Abstract
BACKGROUND
Delirium is common and dangerous, yet under-detected and under-treated. Current screening questionnaires are subjective and ineffectively implemented in busy hospital workflows. Electroencephalography (EEG) can objectively detect the diffuse slowing characteristic of delirium, but it is not suitable for high-throughput screening due to size, cost, and the expertise required for lead placement and interpretation. We hypothesized that we could develop an efficient and reliable point-of-care EEG device for high-throughput screening.
METHODS
This is a prospective study to measure bispectral EEG (BSEEG) from the elderly inpatients to assess their outcomes conducted at the University of Iowa Hospitals and Clinics (UIHC) from January 2016 to October 2017. A BSEEG score was defined based on the distribution of 2938 EEG recordings from the 428 subjects, who were assessed for delirium; primary outcomes measured were hospital length of stay (LOS), discharge disposition, and mortality.
RESULTS
274 patients had BSEEG scores data available for analysis. Delirium and BSEEG score had a significant association (P<0.001). Higher BSEEG scores were significantly correlated with LOS (P<0.001) as well as with discharge not to home (P<0.01). Hazard ratio for survival controlling for age, gender, Charlson Comorbidity Index and delirium status was 1.35 (95% confidence interval = 1.04 to 1.76, P=0.025).
CONCLUSION
We have developed an efficient and reliable device that provides an objective measurement of delirium status. This BSEEG score is significantly associated with pertinent clinical outcomes of mortality, hospital length of stay, and discharge disposition. The BSEEG score better predicts mortality than clinical delirium status. We identified a previously unrecognized sub-population of patients without clinical features of delirium who are at increased mortality risk.
Keywords: EEG, screening, delirium, patient outcomes, mortality, geriatrics
Introduction
Background/rationale
Delirium is common and dangerous in hospitalized elderly patients (1–3). It is also underdiagnosed and therefore undertreated (4–6). It is estimated there are at least 2–3 million cases of delirium per year in the US alone.(1, 7) Delirium is associated with increased mortality (8–11), rate of complications, hospital length of stay (LOS), and institutionalization after discharge.(1–3) Patients with delirium also have a high risk of long-term cognitive impairment.(12) Delirium can add over $60,000 in healthcare costs per patient per year, or more than $150 billion in added healthcare costs in the US per year.(3) Early identification of risk and immediate intervention is a key to reducing poor outcomes associated with delirium.(13–16)
Extensive efforts have been made to identify efficient methods for screening delirium, including the Confusion Assessment Method (16),(17) the CAM for the intensive care unit (CAM-ICU),(18, 19) and the Delirium Rating Scale-Revised-98 (DRS-R-98).(20) Despite these tools, delirium remains underdiagnosed.(4, 6) Although studies show that 30–40% of delirium cases can be prevented using low-technology interventions,(3, 16, 21) these are often challenging to apply because they need to be administered frequently by busy hospital personnel. The questionnaires’ subjective nature makes monitoring change over time difficult, particularly when administered by different evaluators, as is often the case. As a result, these tools have been reported to have suboptimal sensitivity (38~47%) in busy clinical environments such as the ICU.(22, 23) These considerations highlight the critical need for more objective detection of delirium.
Electroencephalography (EEG) has been a useful modality in detecting brain waves characteristic of delirium.(24, 25 ) Several limitations, however, prevent its routine use for screening large volumes of inpatients. First, a standard 20-lead EEG machine is typically large and expensive, thus patient access to such machines is limited. Second, an experienced technician must spend significant time correctly positioning the 20 leads and obtaining the tracings, and a specialized neurologist must interpret the data. This often results in significant delays in initiating appropriate treatment.
Objective
Electrophysiological signals characteristic of delirium are often reported as “diffuse slowing.” The term implies that brain waves across most channels are of a reduced frequency. The emergence of low-frequency waves indicates potential occurrence of delirium. The fact that all channels are able to detect the same reduction in frequency suggests only a small number of channels would be sufficient to obtain the relevant data. Bispectral EEG (BSEEG) utilizes only two channels, and when combined with appropriate signal analysis algorithms, may be easily applied by non-experts, thus greatly facilitating its use as a screening tool. Due to its objective nature, inter-rater reliability does not affect BSEEG and it can be more strongly correlated with patient outcomes. Previously our team showed that BSEEG signals have significant correlation with delirium in general hospital (26) as well as in emergency room (27). Thus, we conducted an expanded study to determine whether BSEEG signals are associated with delirium and whether they predict patient outcomes, including mortality.
Methods
Study Design and Oversight
This is a prospective cohort study to determine an EEG signal feature associated with delirium. Initially, we aimed to establish the association between BSEEG signals and clinical delirium. Also, to test the usefulness of this approach on patient care, the association of BSEEG scores from this algorithm and patient outcomes were investigated. This study protocol was reviewed and approved by the University of Iowa Institutional Review Board prior to enrollment and data collection. Written informed consent was received from participants prior to inclusion in the study.
Setting and Participants
For the purpose of this study, we recruited patients 18–99 years old from the University of Iowa Hospitals and Clinics (UIHC) from January 2016 to October 2017. The study team screened and reviewed potential subjects admitted to UIHC. Eligible patients were assessed for capacity to consent and participate. If the subject was found not to have capacity to provide consent, consent was obtained from their legally authorized representative. If the subject’s capacity to consent was intact, consent was obtained from the subject directly. Subjects with age 18–99 yo were included. Exclusion criteria were patients with droplet or contact precautions, or whose goals of care were comfort measures only. All participants or their legally authorized representative provided written informed consent. Given our aim was to investigate the potential role of brain wave signals associated with delirium and patient outcomes, our analysis focused on an age group 55 yo or older, who are more vulnerable to delirium.
