Abstract
Animal work indicates exposure to air pollutants may alter the composition of the gut microbiota. This study examined relationships between air pollutants and the gut microbiome in young adults residing in Southern California. Our results demonstrate significant associations between exposure to air pollutants and the composition of the gut microbiome using whole-genome sequencing. Higher exposure to 24-hour O3 was associated with lower Shannon diversity index, higher Bacteroides caecimuris, and multiple gene pathways, including L-ornithine de novo biosynthesis as well as pantothenate and coenzyme A biosynthesis I. Among other pollutants, higher NO2 exposure was associated with fewer taxa, including higher Firmicutes. The percent variation in gut bacterial composition that was explained by air pollution exposure was up to 11.2% for O3 concentrations, which is large compared to the effect size for many other covariates reported in healthy populations. This study provides the first evidence of significant associations between exposure to air pollutants and the compositional and functional profile of the human gut microbiome. These results identify O3 as an important pollutant that may alter the human gut microbiome.
Keywords: air pollution, gut microbiome, whole genome sequencing
Introduction
Air pollution has a significant impact on population health and has been identified as the fifth leading risk factor for mortality worldwide (“State of Global Air 2019. Special Report. Boston, MA: Health Effects Institute.,” 2019). Most of the disease burden that is attributable to air pollution results from chronic noncommunicable diseases, including respiratory disease and type 2 diabetes. In addition to exposure-induced effects on mortality, work has shown that long-term exposure to particulate matter with an aerodynamic diameter ≤2.5 micrometers (PM2.5), nitrogen dioxide (NO2), ozone (O3), and nitrogen oxides (NOx) are associated with greater risk for obesity, glucose dysregulation, and type 2 diabetes (Alderete et al., 2018a; Jerrett et al., 2017; 2014; McConnell et al., 2015; Miller et al., 2016; Vella et al., 2015). The mechanisms underlying these associations remain uncertain but are thought to include increased systemic levels of inflammation, alterations to adipose tissue metabolism, as well as impacts on the gut microbiota (Alderete et al., 2018b; Kelishadi et al., 2014; C. Liu et al., 2014; Matthews et al., 2016; Sun et al., 2009). For example, two studies have shown that increased near-roadway air pollution exposure (NOx) was correlated with the relative abundance of gut bacteria that have been associated with obesity and altered metabolism (Alderete et al., 2018b; Ley et al., 2006). Additionally, a recent population-based epidemiological study found that the gut microbiota partially mediated the effect of fine particulate matter (PM) on type 2 diabetes (T. Liu et al., 2019). To date, no human studies have examined the association between exposure to air pollutants with both the composition and functional potential of the gut microbiome using shotgun metagenomic sequencing.
Studies suggest that air pollutants may adversely affect the gastrointestinal tract (Beamish et al., 2011; Lomer et al., 2002) where ultrafine particles may reach the intestine through inhalation and diffusion from the terminal alveoli into systemic circulation or through ingestion of inhaled particles following mucociliary clearance from the airways (Beamish et al., 2011; Elder and Oberdörster, 2006; Salim et al., 2014). Once in the intestine, PM components may alter the composition and function of the gut microbiota by either supporting or inhibiting the growth of specific microbes (Adams et al., 2015; Gao et al., 2016; X. Li et al., 2019; Yasuyuki et al., 2010). Additionally, PM2.5 and O3 have been shown to have extrapulmonary effects that may alter the hypothalamic-pituitary-adrenal (HPA) axis through vagal nerve activation (Gackière et al., 2011) or effects on the hippocampus (Thomson, 2019), which can increase levels of catecholamines and steroid hormones. Animal and human studies have shown that increased exposure to O3 resulted in increased plasma corticosterone levels (Thomas et al., 2018) as well as plasma cortisol and corticosterone concentrations (Miller et al., 2016), respectively. Thus, O3 induced activation of the HPA axis may increase the production of cortisol and norepinephrine, which may alter the composition of the gut microbiota (Bassett et al., 2019; Lyte and Ernst, 1993; Petrosus et al., 2018) via receptors that have similar activity to adrenergic receptors (Hughes et al., 2009; Hughes and Sperandio, 2008). Norepinephrine may also induce changes in the enteric nervous system (Carabotti et al., 2015), which can alter gastrointestinal motility, mucus secretion, and ion transport (Corfield, 2018; Keely et al., 2012; Sandle and Binder, 1987; Schroeder, 2019; Sheppard, 2002; Wiles et al., 2016). Such changes in the luminal environment, could lead to alterations in the gut microbiota. Overall, this hypothesized mechanism is supported by the gut-brain axis, which allows for bidirectional communication (Lyte, 2014) that may result in changes in bacterial proliferation in the presence of norepinephrine (Lyte and Ernst, 1992) at the gut lamina propria.
Studies have shown that the gut microbiota may contribute to the development of obesity and type 2 diabetes (Ley et al., 2006; Qin et al., 2012; Ross et al., 2015; Turnbaugh et al., 2006; Vrieze et al., 2012). Emerging evidence also indicates that air pollutants may impact the relative abundance of gut bacteria (Alderete et al., 2018b; T. Liu et al., 2019). Despite this, it remains unknown whether air pollutants are associated with the composition and functional potential of the gut microbiome. The primary aim of this study was to determine whether residential based estimates of PM2.5, particulate matter with an aerodynamic diameter ≤10 micrometers in diameter (PM10), NO2, O3, or NOx exposures were associated with the gut microbiome in young adults before and after adjusting for potentially important confounding variables, which included body mass index (BMI), age, sex, race/ethnicity, season of testing, diet, and parental education. We hypothesized that higher prior year exposure to air pollutants would be associated with the relative abundance and the functional potential of gut bacteria using shotgun sequencing of stool samples. In an exploratory analysis, we sought to determine whether specific gut bacterial taxa found to be associated with exposures were also associated with BMI, body fat percent, and risk factors for type 2 diabetes (i.e., fasting glucose, fasting insulin, 2-hour glucose and insulin levels, glucose and insulin area under the curve, insulin resistance, and β-cell function) or markers of gut bacterial translocation (i.e., lipopolysaccharide-binding protein (LBP), sCD14).
Materials and Methods
Research Design
This microbiome study was composed of 101 participants who were recruited from the Meta-AIR (Metabolic and Asthma Incidence Research) study between 2014–2017 at the University of Southern California (USC). The Meta-AIR study was a sub study of the Southern California Children’s Health Study (CHS), which was a large school-based cohort that began in 2002–2003. The primary aim of the Meta-AIR study was to investigate the impacts of ambient and near-roadway exposures on metabolic health and adiposity in young adults. Participants in the Meta-AIR study were recruited based on their overweight and obese status, which was determined at school visits in 2011–2012. This study also recruited participants to represent the extremes of residential NOx values in Southern California CHS communities using probability weighted sampling from addresses reported at their last school visit. The inclusion criteria for Meta-AIR included participation in Cohort E of the CHS, as well as age and sex adjusted BMI of ≥ 85th percentile in 2011–2012, and the absence type 1 or type 2 diabetes. The CHS study design has been described in detail previously (Chen et al., 2015; Gauderman et al., 2015; Urman et al., 2014). Briefly, children from Cohort E were followed from kindergarten or first grade to high school graduations with an original enrollment of 3,474 participants (Chen et al., 2015). Smoking was not an exclusion criterion, yet only 9.9% of participants in this study reported smoking in within the last month. Participants were excluded if they were using any medication or diagnosed with a condition known to influence insulin and/or glucose metabolism or body composition. Additionally, participants were excluded from the current study if they used antibiotics in the two weeks prior to their clinical visit. Prior to testing, informed written consent/assent was obtained from the parents/participant. The University of Southern California Institutional Review Board approved this study.
Clinical Assessments
As previously reported (Kim et al., 2019) Meta-AIR study participants underwent thorough phenotyping which included measures indicative of adiposity and metabolic outcomes, which were collected at the USC Diabetes and Obesity Research Institute and Clinical Trials Unit. These clinical measures included height, weight, waist circumference, blood pressure, heart rate and an oral glucose tolerance test (OGTT). BMI was calculated as weight in kilograms over the square of height in meters and body composition was assessed using a dual-energy X-ray absorptiometry (DEXA) scan. The Human Insulin ELISA Kit (EMD Millipore) was used to assay fasting insulin, and a hexokinase-mediated reaction assay with Roche Covas C501 was used to assay glucose tolerance. Insulin resistance was calculated by HOMA-IR [fasting glucose (mg/dL)*fasting insulin (μU/mL)/405]. β-cell function (HOMA-β) was calculated by (360*fasting insulin)/(fasting glucose-63). A subset of 81 participants’ diets were assessed using 24-hour diet recalls, and this nutritional data was analyzed using the Nutrition Data System for Research (version 2014, University of Minnesota).
Regional Ambient and Near-Roadway Air Pollution Exposure Assessment
Residential history was collected during study visits which included move in and move out dates for each respective residence accounting for multiple places of residence. Latitude and longitude coordinates were determined via parcel level geocoding of residential addresses using the Texas A&M geocoder (http://geoservices.tamu.edu/Services/Geocode/). As described in our previous study (Alderete et al., 2018b), the determined latitude and longitude coordinates were used to estimate average prior year residential ambient and near-roadway air pollutant exposure levels. For example, in the case that a resident inhabited more than one residence in the year prior to their study visit, time spent at each residence was determined and exposure estimates were weighted based on this time distribution.
Ambient monitoring stations were used to determine monthly air pollution exposure by downloading hourly air quality data from the U.S. Environmental Protection Agency’s Air Quality System (https://www3.epa.gov/ttn/airs/airsaqs/). Using this method, exposure data for the 12 months prior to each visit were estimated for each participant and used to calculate prior year exposure. These air quality measurements provide a good characterization of relative regional air pollution gradients since monitoring stations are spaced 20–30 kilometers (km) apart. Federal Reference Method (FRM) monitors were used to measure gaseous pollutants, whereas FRM and Federal Equivalent Method (FEM) monitors were used to measure particulates. Monthly averages were calculated from daily data using 75% completeness criteria. To calculate monthly ambient exposures, parcel level data was used in the inverse distance-squared weighting (IDW2) algorithm which spatially interpolated air quality data from up to four monitoring stations within a 50 km radius of the participant’s residence (Wong et al., 2004). Prior work by our group has shown that the IDW2 method in California was robust to a leave one out validation for monthly monitoring AQS site data and performs as well as more sophisticated models that are limited by shorter spatial-temporal coverage (Eckel et al., 2016).
In order to model residential near-roadway exposure for the year prior to each clinical visit, the California Line Source Dispersion Model (CALINE4) was used to estimate levels of nitrogen oxides (NOx) produced by motor vehicles on roads within 5 km of each residence. CALINE4 takes into account traffic counts, road geometry, emission factors, and local meteorology (e.g., wind speed/direction, mixing heights, atmospheric stability) (Benson, 1992). This model obtains traffic counts and road geometry from Caltrans and TeleAtlas/GDT. The CALINE4 model encompasses annual-average traffic impact estimates from all road classes (i.e. freeways or highways, major roadways, and minor roadways). These estimates were based on the Streetmap Premium database (ArcGIS 10.1, Environmental Systems Research Institute Inc., Redlands, CA). Total near-roadway exposures were defined as the sum of freeway and non-freeway sources of NOx. Non-freeway exposures were defined as the sum of NOx from major and minor roadways. Near-roadway air pollution is a complex mixture of particles and gases, including NOx, carbon monoxide, particulate matter, organic compounds, elemental carbon, and polycyclic aromatic hydrocarbons (Fujita et al., 2007) that differ by roadways type and traffic volumes (Clements et al., 2009; Zhu et al., 2009). Due to the complexity of this exposure, NOx was used as a marker for the mixture of near-roadway pollutants. The CALINE4 line source dispersion model has been evaluated against near-road hourly observations in numerous studies (Benson, 1992; Kenty et al., 2007; Levitin et al., n.d.; Yura et al., 2007). Thus, the CALINE4 model has demonstrated reasonable performance for a variety of inert pollutants in different roadway and meteorological setting.
