. Given our sample was predominantly Caucasian, participants’ race was coded as either Caucasian (n = 40) or an Ethnic minority (n = 14). Participants were also asked to report the name, dose, time of day of administration, and time of most recent administration of any medication use. Due to the small number of participants reporting any medication use, medication use was recoded as either Yes (n = 4) or No (n = 50). A research assistant recorded participants’ speech order and group size.
Analytic Plan
We performed descriptive and Pearson correlation analyses on all variables. Next, we performed repeated measures analyses in a mixed modeling framework to test whether the GPST-A elicited a significant change in salivary cortisol, sAA, positive affect, and negative affect across the sampling period. We then tested whether gender, race, medication use, speech order, and group size were associated with salivary cortisol, sAA, positive affect, and negative affect. Only variables associated with the physiological and affect response to the GPST-A were included as covariates in analyses. Time of day was not included as a covariate because all interviews were conducted between 2:30–4pm. Studies using a similar stress paradigm show clear effects of the task on cortisol reactivity with sample sizes of N=20–60 (Dickerson & Kemeny, 2004). SAS Proc Power indicates that, assuming a modest effect size (r = 0.35; Cohen, 1988), to power this study at 80% requires a sample size of N = 52. All measures, manipulations, and exclusions have been included in this manuscript.
We fit a series of growth curve models using SAS software (Version 9.4), estimated with restricted maximum likelihood estimation and an unstructured covariance structure. For salivary cortisol and sAA, we performed two-piece multilevel growth curve modeling with landmark registration with random intercepts and slopes, to examine how peer relationships among group m embers and psychological characteristics relate to individuals’ salivary cortisol and sAA reactivity and recovery to the GPST-A. Landmark registration accounts for individual differences in the time at which participants reach peak cortisol concentrations (Lopez-Duran et al., 2014). Landmark registration was performed by identifying the sample at which each individual peaked in cortisol and creating a Cortisol Inflection (CortInflect) variable, coded as 0 at sample times when the participant was in their reactivity phase and at peak, and coded as 1 when participants were in their recovery phase (Lopez-Duran et al., 2014; Singer & Willett, 2003). Next, CortInflect was used to create the adjusted time variables Cortisol Reactivity and Cortisol Recovery for each participant. When participants were in their reactivity phase (CortInflect = 0), Cortisol Reactivity equals (tpeak – 1)*(−1) where tpeak equals the time in minutes when participants reached their peak cortisol concentration. Cortisol Reactivity was set to 0 for all samples during the recovery phase (CortInflect = 1). When participants were in their recovery phase (CortInflect = 1), Cortisol Recovery equals t – tpeak, where t equals the post-peak sample time. Cortisol Recovery was set to 0 for all samples during the reactivity phase (CortInflect = 0). The same steps were followed to calculate sAA Reactivity and sAA Recovery. For more detailed steps on the calculation of Cortisol Reactivity, Cortisol Recovery, sAA Reactivity, and sAA Recovery, see Katz and Peckins (2017). We then tested whether peer relationships among group members were associated with the salivary cortisol and sAA trajectories. Finally, we tested whether psychological characteristics (positive and negative affect, state and trait rumination, coping and engagement factors from the RSQ, expressive suppression and cognitive reappraisal subscales from the ERQ-CA, and depression and anxiety symptoms) were associated with the salivary cortisol and sAA response to the GPST-A.
For positive affect and negative affect, we fit linear and quadratic growth curve models with random intercepts and slopes. The best fitting growth curve model of positive affect and negative affect had the lowest Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC). We then tested whether peer relationships among group members were associated positive affect and negative affect trajectories.
Results
Descriptive statistics
Descriptive statistics are presented in Table 1 and Pearson correlation analyses were performed on all variables. Cortisol levels were not correlated with peer relationships within the group at any time point (p > .05). Log transformed cortisol levels at Time 1 were positively correlated with positive affect at Time 2 (r = .27, p < .05) and negatively correlated with negative affect at Time 6 (r = −.27, p < .05). Cortisol levels were not correlated with state rumination at any time point; however, cortisol levels at Time 1 were positively correlated with trait rumination (r = .31, p < .05). Cortisol levels were not correlated with any coping or engagement factor from the RSQ or subscale from the ERQ-CA. Time 1 cortisol levels were negatively correlated with anxiety symptoms (r = −.35, p < .05) and cortisol levels at each time point were negatively correlated with depression symptoms (r = −.44 to −.37, p < .01).
Table 1.
Range, mean, and standard deviation of key predictor variables.
| Variable | Range | Mean | SD |
|---|---|---|---|
| Social Connectednessa | |||
| Victimization | 0.0–6.0 | 0.76 | 1.42 |
| Negative Evaluation | 0.0–6.0 | 1.53 | 1.66 |
| Comfort | 0.0–12.0 | 5.04 | 2.94 |
| Connectedness | −5.0–17.0 | 5.88 | 4.68 |
| State Ruminationb | 3.00–56.00 | 30.28 | 13.71 |
| Response to Stressc | |||
| Trait Rumination | 0.03–0.07 | 0.06 | 0.01 |
| Primary Control Coping Factor | 0.12–0.29 | 0.19 | 0.04 |
| Secondary Control Coping Factor | 0.16–0.36 | 0.26 | 0.04 |
| Disengagement Coping Factor | 0.09–0.20 | 0.15 | 0.03 |
| Involuntary Engagement Factor | 0.14–0.30 | 0.25 | 0.04 |
| Involuntary Disengagement Factor | 0.10–0.25 | 0.16 | 0.03 |
| Emotion Regulationd | |||
| Cognitive Reappraisal | 1.20–5.20 | 3.11 | 0.85 |
| Expressive Suppression | 2.25–7.00 | 3.98 | 1.12 |
| Anxiety Symptomse | 0.0–19.00 | 7.28 | 5.84 |
| Depression Symptomsf | 0.0–20.00 | 7.17 | 4.74 |
Note.
Connectedness, Comfort, Negative Evaluation, and Victimization were measured with the Social Connectedness Survey (SCS).
State rumination was measured with the Thoughts Questionnaire.
Responses to stress were measured with the Responses to Stress Questionnaire (RSQ). Univariate statistics are also presented for the trait rumination subscale of the RSQ because of our interest in understanding the effects of state and trait rumination on the physiological response to stress.
Emotion regulation was measured with the Emotion Regulation Questionnaire-Adolescent (ERQ-CA).
Anxiety Symptoms were measured with the Patient Health Questionnaire (PHQ).
Depression Symptoms were measured with the Primary Health Questionnaire (PHQ-8).
Log transformed sAA levels were not correlated with peer relationships within the group at any time point (p > .05). sAA levels were also not correlated with positive affect, negative affect, state rumination, trait rumination, coping and engagement factors from the RSQ, and subscales from the ERQ-CA (p > .05). However, Time 5 sAA levels were negatively correlated with anxiety symptoms (r = −.32, p < .05) yet not correlated with depression symptoms.
Positive affect was not correlated with peer relationships within the group (p > .05). However, negative affect was positively correlated with victimization at each time point (r = .29 to .53, p < .05). Negative affect was also positively correlated with negative evaluation at Time 2 (r = .37, p < .05), Time 3 (r = .31, p < .05), Time 4 (r = .34, p < .05), and Time 6 (r = .39, p < .01).