Variables and Data Sources
Questionnaire instruments and delirium definition
Baseline medical and surgical history and demographic information was obtained from medical records and patient interviews. For measurement of clinical symptoms of delirium, the CAM-ICU (18, 19), the DRS-R-98 (20), and Delirium Observation Screening Score (DOSS) (28, 29) were used. For the assessment of baseline cognitive function the Montreal Cognitive Assessment (MoCA)(30) was used. CAM-ICU and DRS-R-98 were administered to each subject twice daily, unless the subject declined that instance of assessment. DOSS was tabulated by clinical nursing staff during their routine care and was obtained from review of the medical record. Delirium was defined based on any questionnaire screening positive, i.e. CAM-ICU positive, DRS-R-98 ≥19, DOSS ≥3, or clinical documentation of altered mental status or confusion consistent with delirium from the medical record.(31) Each case was reviewed by a weekly research meeting led by a board-certified consult-liaison psychiatrist (G.S.).
BSEEG Data Collection
A hand-held, two-channel EEG device (CMS2100, CONTEC, Qinhuangdao, Hebei, China) was used for brain wave recording. BSEEG was collected twice daily, unless the subject declined that instance of assessment. The trained research assistant cleaned the patient’s forehead with an alcohol swab and placed one electrode on the center of forehead as a ground, two electrodes on the left and right sides of the forehead (Fp), and two electrodes on both sides of the earlobe as references to obtain BSEEG signals for 10 min. The 10 minute duration was chosen a priori as an amount of time that allowed for collection of an adequate amount of EEG data without sacrificing efficiency and throughput, which are essential features of a screening test. The recording was obtained while the patient was at their highest level of consciousness as we roused the patient so that they were as awake and alert as their clinical status allowed. Patients were instructed to keep their eyes closed, jaw relaxed, and remain quiet and still as much as possible. The obtained BSEEG data was converted into spectral density plots, and the signal-processing algorithm was used to produce a BSEEG score.
Spectral Density Analysis and BSEEG Score
Raw EEG signal from each channel was subjected to power spectral density analysis to determine relative presence of “high” and “low” frequency components. Through an iterative approach, a score reflecting the relative presence of high and low frequency activity was developed. From 2938 recordings of EEG scores from all 428 study participants, a mean value and standard deviation (SD) was calculated (Supplemental Figure 1). The BSEEG score is defined as the number of SD from the study population mean.
Outcome Measures
We measured three patient outcomes as follows: 1) hospital LOS; 2) discharge not to home, which included death during hospitalization; and 3) mortality at the time of study conclusion. LOS, discharge outcome, and mortality status were obtained through each subject’s hospital record. Mortality was also assessed by a follow-up phone call interview and obituary record.
Statistical Methods and Analysis
Regression analyses were used to illustrate how the proposed BSEEG score is associated with clinical delirium and patient outcomes such as hospital LOS, discharge not to home, and mortality. Specifically, logistic regression was conducted by treating delirium and discharge not to home as binary response variables, respectively, while linear regression was used to evaluate the relationship between hospital LOS and BSEEG score. In addition, the hazard ratio (32) for mortality was computed through Cox proportional hazards regression analyses. Age, gender, and severity of illness (Charlson Comorbidity Index (CCI)(33)) were controlled in regression analyses. The association between mortality and BSEEG scores was further illustrated by comparing two non-parametric survival functions for BSEEG-positive and BSEEG-negative groups. Note that the R package survminer (v 0.3.1) was utilized to determine a cut point, which maximizes the difference of mortality between two groups. The survival function is a series of the Kaplan-Meier estimators obtained from the number of deaths and the total individuals at risk at the time. The log-rank test was conducted to determine whether the two survival functions differ. Two-sided P-values of 0.05 or less were considered to indicate statistical significance. All analyses were performed with R software, version 3.4.3.
Results
Participants, Descriptive Data and Outcome Data
From January 30, 2016 to October 30, 2017, 820 patients were approached and total of 428 patients were enrolled in the study. We decided to focus on patients 55 yo or above as older age is associated with increased susceptibility to delirium. 337 out of 428 patients were 55 yo or older and 274 out of 337 had BSEEG scores available for analysis. In some cases, BSEEG scores could not be calculated due to poor signal quality of raw EEG data. In the group 55 yo or older, 37.2% of patients were categorized as delirious by questionnaire screening such as CAM-ICU, DRS, and DOSS, or clinical documentation. The study population was also independently divided into two groups, BSEEG-positive, with higher BSEEG scores, indicative of more low-frequency components in their brain waves, and BSEEG-negative, with lower BSEEG scores, indicative of less low-frequency components in their brain waves, based on a threshold to differentiate patient outcomes as described in the following section (Supplemental Figure 2). Otherwise, these cohorts were balanced with respect to overall baseline characteristics (Table 1).
Table 1.