Gut Microbiome
Fecal samples were collected using commercial collection kits (Second Genome, San Francisco, CA) that contain a preservative (Norgen Biotek, Canada) which stabilizes DNA and RNA at ambient temperatures. Within 2.4 days after collection, fecal samples were stored at −80°C until analysis. Genomic DNA was isolated from samples and amplified in accordance with Earth Microbiome Project standard protocols (van Esterik et al., 2015), and Genomic isolates were collected in a final 50 μl extraction volume. Shallow whole-genome sequencing (WGS) was conducted at the University of California, San Diego in Dr. Rob Knight’s laboratory, and used to determine the relative abundance of bacterial taxa. WGS sequencing was performed on the Illumina HiSeq 4000 platform.
Metagenomic DNA sequences were decontaminated from human genome reads using KneadData (http://huttenhower.sph.harvard.edu/kneaddata). Taxonomic classification was performed using Kraken2 (Wood and Salzberg, 2014) through an automated pipeline BioLockJ (https://github.com/msioda/BioLockJ). Average number of reads per sample was 366,499 ± 161,679 (n=101). Abundance of microbial sequence read counts were normalized for each taxonomic group in order to account for varying sequencing depths across samples using the equation (McCafferty et al., 2013) below:
This normalization scheme attempts to mitigate the impact of the pseudo-count on samples of different sequencing depth. Microbial gene families and pathways were profiled using the HUMAnN2 pipeline (Franzosa et al., 2018). Briefly, HUMAnN2 characterizes the abundance of each pathway in a community as a function of the abundance of the pathway’s component reactions and each reaction’s abundance is computed as the sum over abundances of genes that catalyze a reaction. Gene family and pathway abundance reads per kilobase were normalized to copies per million (cpm). On average, 492768 ± 68943 cpm in each sample were uniquely annotated to a gene family using the UniRef90 database (Suzek et al., 2015), and 25,601 ± 3,681 cpm in each sample were functionally annotated to a gene pathway using the MetaCyc database (Caspi et al., 2018). In addition, HUMAnN2 uses the MetaPhlAn2 (Truong et al., 2015) classifier to assign taxonomy to organisms in a metagenomic sample. Taxonomic classification from MetaPhlAn2 classifier was further used to confirm the results from Kraken2.
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation (SD) for continuous variables and as frequency (percentage) for categorical variables. The Shannon diversity index (a measure of richness and evenness) was calculated using the vegan package in the R statistical program. Briefly, a community with a higher number of species and a homogeneous distribution of relative abundances has a higher Shannon diversity index. A higher diversity can be indicative of a healthier microbial ecosystem (Calle, 2019). Linear regression analysis was performed to examine the univariate associations between the gut microbiome and air pollutants (i.e., total NOx, PM2.5, PM10, NO2, O3). Multivariable linear models were then used to study these associations after adjusting for potentially important covariates (i.e., age, BMI, energy intake, macronutrients, Hispanic ethnicity, sex, season of testing (warm/cold), and parental education as a proxy for socioeconomic status). Parental education was categorized as low (n=43), high (n=38), and missing/don’t know (n=20). Low parental education was defined as <12th grade, completed grade 12, and some college or technical school while high parental education was defined as 4 years of college and some graduate training college. There was strong correlation among the air pollutants (Supplemental Table 1); however, sensitivity analyses were performed using multi-pollutant models for O3 and NO2 (Spearman’s rank-order correlation r = −0.42; p<0.001). Following this, Kendall tests were used to determine if findings from the linear models were robust to non-parametric testing. For all univariate linear regression models and Kendall tests, rare taxa or gene pathways that were present in less than 25% in samples were removed. Additional analysis was performed to confirm the primary findings form the linear regression analysis in Songbird (v1.0.1) (Morton et al., 2019). This differential abundance analysis was performed on the species level Kraken2 results with respect to O3. Briefly, Songbird accounts for the compositional nature of microbial data and uses a multinomial regression model to estimate differential ranks. Optimized model parameters were determined (differential prior = 1 and learning rate = 0.01) and compared to a baseline model of 1. The models are then compared by a Q2-value defined as 1 – model CV/baseline CV, which is similar to a R2-value used in ordinary linear regression. A Q2-value close to one and greater than zero ensures that the covariates entered into the formula are improving the model fit. A tutorial on this procedure can be found at https://github.com/biocore/songbird. We obtained a Q2-value of 0.37 when including the covariate O3, indicating good model predictive accuracy. Next, we confirmed differently ranks by generating log-ratios through Qurro (Fedarko et al., n.d.) following the tutorial found at https://github.com/biocore/qurro. Based on this analysis, differential rankings confirmed that Bacteroides was highly ranked with increased O3. From these highly ranked species, the log-ratio of Bacteroides caecimuris versus Leuconostoc spp. was compared to O3. Leuconostoc spp. was selected as the denominator due to a mean rank close to zero, indicating little change compared to O3.
Multidimensional scaling, a statistical technique to visualize dissimilarity between samples in a high dimensional dataset, was performed on WGS sequencing data using the “capscale” function in the vegan package with Bray-Curtis dissimilarity. In addition, the “envfit” function in the vegan package was used to visualize the correlation of air pollutant variables with the gut microbiota composition on the ordination plot. Lastly, the ADONIS test, a permutation-based multivariate analysis of variance using Bray-Curtis dissimilarity matrices with 10,000 permutations, was used to examine associations between the gut microbiota with air pollutants (i.e., total NOx PM2.5, PM10, NO2, O3) and participant characteristics (i.e., BMI, age, sex, Hispanic ethnicity, energy intake, season, and parental education). Effect modification by sex and obesity on the associations between the gut microbiome and air pollutants was further examined using interaction terms in these multivariate models.
For our explanatory aim, associations between gut bacteria and obesity (i.e., BMI, body fat percent), metabolic outcomes (i.e., fasting glucose, fasting insulin, 2-hour glucose and insulin levels, glucose and insulin area under the curves following OGTT, HOMA-IR, and HOMA-β), and markers of gut microbial translocation (i.e., sCD14, LBP, ratio of sCD14/LBP) were examined using both simple univariate and multivariable linear regression models that adjusted for age, BMI, energy intake, Hispanic ethnicity, sex, season, and parental education. P-values from all statistical analyses were adjusted for multiple hypothesis testing using a false discovery rate of 5% with the Benjamini-Hochberg procedure. For each table the total number of hypotheses corrected was the product of all of the independent variables considered and the number of taxa examined. All analyses were conducted in R statistical package version 3.5.1 and figures were produced using RStudio.
Results
This study included 101 participants from the Meta-AIR Study. General characteristics and the average air pollutant exposures are reported in Table 1. The mean age of participants was 19.6 years (range 17.7 – 21.8), 57.4% were male, and approximately 54.5% self-identified as Hispanic ethnicity. On average, participants were overweight with an average BMI of 28.9 kg/m2 (range 17.3 – 47.4). The gut bacterial community composition was dominated by the phylum Bacteroidetes (59.74% ± 13.97), followed by Firmicutes (31.51% ± 10.75), Proteobacteria (4.99% ± 6.34), and Actinobacteria (2.54% ± 2.51).
Table 1.
Baseline Characteristics and Prior Year Ambient and Near-Roadway Concentration in Young Adults from the Meta-AIR Study
| Mean ± SD | Missing Values | |
|---|---|---|
| General Characteristics | n=101 | |
| Age (years) | 19.6 ± 0.9 | 0 |
| Sex (Males/Females, %) | 58/43, 57.4% | 0 |
| Ethnicity (Hispanic/Non-Hispanic, %) | 55/46, 54.5% | 0 |
| BMI (kg/m2) | 28.9 ± 5.4 | 0 |
| Parental Education | ||
| Low (n, %) | 43, 42.6% | 0 |
| High (n, %) | 38, 37.6% | 0 |
| Unknown/Missing (n, %) | 20, 19.8% | - |
| Metabolic Indices | ||
| Fasting Glucose (mg/dL) | 89.8 ± 8.7 | 4 |
| Fasting Insulin (μU/mL) | 10.6 ± 7.6 | 25 |
| Body Fat Percent (%) | 34.6 ± 8.8 | 17 |
| 2-Hour Glucose (mg/dL) | 120.2 ± 28.6 | 19 |
| 2-Hour Insulin (μU/mL) | 102.2 ± 100.6 | 19 |
| Glucose Area Under the Curve | 272.8 ± 46.5 | 19 |
| Insulin Area Under the Curve | 243.8 ± 167.2 | 38 |
| HOMA-IR | 2.4 ± 1.8 | 25 |
| HOMA-B | 163.4 ± 152.4 | 25 |
| Energy Intake and Macronutrients | ||
| Energy Intake (Kcal) | 1994.0 ± 655.9 | 20 |
| Carbohydrates (g) | 241.4 ± 79.1 | 20 |
| Fat (g) | 80.4 ± 34.8 | 20 |
| Protein (g) | 81.6 ± 34.6 | 20 |
| Fiber (g) | 17.6 ± 6.7 | 20 |
| Prior Year Average Exposure to Air Pollutants** | ||
| NO2 (ppb) | 15.5 ± 3.9 | 1 |
| PM10 (μg/m3) | 30.1± 7.1 | 0 |
| PM2.5 (μg/m3) | 12.0 ± 2.5 | 0 |
| O3 (ppb) | 29.8 ± 2.5 | 0 |
| Total NOx (ppb) | 7.1± 7.1 | 1 |
Average prior year exposure to ambient and near-roadway air pollutants based on residential addresses. Nitrogen oxides (NOx) in parts per billion (ppb) were used as a marker of traffic emissions. Data are reported as mean with standard deviation (SD). Parental education was defined as low (n=43), high (n=38), or unknown/missing (n=20). Low was defined as <12th grade, completed grade 12, and some college or technical school. High was defined as 4 years of college and some graduate training college.
Air Pollutants were Associated with the Gut Microbiota using WGS
We first performed multi-dimensional scaling on the WGS data using Bray-Curtis dissimilarity at the species level (Figure 1). The ordination plot shows that O3 exposure was significantly correlated with the first axis of the multi-dimensional scaling analysis, which explains 28% of variation in the data. Using a non-parametric multivariate ADONIS test with 10,000 permutations, we found that exposure to air pollutants explained the largest proportion of the variance in gut bacterial composition (Figure 2). The percent variation that was explained by exposure to air pollutants was 4.0% for total NOx (FDR corrected p = 0.049), 4.4% for NO2 (FDR corrected p = 0.049), and 11.2% for O3 concentrations (FDR corrected p = 0.001), which is large relative to the effect size for many other covariates reported in healthy populations (Falony et al., 2016). At the species level, O3 exposure explained 5.4% of the variation in gut bacterial composition (FDR corrected p = 0.001). By contrast, the metadata variables sex, season, Hispanic ethnicity, BMI, age, parental education, and energy intake from 24-hour diet recalls all explained less than 4% of the variance and were not significantly associated with gut microbial community composition by the ADONIS test at a p <0.05 threshold.
Figure 1. Exposure to O3 was Significantly Correlated with Gut Bacterial Species on the First Axis of the Multi-dimensional Scaling Analysis using Whole Genome Sequencing (WGS).