Cortisol and sAA Response to the GPST-A
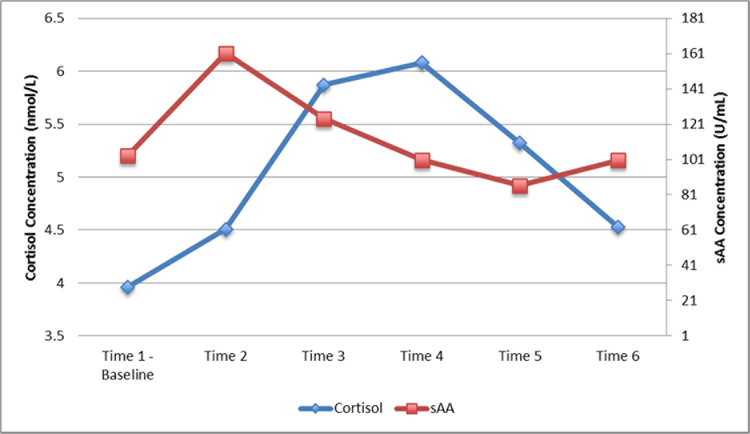
In the present study, 78% of the sample (n = 42) had at least a 10% increase in cortisol and sAA from baseline, which is a commonly accepted indication of HPA axis activation in the literature (Gordis, Granger, Susman, & Trickett, 2006). As reported in Katz and Peckins (2017), in this sample the average cortisol and sAA increase post-stressor was 50% and 115% over baseline levels, respectively. The majority of participants (65%) reached peak cortisol concentrations 21–30 minutes post-stressor (Time 3 or Time 4) and 80% of participants peaked in sAA concentrations 10–24 minutes post-stressor (Time 2 or Time 3). Twenty-four (44%) and 32 (59%) participants returned to their baseline levels of cortisol and sAA by the final sample, respectively (Figure 3). See Katz and Peckins (2017) for more information on the cortisol and sAA response to the GPST-A in this sample of adolescents.
Figure 3.
Average salivary cortisol and salivary alpha-amylase (sAA) response to the GPST-A.
Initial analyses tested whether gender, race, medication use, speech order, and group size were associated with the cortisol and sAA response to the GPST-A using repeated measures analysis in a mixed modeling framework. Only gender was associated with cortisol concentrations. On average, boys started out with significantly greater cortisol concentrations compared to girls (F1,52 = 14.53, p < .01), which is consistent with cortisol research in adults (Nicolson, Storms, Ponds, & Sulon, 1997). Therefore, gender was included as a covariate in all subsequent cortisol models. No covariates were associated with the sAA response to the GPST-A.
There was a significant increase in cortisol from Time 1 to Time 2 (t52 = −2.79, p < .01) and Time 2 to Time 3 (t52 = −3.85, p < .01), and significant decline in cortisol from Time 4 to Time 5 (t52 = 4.93, p < .01) and Time 5 to Time 6 (t52 = 4.96, p < .01). The GPST-A also elicited a significant change in sAA across the study (F5,53 = 19.70, p < .01). There was a significant increase in sAA from Time 1 to Time 2 (t53 = −7.39, p < .01) and decline in sAA from Time 2 to Time 3 (t53 = 2.47, p < .05) and Time 3 to Time 4 (t53 = 3.82, p < .01). Consistent with the results reported in Katz and Peckins (2017), the two-piece multilevel growth curve models with landmark registration revealed a significant linear increase (b = 0.01, p < .01) and decline (b = −0.01, p < .01) in cortisol in response to the GPST-A. There was also a significant linear increase (b = 0.02, p < .01) and decline (b = −0.01, p < .01) in sAA in response to the GPST-A (Katz & Peckins, 2017).
Affect Response to the GPST-A
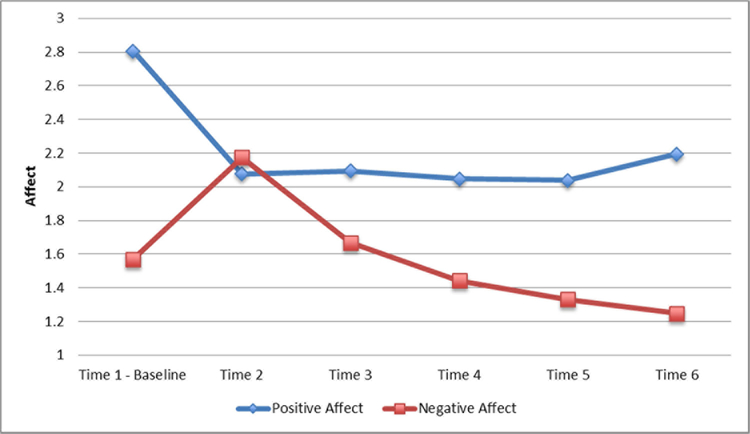
Gender, race, speech order, and group size were not associated with positive and negative affect in response to the GPST-A. Repeated measures analyses revealed there was a significant change in both positive affect (F5,53 = 10.81, p < .01) and negative affect (F5,53 = 37.36, p < .01) over time. Participants reported a significant decrease in positive affect from Time 1 to Time 2 (t53 = 6.16, p < .01) and remained low for the remainder of the study. In contrast, participants reported a significant increase in negative affect from Time 1 to Time 2 (t53 = −7.10, p < .01). Negative affect declined from Time 2 to Time 3 (t53 = 7.93, p < .01) and Time 3 to Time 4 (t53 = 3.93, p < .01), resulting in significantly lower levels of negative affect at Time 6 compared to Time 1 (t53 = 4.06, p < .01) (Figure 4). Linear and quadratic growth curve models were fit to the affect data, and the quadratic models fit the positive and negative affect data best (Table 2).
Figure 4.
Average positive and negative affect response to the GPST-A.
Table 2.
Estimates from Unconditional Linear and Quadratic Growth Curve Models for Positive and Negative Affect
| Positive Affect | Negative Affect | |||||||
|---|---|---|---|---|---|---|---|---|
| Linear | Quadratic | Linear | Quadratic | |||||
| b | SE | b | SE | b | SE | b | SE | |
| Intercept | 2.54** | 0.12 | 3.26** | 0.15 | 2.00** | 0.09 | 1.69** | 0.12 |
| Time | −0.09** | 0.02 | −0.63** | 0.07 | −0.12** | 0.01 | 0.11 | 0.06 |
| Time2 | --- | --- | 0.08** | 0.01 | --- | --- | −0.03** | 0.01 |
| Indices of Model Fit | ||||||||
| AIC | 661.1 | 612.1 | 467.3 | 461.6 | ||||
| BIC | 669.0 | 620.1 | 475.2 | 469.6 | ||||
Note.
p < .01,
p < .05;
AIC = Akaike Information Criterion;
BIC = Bayesian Information Criterion.
Peer Relationships Among GPST-A Group Members
Connectedness, comfort, negative evaluation, and victimization were not associated with the cortisol (ps .32 to 1.00) or sAA (ps .17 to .73) response to the GPST-A (Table A.1), nor were they associated with the positive affect response to the GPST-A (Table 3). However, adolescents’ reports of negative evaluation and victimization were related to change in negative affect over time. Specifically, greater levels of negative evaluation and victimization were each associated with a steeper linear increase (bnegative evaluation = .09, p < .05; bvictimization = .10, p < .05) and steeper quadratic decline (bnegative evaluation = −.01, p < .05; bvictimization = −.01, p < .05) in negative affect in response to the GPST-A (Table 3).
Table 3.