Patient Characteristics
| Classification | Clinical categories | BSEEG categories | ||
|---|---|---|---|---|
| Delirious | Control | Positive | Negative | |
| N | 102 | 172 | 138 | 136 |
| Mean age — yr | 73.8 | 73.3 | 73.4 | 73.6 |
| SD | 9.4 | 9.8 | 8.8 | 10.5 |
| Female sex (n) | 52 | 95 | 74 | 73 |
| % | 51.0% | 55.2% | 53.7% | 53.7% |
| Race | ||||
| White (n) | 101 | 168 | 135 | 134 |
| % | 99.0% | 97.7% | 97.8% | 98.5% |
| Other (n) | 1 | 6 | 4 | 3 |
| % | 1.0% | 3.5% | 2.9% | 2.2% |
| Mean CCI * | 4.2 | 3.4 | 4.2 | 3.3 |
| Mean DRS ** | 16.9 | 6.6 | 12.3 | 8.6 |
| Mean DOSS ** | 6.3 | 0.2 | 3.5 | 1.3 |
| Mean MoCA ** | 14.4 | 23.4 | 18.7 | 22.2 |
Patient Characteristics: Age and gender were not significantly different between delirious and control groups, or between BSEEG positive and negative groups. CCI, DRS, DOSS and MoCA were significantly different between groups.
P<0.05
P < 0.01
Main Results
Association between BSEEG Score and Clinical Delirium
Data from 274 subjects were analyzed to establish association between BSEEG score and clinical delirium. Logistic regression showed significant association between delirium category and BSEEG score (P = 6.39 × 10–6, unadjusted; P = 1.22 × 10–5, adjusted for age, gender, and CCI).
BSEEG Score and Patient Outcomes
To test the usefulness of the BSEEG score in predicting patient outcomes, we used outcome data available from 274 subjects who were 55 yo or older to investigate the association of BSEEG scores obtained at the time of study enrollment with patient outcomes commonly affected by delirium. Specifically, we assessed hospital LOS, discharge disposition, and mortality.
First, LOS and BSEEG scores were significantly associated (P = 0.00099, unadjusted; P = 0.0014, adjusted for age, gender and CCI). A higher BSEEG score coincides with an increase in a patient’s LOS.
Second, we compared the discharge outcome and BSEEG score. When BSEEG was compared between those who were discharged to their home and those discharged not to home, including death during hospitalization, a higher BSEEG score was significantly associated with discharge not to home (P = 0.0038, unadjusted; P = 0.0090, adjusted for age, gender, and CCI).
Third, when we analyzed subject mortality controlling for age, gender, and CCI, the hazard ratio (32) based on 1 SD change of BSEEG score was 1.44 (1.12 to 1.84, P = 0.004). Even after controlling for clinical delirium status in addition to age, gender and CCI, the HR based on BSEEG score remained significant at 1.35 (95% confidence interval = 1.04 to 1.76, P = 0.025).
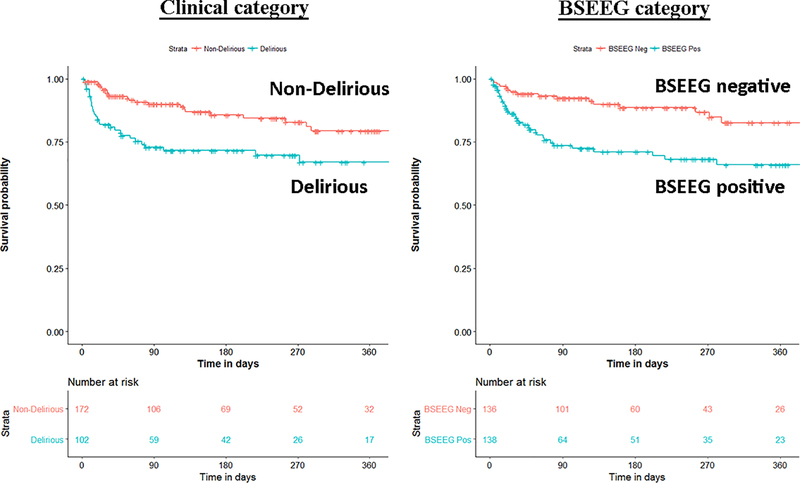
Besides mathematical association, we wanted to determine if the BSEEG could be useful as a measure to assess risk for poor patient outcomes. We divided the study population into a BSEEG-positive group and a BSEEG-negative group, as mentioned above. Then, we assessed if there was also a correlation between groups based on BSEEG scores and all-cause mortality at the end of our study period in patients in our dataset, because association between delirium and mortality has been shown previously in the literature.(8, 9 ) We first assessed overall survival rates among our study participants to confirm that our clinical categorization of delirium is valid enough to replicate well established association between delirium and higher mortality. Our result showed differences in mortality between those with and without clinical delirium (P = 0.0038) (Figure 1). Second, we tested a group difference based on a BSEEG cut-off score and confirmed that the BSEEG-positive group showed worse survival compared to the BSEEG-negative group (P = 0.0032) (Figure 1). This categorization also differentiated other outcomes including LOS and discharge disposition significantly (Table 2).
Figure 1.
Survival curve over 360 days based on clinical delirium status (left panel) and BSEEG score (right panel).
Patients who were clinically delirious had increased mortality compared to those without clinical delirium (P = 20.0038). BSEEG positive patients showed higher mortality than BSEEG negative patients, regardless of clinical delirium status (P = 0.0032). ”Number at Risk” indicates how many subjects in each stratum are followed at each time point to calculate survival probability as shown in the figure.