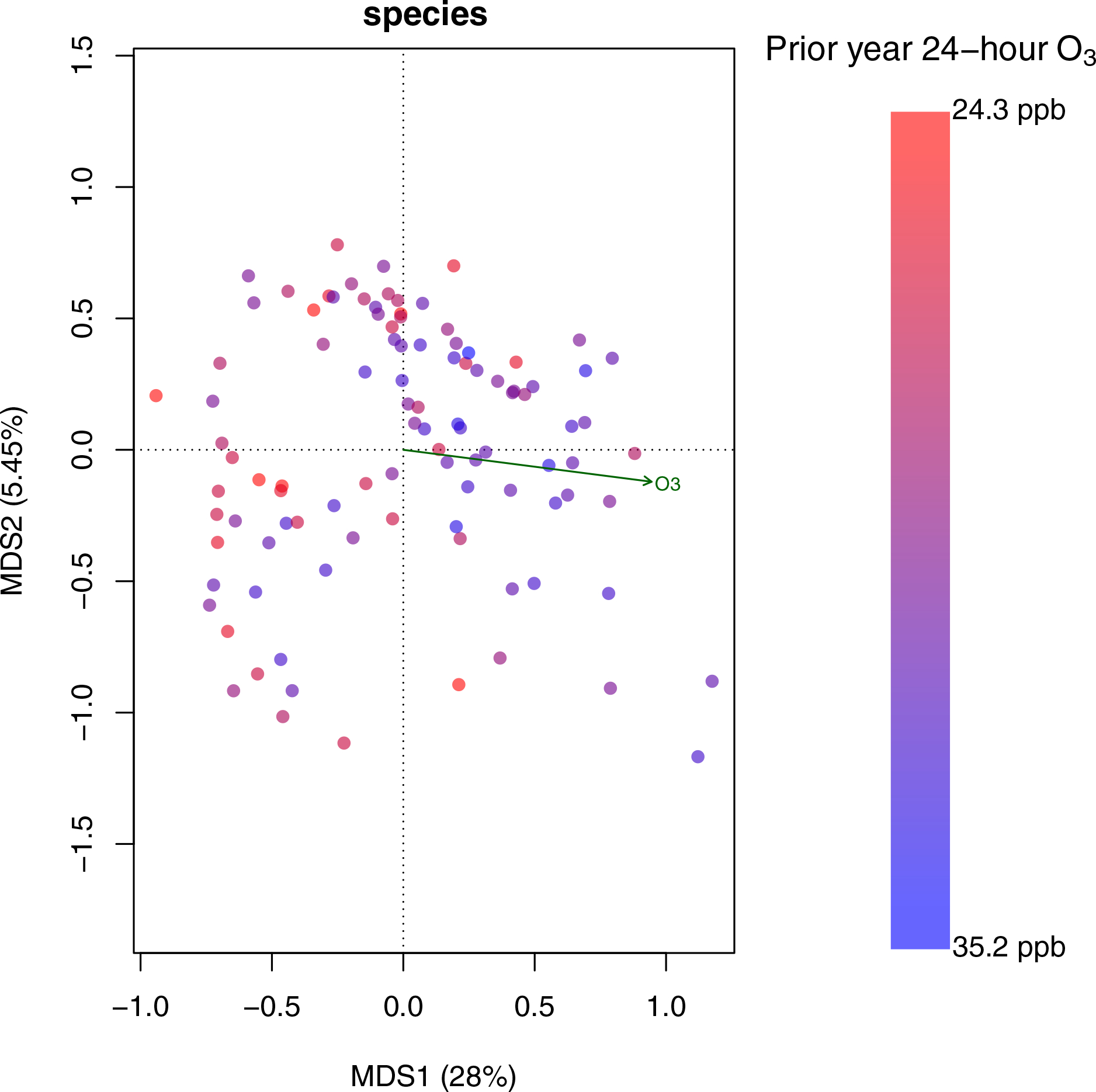
Multi-dimensional scaling on the WGS data was performed using Bray-Curtis dissimilarity at the species level. Each point represents a sample and the gradient of O3 exposure is color coded. This ordination plot shows that O3 exposure was significantly correlated with the first axis of the multi-dimensional scaling analysis, which explains 28% of variation in the data (p<0.001, R2=0.17).
Figure 2. Exposure to Air pollutants was Associated with the Gut Microbiota at the Phylum and Species Level Using Whole Genome Sequencing (WGS).
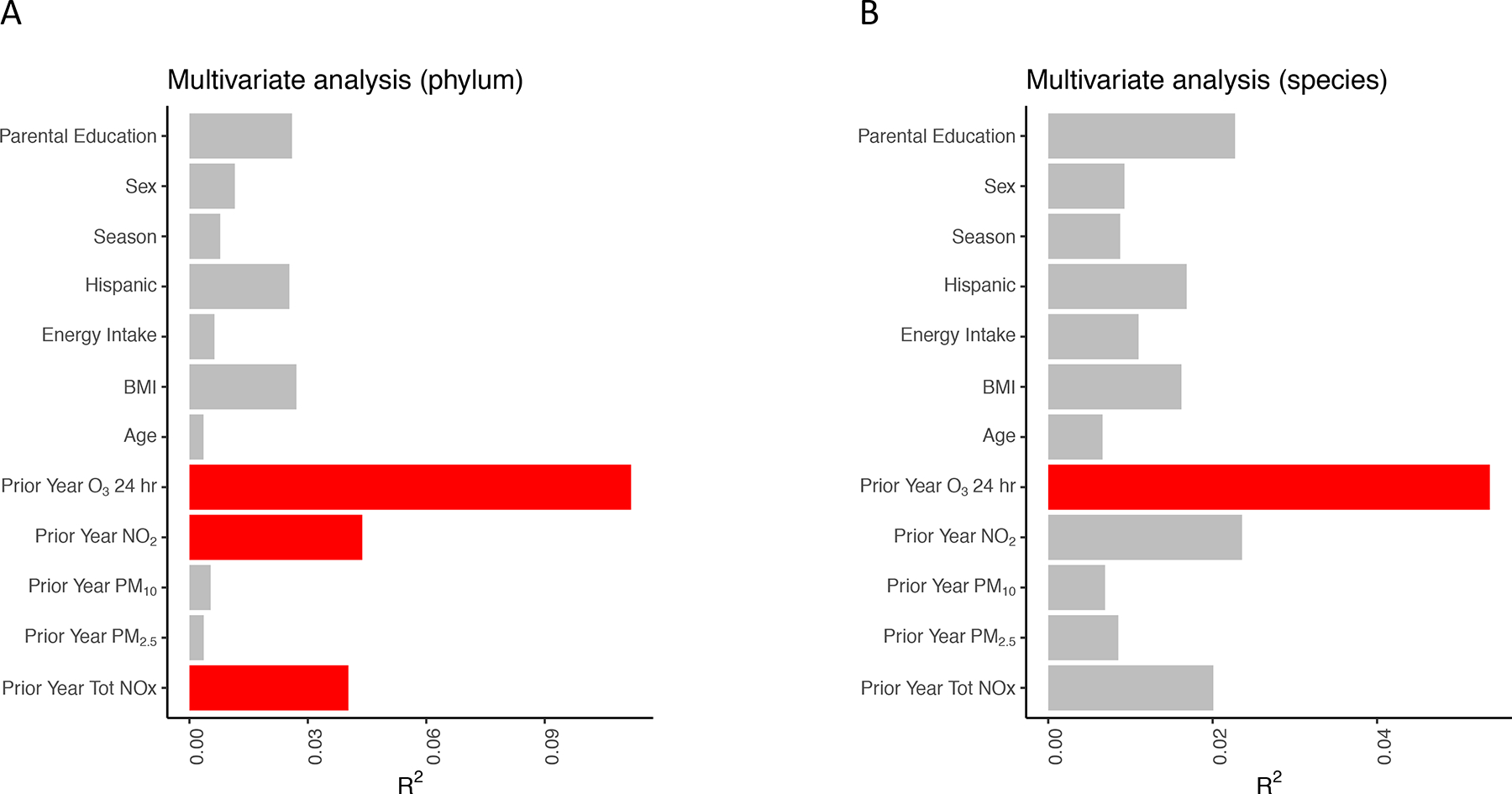
Figure shows the results from a non-parametric multivariate ADONIS test with 10,000 permutations. For each explanatory variable, separate multivariate models were constructed to determine the association between the gut microbiota composition and each explanatory variable at the level of the phylum (A) and species (B). R2 values correspond to the fraction of variation in the gut microbiota composition that is explained by each variable. Red bars indicate statistical significance (FDR<0.05).
The above analysis suggests patterns of association between air pollutants and global measures of the entire microbial community but does not reveal which individual taxa drive these associations. We therefore built simple univariate linear regression models for ambient and near-roadway air pollutants including total NOx, NO2, PM2.5, PM10, and O3 exposure as well as potentially important confounding subject metadata including sex, BMI, age, energy intake, season, parental education, and Hispanic ethnicity (Figure 3). Of all of factors, O3 exposure had the most significant associations at the phylum level (Supplemental Figure 1). Most of these phyla were low abundant taxa with the exception of a few common gut bacterial phyla including Bacteroidetes (FDR corrected p = 0.022, R2 = 0.11), Actinobacteria (FDR corrected p = 0.022, R2 = 0.11) and Proteobacteria (FDR corrected p=0.038, R2=0.10; Supplemental Figure 2A–C). In addition, the phylum Firmicutes was positively associated with NO2 (FDR corrected p = 0.017, R2 = 0.12; Supplemental Figure 2D). Similarly, O3 exposure had the most significant associations at the family level. For example, O3 was negatively associated with Coriobacteriaceae (FDR corrected p = 0.004, R2 = 0.18), Lactobacillaceae (FDR corrected p = 0.004, R2 = 0.17), Acidobacteriaceae (FDR corrected p = 0.005, R2 = 0.16), and Bacillaceae (FDR corrected p = 0.024, R2 = 0.11) and was positively associated with Bacteroidaceae (FDR corrected p = 0.006, R2 = 0.15). A few taxa at the family level were also positively associated with NO2, such as Coriobacteriaceae (FDR corrected p = 0.039, R2 = 0.10) Ruminococcaceae (FDR corrected p = 0.041, R2 = 10), Acidobacteriaceae (FDR corrected p = 0.048, R2 = 0.09). These results were generally robust to the non-parametric Kendall test (Supplemental Figure 3).
Figure 3. Exposure to O3 was Associated with the Gut Microbiota at the Phylum Level Using Whole Genome Sequencing (WGS).