Estimates from Quadratic Growth Models for Positive, Negative Affect, and Peer Relationships
| Positive Affect | Peer Relationship Construct | |||||||
|---|---|---|---|---|---|---|---|---|
| Connectedness | Comfort | Negative Evaluation | Victimization | |||||
| b | SE | b | SE | b | SE | b | SE | |
| Intercept | 3.23** | 0.15 | 3.21** | 0.16 | 3.21** | 0.16 | 3.21** | 0.16 |
| Time | −0.61** | 0.07 | −0.59** | 0.07 | −0.59** | 0.07 | −0.59** | 0.07 |
| Time2 | 0.07** | 0.01 | 0.07** | 0.01 | 0.07** | 0.01 | 0.07** | 0.01 |
| Peer Relationship Constructa | 0.03 | 0.03 | 0.05 | 0.05 | −0.08 | 0.10 | −0.09 | 0.11 |
| Time x Peer Relationship Constructa | −0.01 | 0.02 | −0.02 | 0.02 | 0.05 | 0.04 | 0.03 | 0.05 |
| Time2 x Peer Relationship Constructa | 0.003 | 0.002 | 0.005 | 0.003 | −0.007 | 0.006 | −0.003 | 0.01 |
| Indices of Model Fit | ||||||||
| AIC | 615.0 | 565.7 | 564.3 | 564.0 | ||||
| BIC | 622.8 | 573.2 | 571.9 | 571.6 | ||||
| Negative Affect | Connectedness | Comfort | Negative Evaluation | Victimization | ||||
| b | SE | b | SE | b | SE | b | SE | |
| Intercept | 1.70** | 0.13 | 1.66** | 0.12 | 1.66** | 0.12 | 1.66** | 0.12 |
| Time | 0.10 | 0.06 | 0.10 | 0.06 | 0.10 | 0.06 | 0.10 | 0.06 |
| Time2 | −0.03** | 0.01 | −0.03** | 0.01 | −0.03** | 0.01 | −0.03** | 0.01 |
| Peer Relationship Constructa | −0.02 | 0.03 | −0.01 | 0.04 | −0.03 | 0.07 | 0.02 | 0.08 |
| Time x Peer Relationship Constructa | −0.002 | 0.01 | 0.03 | 0.02 | 0.09* | 0.04 | 0.10* | 0.05 |
| Time2 x Peer Relationship Constructa | 4.88×10−4 | 0.002 | −0.004 | 0.003 | −0.01* | 0.01 | −0.01* | 0.01 |
| Indices of Model Fit | ||||||||
| AIC | 463.3 | 418.7 | 407.4 | 397.8 | ||||
| BIC | 471.1 | 424.4 | 413.1 | 403.4 | ||||
Note.
p < .01,
p < .05.
Peer relationship construct is a placeholder for either connectedness, comfort, negative evaluation, or victimization;
AIC = Akaike Information Criterion;
BIC = Bayesian Information Criterion.
Psychological Characteristics and the Cortisol and sAA Response to the GPST-A
Estimates for the two-piece multilevel growth curve models with landmark registration are presented in Table 4 for cortisol and Table 5 for sAA. Results are only presented in tables for psychological predictors that were significantly associated with the cortisol or sAA response to the GPST-A.
Table 4.
Estimates from Two-piece Multilevel Growth Curve Models with Landmark Registration for Cortisol and Significant Psychological Predictors
| Psychological Construct | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cortisol | Positive Affect | Negative Affect | State Rumination | Trait Rumination | Depression Symptoms | |||||
| b | SE | b | SE | b | SE | b | SE | b | SE | |
| Intercept | 1.70** | 0.09 | 1.70** | 0.09 | 1.70** | 0.09 | 1.69** | 0.09 | 1 73** | 0.09 |
| Psychological Constructa | −0.03 | 0.03 | 0.06 | 0.03 | 0.003 | 0.01 | −8.04 | 7.22 | −0.04* | 0.02 |
| Gender | 0.62** | 0.17 | 0.60** | 0.17 | 0.61** | 0.17 | 0.65** | 0.17 | 0.48** | 0.17 |
| Estimates for Reactivityb | ||||||||||
| Intercept | 0.01** | 0.003 | 0.01** | 0.002 | 0.01** | 0.002 | 0.01** | 0.002 | 0.01** | 0.003 |
| Psychological Constructa | −0.004* | 0.001 | 0.01** | 0.002 | 3.57×10−4* | 1.53×10−4 | −0.48* | 0.20 | −6.00×10−5 | 0.001 |
| Gender | 0.01 | 0.01 | 0.001 | 0.005 | 0.004 | 0.005 | 0.005 | 0.005 | 0.003 | 0.005 |
| Estimates for Recoveryc | ||||||||||
| Intercept | −0.01** | 0.001 | −0.01** | 0.002 | −0.01** | 0.001 | −0.01** | 0.001 | −0.01** | 0.001 |
| Psychological Constructa | 7.24×10−4 | 0.001 | −0.002 | 0.002 | −1.20×10−4 | 8.50×10−5 | 0.04 | 0.11 | 2.62×10−4 | 0.31 |
| Gender | −0.01** | 0.003 | −0.01** | 0.003 | −0.01** | 0.003 | −0.01** | 0.003 | −0.01** | 0.003 |
| Indices of Model Fit | ||||||||||
| AIC | −8.1 | −28.0 | 3.7 | −38.7 | 4.4 | |||||
| BIC | 5.9 | −14.1 | 17.6 | −24.8 | 18.1 | |||||
Note.
p < .01,
p < .05;
Psychological Construct is a placeholder for either positive affect, negative affect, state rumination, trait rumination, or depression symptoms;
Estimates for slope from baseline to peak.
Estimates for slope from peak to end;
AIC = Akaike Information Criterion;
BIC = Bayesian Information Criterion.
Table 5.
Estimates from Two-piece Multilevel Growth Curve Models with Landmark Registration for sAA and Significant Psychological Predictors
| Psychological Construct | ||||
|---|---|---|---|---|
| sAA | Negative Affect | Disengagement Coping | ||
| b | SE | b | SE | |
| Intercept | 4.69** | 0.09 | 4.76** | 0.08 |
| Psychological Constructa | 0.24** | 0.06 | −6.49* | 3.12 |
| Estimates for Reactivityb | ||||
| Intercept | 0.02** | 0.004 | 0.02** | 0.004 |
| Psychological Constructa | 0.02** | 0.01 | 0.02 | 0.17 |
| Estimates for Recoveryc | ||||
| Intercept | −0.01** | 0.002 | −0.01** | 0.002 |
| Psychological Constructa | −0.01 | 0.003 | 0.01 | 0.06 |
| Indices of Model Fit | ||||
| AIC | 434.7 | 423.4 | ||
| BIC | 448.6 | 431.3 | ||
Note.
p < .01,
p < .05;
Psychological Construct is a placeholder for either negative affect or disengagement coping;
Estimates for slope from baseline to peak.
Estimates for slope from peak to end;
AIC = Akaike Information Criterion;
BIC = Bayesian Information Criterion.
Cortisol, sAA, and Affect.
Positive affect was negatively associated with the cortisol response to the GPST-A even after controlling for gender. Specifically, greater levels of positive affect were associated with a less steep increase in cortisol from baseline to peak (b = −.004, p < .05), yet not associated with peak activation or cortisol recovery slope. In contrast, negative affect was associated with a heightened cortisol response to the GPST-A. Greater levels of negative affect were associated with a steeper increase in cortisol (b = .01, p < .05) from baseline to peak. Negative affect was not associated with peak activation or the recovery phase of the cortisol response to the GPST-A (Table 4).
Positive affect was not associated with any phase of the sAA response to the GPST-A, yet negative affect was associated with a heightened sAA response (Table 5). Greater levels of negative affect were associated with a steeper increase in sAA from baseline to peak (b = .24, p < .01) and elevated peak activation (b = .02, p < .01). Negative affect was not associated with the recovery phase of the sAA response to the GPST-A.
Cortisol, sAA, and State Rumination.
State rumination was associated with the cortisol response to the GPST-A when controlling for gender. Greater levels of state rumination were associated with a steeper increase in cortisol from baseline to peak (b = 3.57 × 10−4, p < .05). However, state rumination was not associated with peak activation or the recovery phase of the cortisol response (Table 4).
State rumination was not associated with the sAA response to the GPST-A.
Cortisol, sAA, Response to Stress, and Emotion Regulation.
Of the subscales and factors from the RSQ and ERQ-CA that were examined, only trait rumination was associated with the cortisol response to the GPST-A. Greater levels of trait rumination were associated with a less steep increase in cortisol from baseline to peak (b = −.48, p < .05) and not associated with peak activation or the recovery phase of the cortisol response (Table 4). The five coping and engagement factors from the RSQ and subscales from the ERQ-CA, including primary control coping, secondary control coping, disengagement coping, involuntary engagement, involuntary disengagement, expression suppression, and cognitive reappraisal, were not associated with the cortisol response to the GPST-A.
