Abstract
Background
Nephrotoxins contribute to 20%–40% of acute kidney injury (AKI) cases in the intensive care unit (ICU). The combination of piperacillin-tazobactam (PTZ) and vancomycin (VAN) has been identified as nephrotoxic, but existing studies focus on extended durations of therapy rather than the brief empiric courses often used in the ICU. The current study was performed to compare the risk of AKI with a short course of PTZ/VAN to with the risk associated with other antipseudomonal β-lactam/VAN combinations.
Methods
The study included a retrospective cohort of 3299 ICU patients who received ≥24 but ≤72 hours of an antipseudomonal β-lactam/VAN combination: PTZ/VAN, cefepime (CEF)/VAN, or meropenem (MER)/VAN. The risk of developing stage 2 or 3 AKI was compared between antibiotic groups with multivariable logistic regression adjusted for relevant confounders. We also compared the risk of persistent kidney dysfunction, dialysis dependence, or death at 60 days between groups.
Results
The overall incidence of stage 2 or 3 AKI was 9%. Brief exposure to PTZ/VAN did not confer a greater risk of stage 2 or 3 AKI after adjustment for relevant confounders (adjusted odds ratio [95% confidence interval] for PTZ/VAN vs CEF/VAN, 1.11 [.85–1.45]; PTZ/VAN vs MER/VAN, 1.04 [.71–1.42]). No significant differences were noted between groups at 60-day follow-up in the outcomes of persistent kidney dysfunction (P = .08), new dialysis dependence (P = .15), or death (P = .09).
Conclusion
Short courses of PTZ/VAN were not associated with a greater risk of short- or 60-day adverse renal outcomes than other empiric broad-spectrum combinations.
Keywords: acute kidney injury, piperacillin-tazobactam, vancomycin, critically ill, nephrotoxicity
Findings suggest that nephrotoxicity occurs after 3–5 days of piperacillin-tazobactam/vancomycin therapy. We found in a large observational study of intensive care unit patients that brief (<72-hour) empiric use of this combination was no more nephrotoxic than other such combinations.
Acute kidney injury (AKI) is common in critically ill patients and confers a nearly 3.5-fold increased risk of death [1–3]. Nephrotoxin exposure contributes to approximately 20%–40% of AKI cases in critically ill patients and represents one of the few modifiable risk factors in this high-risk population [4–6]. Vancomycin (VAN) and piperacillin-tazobactam (PTZ) each heighten the risk for drug-associated AKI, and their combination has been suggested to be particularly nephrotoxic relative to alternative broad-spectrum combinations [7–11].
To date, studies have only provided limited descriptions of the temporal association between PTZ/VAN exposure and AKI. The majority of studies included patients receiving PTZ/VAN for ≥48 hours and found that AKI onset coincided with 3–5 days of PTZ/VAN exposure [10, 12]. Informed by modern diagnostics and antimicrobial stewardship practices, intensive care unit (ICU) patients exposed to empiric broad-spectrum antibiotics often undergo deescalation well before this threshold of sustained exposure occurs [13].
Findings suggest that the median VAN duration in patients treated empirically for pneumonia may be as short as 27 hours [14]. It is unknown whether these short exposures to combination PTZ/VAN in the ICU confer a similarly heightened risk of AKI as has been previously reported. In addition, the association between PTZ/VAN and AKI in critically ill patients has been modest if even present at all, compared with less critically ill hospitalized patients [7, 15, 16]. In the critical care setting, there is a high baseline risk of AKI that may be unaffected by additional risk from this antibiotic combination. Prior studies in the critical care setting may have inadequately adjusted for confounders or used alternate AKI definitions. Therefore, the purpose of the current study was to determine whether short courses of PTZ/VAN predicted an increased risk of AKI compared with other broad-spectrum antibiotic combinations in critically ill patients.
METHODS
Study Design and Setting
Eligible individuals were adults (aged ≥18 years) hospitalized in any of the ICUs at Mayo Clinic in Rochester, Minnesota, between 1 January 2006 and 31 December 2016 with Minnesota Research Authorization [17]. For patients admitted on multiple occasions during the study time frame, the last admission was used. The protocol was approved by the Mayo Clinic Institutional Review Board with a waiver of informed consent.