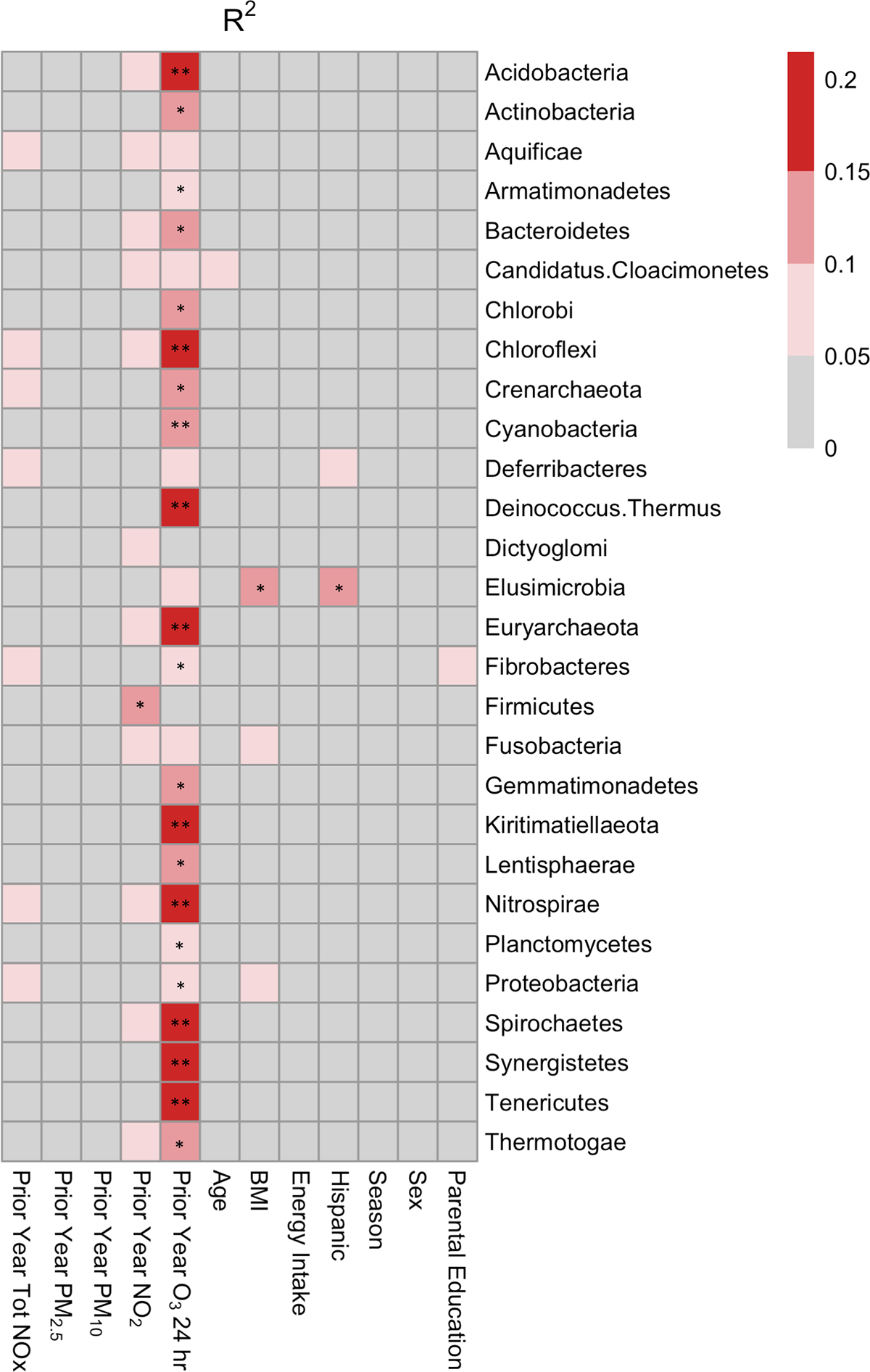
Univariate linear regression was used to determine the association between the relative abundance of gut bacterial phyla and each explanatory variable. The heatmap shows R2 values, which are indicative of the amount of variation explained by each explanatory variable in univariate regression models. ** FDR-corrected p-value < 0.01, * FDR-corrected p-value < 0.05.
At the genus level, WGS sequencing demonstrated significant positive associations between Bacteroides and O3 (p = 0.009, R2 = 0.16; Figure 4A). Utilizing the increased taxonomic resolution of WGS, we built linear models at the species level. Within Bacteroides, higher abundances of the bacterial species Bacteroides caecimuris were associated with higher exposure to O3 (p = 0.037, R2 = 0.15; Figure 4B). Overall, 128 bacterial species (R2 = 0.14 to 0.25) were associated with O3 (pall<0.05). While only 4 (R2= 0.14 to 0.17) and 5 (R2 = 0.14 to 0.16) bacterial species were associated with NO2 and total NOx, respectively (pall<0.05) (Supplemental Figure 4). Differential abundance analysis using Songbird reveled that these findings were not driven by compositional artifacts (Morton et al., 2019). Specifically, differential rankings showed that at the genus level, Bacteroides was highly ranked with an increase in O3 and that the log-ratio of the Bacteroides caecimuris versus Leuconostoc spp. had a significant relationship by Pearson correlation to O3 (R2 = 0.31; p = 0.007) but not to NO2 (R2 = −0.18; p = 0.123) (Supplemental Figure 5).
Figure 4. Exposure to O3 was Positively Associated with the Relative Abundance of Taxa Belonging to the Bacteroides Genus and was Negatively Associated with Gut Bacterial Diversity using Whole Genome Sequencing (WGS) Taxonomically Classified by Kraken2.

Higher exposure to O3 was positively associated with the relative abundance of the genus Bacteroides (A) and the relative abundance of the bacterial species Bacteroides caecimuris (B). Higher exposure to O3 was associated with a lower bacterial Shannon diversity index (C) and evenness (D) at the species level.
WGS also revealed that higher exposure to O3 was associated with a lower bacterial evenness (p<0.001; R2 = 0.15) and a lower Shannon diversity index (p<0.001, R2 = 0.15) at the species level (Figure 4C and D). Results from our univariate linear regression modeling also indicated that age, sex, season, Hispanic ethnicity, energy intake, parental education, and BMI were not strongly associated with the relative abundance of gut bacterial taxa (Figure 3). Likewise, associations between the gut microbiota composition and bacterial diversity and O3 exposure were unchanged after adjusting for each of these respective variables as well as dietary macronutrients (i.e., protein, fat, carbohydrate, fiber) using multivariable linear regression models (Supplemental Tables 2 and 3). Overall, there was no evidence to support differences in effects of exposure to air pollutants by sex (males versus females) or overweight/obese status (overweight/obese versus normal BMI) (Supplemental Table 4). Lastly, gut bacteria found to be associated with O3 were not associated with BMI, glucose metabolism, insulin resistance, or markers of gut microbial translocation before or after adjusting for covariates, which included sex, BMI, age, energy intake, season, parental education, and Hispanic ethnicity (Supplemental Table 5 and 6).
Finally, in order to validate the results obtained from WGS data that were taxonomically classified by Kraken2, we compared the results to those obtained from MetaPhlAn2, which relies on a set of marker genes as opposed to the Kraken2 that uses all available sequences (Lindgreen et al., 2016). As we might expect from previous literature, fewer taxa were identified by MetaPhlAn2 when compared to Kraken2 (68 versus 1184 genera). However, despite differences in taxonomy, similar results were observed with O3. Prior year O3 exposure was positively associated with Bacteroides (FDR corrected p = 0.014, R2 = 0.15) and was negatively associated with Shannon diversity (FDR corrected p<0.001, R2 = 0.15) and evenness (FDR corrected p<0.001, R2 = 0.16) at the genus level (Supplemental Figure 6). Collectively, these results demonstrate that associations between O3 and microbial composition and diversity are robust to different analysis pipelines (Kraken2 vs. MetaPhlAn2).
Air Pollutants were Associated with Gut Bacterial Function using WGS
We next expanded our analysis beyond taxa identification by using the HUMAnN2 pipeline on the WGS data to investigate which gene pathways were associated with increased exposure to O3 (Figure 5A). Functional gene pathways related to cell growth and insulin release were found to be associated with O3 exposure. These include pathways involved in coenzyme A biosynthesis, pantothenate and coenzyme A biosynthesis, L-ornithine de novo biosynthesis, glycolysis, mannitol cycle, and gondoate biosynthesis were positively associated with O3 (FDR corrected p<0.05, R2 = 0.14–0.18; Figure 5B). These associations were largely unchanged after adjusting for NO2, BMI, age, sex, energy intake, season, Hispanic ethnicity, dietary macronutrients, and parental education (Supplemental Table 7). Further, simple linear regression models did not display functional associations with any of the other air pollutants, BMI, age, sex, energy intake, season, parental education, or Hispanic ethnicity (Figure 7A).
Figure 5. Exposure to O3 was Significantly Associated with Multiple Gene Pathways Using Whole Genome Sequencing (WGS).
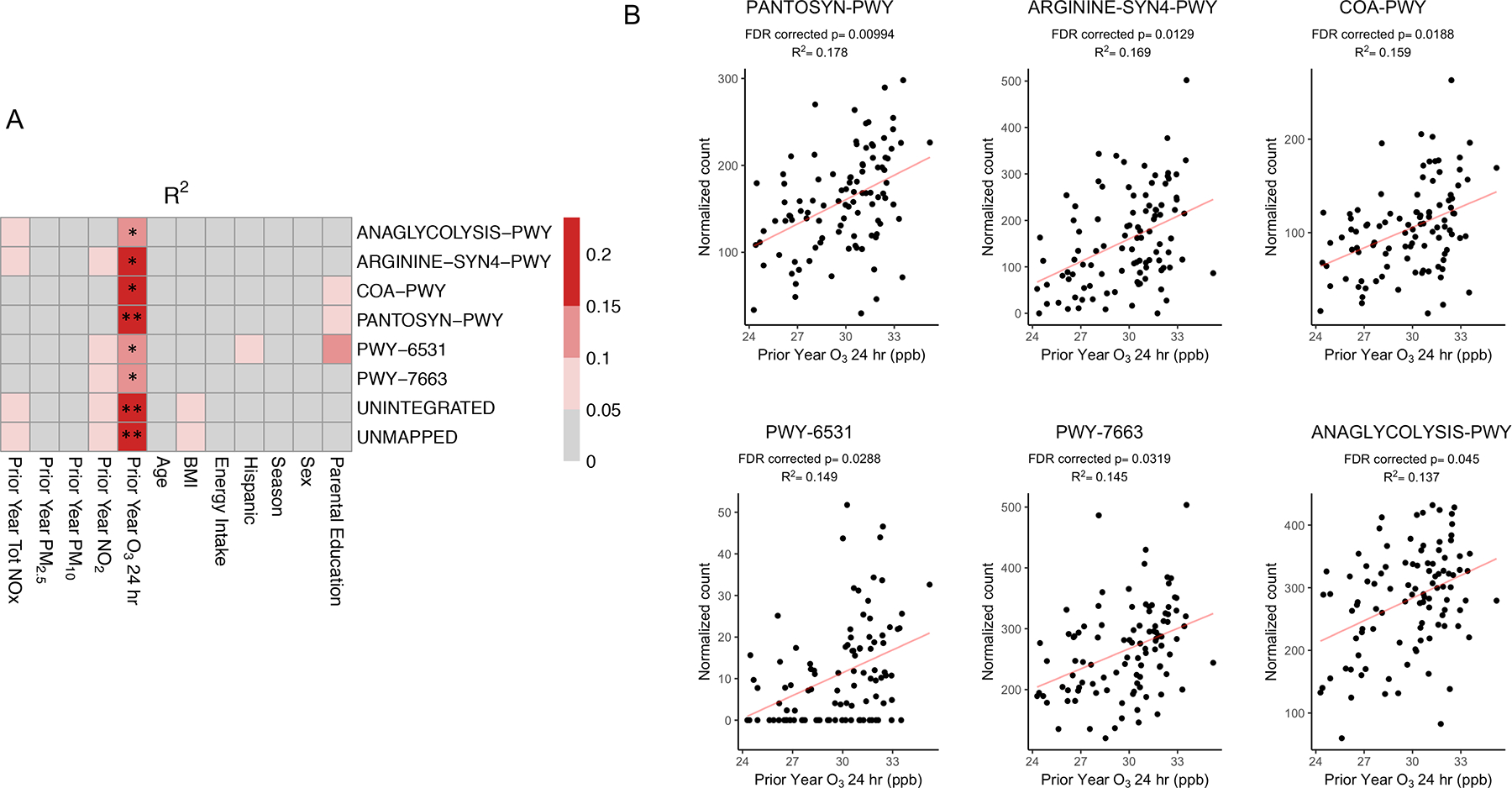
Univariate linear regression analysis was used to determine the associations between the relative abundance of microbial gene pathways and each explanatory variable. The heatmaps shows R2 values (A), which are indicative of the amount of variation explained by each explanatory variable. ** FDR-corrected p-value < 0.01, * FDR-corrected p-value < 0.05. Scatter plots (B) show the associations between the relative abundance of six gene pathways and O3 exposure using univariate linear models with the corresponding R2 and FDR corrected p-values. PANTOSYN-PWY: pantothenate and coenzyme A biosynthesis I, ARGININE-SYN4-PWY: L-ornithine de novo biosynthesis, COA-PWY: coenzyme A biosynthesis I, PWY-6531: mannitol cycle, PWY-7663: gondoate biosynthesis (anaerobic), ANAGLYCOLYSIS-PWY: glycolysis III (from glucose). Pathway abundance is normalized to copies per million (cpm).