Only disengagement coping was associated with the sAA response to the GPST-A. Greater levels of disengagement coping predicted lower peak sAA activation (b = −6.49, p < .05) and was not associated with the reactivity or recovery phase of the sAA response (Table 5). No other factors or subscales from the RSQ and ERQ-CA were associated with the sAA response to the GPST-A.
Cortisol, sAA, Anxiety, and Depression Symptoms.
Anxiety symptoms were not associated with the cortisol response; however, depression symptoms were associated with lower peak activation (b = −.04, p < .05). Depression symptoms were not associated with the reactivity or recovery phases of the cortisol response to the GPST-A (Table 4).
Anxiety and depression symptoms were not related to the sAA response to the GPST-A.
Discussion
In a previous study, we determined that the GPST-A administered among peers was effective at eliciting cortisol and sAA responses to the stressor (Katz & Peckins, 2017). In the present study, we expanded on this work by testing how peer relationships among members of GPST-A groups affected the cortisol, sAA, and affect response to the GPST-A due to the increased social sensitivity indicative of adolescence (Somerville, 2013). We also tested whether the GPST-A evoked a change in affect and examined how different measures of psychological characteristics, including affect during the visit and coping styles, impacted the cortisol and sAA response to the GPST-A. In support of our hypotheses, the GPST-A administered in a school setting among peers was both feasible and successful at eliciting a decrease in positive affect and increase in negative affect. Furthermore, positive affect was associated with blunted cortisol reactivity while negative affect was associated with increased cortisol and sAA reactivity to the GPST-A. With the exception of rumination, involuntary action, and depression severity, none of the measures of psychological characteristics were related to the cortisol or sAA response to the GPST-A. Only negative evaluation and victimization were associated with a steeper increase and decline in negative affect across the protocol.
Peer Relationships
A unique aspect of this study was the utilization of whole-network social data collected from participants to examine how the social relationships among members of the group may impact their physiological and affect response. Interestingly, reports of positive feelings (comfort and connectedness) among group members did not have an impact on participants’ reactions to the stressor but negative affiliations (negative evaluation and victimization) did. Feelings of negative evaluation and victimization from group members were associated with a steeper increase in negative affect following onset of the GPST-A, followed by a steeper return to baseline. This may indicate that adolescents who are in a stressful situation coupled with negative peer relationships could be more psychologically reactive to experiences of stress. The lack of association between physiological measures and peer relationships was surprising, but understandable given that the majority of participants reported experiencing no victimization by group members. The limited reports of peer victimization and negative evaluation was likely not severe in our sample because we recruited participants from the general student body and did not target students who experienced high rates of negative peer interactions. A larger sample size would likely contain higher numbers of adolescents with these negative peer experiences. Nonetheless, although findings for social relationships were limited, the approach described in the present study provides a model for future studies to test the impact of social relationships among group members on the response to stress, a facet of group protocols that has been largely ignored. This is a particularly important strategy when exploring the stress response among adolescents because of the profound impact that peers have on one another during this developmental period (Somerville, 2013).
Psychological Characteristics
Positive and Negative Affect.
Subjective ratings of positive and negative affect changed in the expected directions, with adolescents initially decreasing in positive affect and increasing in negative affect following the GPST-A, and eventually returning to baseline levels. This indicates that the participants experienced some distress as a result of the GPST-A. Our findings are consistent with a previous study that showed similar asymmetric patterns of positive and negative affect in response to the GPST-A (Hostinar et al., 2014). However, Hostinar and colleagues (2014) only measured positive and negative affect at two time points, before and after the GPST-A, whereas we assessed positive and negative affect each time a saliva sample was collected. Thus, we were able to model the time course of change in affect across the entire protocol and include affect as a time-varying predictor of the cortisol and sAA response to the GPST-A.
A concern with studies utilizing stress paradigms such as the GPST-A is that the task will be too distressing, leading to participant non-adherence to the protocol or withdrawal from the study. All participants in the present study were given the option to refuse participation in any portion of the protocol or withdraw from the study at any time; however, none refused to perform the task or provide saliva samples. Consistent with the finding that adolescents were only mild to moderately stressed as evidenced by a decline in cortisol ad sAA during the recovery period (Katz & Peckins, 2017), this suggests administering the GPST-A in a school among peers is not too extreme of a stressor and does not contribute to attrition or missing data. On average, levels of negative affect were lower at Time 6 than Time 1, indicating that participants recovered both physiologically and psychologically from the stressor. Together, these results alleviate concerns that the group testing with classmates may have been too upsetting or stressful due to adolescents’ enhanced sensitivity to social evaluation by peers (Somerville, 2013).
Coping styles.
The vast majority of neuroendocrine research has focused on how types of stressors shape the stress response profile (Gunnar & Quevedo, 2007), rather than the impact of coping strategies on the physiological response to stress. Although limited research on this topic is available, animal models and studies of humans have shown that engagement and disengagement coping styles have distinct physiological responses (i.e., through cognitive appraisals; Kemeny, 2003; Wood & Bhatnagar, 2015) and moderate the association between adolescents’ perception of daily stress and cortisol levels (Sladek, Doane, Luecken, & Eisenberg, 2016). More recently, Janson and Rohlender (2017) found that in adults, denial coping was associated with higher peak cortisol levels following stress, whereas distraction coping predicted steeper cortisol recovery. Thus, it was surprising that there were few associations between the phases of the physiological response to the GPST-A, self-reported coping strategies, and response styles in the present study. One possible explanation for our findings may be that adolescents are still establishing their own coping style patterns. Research examining adolescent coping shows that adolescents are inconsistent, using a variety of coping strategies when faced with the same stressor on different days (Sladek et al., 2016). This suggests that associations between coping responses and the physiological response to stress may not be crystallized until after coping strategies have been established in adulthood.
There is some evidence that rumination after stressful events may prolong recovery of the physiological response to stress, but methodological differences between studies prevent clear conclusions to be made (Stewart et al., 2013; Zoccola & Dickerson, 2012). Interestingly, two different dimensions of rumination – state and trait – were associated with the reactivity phase of the cortisol response but in opposite ways. Increased reports of state rumination, as measured by the Thoughts Questionnaire at the end of the visits, were associated with a steeper reactivity slope whereas trait rumination, measured by the rumination subscale of the RSQ, was associated with less steep cortisol reactivity. While it is surprising that these two measures of rumination showed different trends with adolescents’ cortisol response to the stressor, these results are consistent with findings from Zoccola et al. (2008) who found the same divergent cortisol patterns with state and trait rumination measures. While it is still not clear what the replication of this finding means for the field of psychology, it gives further support to the importance of measuring state tendencies alongside trait tendencies in psychological coping research with adolescents.
Symptoms of Anxiety and Depression.
Within the child and adolescent literature, links between cortisol and symptoms of psychopathology have not been consistently found across all studies, especially in community based samples of youth (Klimes-Dougan et al., 2001). Associations that have been observed between cortisol reactivity and depression have mostly been found in samples reporting borderline or clinical levels of internalizing and externalizing behaviors (Angold 2003; Klimes- Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001). Thus, it was noteworthy that we found increased reports of depressive symptoms to be associated with lower peak levels of cortisol in our non-clinical sample of youth, which is consistent with previous literature showing a blunting of the cortisol response in subjects with depression (Burke et al., 2005). In the present study, the effect of depression was only on peak cortisol levels and not the reactivity or recovery phases of the stress response, suggesting adolescents with increased depressive symptoms may start out with lower levels of cortisol levels but mount a similar cortisol response to the GPST-A. This explanation is supported by our finding that depressive symptoms were negatively correlated with cortisol concentrations at each of the six sampling times; however, we were not able to directly test this hypothesis with data available from the present study.