Table 2.
Patient Outcomes
| Patient outcomes | Delirious | Control | BSEEG positive | BSEEG negative | P Value | |
|---|---|---|---|---|---|---|
| N | 102 | 172 | 138 | 136 | ||
| Delirious vs. Control | BSEEG(+) vs. BSEEG(–) | |||||
| Deceased (n) | 29 | 23 | 36 | 16 | < 0.005 | < 0.005 |
| % | 28.4% | 13.4% | 26.1% | 11.8% | ||
| Mean Length of Stay— day | 10 | 5.2 | 8.1 | 5.8 | < 0.001 | < 0.005 |
| Discharge not to home (n) | 74 | 60 | 76 | 58 | < 0.001 | 0.041 |
| % | 72.5% | 34.9% | 55.1% | 42.6% | ||
Patient Outcomes: BSEEG(+): BSEEG-positive group, indicative of more low-frequency brain waves; BSEEG(–): BSEEG-negative group, indicative of less low-frequency brain waves. For comparing patient outcomes between two groups, Student’s t-test was used for LOS, while Fisher’s exact test was used for mortality and discharge not to home. Results show that all outcomes significantly differ between the two groups.
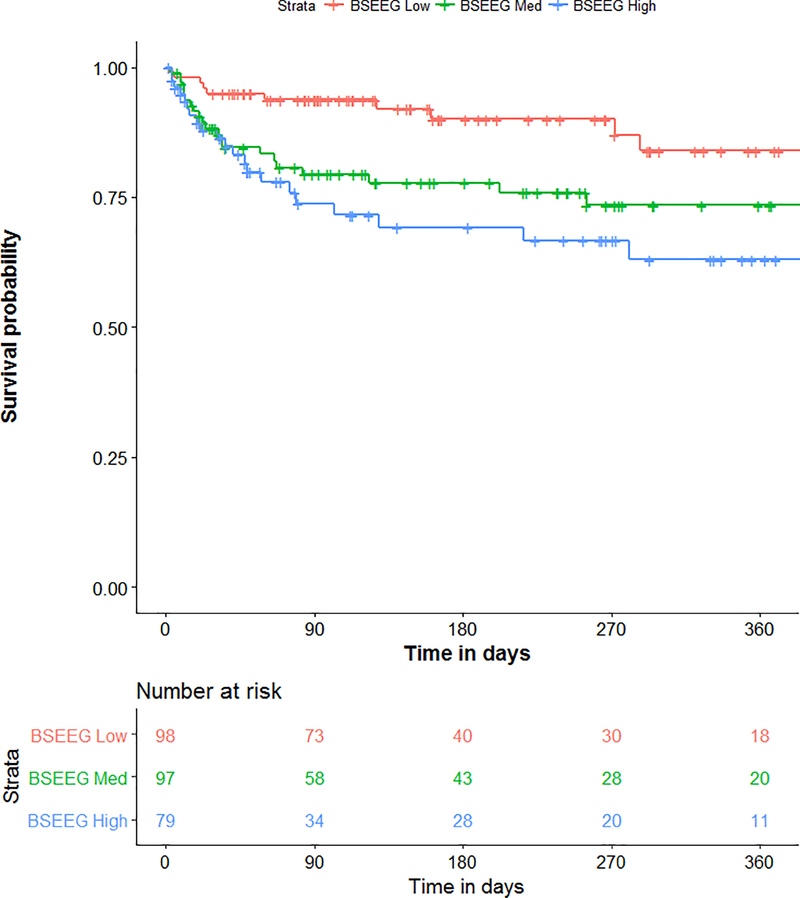
We also hypothesized that BSEEG score not only measures the presence of delirium, but represents delirium severity. We therefore divided subjects in three groups based on BSEEG score: BSEEG high, BSEEG intermediate, and BSEEG low. The survival curve showed a “dose-dependent” relationship of increasing mortality with increasing BSEEG score (Figure 2, P = 0.005), suggesting a strong relationship between BSEEG score and mortality.
Figure 2.
Survival curve over 360 days based on three BSEEG categories.
Mortality was directly proportional to the BSEEG score, with higher BSEEG score group associated with higher mortality (P= 0.005). ”Number at Risk” indicates how many subjects in each stratum are followed at each time point to calculate survival probability as shown in the figure.