Discussion
In this cohort of young adults from Southern California, our results demonstrate significant associations between exposure to air pollutants and the composition of the gut microbiome using WGS. These findings were robust to adjusting for confounders such as age, sex, Hispanic ethnicity, BMI, season of study visit, parental education (proxy for socioeconomic status), energy intake, and macronutrients (i.e., protein, fat, carbohydrates, fiber). Notably, the percent variation that was explained by exposure to air pollutants was 4.0% for total NOx, 4.4% for NO2, and 11.2% for O3 concentrations. These air pollutants explained the highest amount of variation in the gut when compared to other factors known to impact the gut microbiome (i.e. BMI, and energy intake); however, this may be partially explained by the relatively small range of BMI, age, energy intake, and race/ethnicity in our study population. Additionally, we observed that the phylum Firmicutes was positively associated with NO2, yet O3 exposure had the most significant associations at the phylum and species level. This study also found that gene pathways involved in fatty acid synthesis/degradation, were enriched with higher O3 exposure. These included L-ornithine de novo biosynthesis and pantothenate and coenzyme A biosynthesis pathways, which may play a role in gut barrier integrity (Leonardi and Jackowski, 2007) as well as obesity and type 2 diabetes (Bandyopadhyay et al., 2006; Davaapil et al., 2014; Webster et al., 2008). Collectively, these results provide novel evidence for significant associations between exposure to air pollutants with both the composition and functional potential of the human gut microbiome.
A growing body of work has found that increased exposure to air pollutants is associated with increased obesity, dysregulated glucose metabolism (Alderete et al., 2017; Lucht et al., 2018; Thiering et al., 2013; Toledo-Corral et al., 2018; Yang et al., 2018), and the composition of the gut microbiota (Alderete et al., 2018b; Kish et al., 2013; R. Li et al., 2017; T. Liu et al., 2019; Mutlu et al., 2018; Ribière et al., 2016; Wang et al., 2018). Additionally, the gut microbiota has also been linked with these adverse health outcomes (Ley et al., 2006; Qin et al., 2012; Ross et al., 2015; Suez et al., 2014; Turnbaugh et al., 2006; Vrieze et al., 2012; Walters et al., 2014). While studies in rodent models demonstrate significant associations between inhaled and ingested air pollutants with the composition of the gut microbiota (Kish et al., 2013; R. Li et al., 2017; Mutlu et al., 2018; Ribière et al., 2016; Wang et al., 2018), only two studies have examined these associations in humans (Alderete et al., 2018b; T. Liu et al., 2019). One of these was our previous pilot study that examined 43 participants from the Meta-AIR cohort using 16S rRNA sequencing. Results from this study found that increased near-roadway exposure (NOx) was correlated with the relative abundance of gut bacteria that have been associated with obesity and altered metabolism (e.g., Coriobacteriaceae and Bacteroidaceae) (Alderete et al., 2018b). Additionally, gut microbial taxa that were correlated with near-roadway air pollution exposure accounted for 24% and 29% of the correlation between exposure to air pollutants and fasting glucose levels (Alderete et al., 2018b). The second study examined 6,627 adults from south China and found that exposure to PM2.5 and PM10 were associated with a decreased gut microbial alpha diversity. PM exposure was also negatively associated with Firmicutes, Proteobacteria, and Verrucomicrobia and was associated with several taxa within the Bacteroidetes phyla. Additionally, impaired fasting glucose was positively associated with PM2.5 and PM10 exposure and these associations were partially mediated by gut microbial diversity (T. Liu et al., 2019).
The current study found that exposure to air pollutants was associated with reduced gut bacterial diversity and numerous gut bacterial species. This could have important implications as several studies have shown that decreased gut bacterial diversity is associate with poor health outcomes (Turnbaugh and Gordon, 2009; Turnbaugh et al., 2009; Vangay et al., 2018). We also found that 128 bacterial species were associated with O3 and 4 and 5 bacterial species were associated with NO2 and total NOx, respectively. Most of these phyla were low abundant taxa with the exception of a few common gut bacterial phyla including Bacteroidetes, Actinobacteria, and Proteobacteria. Further, within Bacteroides, higher abundances of the bacterial species Bacteroides caecimuris were associated with higher exposure to O3. In addition, the phylum Firmicutes was positively associated with NO2. Similar to our findings, the Liu et al. study found that a lower gut microbial diversity was associated with higher PM2.5 and PM10 exposure (T. Liu et al., 2019). In our previous study, we found that a lower abundance of Bacteroidaceae and a higher abundance of Coriobacteriaceae was associated with higher NOx exposure (Alderete et al., 2018b). In the current study, a lower abundance of Bacteroidaceae was also associated with higher NOx exposure, but this association did not remain significant after adjusting for multiple comparisons (data not shown). Additionally, in the current study, higher NO2 was associated with a higher abundance of Coriobacteriaceae and higher O3 exposure was associated with a higher abundance bacterial species belonging to the Bacteroidaceae family. These mixed findings may be due to differences in sample size, air pollutants examined, or different sequencing methods that allow for varying levels of taxonomic resolution to be examined.
Studies have shown that these bacterial taxa and reduced gut microbial diversity have been linked with obesity and type 2 diabetes (Qin et al., 2012; Turnbaugh et al., 2006; Vrieze et al., 2012). Despite these findings, we did not observe any significant associations between the gut microbiome and metabolic outcomes either before or after adjusting for important confounders (BMI, age, sex, energy intake, season, parental education, or Hispanic ethnicity). This may be partially due to the fact that our study included young adults that, despite being overweight, were largely metabolically healthy. This study builds on previous work by examining the functional potential of the gut microbiome using WGS. Our results show that increased exposure to average prior year O3 was associated with enrichment of multiple gene pathways that have the potential to impact gut barrier integrity and/or host metabolism, including L-ornithine de novo biosynthesis and pantothenate and coenzyme A biosynthesis I. L-ornithine is a non-essential amino acid and precursor for polyamines and nitric acid (Selamnia et al., 1998) and polyamines are necessary for cell growth (Hölttä et al., 1993) and nitric acid has antiproliferative properties (Kumar and Kashyap, 2015). Additionally, pantothenate and CoA biosynthesis play a role in fatty acid synthesis/degradation, phospholipids synthesis, and serve as a cofactor for cell growth (Leonardi and Jackowski, 2007). CoA derivatives have also been shown to inhibit insulin release (Davaapil et al., 2014; Webster et al., 2008) and are elevated in obesity and type 2 diabetes (Bandyopadhyay et al., 2006). Thus, enrichment of these pathways may have important implications in gut bacterial communities and gut permeability through cell growth and cytotoxicity as well as changes in fatty acid and/or phospholipid synthesis and degradation.
PM2.5 and O3 have been shown to have extrapulmonary effects that may alter the HPA axis through vagal nerve activation (Gackière et al., 2011) or effects on the hippocampus (Thomson, 2019), which can increase levels of catecholamines and steroid hormones. While previous work has largely focused on the effects of PM or near-roadway exposure on the gut microbiota (Alderete et al., 2018b; Kish et al., 2013; R. Li et al., 2017; T. Liu et al., 2019; Ribière et al., 2016), O3 has been largely overlooked. In the current study, we found that O3 exposure was consistently and strongly associated with the composition and functional potential of the gut microbiome. While the mechanisms that link increased O3 exposure and the gut microbiome have yet to be fully characterized, animal and human studies have shown that O3 exposure increases plasma corticosterone levels (Thomas et al., 2018) as well as plasma cortisol and corticosterone concentrations (Miller et al., 2016), respectively. Thus, activation of the HPA axis may increase the production of cortisol and norepinephrine, which may induce changes in the gut microbiota (Petrosus et al., 2018). Additionally, the gut-brain axis allows for bidirectional communication (Lyte, 2014) that may result in changes in bacterial proliferation in the presence of norepinephrine (Lyte and Ernst, 1992).
Results from this study provide early evidence that elevated exposure to air pollutants may impact the composition and functional potential of the human gut microbiome. These findings were unchanged after adjusting for potentially important confounders, including age, sex, Hispanic ethnicity, BMI, season, parental education, and dietary factors known to influence the composition of the gut microbiota. Despite these study strengths, this work was limited by the moderate sample size of 101, which may have reduced our statistical power. However, this study was able to detect 128 bacterial species that were associated with O3 exposure as well as 6 functional pathways. The current study may also be limited by participant selection, which was based on being overweight or obese at their previous visit. This may limit the generalizability of the current study, be a potential source of selection bias, and may have reduced our ability to find associations between gut bacteria and metabolic markers. However, at the time of the current study, we observed a range of BMI values that included normal weight, overweight, and obese young adults. While exploratory analysis using multi-pollutant models showed that our significant findings from single pollutant models for O3 were robust to NO2 adjustment, a known limitation air exposure research is the moderate to high correlation among exposure variables. As such, our multi-pollutant models may be limited by bias amplification (Weisskopf et al., 2018). Residential based estimates of air pollution exposure may have resulted in exposure misclassification, yet exposure misclassification should be random across participants (non-differential) and likely would bias estimates towards the null (Nerriere et al., 2005). Although compositional data analysis used in human microbiome research may contribute to type I errors, we verified the main findings using Songbird that is a compositionally aware method (Morton et al., 2019). Lastly, differences in processing pipelines could impact the specific gut bacterial taxa found to be associated with air pollution exposure. However, we utilized two different classifiers for taxonomic assignment of WGS data and found that both of these pipelines identified significant associations between the gut microbiota and exposure to O3.
Conclusions
Our results show that increased exposure to air pollution has the potential to impact the human gut microbiome, which remained robust after adjusting for potentially important confounders. These early findings, coupled with other epidemiological and animal studies, suggest that exposure to air pollutants may increase risk for obesity and type 2 diabetes through alterations to the gut microbiome. These findings have significant public health relevance since air pollution remains a challenge despite extensive efforts to improve air quality and O3 pollution has worsened in much of the nation. Despite this, additional epidemiological and mechanistic studies are needed in order to examine the exact mechanisms by which pollutants impact the human gut microbiome and increase human disease risk.
Supplementary Material
Supplemental Figure Titles and Legends
Supplemental Figure 1 Title: Exposure to O3 was Significantly Associated with Gut Bacterial Phyla Using Whole Genome Sequencing (WGS) Taxonomically Classified by Kraken2 Higher exposure to O3 was negatively associated with 20 bacterial phyla and positively associated with one bacterial phylum (Bacteroidetes).
Supplemental Figure 2 Title: Exposure to O3 and NO2 was Associated with Gut Bacterial Phyla Using Whole Genome Sequencing (WGS) Taxonomically Classified by Kraken2 Scatter plots show associations between gut bacterial phyla and O3 exposure using univariate linear models. Plots show the R2 and FDR corrected p-values. Panels A-C show common gut bacterial phyla that were associated with O3 exposure, including Bacteroidetes, Actinobacteria, and Proteobacteria. In addition, panel D shows the phylum Firmicutes, which was positively associated with NO2 exposure.
Supplemental Figure 3 Title: Exposure to O3 was Significantly Correlated with Gut Bacterial Phyla Using Non-Parametric Kendall Tests and Whole Genome Sequencing (WGS) The heatmap shows the FDR corrected p-values based on non-parametric Kendall tests between exposure variables and gut microbial taxa at the phylum level.
Supplemental Figure 4 Title: Exposure to O3, NO2, and Total NOx was Significantly Associated with Gut Bacterial Scatter plots show the significant associations between the relative abundance of gut bacterial species with O3, NO2, and total NOx exposures using univariate linear models with the corresponding R2 and FDR corrected p-values. Overall, 128 bacterial species were significantly associated with O3 while only 4 and 5 bacterial species were significantly associated with NO2 and total NOx, respectively.