Patient Selection
Patients were included if they received ≥24 and ≤72 hours of continuous concurrent therapy with VAN and either PTZ, cefepime (CEF), or meropenem (MER). In current clinical practice, patients are administered empiric combination therapy and subsequently transitioned to directed therapy once culture data are returned. Clinical pharmacists are responsible for dosing and monitoring VAN and β-lactam dose adjustments for kidney dysfunction. Patients were excluded if they received >1 antipseudomonal β-lactam within 7 days, received concomitant antibiotics >24 hours before being admitted to the ICU, developed stage 2 or 3 AKI before or ≤24 hours after concomitant antibiotic therapy initiation, had end-stage renal disease defined as requiring renal replacement therapy, died within 48 hours of combination antibiotic therapy initiation, or were pregnant.
Data Collection and Definitions
Data extracted from the electronic health record included demographic information, comorbid conditions, Acute Physiology and Chronic Health Evaluation (APACHE III) score, Sequential Organ Failure Assessment (SOFA) score, microbiological data, and antibiotic use. AKI was identified with an electronic alert (“AKI sniffer”) that continuously applied the Acute Kidney Injury Network (AKIN) AKI criteria to hourly urine output measurements and all serum creatinine values [18]. This electronic surveillance tool was developed and validated against AKI confirmed by means of manual medical record review [19, 20]. Baseline serum creatinine was calculated as the median of all creatinine values in the 6 months preceding the index admission, or if unavailable, from back-calculation using Modification of Diet in Renal Disease equation with an estimated glomerular filtration rate of 60 mL/min per 1.73 m2 body surface area [21].
End Points
The primary end point was the incidence of AKI, defined as AKIN stage 2 or 3 AKI [18]. The occurrence of AKI was assessed beginning 24 hours after initiation of combination antibiotic treatment. Follow-up continued until 7 days after 1 or both antibiotics in the combination were discontinued. Secondary end points included incidence of maximum-stage AKI, defined as AKIN stage 1, 2, or 3 AKI, and major acute kidney events at 60 days (MAKE60) [18], which included a persistent doubling of serum creatinine level from baseline, a new requirement of renal replacement therapy, or death [22].
Statistical Analysis
Continuous data were summarized as means with standard deviation (SD) or medians with interquartile range (IQR), depending on the normality of the distribution. Percentages were used for categorical data. Continuous data were compared across groups using analysis of variance or Kruskal-Wallis tests, and categorical data using χ2 or Fisher exact tests. Logistic regression models were fit to compare the odds of AKI development between PTZ/VAN and other antibiotic combinations.
One model adjusted for baseline AKI risk using a validated prediction score for critically ill patients. This AKI risk score assigns points (minimum score, 0; maximum score, 21) for chronic conditions (chronic kidney disease, chronic liver disease, congestive heart failure, hypertension, and atherosclerotic cardiovascular disease) and acute disease states and exposures (pH ≤7.3, nephrotoxin exposure, sepsis [defined according to Sepsis-3 criteria], mechanical ventilation requirement, and anemia) [23, 24]. The second model was inclusive of all patient and treatment characteristics thought to affect AKI risk. The incidence of MAKE60 was compared in the patients with available serum creatinine follow-up data in our electronic health record at 60 ± 30 days. A 2-sided alpha of 0.05 was considered statistically significant. All analyses were performed with JMP 13.0.0 software (SAS Institute) and R software (version 3.4.2; R Core Team, R Foundation for Statistical Computing).
RESULTS
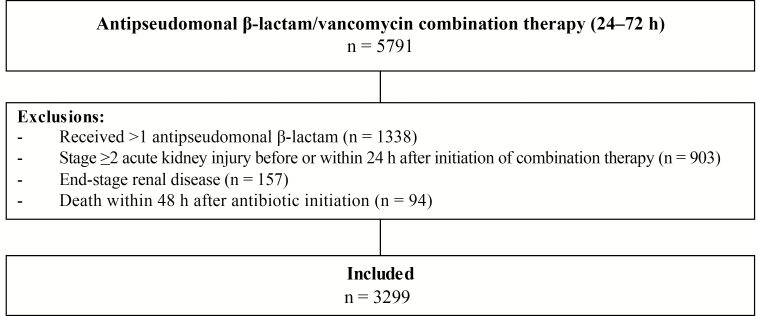
Of the 5791 patients screened for eligibility, 3299 were included (Figure 1). The primary reasons for exclusion were the use of >1 antipseudomonal β-lactam drug (n = 1338) or the presence of stage 2 or 3 AKI before combination therapy initiation (n = 903). Antibiotic combination therapy began within a median (IQR) of 3.2 (−0.4 to 12) hours after ICU admission. The duration of concomitant antibiotic therapy decreased each successive year. In the final year of the study time frame (2016), which most closely approximates contemporary practice, the median (IQR) duration was 1.5 (0.9–2.6) days.