As neuroendocrine research becomes more accessible and statistical methods become more sophisticated, more research in this area is needed to determine if patterns that may exist between psychological constructs and physiological responses persist across stressors and populations. In their review of the literature on cortisol reactivity and challenge, Kudielka and colleagues (2009) point out that identifying mechanisms that regulate cortisol responses in humans is challenging because there are a variety of confounding factors, many of which might be relevant to our study– underlying chronic stress experienced by participants, methodological limitations of comparable research, early life experiences, and genetic differences. This is only magnified in adolescence when individuals have not necessarily settled into patterns of coping and they are experiencing many biological changes.
Limitations
Although there are many strengths to this study, the findings should be considered within the context of its limitations. First, the sample size was small (54 participants, 12 groups) and participants were ethnically homogenous. Therefore, findings from the present study are only generalizable to Caucasian adolescents living in suburban towns. Second, there are elements of the GPST-A that may have influenced participants’ responses to stress that remain unexplored. For example, the judges were both female and different ages and races (Caucasian 25-year old and African American 56-year old). Ideally, studies should include one male and one female judge to control for participants’ potential gender biases (Kirschbaum et al., 1993). Additionally, while a strength of the study is that it was done in the school among classmates, thus improving ecological validity, this is also a limitation. Laboratory-based paradigms focusing on individuals have the ability to control for many more possible confounds including anonymity among participants. In the present study, the anonymity of the audience may be important for detecting individual differences in the stress response. In the current design, this is confounded by the fact that the group members know one another. In turn, this protocol cannot be used in place of the individual TSST, but when the research question warrants it.
Third, while sAA is a validated measure of SNS activity, its timing is faster than the HPA axis and related to salivary flow (Nagy et al., 2015). In turn, subsequent studies seeking to accurately model the SNS and HPA axis simultaneously, should consider collecting more saliva samples earlier in the protocol.
Lastly, there are potential challenges related to classmates participating in GPST-A. There are multiple sources of variability when participants are peers – the social evaluation may vary, groups will differ on how well participants know and like/dislike one another, which all may lead to variability in the stress response. While the whole network tries to capture and explore these impacts, it is very challenging to account for immense variability in social relationships. Also, there may be confidentiality issues with participants sharing content from other participants’ speeches and there is no way to prevent students from telling future participants about the study, thus reducing the novelty of the stressor. However, we do not believe this was an issue in the present study because interview date was not associated with the cortisol or sAA response to the GPST-A.
Strengths
Many fields of research are beginning to utilize measures of physiological functioning to better understand biopsychosocial processes related to health and behavior. With salivary biomarkers becoming more accessible, successfully conducting the GPST-A in a school context demonstrates that this stressor protocol that was previously performed in a laboratory setting is portable and efficient.
There were several strengths of the study presented here. First, examining physiological measures in conjunction with psychological constructs gives us a better understanding of the complexity of adolescents’ response to stress. This is important because there are mixed findings in the stress literature with respect to the association between physiological measures and self-reported appraisals of stress and/or affect - sometimes they are associated (e.g. in adolescents Oldehinkel et al., 2011), but often times are not associated (Gerin et al., 1999). There have been a variety of hypotheses about why this discrepancy exists. One hypothesis is that participants’ affect is often measured only once at the end of a study period and physiology is often measured repeatedly during a session (Feldman et al., 1999). Similarly, to test if levels of affect covary with physiology over time, some studies use between-person analyses when it is a within-person question (Zawadzki, Smyth, Sliwinski, Ruiz & Gern, 2017). The model presented in the present study addresses these issues. We use a within-person analysis to test whether participants’ change in affect or rumination during impacts different phases of the stress response. Continuing to explore the relationships between psychological and physiological measures with more accurate methodology as illustrated in the present study is a necessity for the advancement of stress research.
Also, to our knowledge, this is the first study of its kind to take place in a school. Traditionally, group social stress paradigms are an efficient method to effectively elicit a cortisol response in adults (Von Dawans et al., 2011) and adolescents in a laboratory setting (Hostinar et al., 2014). However, stress paradigms administered in a laboratory setting often lack ecological validity and limit the sample to participants who are able and willing to travel to the lab, which is doubly limiting with adolescent populations because of required parental involvement for transportation needs. The ecological validity of the GPST-A was twofold; the task was performed in the adolescents’ school building among classmates - making both the paradigm and the setting realistic for participants. The stress paradigm was developmentally appropriate and a realistic stressor for adolescents because introducing oneself and speaking in front of groups of adults and peers are typical scenarios in school settings. Performing the GPST-A in a classroom at the participants’ school at the end of a regular school day has the potential to be more efficient and inclusive of high-risk or hard to reach participants who may not otherwise participate in a lab-based study. This study shows the portability of this task and encourages neuroendocrine researchers to conduct research in schools so they can recruit larger and more diverse samples of adolescents.
Lastly, results from this study alleviate possible concerns researchers might have about using a stress paradigm among adolescent peers. Some may worry that peer relationships could affect adolescents’ stress response, limiting the reliability of the stressor to elicit a cortisol response. However, results from this study do not support this hypothesis. In fact, the social network data collected in this protocol function as control variables (particularly connectedness and comfort). Results here show that even after taking into consideration positive peer relationships among group members, we still saw a significant change in cortisol and sAA over time in response to the GPST-A (reactivity, peak activation represented by the “Intercept”, and recovery phases of cortisol and sAA were significant in all of the models; Table A.1). These findings point to the strength of this approach for studying the stress response in adolescents who are among their peers.
Future Directions and Translational Implications
While this study did not render provocative findings regarding patterns in psychological thinking with stress response profiles, it provides a solid model for future studies seeking to examine the physiological, psychological and social factors that impact adolescents’ experience of stress. Future studies utilizing the GPST-A should adopt a multisystem and multiphase analytic approach as modeled here. By measuring both the reactivity, peak activation, and recovery phases of the stress response, we may gain insight into the impact that both adaptive and maladaptive coping strategies have on individual physiology. Additionally, a multisystem approach gives us the ability to look at patterns within individual regulatory systems. The same pattern of individual differences may not be found across regulatory systems and instead, it could be the coordination between systems that is the key to understanding individual differences in psychological, behavioral, and health outcomes. For example, Gordis and colleagues (2008) found the asymmetry between cortisol and salivary alpha amylase and not the trajectory of either biomarker alone, was related to aggressive behavior in adolescents. The GPST-A performed in a school among peers has the potential to elicit a sAA response as the autonomic nervous system has been shown to be more sensitive to tasks involving peers than to the traditional TSST without peers present (Stroud et al., 2009).
There are a variety of ways that social network data could be utilized in the GPST-A. With a larger sample size, researcher could explore the social network data in conjunction with the physiological data. Full network data could be used to examine the network of relationships among the participants or holistically, by examining the overall ‘density’ of social constructs. With a larger number of groups than the present study, network analysis could include testing how the pattern of individual choices gives rise to more holistic patterns between relationships and physiological reactivity to stressful experiences. Future studies could explore how peer relationships might act as a buffer against or exacerbate the negative consequences of stress.
Lastly, future prevention science research may benefit from utilizing the GPST-A as a pre- and post- measure during intervention studies. For example, many school-based social emotional learning interventions teach adaptive emotion regulation strategies and stress management skills with the hope that students will be able to utilize them during stressors they encounter on a day-to-day basis (e.g. Learning to Breathe; Metz et. al., 2013) and there is a foundation of evidence on which to hypothesize that these programs may have positive impacts on participants’ regulatory systems (Greenberg, Katz & Klein, 2015). The GPST-A administered in a school among peers would allow researchers to determine if students are utilizing skills learned through intervention during an ecologically valid stressor and if utilizing those skills affects their physiological and psychological response to stressors.
Funding:
This work was supported by the Institute of Education Sciences [grant R305B90007]. The second author was supported by a National Institute of Child Health and Human Development T32 Fellowship in Developmental Psychology, Department of Psychology, University of Michigan [2T32HD007109–36]. The views expressed here are ours and do not represent the granting agencies.
Appendix
Table A.1.