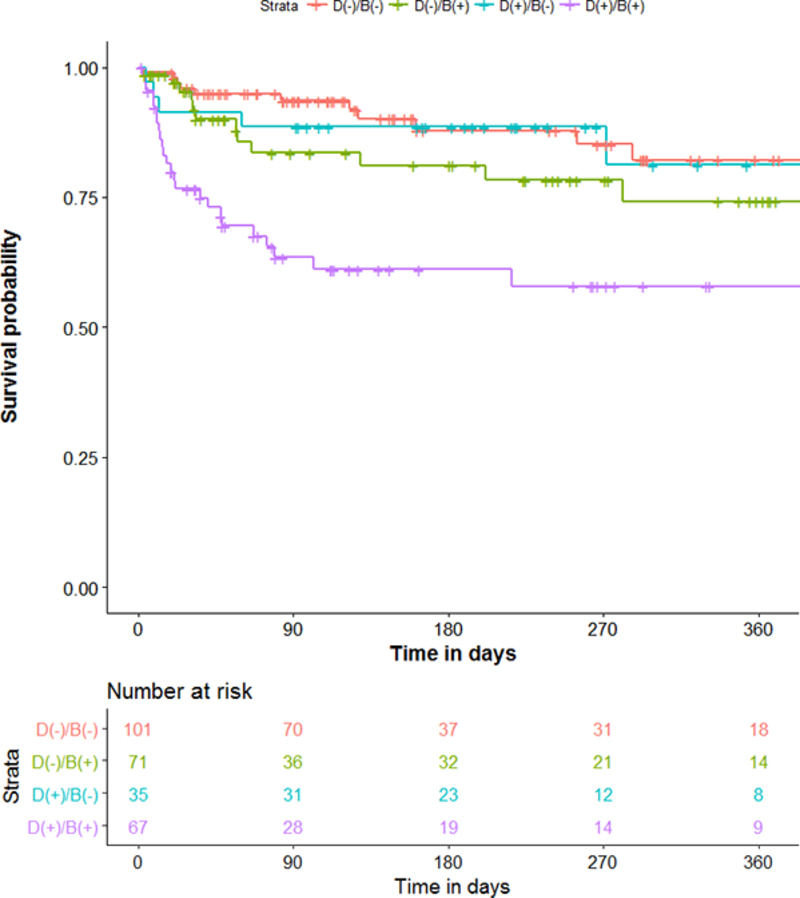
We wondered about outcomes of subjects in whom clinical assessment and BSEEG score were discordant. We therefore divided the cohort into four groups based on clinical delirium diagnosis and BSEEG. Clinically delirious subjects with positive BSEEG scores showed the highest mortality. In contrast, those patients categorized as clinically delirious but with a negative BSEEG score had lower mortality, similar to that of non-delirious subjects with negative BSEEG scores. Moreover, those thought to be non-delirious subjects based on results of clinical assessment but with positive BSEEG scores had a higher mortality, even compared to those patients with clinical delirium but with a negative BSEEG score (Figure 3).
Figure 3.
Subgroup analysis of mortality based on both clinical delirium status and BSEEG category.
Patients who were both clinical delirious and BSEEG positive showed the highest mortality (purple line). Patients who were clinically delirious but BSEEG negative had lower mortality (blue line), almost as low as patients who were both clinically non-delirious and BSEEG negative (orange line). In contrast, patients who were clinically non-delirious, but BSEEG positive, had higher mortality rates (green line), indicating that BSEEG score was a better predictor of mortality than clinical delirium status. ”Number at Risk” indicates how many subjects in each stratum are followed at each time point to calculate survival probability as shown in the figure.
Discussion
Key results and Interpretation
The data presented here show that the BSEEG score was significantly associated with the clinical presence of delirium, even after controlling for age, gender, and CCI. More importantly, our results indicate that the BSEEG score was strongly associated with patient outcomes, including hospital LOS, discharge disposition, and mortality among hospitalized patients. Importantly, this association was based on a BSEEG score obtained at the time of enrollment, often within 24 hr of admission. These results suggest that a single BSEEG score obtained at the beginning of hospitalization can predict patient outcomes. This result also indicates that among patients who cannot be clinically identified as delirious, a subset is at high risk of death that is distinguishable by differences in brain wave activity as detected by BSEEG. We have termed this state as subclinical brain failure (SBF), a finding which warrants further investigation and more attention. Thus, identification of this population with the BSEEG method could lead to early intervention and potentially improved survival rates.
The search for EEG-based biomarkers of delirium has a long history involving several research groups. Since the 1940s it has been reported that EEG patterns detected from only two channels can be used to distinguish marked delirium from normal awareness.(24) However, currently available technologies using a small number of EEG leads are not “tuned” for delirium screening and lack a form factor appropriate for mass screening. In the area of anesthesiology in the last two decades, new devices utilizing EEG signals obtained from a few leads attached to a patient’s forehead have been used to monitor the depth of anesthesia. This approach has gained much popularity and is commonly used during surgical operations.(34–36) Although this approach is similar to the one proposed here, the algorithm is trained only to measure depth of anesthesia and the equipment used is heavy and embedded in a large anesthesia machine; thus the cost is quite high and it is unsuitable for high-throughput screening of the general hospitalized population. Two-channel brain wave monitoring has also been used in psychiatric treatment involving electro-convulsive therapy (ECT).(37) The ECT machine includes an EEG function, with two leads to monitor seizure activity. However, it also lacks delirium screening technology and has an inappropriate form factor for mass point-of-care screening use. Nevertheless, these applications demonstrate that obtaining EEG signals from a limited number of leads is established technology. The usefulness of an EEG obtained using a limited number of electrodes for detecting delirium has only recently been demonstrated; one study confirmed that the sensitivity and specificity of limited EEG leads are excellent and comparable to machines with the traditional 20 leads for this purpose(38). However that report did not provide information about the relationship of limited-lead EEG screening for delirium with patient outcomes.
Although previous literature supports the notion that EEG is useful for detecting delirium, no investigation has determined the effects of using a point-of-care BSEEG device to identify biomarkers of delirium on a large number of patients, nor has any study tested the association of such a screening method with patient outcomes, such as hospital LOS, discharge disposition, and mortality, or the effect of intervention on these outcomes. There are only a limited number of studies investigating the association of conventional EEG data and mortality, although in general those EEG recordings were limited to those subjects who were thought to need EEG examination for another medical indication such as altered mental status, and thus a broader range of general inpatients has never been studied (32, 39, 40).