Figure 1.
Study design and patient inclusion.
At baseline, the PTZ/VAN, CEF/VAN, and MER/VAN groups compared well. Patients receiving MER/VAN had a greater acuity of illness, as evidenced by higher APACHE III and SOFA scores; the mean (SD) APACHE III scores for the MER/VAN, PTZ/VAN, and CEF/VAN groups were 72 (25), 67 (22), and 64 (23), respectively, and the mean SOFA scores were, 5.8 (3.3), 5.2 (3.1), and 4.8 (3.1) (both P ≤ .001). The MER/VAN group also had a greater frequency of acidosis, anemia, sepsis, and need for mechanical ventilation. A 150%–200% increase in serum creatinine level from baseline or urine output <0.5 mL/kg/h for >6 hours (stage 1 AKI) was present in one-third of patients when combination antibiotic therapy was started. The use of other nephrotoxic drugs at baseline was infrequent. No other clinically significant differences were observed between the groups (Table 1).
Table 1.
Baseline Patient Demographic and Clinical Characteristics
| Description | PTZ/VAN (n = 1540) |
CEF/VAN (n = 1373) |
MER/VAN (n = 386) |
P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 64 (17) | 63 (17) | 64 (17) | .18 |
| Male sex, No. (%) | 943 (61) | 801 (58) | 212 (55) | .050 |
| White race, No. (%) | 1405 (91) | 1240 (90) | 351 (91) | .69 |
| Height, mean (SD), cm | 170 (11) | 170 (11) | 169 (11) | .02 |
| Weight, mean (SD), kg | 85 (26) | 84 (24) | 83 (27) | .20 |
| Body mass index, mean (SD), kg/m2 | 29 (8) | 29 (7) | 29 (8) | .65 |
| APACHE III score, mean (SD) | 67 (22) | 64 (23) | 72 (25) | <.001 |
| SOFA score, mean (SD), | 5.2 (3.1) | 4.8 (3.1) | 5.8 (3.3) | <.001 |
| Charlson Comorbidity Index, mean (SD) | 5.9 (3.7) | 5.8 (3.7) | 6.1 (3.6) | .26 |
| Vasopressor use, No. (%) | 664 (43) | 540 (39) | 172 (45) | .057 |
| Lactate, mean (SD), mmol/La | 1.9 (1.6) | 1.9 (1.4) | 1.8 (1.2) | .16 |
| Baseline serum creatinine, median (IQR), mg/dL | 1.0 (0.8– 1.3) | 1.0 (0.8–1.3) | 1.0 (0.7– 1.4) | .61 |
| Renal parameters at antibiotic initiation | ||||
| Serum creatinine, mean (SD), mg/dL | 1.1 (0.5) | 1.0 (0.5) | 1.0 (0.5) | <.001 |
| Creatinine clearance, mean (SD), mL/min | 64.0 (39.4) | 67.6 (37.6) | 66.2 (39.1) | .003 |
| AKI stage 1 at antibiotic initiation, No. (%) | 457 (30) | 315 (23) | 108 (28) | <.001 |
| AKI risk prediction score at antibiotic initiation, No. (%) of each component)b | 6.0 (3.0) | 5.8 (3.2) | 6.5 (3.2) | <.001 |
| Chronic kidney disease | 205 (13) | 171 (13) | 59 (13) | .79 |
| Chronic liver disease | 32 (2) | 20 (2) | 9 (2) | .35 |
| Congestive heart failure | 216 (14) | 184 (13) | 54 (14) | .88 |
| Hypertension | 871 (57) | 697 (51) | 201 (52) | .006 |
| Atherosclerotic cardiovascular disease | 433 (28) | 373 (27) | 118 (31) | .42 |
| pH ≤7.3 | 286 (19) | 279 (20) | 107 (28) | <.001 |
| Mechanical ventilation | 830 (54) | 726 (53) | 217 (56) | .50 |
| Anemia (hemoglobin <9 g/dL) | 681 (44) | 588 (43) | 194 (50) | .03 |
| Sepsis | 1132 (74) | 910 (66) | 299 (78) | <.001 |
| Nephrotoxin exposure, No. (%)c | ||||
| Amphotericin B | 4 (0) | 4 (0) | 5 (1) | .03 |
| Aminoglycosides | 8 (1) | 13 (1) | 5 (1) | .21 |
| Iodinated contrast | 14 (1) | 2 (0) | 1 (0) | .01 |
| Chemotherapy (nephrotoxic) | 3 (0) | 3 (0) | 1 (0) | >.99 |
| Antiretroviral medications | 0 (0) | 3 (0) | 1 (0) | .13 |
| Culture-positive infection, No. (%) | 468 (30) | 351 (26) | 127 (33) | .002 |
| VAN dosage, mean (SD) | ||||
| Dosage, mg/d | 2125 (797) | 2181 (829) | 2017 (801) | .002 |
| Dosage, mg/kg/d | 25.4 (7.7) | 26.2 (8.1) | 24.8 (7.5) | .001 |
Abbreviations: AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation; CEF, cefepime; IQR, interquartile range; MER, meropenem; PTZ, piperacillin-tazobactam; SD, standard deviation; SOFA, Sequential Organ Failure Assessment; VAN, vancomycin.