Estimates from Two-piece Multilevel Growth Curve Models with Landmark Registration for Cortisol, sAA, and Peer Relationships
| Cortisol | Peer Relationship Construct | |||||||
|---|---|---|---|---|---|---|---|---|
| Connectedness | Comfort | Negative Evaluation | Victimization | |||||
| b | SE | b | SE | b | SE | b | SE | |
| Intercept | 1 71** | 0.09 | 1 71** | 0.10 | 1.70** | 0.10 | 1 71** | 0.09 |
| Peer Relationship Constructa | −0.01 | 0.02 | −0.03 | 0.03 | −0.02 | 0.05 | −0.06 | 0.06 |
| Gender | 0.58 | 0.18 | 0.56** | 0.20 | 0.57** | 0.20 | 0.57** | 0.20 |
| Estimates for Reactivityb | ||||||||
| Intercept | 0.01** | 0.003 | 0.01** | 0.003 | 0.01** | 0.003 | 0.01** | 0.003 |
| Peer Relationship Constructa | −3.70×10−4 | 4.99×10−4 | −2.60×10−4 | 0.001 | 7.47×10−6 | 0.001 | 0.002 | 0.002 |
| Gender | 0.003 | 0.01 | .002 | 0.01 | 0.002 | 0.01 | 0.002 | 0.01 |
| Estimates for Recoveryc | ||||||||
| Intercept | −0.01** | 0.001 | −0.01** | 0.001 | −0.01** | 0.001 | −0.01** | 0.001 |
| Peer Relationship Constructa | −1.82×10−6 | 2.55×10−4 | −1.91×10−7 | 4.07×10−4 | 6.22×10−4 | 6.96×10−4 | 4.78×10−4 | 8.29×10−4 |
| Gender | −0.01** | 0.003 | −0.01* | 0.003 | −0.01* | 0.003 | −0.01* | 0.003 |
| Indices of Model Fit | ||||||||
| AIC | 7.4 | 0.6 | −2.1 | −7.8 | ||||
| BIC | 21.1 | 13.8 | 11.1 | 5.4 | ||||
| sAA | Connectedness | Comfort | Negative Evaluation | Victimization | ||||
| b | SE | b | SE | b | SE | b | SE | |
| Intercept | 4.78** | 0.08 | 4.76** | 0.08 | 4.76** | 0.08 | 4.76** | 0.08 |
| Peer Relationship Constructa | −0.01 | 0.02 | −0.04 | 0.03 | −0.04 | 0.05 | −0.03 | 0.06 |
| Estimates for Reactivityb | ||||||||
| Intercept | 0.02** | 0.004 | 0.02** | 0.004 | 0.02** | 0.004 | 0.02** | 0.004 |
| Peer Relationship Constructa | 6.37×10−4 | 8.73×10−4 | 5.33×10−4 | 0.002 | −0.002 | 0.003 | 0.004 | 0.003 |
| Estimates for Recoveryc | ||||||||
| Intercept | −0.01** | 0.002 | −0.02** | 0.002 | −0.02** | 0.002 | −0.02** | 0.002 |
| Peer Relationship Constructa | 2.04×10−4 | 3.67×10−4 | 7.52×10−4 | 0.001 | 0.001 | 0.001 | −4.90×10−4 | 0.001 |
| Indices of Model Fit | ||||||||
| AIC | 447.0 | 423.2 | 421.0 | 420.1 | ||||
| BIC | 454.8 | 430.8 | 428.6 | 427.7 | ||||
Note.
p < .01,
p < .05;
Peer relationship construct is a placeholder for either connectedness, comfort, negative evaluation, or victimization;
Estimates for slope from baseline to peak.
Estimates for slope from peak to end; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Till-Hoyt L, & Granger DA (2011). Diurnal alpha amylase patterns in adolescents: associations with puberty and momentary mood states. Biological Psychology, 88, 170–173. doi: 10.1016/j.biopsycho.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agorastos A, Pervanidou P, Chrousos GP, & Kolaitis G (2018). Early life stress and trauma: Developmental neuroendocrine aspects of prolonged stress system dysregulation. Hormones, 17, 507–520. doi: 10.1007/s42000-018-0065-x [DOI] [PubMed] [Google Scholar]
- Albert D, Chein J, & Steinberg L (2013). The teenage brain: Peer influences on adolescent decision making. Current Directions in Psychological Science, 22, 114–120. doi: 10.1177/0963721412471347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, & Teicher MH (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences, 31, 183–191. doi: 10.1016/j.tins.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Angold A (2003). Adolescent depression, cortisol, and DHEA. Psychological Medicine, 33, 573–581. doi: 10.1017/S003329170300775X [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, & Boyce WT (2002). Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental and Behavioral Pediatrics, 23, 102–113. doi: 10.1097/00004703-200204000-00007 [DOI] [PubMed] [Google Scholar]
- Boyce WT, & Ellis BJ (2005). Biological sensitivity to context: I. An evolutionary developmental theory of the origins and functions of stress reactivity. Developmental Psychopathology, 17, 271–301. doi: 10.10170S0954579405050145 [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, & Mohr DC (2005). Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology, 30, 846–856. [DOI] [PubMed] [Google Scholar]
- Cameron CA, McKay S, Susman EJ, Wynne-Edwards K, Wright JM, & Weinberg J (2017). Cortisol stress response variability in early adolescence: Attachment, affect and sex. Journal of Youth and Adolescence, 46, 104–120. doi: 10.1007/s10964-016-0548-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB (1932). The Wisdom of the Body. New York, NY: W W Norton & Co. [Google Scholar]
- Carver CS, & Scheier MF (1999). Stress, coping, and self-regulatory processes In Pervin LA & John OP (Eds.), Handbook of personality: Theory and research (2nd ed., pp. 553–575). New York: Guilford Press. [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Lawrence Earlbaum Associates. [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thomsen AH, & Wadsworth ME (2001). Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin, 127, 87–127. doi: 10.1037//0033-2909.127.1.87 [DOI] [PubMed] [Google Scholar]
- Dahl RE, & Gunnar MR (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Development and Psychopathology, 21, 1–6. doi: 10.1017/S0954579409000017 [DOI] [PubMed] [Google Scholar]
- Denson TF, Spanovic M, & Miller N (2009). Cognitive appraisals and emotions predict cortisol and immune responses: A meta-analysis of acute laboratory social stressors and emotion inductions. Psychological Bulletin, 135, 823–853. doi: 10.1037/a0016909 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Kemeny ME (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–391. doi: 10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Dickerson SS, & Zoccola PM (2013). Cortisol responses to social exclusion In DeWall CN (Ed.) The Oxford Handbook of Social Exclusion (pp. 143–151). New York: Oxford University Press. doi: 10.1093/oxfordhb/9780195398700.013.0014 [DOI] [Google Scholar]
- Edwards SL, Rapee RM, & Franklin J (2003). Post-event rumination and recall bias for a social performance event in high and low socially anxious individuals. Cognitive Therapy and Research, 27, 603–617. doi: 10.1023/A:1026395526858 [DOI] [Google Scholar]
- Egloff B, Schmukle SC, Burns LR, & Schwerdtfeger A (2006). Spontaneous emotion regulation during evaluated speaking tasks: Associations with negative affect, anxiety expression, memory, and physiological responding. Emotion, 6, 356–366. doi: 10.1037/1528-3542.6.3.356 [DOI] [PubMed] [Google Scholar]
- Feldman PJ, Cohen S, Lepore SJ, Matthews KA, Kamarck TW, & Marsland AL (1999). Negative emotions and acute physiological responses to stress. Annals of Behavioral Medicine, 21, 216–222. doi: 10.1007/BF02884836 [DOI] [PubMed] [Google Scholar]
- Gianferante D, Thoma MV, Hanlin L, Chen X, Breines JG, Zoccola PM, & Rohleder N (2014). Post-stress rumination predicts HPA axis responses to repeated acute stress. Psychoneuroendocrinology, 49, 244–52. doi: 10.1016/j.psyneuen.2014.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerin W, Bovbjerg DH, Glynn L, Davidson K, Sanders M, Sheffield D, & Christenfeld N (1999). Comment on ‘negative emotions and acute cardiovascular responses to laboratory challenges’. Annals of Behavioral Medicine, 21, 223–224. doi: 10.1007/BF02884837 [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, & Trickett PK (2006). Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology, 31, 976–987. doi: 10.1016/j.psyneuen.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, & Trickett PK (2008). Salivary alpha amylase-cortisol asymmetry in maltreated youth. Hormones and Behavior, 53, 96–103. doi: 10.1016/j.yhbeh.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85, 348–362. doi: 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Gross J, & Thompson RA (2007). Conceptual foundations for the field In Gross J (Ed.), Handbook of Emotion Regulation (pp. 3–24). New York: Guilford. [Google Scholar]