Supplemental Figure 5 Title: Exposure to O3 was Significantly Associated with the Log Ratio of Bacteroides caecimuris vs. Leuconostoc spp using Songbird Differential abundance shows a high ranking of Bacteroides related to increases in O3. A boxplot of the differential rankings from Songbird with respect to O3 (A). Regression plots between the log-ratio of Bacteroides caecimuris vs. Leuconostoc spp. and O3 (B) and NO2 (C) with a Pearson correlation included in the upper-left and lower-left box.
Supplemental Figure 6 Title: Exposure to O3 was Significantly Associated with a Higher Relative Abundance of Bacteroides and a Lower Diversity and Evenness Using Whole Genome Sequencing (WGS) Data that were Taxonomically Classified by MetaPhlAn2 Scatter plots show the associations between the relative abundance of Bacteroides, Shannon Diversity Index, and Evenness with O3 exposure using univariate linear models with the corresponding R2 and FDR corrected p-values. O3 was positively associated with Bacteroides (A) and was negatively associated with Shannon Diversity (B) Index and evenness (C).
Supplemental Table Titles and Legends
Supplemental Table 1. Correlations Between Air Pollutants among Young Adults from the Meta-AIR Study Spearman’s rank-order correlation coefficients between prior year exposure to air pollutants are shown. Statistically significant correlations are denoted as *p-value<0.05; **p-value<0.01; ***p-value<0.001
Supplemental Table 2. Ozone Findings were Largely Unchanged After Adjusting for Potentially Important Confounders Multivariable linear regression models (taxa ~ O3 + covariant) were built to control for 24-hour NO2, BMI, age, energy intake, Hispanic ethnicity, sex, season, parental education, carbohydrate intake, fat intake, fiber intake, and protein intake. FDR corrected p-values corresponding to 24-hour O3 from each model are shown in the table.
Supplemental Table 3. Associations Between Prior Year Exposure to Air Pollutants and Gut Bacterial Diversity and Evenness at the Species Level in Young Adults from the Meta-AIR Study R2 and FDR-corrected p-values from univariate linear regression between each ambient and near-roadway air pollutant and measures of gut bacterial diversity (Shannon Diversity Index) and evenness are shown. Models were adjusted for aNO2, bBMI, cage, denergy intake, eHispanic ethnicity, fsex, gseason, hcarbohydrate intake, ifat intake, jfiber intake, kprotein intake, and lparental education as indicated.
Supplemental Table 4. No Evidence for Effect Modification by Obesity Status or Sex was Found for the Association Between O3 and the Gut Bacteria Using Whole Genome Sequencing (WGS) Multivariate analysis (ADONIS test) was used to examine the associations between the gut microbiota composition at the genus level and O3. Separate models with interaction terms included were constructed to examine the effect modification by obesity status (normal weight versus overweight and obese) and sex.
Supplemental Table 5. No Significant Association between the Gut Microbiota and Metabolic Indices were Found at the Species Level Using Whole Genome Sequencing (WGS) A. Simple univariate regression models were built to examine the association between microbial species and metabolic indices. FDR-corrected p-values are shown in the table. B. Multivariable linear regression models were built to examine the association between microbial species and metabolic indices after adjusting for age, BMI, energy intake, Hispanic ethnicity, sex, season (warm/cold), and parental education. FDR-corrected p-values are shown in the table.
Supplemental Table 6. No Significant Association between the Gut Microbiota and Bacterial Translocation Factors were Found at the Species Level Using Whole Genome Sequencing (WGS) A. Simple univariate regression models were built to examine the association between microbial species and bacterial translocation factors, including sCD14, lipopolysaccharide-binding protein (LBP), and LBP/sCD14 ratio. FDR-corrected p-values are shown in the table. B. Multivariable linear regression models were built to examine the association between microbial species and bacterial translocation factors, including sCD14, lipopolysaccharide-binding protein (LBP), and LBP/sCD14 ratio after adjusting for age, BMI, energy intake, Hispanic ethnicity, sex, season (warm/cold), and parental education. FDR-corrected p-values are shown in the table.
Supplemental Table 7. Significant Associations between O3 and Microbial Gene Pathways were Largely Unchanged After Adjusting for Potentially Important Confounders Multivariable linear regression models (gene pathway ~ O3 + covariant) were built to control for 24-hour NO2, BMI, age, energy intake, Hispanic ethnicity, sex, season (warm/cold), parental education, carbohydrate intake, fat intake, fiber intake, and protein intake. FDR corrected p-values corresponding to 24-hour O3 from each model are shown in the table.
Highlights.
Exposure to air pollutants was significantly associated with the composition of the gut microbiome using whole-genome sequencing.
Higher exposure to 24-hour O3 was associated with lower Shannon diversity index, higher Bacteroides caecimuris and multiple gene pathways, including L-ornithine de novo biosynthesis and pantothenate and coenzyme A biosynthesis I.
This study provides the first evidence of significant associations between exposure to air pollutants and the compositional and functional profile of the human gut microbiome.
Funding
This work was supported by the following agencies: National Institutes of Health (NIH) (grant R00ES027853), Southern California Environmental Health Sciences Center from NIH NIEHS (grants 5P30ES07048 and P30ES007048), Southern California Children’s Environmental Health Center from NIH and EPA (grant P01ES022845 and RD-83544101-0), NIH T32 Environmental Genomics Training grant from (grant T32ES013678), and the Hastings Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests statement
The authors declare no competing interests.
References
- Adams K, Greenbaum DS, Shaikh R, van Erp AM, Russell AG, 2015. Particulate matter components, sources, and health: Systematic approaches to testing effects. J Air Waste Manag Assoc 65, 544–558. doi: 10.1080/10962247.2014.1001884 [DOI] [PubMed] [Google Scholar]
- Alderete TL, Chen Z, Toledo-Corral CM, Contreras ZA, Kim JS, Habre R, Chatzi L, Bastain T, Breton CV, Gilliland FD, 2018a. Ambient and Traffic-Related Air Pollution Exposures as Novel Risk Factors for Metabolic Dysfunction and Type 2 Diabetes. Curr Epidemiol Rep 5, 79–91. doi: 10.1007/s40471-018-0140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete TL, Habre R, Toledo-Corral CM, Berhane K, Chen Z, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD, 2017. Longitudinal Associations Between Ambient Air Pollution With Insulin Sensitivity, β-Cell Function, and Adiposity in Los Angeles Latino Children. Diabetes 66, 1789–1796. doi: 10.2337/db16-1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete TL, Jones RB, Chen Z, Kim JS, Habre R, Lurmann F, Gilliland FD, Goran MI, 2018b. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ. Res 161, 472–478. doi: 10.1016/j.envres.2017.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM, 2006. Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55, 2277–2285. doi: 10.2337/db06-0062 [DOI] [PubMed] [Google Scholar]
- Bassett SA, Young W, Fraser K, Dalziel JE, Webster J, Ryan L, Fitzgerald P, Stanton C, Dinan TG, Cryan JF, Clarke G, Hyland N, Roy NC, 2019. Metabolome and microbiome profiling of a stress-sensitive rat model of gut-brain axis dysfunction. Sci Rep 9, 14026–13. doi: 10.1038/s41598-019-50593-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamish LA, Osornio-Vargas AR, Wine E, 2011. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis 5, 279–286. doi: 10.1016/j.crohns.2011.02.017 [DOI] [PubMed] [Google Scholar]
- Benson PE, 1992. A review of the development and application of the CALINE3 and 4 models. Atmospheric Environment Part B Urban Atmosphere 26, 379–390. doi: 10.1016/0957-1272(92)90013-I [DOI] [Google Scholar]
- Calle ML, 2019. Statistical Analysis of Metagenomics Data. Genomics Inform 17, e6–9. doi: 10.5808/GI.2019.17.1.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C, 2015. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28, 203–209. [PMC free article] [PubMed] [Google Scholar]
- Caspi R, Billington R, Fulcher CA, Keseler IM, Kothari A, Krummenacker M, Latendresse M, Midford PE, Ong Q, Ong WK, Paley S, Subhraveti P, Karp PD, 2018. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 46, D633–D639. doi: 10.1093/nar/gkx935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Eckel SP, Breton CV, Gilliland FD, 2015. Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children’s Health Study. J Thorac Dis 7, 46–58. doi: 10.3978/j.issn.2072-1439.2014.12.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AL, Jia Y, DenBleyker A, McDonald-Buller E, Fraser MP, Allen DT, Collins DR, Michel E, Pudota J, Sullivan D, Zhu Y, 2009. Air pollutant concentrations near three Texas roadways, part II: Chemical characterization and transformation of pollutants. Atmospheric Environment 43, 4523–4534. doi: 10.1016/j.atmosenv.2009.06.044 [DOI] [Google Scholar]
- Corfield AP, 2018. The Interaction of the Gut Microbiota with the Mucus Barrier in Health and Disease in Human. Microorganisms 6, 78. doi: 10.3390/microorganisms6030078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davaapil H, Tsuchiya Y, Gout I, 2014. Signalling functions of coenzyme A and its derivatives in mammalian cells. Biochem. Soc. Trans 42, 1056–1062. doi: 10.1042/BST20140146 [DOI] [PubMed] [Google Scholar]
- Eckel SP, Cockburn M, Shu Y-H, Deng H, Lurmann FW, Liu L, Gilliland FD, 2016. Air pollution affects lung cancer survival. Thorax 71, 891–898. doi: 10.1136/thoraxjnl-2015-207927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder A, Oberdörster G, 2006. Translocation and effects of ultrafine particles outside of the lung. Clin Occup Environ Med 5, 785–796. doi: 10.1016/j.coem.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, Kurilshikov A, Bonder MJ, Valles-Colomer M, Vandeputte D, Tito RY, Chaffron S, Rymenans L, Verspecht C, De Sutter L, Lima-Mendez G, D’hoe K, Jonckheere K, Homola D, Garcia R, Tigchelaar EF, Eeckhaudt L, Fu J, Henckaerts L, Zhernakova A, Wijmenga C, Raes J, 2016. Population-level analysis of gut microbiome variation. Science 352, 560–564. doi: 10.1126/science.aad3503 [DOI] [PubMed] [Google Scholar]
- Fedarko MW, Martino C, Morton JT, bioRxiv AG, 2019, n.d. Visualizing’omic feature rankings and log-ratios using Qurro. biorxiv.org doi: 10.1101/2019.12.17.880047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzosa EA, McIver LJ, Rahnavard G, Thompson LR, Schirmer M, Weingart G, Lipson KS, Knight R, Caporaso JG, Segata N, Huttenhower C, 2018. Species-level functional profiling of metagenomes and metatranscriptomes. Nat Meth 15, 962–968. doi: 10.1038/s41592-018-0176-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita EM, Zielinska B, Campbell DE, Arnott WP, Sagebiel JC, Mazzoleni L, Chow JC, Gabele PA, Crews W, Snow R, Clark NN, Wayne WS, Lawson DR, 2007. Variations in speciated emissions from spark-ignition and compressionignition motor vehicles in California’s south coast air basin. J Air Waste Manag Assoc 57, 705–720. doi: 10.3155/1047-3289.57.6.705 [DOI] [PubMed] [Google Scholar]
- Gackière F, Saliba L, Baude A, Bosler O, Strube C, 2011. Ozone inhalation activates stress-responsive regions of the CNS. Journal of Neurochemistry 117, 961–972. doi: 10.1111/j.1471-4159.2011.07267.x [DOI] [PubMed] [Google Scholar]