Our data demonstrated here shows the usefulness of the BSEEG score in identifying patients at risk for delirium and in predicting patient outcomes, such as hospital LOS, discharge disposition, and mortality, among elderly hospitalized patients. This is the first study using a point-of-care EEG device to demonstrate the association between BSEEG biomarker signals and their association with delirium and patient outcomes. BSEEG was used not only for patients with obvious mental status change, but also for a broader cohort of inpatients. Because BSEEG method would not require extensive training for lead placement or interpretation by a neurology specialist, application for screening large number of patients even without notable mental status change becomes possible.
With additional clinical validation, such BSEEG-based biomarkers will enable early intervention and will potentially improve the current practice of medicine and surgery for patients at risk of delirium. For example, BSEEG analysis may be an important factor in the decision to perform elective surgery or be used for heightened monitoring after surgery. When high-risk patients are identified through BSEEG analysis, it is then possible to direct hospital resources more efficiently and effectively compared to the current standard of care.
BSEEG monitoring may also be applicable in additional settings such as the primary care clinic, emergency department, and in nursing home or home-care settings. Delirium is particularly dangerous when patients experience it outside of hospitals because of the lack of recognition and resources to manage it. The simple, noninvasive nature of this test makes it ideal for routine screening. BSEEG has the potential to be a fast, easy, non-invasive, and objective assessment tool, similar to the measurement of vital signs that can be used to monitor or screen for delirium in appropriate populations. A positive result would raise an early alarm and trigger more comprehensive work-up for an acute illness. The BSEEG may be more clinically relevant and would provide an objective and quantitative replacement for currently used criteria of “altered mental status” in other prognostic models such as the Sequential Organ Failure Assessment (SOFA (41).As the aging population is expanding rapidly, efficient modalities for delirium screening such as BSEEG is predicted to be in high demand.
Limitations
We introduced constraints into this study by limiting electrode placement to the forehead. This was intentional due to our ultimate goal of developing a user-friendly screening method for hospital staff. Other lead placement conformations may result in better performance, but could hinder broad usability. The usefulness of BSEEG score for delirium screening depends on the assumption that EEG changes are generalized or diffuse. Therefore, focal change, either by slowing or seizure activity, or a structural brain abnormality, may confound results. Another limitation is that we do not have information about several patient characteristics that could potentially affect risk for delirium and thus patient outcomes. Those include rates of dementia or mild cognitive impairment, past history of delirium, admission diagnosis, habitual use of alcohol or benzodiazepine receptor agonists, and other prescribed medications such as opioids and corticosteroids. An additional limitation of this study is that it was conducted at a single institution in the Midwest region of the US, where most of the patient population consists of non-Hispanic Whites. Thus, generalizability needs to be tested with a future multicenter study based on the presented results. Nevertheless, we have developed a useful algorithm to differentiate delirium and associate the BSEEG score with patient outcomes.
Our future investigations will determine whether certain BSEEG-triggered preemptive interventions improve outcomes. For example, new studies have shown that both ramelteon(42) and suvorexant(43) can decrease the risk of delirium. Thus, exploring the effect of these treatments in conjunction with BSEEG monitoring will allow us to determine the impact of certain medications on BSEEG score and outcomes. These provide new avenues to explore better treatments for delirium and, ultimately, improve patient outcomes.
Implications for Practice
In conclusion, we demonstrate that simple, noninvasive, point-of-care EEG collection combined with BSEEG scoring was able to predict adverse patient outcomes, including mortality, in an elderly population. Importantly, we identified a certain patient population who cannot be identified by current clinical assessments, but are at high risk for mortality, that warrants further investigation to see if early detection and intervention can modify their mortality.
Supplementary Material
Supplemental Figure 1. Distribution of BSEEG Scores
Scores are based on a total of 2938 recordings from 428 patients of all age groups. The BSEEG score is defined as the number of standard deviations from the mean, with the mean being a score of 0.
Supplemental Figure 2. Study participant Enrollment Flow Chart.
The study population was categorized in two ways: clinical delirium status and BSEEG score.
Clinical Points.
Diagnosing delirium is difficult in busy clinical settings, and delirium remain underdiagnosed and undertreated, leading to poor patient outcomes including mortality.
We aimed to investigate whether bispectral EEG (BSEEG) can detect delirium and predict patient outcomes including mortality.
BSEEG score was significantly associated with clinical presence of delirium as well as patient outcomes, including hospital length of stay, discharge disposition, and mortality.
Acknowledgment
Authors thank patients who participated to this study.
Financial Support: This study was supported by the University of Iowa Research Foundation GAP funding award for Gen Shinozaki and John Cromwell. Gen Shinozaki received grant support from the National Science Foundation, award number 1664364. Aubrey Chan was supported by the National Institute of Mental Health under award number T32MH019113.
Footnotes
Disclaimer: Drs. Shinozaki and Cromwell are co-founders of Predelix Medical LLC and have a patent “Non-Invasive Device for Predicting and Screening Delirium” in the PCT Application No. PCT/US2016/064937, and in U.S. Provisional Patent No. 62/263,325 pending. All other authors have declared that no conflict of interest exists.