- Greenberg MT, Katz DA, & Klein LC (2015). The potential effects of social and emotional learning on biomarkers and health outcomes: A promissory note In Durlak JA, Gullotta T, Domitrovich C, Goren P, & Weissberg RP (Eds), Handbook of Social and Emotional Learning. New York: Guilford. [Google Scholar]
- Gullone E, & Taffe J (2012). The Emotion Regulation Questionnaire for Children and Adolescents (ERQ–CA): A psychometric evaluation. Psychological Assessment, 24, 409–417. doi: 10.1037/a0025777 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, & Herrera A (2009a). Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology, 34, 953–967. doi: 10.1016/j.psyneuen.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, & Vazquez D (2006). Stress neurobiology and developmental psychopathology In Cicchetti D & Cohen D (Eds.), Developmental Neuroscience (Vol. 2, pp. 533–577). New York, NY: Wiley. doi: 10.1002/9780470939390.ch13 [DOI] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, & Griggs C (2009b). Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and Psychopathology, 21, 69–85. doi: 10.1017/S0954579409000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–73. doi: 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Harris CR (2001). Cardiovascular responses of embarrassment and effects of emotional suppression in a social setting. Journal of Personality and Social Psychology, 81, 886–897. doi: 10.1037/0022-3514.81.5.886 [DOI] [PubMed] [Google Scholar]
- Häusser JA, Kattenstroth M, & Mojzisch A (2012). “We” are not stressed: Social identity in groups buffers neuroendocrine stress reactions. Journal of Experimental Social Psychology, 48, 973–977. doi: 10.1016/j.jesp.2012.02.020. [DOI] [Google Scholar]
- Hellhammer DH, Wüst S, & Kudielka BM (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology, 34, 163–171. doi: 10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, & Adam EK (2014). Cortisol responses to a group public speaking task for adolescents: Variations by age, gender, and race. Psychoneuroendocrinology, 50, 155–166. doi: 10.1016/j.psyneuen.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, Sindi S, Marin MF, Perna A, Hashemi A, Pruessner JC, & Lupien SJ (2011). A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology, 36, 797–805. doi: 10.1016/j.psyneuen.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Janson J, & Rohleder N (2017). Distraction coping predicts better cortisol recovery after acute psychosocial stress. Biological Psychology, 128, 117–124. doi: 10.1016/j.biopsycho.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Katz DA, & Peckins MK (2017). Cortisol and salivary alpha-amylase trajectories following a group social-evaluative stressor with adolescents. Psychoneuroendocrinology, 86, 8–16. doi: 10.1016/j.psyneuen.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny ME (2003). The psychobiology of stress. Current directions in psychological science, 12, 124–129. doi: 10.1111/1467-8721.01246 [DOI] [Google Scholar]
- Kirschbaum C, & Hellhammer DH (1989). Salivary cortisol in psychobiological research: An overview. Neuropsychobiology, 22, 150–69. doi: 10.1159/000118611 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, & Hellhammer DH (1995). Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine, 57, 23–31. doi: 10.1097/00006842-199501000-00004 [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, & Hellhammer DH (1993). The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. doi: 10.1159/000119004 [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, & Zahn-Waxler C (2001). Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development & Psychopathology, 13, 695–719. doi: 10.1017/S0954579401003157 [DOI] [PubMed] [Google Scholar]
- Knack JM, Jensen-Campbell LA, & Baum A (2011). Worse than sticks and stones? Bullying is associated with altered HPA axis functioning and poorer health. Brain and Cognition, 77, 183–90. doi: 10.1016/j.bandc.2011.06.011 [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, & Wüst S (2009). Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology, 34, 2–18. doi: 10.1016/j.psyneuen.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Kumsta R, Chen FS, Pape HC, & Heinrichs M (2013). Neuropeptide S receptor gene is associated with cortisol responses to social stress in humans. Biological Psychology, 93, 304–307. doi: 10.1016/j.biopsycho.2013.02.018 [DOI] [PubMed] [Google Scholar]
- La Greca AM & Stone WL (1993). The Social Anxiety Scale for Children – Revised: Factor structure and concurrent validity. Journal of Clinical Child Psychology, 22, 17–27. doi: 10.1207/s15374424jccp2201_2 [DOI] [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, & Zaldivar F (2009). Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology, 34, 1355–1362. doi: 10.1016/j.psyneuen.2009.04.006 [DOI] [PubMed] [Google Scholar]
- Laurent HK, Powers SI, & Granger DA (2013). Refining the multisystem view of the stress response: Coordination among cortisol, alpha-amylase, and subjective stress in response to relationship conflict. Physiology and Behavior, 119, 52–60. doi: 10.1016/j.physbeh.2013.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS (1966). Psychological Stress and the Coping Process. New York: McGraw Hill. [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping. Springer Publishing Company, New York. [Google Scholar]
- Lopez-Duran NL, Mayer SE, & Abelson JL (2014). Modeling neuroendocrine stress reactivity in salivary cortisol: Adjusting for peak latency variability. Stress, 17, 285–295. doi: 10.3109/10253890.2014.915517 [DOI] [PubMed] [Google Scholar]
- Löwe B, Decker O, Müller S, Brähler E, Schellberg D, Herzog W, & Herzberg PY (2008). Validation and standardization of the Generalized Anxiety Disorder Screener (GAD-7) in the general population. Medical Care, 46, 266–274. doi: 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- Massey AR, Byrd-Craven J, Auer B, & Swearingen CSL (2015). Climbing the social ladder: Physiological response to social status in adolescents. Adaptive Human Behavior and Physiology, 1, 72–92. doi: 10.1007/s40750-014-0009-x [DOI] [Google Scholar]
- Mauss IB, Cook CL, Cheng JYJ, & Gross JJ (2007). Individual differences in cognitive reappraisal: experiential and physiological responses to an anger provocation. International Journal of Psychophysiology, 66, 116–124. doi: 10.1016/j.ijpsycho.2007.03.017 [DOI] [PubMed] [Google Scholar]
- McClure EB, & Pine DS (2007). Social stress, affect, and neural function in adolescence In Romer D & Walker EF (Eds.), Adolescent psychopathology and the developing brain: Integrating brain and prevention science (pp. 441–462). New York: Oxford University Press. [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. The New England Journal of Medicine, 338, 171–179. doi: 10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- Metz SM, Frank JL, Reibel D, Cantrell T, Sanders R, & Broderick PC (2013). The effectiveness of the learning to BREATHE program on adolescent emotion regulation. Research in Human Development, 10, 252–272. doi: 10.1080/15427609.2013.818488 [DOI] [Google Scholar]
- Nagy T, van Lien R, Willemsen G, Proctor G, Efting M, Fülöp M, … Bosch JA (2015). A fluid response: Alpha-amylase reactions to acute laboratory stress are related to sample timing and saliva flow rate. Biological Psychology, 109, 111–119. doi: 10.1016/j.biopsycho.2015.04.012 [DOI] [PubMed] [Google Scholar]
- Nater UM, & Rohleder N (2009). Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology, 34, 486–496. doi: 10.1016/j.psyneuen.2009.01.014 [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, & Pine DS (2004). The social reorientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–174. doi: 10.1017/S0033291704003915 [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, & Pine DS (2005). The social reorientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35, 163–174. doi: 10.1017/S0033291704003915 [DOI] [PubMed] [Google Scholar]