- Gao B, Chi L, Mahbub R, Bian X, Tu P, Ru H, Lu K, 2016. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem. Res. Toxicol 30, 996–1005. doi: 10.1021/acs.chemrestox.6b00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, Chang R, Lurmann F, Gilliland F, 2015. Association of improved air quality with lung development in children. N. Engl. J. Med 372, 905–913. doi: 10.1056/NEJMoa1414123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölttä E, Auvinen M, Andersson LC, 1993. Polyamines are essential for cell transformation by pp60v-src: delineation of molecular events relevant for the transformed phenotype. J. Cell Biol 122, 903–914. doi: 10.1083/jcb.122.4.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DT, Clarke MB, Yamamoto K, Rasko DA, Sperandio V, 2009. The QseC adrenergic signaling cascade in Enterohemorrhagic E. coli (EHEC). PLoS Pathog. 5, e1000553. doi: 10.1371/journal.ppat.1000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DT, Sperandio V, 2008. Inter-kingdom signalling: communication between bacteria and their hosts. Nat. Rev. Microbiol 6, 111–120. doi: 10.1038/nrmicro1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Brook R, White LF, Burnett RT, Yu J, Su J, Seto E, Marshall J, Palmer JR, Rosenberg L, Coogan PF, 2017. Ambient ozone and incident diabetes: A prospective analysis in a large cohort of African American women. Environ Int 102, 42–47. doi: 10.1016/j.envint.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, McConnell R, Wolch J, Chang R, Lam C, Dunton G, Gilliland F, Lurmann F, Islam T, Berhane K, 2014. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health 13, 49. doi: 10.1186/1476-069X-13-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely S, Kelly CJ, Weissmueller T, Burgess A, Wagner BD, Robertson CE, Harris JK, Colgan SP, 2012. Activated fluid transport regulates bacterial-epithelial interactions and significantly shifts the murine colonic microbiome. Gut Microbes 3, 250–260. doi: 10.4161/gmic.20529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi R, Hashemi M, Javanmard SH, Mansourian M, Afshani M, Poursafa P, Sadeghian B, Fakhri M, 2014. Effect of particulate air pollution and passive smoking on surrogate biomarkers of endothelial dysfunction in healthy children. Paediatr Int Child Health 34, 165–169. doi: 10.1179/2046905513Y.0000000104 [DOI] [PubMed] [Google Scholar]
- Kenty KL, Poor ND, Kronmiller KG, McClenny W, King C, Atkeson T, Campbell SW, 2007. Application of CALINE4 to roadside NO/NO2 transformations. Atmospheric Environment 41, 4270–4280. doi: 10.1016/j.atmosenv.2006.06.066 [DOI] [Google Scholar]
- Kim JS, Chen Z, Alderete TL, Toledo-Corral C, Lurmann F, Berhane K, Gilliland FD, 2019. Associations of air pollution, obesity and cardiometabolic health in young adults: The Meta-AIR study. Environ Int 133, 105180. doi: 10.1016/j.envint.2019.105180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Gänzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, 2013. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS ONE 8, e62220. doi: 10.1371/journal.pone.0062220.s003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kashyap P, 2015. Antiproliferative activity and nitric oxide production of a methanolic extract of Fraxinus micrantha on Michigan Cancer Foundation-7 mammalian breast carcinoma cell line. J Intercult Ethnopharmacol 4, 109–113. doi: 10.5455/jice.20150129102013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi R, Jackowski S, 2007. Biosynthesis of Pantothenic Acid and Coenzyme A. EcoSal Plus 2. doi: 10.1128/ecosalplus.3.6.3.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitin J, Härkönen J, Kukkonen J, Environment JNA, 2005, n.d. Evaluation of the CALINE4 and CAR-FMI models against measurements near a major road. Elsevier. doi: 10.1016/j.atmosenv.2005.03.046 [DOI] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI, 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023. doi: 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- Li R, Yang J, Saffari A, Jacobs J, Baek KI, Hough G, Larauche MH, Ma J, Jen N, Moussaoui N, Zhou B, Kang H, Reddy S, Henning SM, Campen MJ, Pisegna J, Li Z, Fogelman AM, Sioutas C, Navab M, Hsiai TK, 2017. Ambient Ultrafine Particle Ingestion Alters Gut Microbiota in Association with Increased Atherogenic Lipid Metabolites. Sci Rep 7, 42906. doi: 10.1038/srep42906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Brejnrod AD, Ernst M, Rykær M, Herschend J, Olsen NMC, Dorrestein PC, Rensing C, Sørensen SJ, 2019. Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ Int 126, 454–467. doi: 10.1016/j.envint.2019.02.048 [DOI] [PubMed] [Google Scholar]
- Lindgreen S, Adair KL, Gardner PP, 2016. An evaluation of the accuracy and speed of metagenome analysis tools. Sci Rep 6, 19233. doi: 10.1038/srep19233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xu X, Bai Y, Wang T-Y, Rao X, Wang A, Sun L, Ying Z, Gushchina L, Maiseyeu A, Morishita M, Sun Q, Harkema JR, Rajagopalan S, 2014. Air Pollution–Mediated Susceptibility to Inflammation and Insulin Resistance: Influence of CCR2 Pathways in Mice 122, 17–26. doi: 10.1289/ehp.1306841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Chen X, Xu Y, Wu W, Tang W, Chen Z, Ji G, Peng J, Jiang Q, Xiao J, Li X, Zeng W, Xu X, Hu J, Guo Y, Zou F, Du Q, Zhou H, He Y, Ma W, 2019. Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: Evidence from a population-based epidemiological study. Environ Int 130, 104882. doi: 10.1016/j.envint.2019.05.076 [DOI] [PubMed] [Google Scholar]
- Lomer MCE, Thompson RPH, Powell JJ, 2002. Fine and ultrafine particles of the diet: influence on the mucosal immune response and association with Crohn’s disease. Proc Nutr Soc 61, 123–130. [DOI] [PubMed] [Google Scholar]
- Lucht SA, Hennig F, Matthiessen C, Ohlwein S, Icks A, Moebus S, Jöckel K-H, Jakobs H, Hoffmann B, 2018. Air Pollution and Glucose Metabolism: An Analysis in Non-Diabetic Participants of the Heinz Nixdorf Recall Study. Environ. Health Perspect 126, 047001. doi: 10.1289/EHP2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyte M, 2014. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol 817, 3–24. doi: 10.1007/978-1-4939-0897-4_1 [DOI] [PubMed] [Google Scholar]
- Lyte M, Ernst S, 1993. Alpha and beta adrenergic receptor involvement in catecholamine-induced growth of gram-negative bacteria. Biochemical and Biophysical Research Communications 190, 447–452. doi: 10.1006/bbrc.1993.1068 [DOI] [PubMed] [Google Scholar]
- Lyte M, Ernst S, 1992. Catecholamine induced growth of gram negative bacteria. Life Sci. 50, 203–212. doi: 10.1016/0024-3205(92)90273-r [DOI] [PubMed] [Google Scholar]
- Matthews NC, Pfeffer PE, Mann EH, Kelly FJ, Corrigan CJ, Hawrylowicz CM, Lee TH, 2016. Urban Particulate Matter-Activated Human Dendritic Cells Induce the Expansion of Potent Inflammatory Th1, Th2, and Th17 Effector Cells. Am. J. Respir. Cell Mol. Biol 54, 250–262. doi: 10.1165/rcmb.2015-0084OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty J, Mühlbauer M, Gharaibeh RZ, Arthur JC, Perez-Chanona E, Sha W, Jobin C, Fodor AA, 2013. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J 7, 2116–2125. doi: 10.1038/ismej.2013.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang C-C, Lurmann F, Berhane K, 2015. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children’s Health Study. Environ. Health Perspect 123, 360–366. doi: 10.1289/ehp.1307031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Ghio AJ, Karoly ED, Bell LN, Snow SJ, Madden MC, Soukup J, Cascio WE, Gilmour MI, Kodavanti UP, 2016. Ozone Exposure Increases Circulating Stress Hormones and Lipid Metabolites in Humans. Am. J. Respir. Crit. Care Med 193, 1382–1391. doi: 10.1164/rccm.201508-1599OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JT, Marotz C, Washburne A, Silverman J, Zaramela LS, Edlund A, Zengler K, Knight R, 2019. Establishing microbial composition measurement standards with reference frames. Nat Commun 10, 2719. doi: 10.1038/s41467-019-10656-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu EA, Comba IY, Cho T, Engen PA, Yazıcı C, Soberanes S, Hamanaka RB, Nigdelioglu R, Meliton AY, Ghio AJ, Budinger GRS, Mutlu GM, 2018. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut 240, 817–830. doi: 10.1016/j.envpol.2018.04.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerriere É, Zmirou-Navier D, Blanchard O, Momas I, Ladner J, Le Moullec Y, Personnaz M-B, Lameloise P, Delmas V, Target A, Desqueyroux H, 2005. Can we use fixed ambient air monitors to estimate population long-term exposure to air pollutants? The case of spatial variability in the Genotox ER study. Environ. Res 97, 32–42. doi: 10.1016/j.envres.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Petrosus E, Silva EB, Lay D, Eicher SD, 2018. Effects of orally administered cortisol and norepinephrine on weanling piglet gut microbial populations and Salmonella passage. J. Anim. Sci 96, 4543–4551. doi: 10.1093/jas/sky312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, 2012. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. doi: 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- Ribière C, Peyret P, Parisot N, Darcha C, Déchelotte PJ, Barnich N, Peyretaillade E, Boucher D, 2016. Oral exposure to environmental pollutant benzo[a]pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci Rep 6, 31027. doi: 10.1038/srep31027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross MC, Muzny DM, McCormick JB, Gibbs RA, Fisher-Hoch SP, Petrosino JF, 2015. 16S gut community of the Cameron County Hispanic Cohort. Microbiome 3, 7. doi: 10.1186/s40168-015-0072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim SY, Kaplan GG, Madsen KL, 2014. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes 5, 215–219. doi: 10.4161/gmic.27251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandle GI, Binder HJ, 1987. Corticosteroids and intestinal ion transport. Gastroenterology 93, 188–196. doi: 10.1016/0016-5085(87)90333-7 [DOI] [PubMed] [Google Scholar]
- Schroeder BO, 2019. Fight them or feed them: how the intestinal mucus layer manages the gut microbiota. Gastroenterol Rep (Oxf) 7, 3–12. doi: 10.1093/gastro/goy052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selamnia M, Robert V, Mayeur C, Delpal S, Blachier F, 1998. De novo synthesis of arginine and ornithine from citrulline in human colon carcinoma cells: metabolic fate of L-ornithine. Biochim. Biophys. Acta 1425, 93–102. doi: 10.1016/s0304-4165(98)00056-7 [DOI] [PubMed] [Google Scholar]
- Sheppard KE, 2002. Nuclear receptors. II. Intestinal corticosteroid receptors. Am. J. Physiol. Gastrointest. Liver Physiol 282, G742–6. doi: 10.1152/ajpgi.00531.2001 [DOI] [PubMed] [Google Scholar]
- State of Global Air 2019. Special Report. Boston, MA: Health Effects Institute, 2019. State of Global Air 2019. Special Report. Boston, MA: Health Effects Institute. 1–24. [Google Scholar]
- Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E, 2014. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 1–17. doi: 10.1038/nature13793 [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S, 2009. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. 119, 538–546. doi: 10.1161/CIRCULATIONAHA.108.799015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzek BE, Wang Y, Huang H, McGarvey PB, Wu CH, UniProt Consortium, 2015. UniRef clusters: a comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics 31, 926–932. doi: 10.1093/bioinformatics/btu739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Cyrys J, Kratzsch J, Meisinger C, Hoffmann B, Berdel D, Berg, von A, Koletzko S, Bauer C-P, Heinrich J, 2013. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia 56, 1696–1704. doi: 10.1007/s00125-013-2925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Guénette J, Thomson EM, 2018. Stress axis variability is associated with differential ozone-induced lung inflammatory signaling and injury biomarker response. Environ. Res 167, 751–758. doi: 10.1016/j.envres.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Thomson EM, 2019. Air Pollution, Stress, and Allostatic Load: Linking Systemic and Central Nervous System Impacts. Journal of Alzheimer’s Disease 69, 597–614. doi: 10.3233/JAD-190015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Corral CM, Alderete TL, Habre R, Berhane K, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD, 2018. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes 13, 54–62. doi: 10.1111/ijpo.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong DT, Franzosa EA, Tickle TL, Scholz M, Weingart G, Pasolli E, Tett A, Huttenhower C, Segata N, 2015. MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Meth 12, 902–903. doi: 10.1038/nmeth.3589 [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Gordon JI, 2009. The core gut microbiome, energy balance and obesity. J Physiol (Lond) 587, 4153–4158. doi: 10.1113/jphysiol.2009.174136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI, 2009. A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi: 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI, 2006. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- Urman R, McConnell R, Islam T, Avol EL, Lurmann FW, Vora H, Linn WS, Rappaport EB, Gilliland FD, Gauderman WJ, 2014. Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax 69, 540–547. doi: 10.1136/thoraxjnl-2012-203159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Esterik JCJ, Verharen HW, Hodemaekers HM, Gremmer ER, Nagarajah B, Kamstra JH, Dollé MET, Legler J, van der Ven LTM, 2015. Compound- and sex-specific effects on programming of energy and immune homeostasis in adult C57BL/6JxFVB mice after perinatal TCDD and PCB 153. Toxicol. Appl. Pharmacol 289, 262–275. [DOI] [PubMed] [Google Scholar]