Role of the Sponsors: The supporters had no role in the design, analysis, interpretation, or publication of this study
References
- 1.Inouye SK (2006). Delirium in older persons. N Engl J Med, 354(11), 1157–65. PMID: 16540616. [DOI] [PubMed] [Google Scholar]
- 2.Fong TG, Tulebaev SR, Inouye SK (2009). Delirium in elderly adults: diagnosis, prevention and treatment. Nature reviews Neurology, 5(4), 210–20. PMID: 19347026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS (2014). Delirium in elderly people. Lancet, 383(9920), 911–22. PMID: 23992774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ely EW, Stephens RK, Jackson JC, Thomason JW, Truman B, Gordon S, Dittus RS, Bernard GR (2004). Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: a survey of 912 healthcare professionals. Crit Care Med, 32(1), 106–12. PMID: 14707567. [DOI] [PubMed] [Google Scholar]
- 5.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK (2008). One-year health care costs associated with delirium in the elderly population. Arch Intern Med, 168(1), 27–32. PMID: 18195192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spronk PE, Riekerk B, Hofhuis J, Rommes JH (2009). Occurrence of delirium is severely underestimated in the ICU during daily care. Intensive Care Med, 35(7), 1276–80. PMID: 19350214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pisani MA, McNicoll L, Inouye SK (2003). Cognitive impairment in the intensive care unit. Clinics in chest medicine, 24(4), 727–37. PMID: 14710700. [DOI] [PubMed] [Google Scholar]
- 8.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E (2002). Delirium predicts 12-month mortality. Arch Intern Med, 162(4), 457–63. PMID: 11863480. [DOI] [PubMed] [Google Scholar]
- 9.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr.,, Inouye SK, Bernard GR, Dittus RS (2004). Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. Jama, 291(14), 1753–62. PMID: 15082703. [DOI] [PubMed] [Google Scholar]
- 10.Ouimet S, Kavanagh BP, Gottfried SB, Skrobik Y (2007). Incidence, risk factors and consequences of ICU delirium. Intensive Care Med, 33(1), 66–73. PMID: 17102966. [DOI] [PubMed] [Google Scholar]
- 11.Kakuma R, du Fort GG, Arsenault L, Perrault A, Platt RW, Monette J, Moride Y, Wolfson C (2003). Delirium in older emergency department patients discharged home: effect on survival. J Am Geriatr Soc, 51(4), 443–50. PMID: 12657062. [DOI] [PubMed] [Google Scholar]
- 12.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW, Investigators B-IS (2013). Long-term cognitive impairment after critical illness. N Engl J Med, 369(14), 1306–16. PMID: 24088092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez M, Martinez G, Calderon J, Villarroel L, Yuri F, Rojas C, Jeria A, Valdivia G, Marin PP, Carrasco M (2009). Impact of delirium on short-term mortality in elderly inpatients: a prospective cohort study. Psychosomatics, 50(3), 234–8. PMID: 19567762. [DOI] [PubMed] [Google Scholar]
- 14.Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS, Robbins T, Garpestad E (2010). Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Critical care medicine, 38(2), 419–27. PMID: 19915454. [DOI] [PubMed] [Google Scholar]
- 15.Hshieh TT, Yue J, Oh E, Puelle M, Dowal S, Travison T, Inouye SK (2015). Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med, 175(4), 512–20. PMID: 25643002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inouye SK, Bogardus ST Jr.,, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM (1999). A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med, 340(9), 669–76. PMID: 10053175. [DOI] [PubMed] [Google Scholar]
- 17.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI (1990). Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med, 113(12), 941–8. PMID: 2240918. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, Truman B, Speroff T, Gautam S, Margolin R, Hart RP, Dittus R (2001). Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA, 286(21), 2703–10. PMID: 11730446. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK (2001). Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med, 29(7), 1370–9. PMID: 11445689. [DOI] [PubMed] [Google Scholar]
- 20.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N (2001). Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci, 13(2), 229–42. PMID: 11449030. [DOI] [PubMed] [Google Scholar]
- 21.Marcantonio ER, Flacker JM, Wright RJ, Resnick NM (2001). Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc, 49(5), 516–22. PMID: 11380742. [DOI] [PubMed] [Google Scholar]
- 22.van Eijk MM, van den Boogaard M, van Marum RJ, Benner P, Eikelenboom P, Honing ML, van der Hoven B, Horn J, Izaks GJ, Kalf A, Karakus A, Klijn IA, Kuiper MA, de Leeuw FE, de Man T, van der Mast RC, Osse RJ, de Rooij SE, Spronk PE, van der Voort PH, van Gool WA, Slooter AJ (2011). Routine use of the confusion assessment method for the intensive care unit: a multicenter study. American journal of respiratory and critical care medicine, 184(3), 340–4. PMID: 21562131. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Yokoyama K, Yamauchi N, Koizumi M, Harasawa N, Yasuda T, Mimura C, Igita H, Suzuki E, Uchiide Y, Seino Y, Nomura M, Yamazaki K, Ishigooka J, investigators T (2016). Sensitivity and specificity of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC) for detecting post-cardiac surgery delirium: A single-center study in Japan. Heart & lung : the journal of critical care, 45(1), 15–20. PMID: 26685069. [DOI] [PubMed] [Google Scholar]