- Nicolson N, Storms C, Ponds R, & Sulon J (1997). Salivary cortisol levels and stress reactivity in human aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 52, M68–M75. doi: 10.1093/gerona/52A.2.M68 [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Ormel J, Bosch NM, Bouma EMC, Van Roon AM, Rosmalen JGM, & Riese H (2011). Stressed out? Associations between perceived and physiological stress responses in adolescents: The TRAILS study. Psychophysiology, 48, 441–452. doi: 10.1111/j.1469-8986.2010.01118.x [DOI] [PubMed] [Google Scholar]
- Olweus W (1991). Bully/victim problems among schoolchildren: Basic facts and effects of a school-based intervention program In Pepler D & Rubin K (Eds.), The development and treatment of childhood aggression (pp. 411–448). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Out D, Granger DA, Sephton SE, & Segerstrom SC (2013). Disentangling sources of individual differences in diurnal salivary alpha-amylase: Reliability, stability, and sensitivity to context. Psychoneuroendocrinology, 38, 367–375. doi: 10.1016/j.psyneuen.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Keshavan M, & Giedd JN (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews: Neuroscience, 9, 947–958. doi: 10.1038/nrn2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne LA, Hibel LC, Granger DA, Tsao JC, & Zeltzer LK (2014). Relationship of salivary alpha amylase and cortisol to social anxiety in healthy children undergoing laboratory pain tasks. Journal of Child and Adolescent Behavior, 2, 100–129. doi: 10.4172/jcalb.1000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzi D, Muehlenbein MP, Geary DC, & Flinn MV (2016). Cortisol, salivary alpha-amylase and children’s perceptions of their social networks. Social Neuroscience, 11, 164–174. doi: 10.1080/17470919.2015.1045988 [DOI] [PubMed] [Google Scholar]
- Roberts AG, & Lopez-Duran NL (2019). Developmental influences on stress response systems : Implications for psychopathology vulnerability in adolescence. Comprehensive Psychiatry, 88, 9–21. doi: 10.1016/j.comppsych.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, & McEwen BS (2006). Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology, 147, 1664–1674. doi: 10.1210/en.2005-1432 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, & Munck AU (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21, 55–89. doi: 10.1210/edrv.21.1.0389 [DOI] [PubMed] [Google Scholar]
- Scheifelbein VL, & Susman E (2006). Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. Journal of Early Adolescence, 26, 397–413. doi: 10.1177/0272431606291943 [DOI] [Google Scholar]
- Scherf KS, Smyth JM, & Delgado MR (2013). The amygdala: An agent of change in adolescent neural networks. Hormones and Behavior, 64, 298–313. doi: 10.1016/j.yhbeh.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D, Farver JM, Chang L, & Lee-Shin Y (2002). Victimization in South Korean children’s peer groups. Journal of Abnormal Child Psychology, 30, 113–128. doi: 10.1023/A:1014749131245 [DOI] [PubMed] [Google Scholar]
- Singer JD & Willett JB (2003). Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press. [Google Scholar]
- Sladek MR, Doane LD, Luecken LJ, & Eisenberg N (2016). Perceived stress, coping, and cortisol reactivity in daily life : A study of adolescents during the first year of college. Biological Psychology, 117, 8–15. doi: 10.1016/j.biopsycho.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH (2013). Special issue on the teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science, 22, 121–127. doi: 10.1177/0963721413476512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer R, Kroenke K, & Williams J (1999). Validation and utility of a self-report version of PRIME-MD: The PHQ primary care study. Primary care evaluation of mental disorders patient health questionnaire. Journal of the American Medical Association, 282, 1727–1744. doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JW, Löwe B, & Kroenke K (2006). A brief measure for assessing generalized anxiety disorder: The GAD-7. Archives of Internal Medicine, 166, 1092–1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- Spear LP (2009). Heightened stress responsivity and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21, 87–97. doi: 10.1017/S0954579409000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, & Lerner RM (2004). The scientific study of adolescence: A brief history. Journal of Early Adolescence, 24, 45–54. doi: 10.1177/0272431603260879 [DOI] [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards K, & Harkness KL (2013). Rumination and impaired cortisol recovery following a social stressor in adolescent depression. Journal of Abnormal Child Psychology, 41, 1015–1026. doi: 10.1007/s10802-013-9740-1 [DOI] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, & Niaura R (2009). Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology, 21, 47–68. doi: 10.1017/S0954579409000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Miers a C., Van Pelt J, & Westenberg PM. (2010). Age and puberty differences in stress responses during a public speaking task: Do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology, 35, 1510–1516. doi: 10.1016/j.psyneuen.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Granger DA, Blades KT, Randazzo W, Heaton JA, & Dorn LD (2010). Cortisol and alpha amylase reactivity and timing of puberty: Vulnerabilities for antisocial behaviour in young adolescents. Psychoneuroendocrinology, 35, 557–569. doi: 10.1016/j.psyneuen.2009.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Houts RM, Steinberg L, Belsky J, Cauffman E, Early N, & Care C (2010). Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 ½ and 15 ½ years. Archives of Pediatrics and Adolescent Medicine, 164, 166–173. doi: 10.1001/archpediatrics.2009.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, & Chrousos GP (2002). Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research, 53, 865–871. doi: 10.1016/S0022-3999(02)00429-4 [DOI] [PubMed] [Google Scholar]
- Van den Bos E, de Rooij M, Miers AC, Bokhorst CL, & Westenberg PM (2014). Adolescents’ increasing stress response to social evaluation: Pubertal effects on cortisol and alpha-amylase during public speaking. Child Development, 85, 220–236. doi: 10.1111/cdev.12118 [DOI] [PubMed] [Google Scholar]
- Von Dawans B, Kirschbaum C, & Heinrichs M (2011). The Trier Social Stress Test for Groups (TSST-G): A new research tool for controlled simultaneous social stress exposure in a group format. Psychoneuroendocrinology, 36, 514–522. doi: 10.1016/j.psyneuen.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Vrijen C, van Roekel E, & Oldehinkel AJ (2018). Alpha-amylase reactivity and recovery patterns in anhedonic young adults performing a tandem skydive. PLoS ONE, 13, 1–17. doi: 10.1371/journal.pone.0204556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EF, Sabuwalla Z, & Huot R (2004). Pubertal neuromaturation, stress sensitivity, and psychopathology. Development and Psychopathology, 16, 807–824. doi: 10.1017/S0954579404040027 [DOI] [PubMed] [Google Scholar]
- Wood SK, & Bhatnagar S (2015). Resilience to the effects of social stress: Evidence from clinical and preclinical studies on the role of coping strategies. Neurobiology of Stress, 1, 164–173. doi: 10.1016/j.ynstr.2014.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawadzki MJ, Smyth JM, Sliwinski MJ, Ruiz JM, & Gerin W (2017). Revisiting the lack of association between affect and physiology: Contrasting between-person and within-person analyses. Health Psychology, 36, 811–818. doi: 10.1037/hea0000466 [DOI] [PubMed] [Google Scholar]
- Zoccola PM, & Dickerson SS (2012). Assessing the relationship between rumination and cortisol: A review. Journal of Psychosomatic Research, 73, 1–9. doi: 10.1016/j.jpsychores.2012.03.007 [DOI] [PubMed] [Google Scholar]
- Zoccola PM, Dickerson SS, & Zaldivar FP (2008). Rumination and cortisol responses to laboratory stressors. Psychosomatic Medicine, 70, 661–667. doi: 10.1097/PSY.0b013e31817bbc77 [DOI] [PubMed] [Google Scholar]