aValue not obtained during intensive care unit stay for 32% of patients.
bThis score assigns points for increasing risk related to chronic conditions (chronic kidney disease, chronic liver disease, congestive heart failure, hypertension, atherosclerotic cardiovascular disease) and acute disease states and exposures (pH ≤7.3, nephrotoxin exposure, sepsis [defined according to Sepsis-3 criteria], mechanical ventilation requirement, and anemia) [23, 24]. Scores range from 0 to 21, with higher scores indicating increased risk.
cNephrotoxin exposure was evaluated from 7 days before hospital admission to 48 hours after intensive care unit admission.
Overall Incidence of AKI Between Treatment Groups
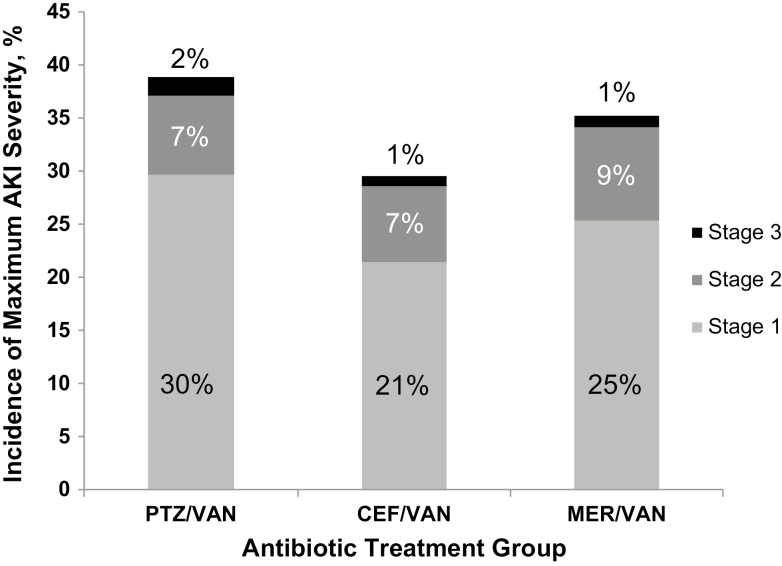
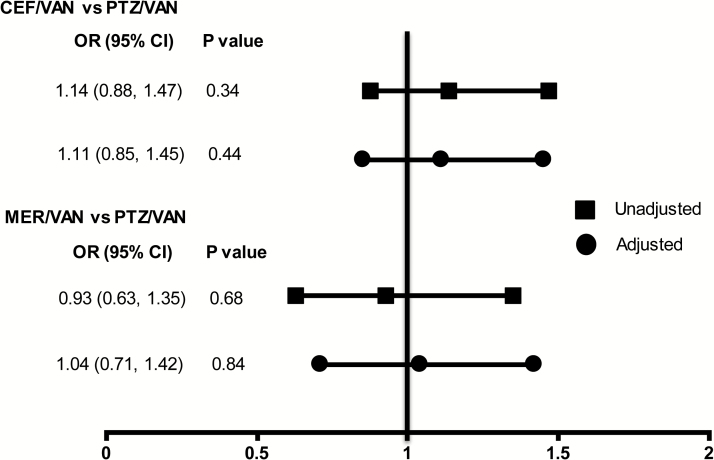
We found an 8.8% overall incidence of stage 2 or 3 AKI with a median (IQR) time to onset of 0.9 (0.4–1.9) days after initiation of combination therapy (Figure 2). In unadjusted analyses, no increased risk of stage 2 or 3 AKI was observed when PTZ/VAN was compared with CEF/VAN or to MER/VAN (Figure 3). Similar results were noted after adjustment for baseline AKI risk score. The risk of AKI development was not affected by the duration of combination therapy (Supplemental Appendix 1).