- Vangay P, Johnson AJ, Ward TL, Al-Ghalith GA, Shields-Cutler RR, Hillmann BM, Lucas SK, Beura LK, Thompson EA, Till LM, Batres R, Paw B, Pergament SL, Saenyakul P, Xiong M, Kim AD, Kim G, Masopust D, Martens EC, Angkurawaranon C, McGready R, Kashyap PC, Culhane-Pera KA, Knights D, 2018. US Immigration Westernizes the Human Gut Microbiome. Cell 175, 962–972.e10. doi: 10.1016/j.cell.2018.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella RE, Pillon NJ, Zarrouki B, Croze ML, Koppe L, Guichardant M, Pesenti S, Chauvin M-A, Rieusset J, Géloën A, Soulage CO, 2015. Ozone Exposure Triggers Insulin Resistance Through Muscle c-Jun N-Terminal Kinase Activation. Diabetes 64, 1011–1024. doi: 10.2337/db13-1181 [DOI] [PubMed] [Google Scholar]
- Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JET, Bloks VW, Groen AK, Heilig HGHJ, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JBL, Nieuwdorp M, 2012. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913–6.e7. doi: 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- Walters WA, Xu Z, Knight R, 2014. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 588, 4223–4233. doi: 10.1016/j.febslet.2014.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhou J, Chen M, Huang X, Xie X, Li W, Cao Q, Kan H, Xu Y, Ying Z, 2018. Exposure to concentrated ambient PM2.5 alters the composition of gut microbiota in a murine model 1–13. doi: 10.1186/s12989-018-0252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster NJ, Searle GJ, Lam PPL, Huang Y-C, Riedel MJ, Harb G, Gaisano HY, Holt A, Light PE, 2008. Elevation in intracellular long-chain acyl-coenzyme A esters lead to reduced beta-cell excitability via activation of adenosine 5’-triphosphate-sensitive potassium channels. Endocrinology 149, 3679–3687. doi: 10.1210/en.2007-1138 [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Seals RM, Webster TF, 2018. Bias Amplification in Epidemiologic Analysis of Exposure to Mixtures. Environ. Health Perspect 126, 047003. doi: 10.1289/EHP2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles TJ, Jemielita M, Baker RP, Schlomann BH, Logan SL, Ganz J, Melancon E, Eisen JS, Guillemin K, Parthasarathy R, 2016. Host Gut Motility Promotes Competitive Exclusion within a Model Intestinal Microbiota. PLoS Biol. 14, e1002517. doi: 10.1371/journal.pbio.1002517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DW, Yuan L, Perlin SA, 2004. Comparison of spatial interpolation methods for the estimation of air quality data. J Expo Sci Environ Epidemiol 14, 404–415. doi: 10.1038/sj.jea.7500338 [DOI] [PubMed] [Google Scholar]
- Wood DE, Salzberg SL, 2014. Kraken: ultrafast metagenomic sequence classification using exact alignments. Genome Biol 15, R46–12. doi: 10.1186/gb-2014-15-3-r46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B-Y, Qian ZM, Li S, Chen G, Bloom MS, Elliott M, Syberg KW, Heinrich J, Markevych I, Wang S-Q, Chen D, Ma H, Chen D-H, Liu Y, Komppula M, Leskinen A, Liu K-K, Zeng X-W, Hu L-W, Guo Y, Dong GH, 2018. Ambient air pollution in relation to diabetes and glucose-homoeostasis markers in China: a cross-sectional study with findings from the 33 Communities Chinese Health Study. Lancet Planet Health 2, e64–e73. doi: 10.1016/S2542-5196(18)30001-9 [DOI] [PubMed] [Google Scholar]
- Yasuyuki M, Kunihiro K, Kurissery S, Kanavillil N, Sato Y, Kikuchi Y, 2010. Antibacterial properties of nine pure metals: a laboratory study using Staphylococcus aureus and Escherichia coli. Biofouling 26, 851–858. doi: 10.1080/08927014.2010.527000 [DOI] [PubMed] [Google Scholar]
- Yura EA, Kear T, Niemeier D, 2007. Using CALINE dispersion to assess vehicular PM2.5 emissions. Atmospheric Environment 41, 8747–8757. doi: 10.1016/j.atmosenv.2007.07.045 [DOI] [Google Scholar]
- Zhu Y, Pudota J, Collins D, Allen D, Clements A, DenBleyker A, Fraser M, Jia Y, McDonald-Buller E, Michel E, 2009. Air pollutant concentrations near three Texas roadways, Part I: Ultrafine particles. Atmospheric Environment 43, 4513–4522. doi: 10.1016/j.atmosenv.2009.04.018 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure Titles and Legends
Supplemental Figure 1 Title: Exposure to O3 was Significantly Associated with Gut Bacterial Phyla Using Whole Genome Sequencing (WGS) Taxonomically Classified by Kraken2 Higher exposure to O3 was negatively associated with 20 bacterial phyla and positively associated with one bacterial phylum (Bacteroidetes).
Supplemental Figure 2 Title: Exposure to O3 and NO2 was Associated with Gut Bacterial Phyla Using Whole Genome Sequencing (WGS) Taxonomically Classified by Kraken2 Scatter plots show associations between gut bacterial phyla and O3 exposure using univariate linear models. Plots show the R2 and FDR corrected p-values. Panels A-C show common gut bacterial phyla that were associated with O3 exposure, including Bacteroidetes, Actinobacteria, and Proteobacteria. In addition, panel D shows the phylum Firmicutes, which was positively associated with NO2 exposure.
Supplemental Figure 3 Title: Exposure to O3 was Significantly Correlated with Gut Bacterial Phyla Using Non-Parametric Kendall Tests and Whole Genome Sequencing (WGS) The heatmap shows the FDR corrected p-values based on non-parametric Kendall tests between exposure variables and gut microbial taxa at the phylum level.
Supplemental Figure 4 Title: Exposure to O3, NO2, and Total NOx was Significantly Associated with Gut Bacterial Scatter plots show the significant associations between the relative abundance of gut bacterial species with O3, NO2, and total NOx exposures using univariate linear models with the corresponding R2 and FDR corrected p-values. Overall, 128 bacterial species were significantly associated with O3 while only 4 and 5 bacterial species were significantly associated with NO2 and total NOx, respectively.
Supplemental Figure 5 Title: Exposure to O3 was Significantly Associated with the Log Ratio of Bacteroides caecimuris vs. Leuconostoc spp using Songbird Differential abundance shows a high ranking of Bacteroides related to increases in O3. A boxplot of the differential rankings from Songbird with respect to O3 (A). Regression plots between the log-ratio of Bacteroides caecimuris vs. Leuconostoc spp. and O3 (B) and NO2 (C) with a Pearson correlation included in the upper-left and lower-left box.
Supplemental Figure 6 Title: Exposure to O3 was Significantly Associated with a Higher Relative Abundance of Bacteroides and a Lower Diversity and Evenness Using Whole Genome Sequencing (WGS) Data that were Taxonomically Classified by MetaPhlAn2 Scatter plots show the associations between the relative abundance of Bacteroides, Shannon Diversity Index, and Evenness with O3 exposure using univariate linear models with the corresponding R2 and FDR corrected p-values. O3 was positively associated with Bacteroides (A) and was negatively associated with Shannon Diversity (B) Index and evenness (C).
Supplemental Table Titles and Legends
Supplemental Table 1. Correlations Between Air Pollutants among Young Adults from the Meta-AIR Study Spearman’s rank-order correlation coefficients between prior year exposure to air pollutants are shown. Statistically significant correlations are denoted as *p-value<0.05; **p-value<0.01; ***p-value<0.001
Supplemental Table 2. Ozone Findings were Largely Unchanged After Adjusting for Potentially Important Confounders Multivariable linear regression models (taxa ~ O3 + covariant) were built to control for 24-hour NO2, BMI, age, energy intake, Hispanic ethnicity, sex, season, parental education, carbohydrate intake, fat intake, fiber intake, and protein intake. FDR corrected p-values corresponding to 24-hour O3 from each model are shown in the table.
Supplemental Table 3. Associations Between Prior Year Exposure to Air Pollutants and Gut Bacterial Diversity and Evenness at the Species Level in Young Adults from the Meta-AIR Study R2 and FDR-corrected p-values from univariate linear regression between each ambient and near-roadway air pollutant and measures of gut bacterial diversity (Shannon Diversity Index) and evenness are shown. Models were adjusted for aNO2, bBMI, cage, denergy intake, eHispanic ethnicity, fsex, gseason, hcarbohydrate intake, ifat intake, jfiber intake, kprotein intake, and lparental education as indicated.
Supplemental Table 4. No Evidence for Effect Modification by Obesity Status or Sex was Found for the Association Between O3 and the Gut Bacteria Using Whole Genome Sequencing (WGS) Multivariate analysis (ADONIS test) was used to examine the associations between the gut microbiota composition at the genus level and O3. Separate models with interaction terms included were constructed to examine the effect modification by obesity status (normal weight versus overweight and obese) and sex.
Supplemental Table 5. No Significant Association between the Gut Microbiota and Metabolic Indices were Found at the Species Level Using Whole Genome Sequencing (WGS) A. Simple univariate regression models were built to examine the association between microbial species and metabolic indices. FDR-corrected p-values are shown in the table. B. Multivariable linear regression models were built to examine the association between microbial species and metabolic indices after adjusting for age, BMI, energy intake, Hispanic ethnicity, sex, season (warm/cold), and parental education. FDR-corrected p-values are shown in the table.
Supplemental Table 6. No Significant Association between the Gut Microbiota and Bacterial Translocation Factors were Found at the Species Level Using Whole Genome Sequencing (WGS) A. Simple univariate regression models were built to examine the association between microbial species and bacterial translocation factors, including sCD14, lipopolysaccharide-binding protein (LBP), and LBP/sCD14 ratio. FDR-corrected p-values are shown in the table. B. Multivariable linear regression models were built to examine the association between microbial species and bacterial translocation factors, including sCD14, lipopolysaccharide-binding protein (LBP), and LBP/sCD14 ratio after adjusting for age, BMI, energy intake, Hispanic ethnicity, sex, season (warm/cold), and parental education. FDR-corrected p-values are shown in the table.
Supplemental Table 7. Significant Associations between O3 and Microbial Gene Pathways were Largely Unchanged After Adjusting for Potentially Important Confounders Multivariable linear regression models (gene pathway ~ O3 + covariant) were built to control for 24-hour NO2, BMI, age, energy intake, Hispanic ethnicity, sex, season (warm/cold), parental education, carbohydrate intake, fat intake, fiber intake, and protein intake. FDR corrected p-values corresponding to 24-hour O3 from each model are shown in the table.