- 24.Romano J, Engel GL (1944). Delirium: I. Electroencephalographic data. Archives of Neurology & Psychiatry, 51(4), 356–77. [Google Scholar]
- 25.Jacobson SA, Leuchter AF, Walter DO (1993). Conventional and quantitative EEG in the diagnosis of delirium among the elderly. Journal of neurology, neurosurgery, and psychiatry, 56(2), 153–8. PMID: 8437004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shinozaki G, Chan AC, Sparr NA, Zarei K, Gaul LN, Heinzman JT, Robles J, Yuki K, Chronis TJ, Ando T, Wong T, Sabbagh S, Weckmann MT, Lee S, Yamada T, Karam MD, Noiseux NO, Shinozaki E, Cromwell JW (2018). Delirium detection by a novel bispectral electroencephalography device in general hospital. Psychiatry and clinical neurosciences, PMID: 30246448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Yuki K, Chan A, Cromwell J, Shinozaki G (2018). The point-of-care EEG for delirium detection in the emergency department. The American journal of emergency medicine, PMID: 30340987. [DOI] [PubMed] [Google Scholar]
- 28.Schuurmans MJ, Shortridge-Baggett LM, Duursma SA (2003). The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract, 17(1), 31–50. PMID: 12751884. [DOI] [PubMed] [Google Scholar]
- 29.Gavinski K, Carnahan R, Weckmann M (2016). Validation of the delirium observation screening scale in a hospitalized older population. Journal of hospital medicine, 11(7), 494–7. PMID: 26970312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 53(4), 695–9. PMID: 15817019. [DOI] [PubMed] [Google Scholar]
- 31.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr.,, Leslie DL, Agostini JV (2005). A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc, 53(2), 312–8. PMID: 15673358. [DOI] [PubMed] [Google Scholar]
- 32.Azabou E, Magalhaes E, Braconnier A, Yahiaoui L, Moneger G, Heming N, Annane D, Mantz J, Chretien F, Durand MC, Lofaso F, Porcher R, Sharshar T, Groupe d’Explorations Neurologiques en R (2015). Early Standard Electroencephalogram Abnormalities Predict Mortality in Septic Intensive Care Unit Patients. PLoS One, 10(10), e0139969 PMID: 26447697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charlson M, Szatrowski TP, Peterson J, Gold J (1994). Validation of a combined comorbidity index. J Clin Epidemiol, 47(11), 1245–51. PMID: 7722560. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Singh H, White PF (1997). Electroencephalographic bispectral index correlates with intraoperative recall and depth of propofol-induced sedation. Anesthesia and analgesia, 84(1), 185–9. PMID: 8989022. [DOI] [PubMed] [Google Scholar]
- 35.Schmidlin D, Hager P, Schmid ER (2001). Monitoring level of sedation with bispectral EEG analysis: comparison between hypothermic and normothermic cardiopulmonary bypass. British journal of anaesthesia, 86(6), 769–76. PMID: 11573582. [DOI] [PubMed] [Google Scholar]
- 36.Powers KS, Nazarian EB, Tapyrik SA, Kohli SM, Yin H, van der Jagt EW, Sullivan JS, Rubenstein JS (2005). Bispectral index as a guide for titration of propofol during procedural sedation among children. Pediatrics, 115(6), 1666–74. PMID: 15930231. [DOI] [PubMed] [Google Scholar]
- 37.Scott AI (2007). Monitoring electroconvulsive therapy by electroencephalogram: an update for ECT practitioners. Advances in Psychiatric Treatment, 13(4), 298–304. [Google Scholar]
- 38.van der Kooi AW, Zaal IJ, Klijn FA, Koek HL, Meijer RC, Leijten FS, Slooter AJ (2015). Delirium detection using EEG: what and how to measure. Chest, 147(1), 94–101. PMID: 25166725. [DOI] [PubMed] [Google Scholar]
- 39.Stecker MM (2009). The EEG as an independent indicator of mortality and healthcare utilization. Clin Neurophysiol, 120(10), 1777–81. PMID: 19699144. [DOI] [PubMed] [Google Scholar]
- 40.Gilmore EJ, Gaspard N, Choi HA, Cohen E, Burkart KM, Chong DH, Claassen J, Hirsch LJ (2015). Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med, 41(4), 686–94. PMID: 25763756. [DOI] [PubMed] [Google Scholar]
- 41.Mettlin C (1989). Trends in years of life lost to cancer: 1970 to 1985. CA Cancer J Clin, 39(1), 33–9. PMID: 2492875. [DOI] [PubMed] [Google Scholar]
- 42.Hatta K, Kishi Y, Wada K, Takeuchi T, Odawara T, Usui C, Nakamura H, Group D-J (2014). Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry, 71(4), 397–403. PMID: 24554232. [DOI] [PubMed] [Google Scholar]
- 43.Hatta K, Kishi Y, Wada K, Takeuchi T, Ito S, Kurata A, Murakami K, Sugita M, Usui C, Nakamura H, Group D-J (2017). Preventive Effects of Suvorexant on Delirium: A Randomized Placebo-Controlled Trial. J Clin Psychiatry, PMID: 28767209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Distribution of BSEEG Scores
Scores are based on a total of 2938 recordings from 428 patients of all age groups. The BSEEG score is defined as the number of standard deviations from the mean, with the mean being a score of 0.
Supplemental Figure 2. Study participant Enrollment Flow Chart.
The study population was categorized in two ways: clinical delirium status and BSEEG score.