Figure 2.
Incidence of maximum acute kidney (AKI) injury stage achieved across 3 antibiotic combinations: piperacillin-tazobactam (PTZ)/vancomycin (VAN), cefepime (CEF)/VAN, and meropenem (MER)/VAN. The overall incidences of stage 1, 2, and 3 AKI were 26%, 7%, and 1%, respectively. The incidences of stage 1 AKI development by serum creatinine in the PTZ/VAN, CEF/VAN, and MER/VAN groups were 62%, 51%, and 50%, respectively.
Figure 3.
Risk of stage 2 or 3 acute kidney injury (AKI) between piperacillin-tazobactam (PTZ)/vancomycin (VAN) and other antibiotic combinations. No differences were observed between groups in unadjusted analyses or analyses adjusted for baseline AKI risk score. Reference antibiotic groups for the models were cefepime (CEF)/VAN and meropenem (MER)/VAN, respectively. Abbreviations: CI, confidence interval; OR, odds ratio.
In patients who developed stage 2 or 3 AKI based on serum creatinine criteria, we analyzed AKI duration [25]. Recovery to <2-fold baseline levels by discharge occurred in 60% of patients receiving PTZ/VAN (28 of 46 patients) with a median (IQR) duration of stage 2 or 3 AKI of 2.1 (0.6–3.7) days. This compared with 38% in the CEF/VAN group (12 of 26) and 80% (4 of 5) in the MER/VAN group, with median (IQR) stage 2 or 3 AKI durations of 3.5 (1.1–5.7) and 2.5 (0.8–3.8) days, respectively.
In our multivariable model, which included all factors thought to contribute to an increased AKI risk, antibiotic group was not associated with AKI (Table 2). Stratified analyses were conducted according to the presence of stage 1 AKI at antibiotic initiation. In both the 880 patients with stage 1 AKI at combination antibiotic initiation, and in the 2419 patients without any AKI at this point, the empiric antibiotic choice did not affect the risk for new or worsening AKI (Table 2).
Table 2.
Multivariate Model of Risk Factors for Stage 2–3 Acute Kidney Injury in the Full Cohort as and Stratified According to Baseline Presence of Stage 1 Acute Kidney Injury
| Antibiotic Treatment Groupa | Full Cohort | No AKI at Baseline | Stage 1 AKI at Baseline | |||
|---|---|---|---|---|---|---|
| OR (95% CI)b | P Value | OR (95% CI) | P Value | OR (95% CI)b |
P
Value |
|
| PTZ/VAN vs CEF/VAN | 1.09 (.83–1.43) | .57 | 1.41 (.95– 2.04) | .085 | 0.75 (.50–1.12) | .17 |
| PTZ/VAN vs MER/VAN | 1.50 (.78–1.72) | .49 | 1.22 (.71– 2.08) | .48 | 0.88 (.48–1.61) | .69 |
Abbreviations: AKI, acute kidney injury; CEF, cefepime; CI, confidence interval; MER, meropenem; OR, odds ratio; PTZ, piperacillin-tazobactam; VAN, vancomycin.
aThe reference antibiotic groups for the 2 models are CEF/VAN and MER/VAN, respectively.
bModel adjusted for the following covariates: age, sex, body mass index, Acute Physiology and Chronic Health Evaluation III score, Charlson Comorbidity Index, creatinine level at antibiotic initiation, creatinine clearance based on the Cockroft-Gault equation at antibiotic initiation, pH ≤7.3, use of mechanical ventilation, hemoglobin level <9 g/dL, sepsis defined according to Sepsis-3 criteria [24], vasopressor use, and nephrotoxin exposure. Consistent with the methods of Malhotra et al [23], nephrotoxin exposure reflects exposure to amphotericin B, aminoglycosides, iodinated contrast medium, nephrotoxic chemotherapy, nonsteroidal anti-inflammatory drugs (excluding low-dose maintenance aspirin), and nephrotoxic antiretroviral therapy in the 7 days before hospital admission through 48 hours after the index intensive care unit admission.
Secondary End Points
In the 1217 (patients 37%) with a follow-up serum creatinine level available in the electronic health record within 60 ± 30 days of the study antibiotic course, no significant difference was noted between groups in the incidence of a persistently elevated serum creatinine ≥2-fold the baseline level (P = .08), new requirement for renal replacement therapy (P = .15), or death (P = .09). When MAKE60 was analyzed as a composite event, there was a significant difference across treatment groups (P = .02), driven by an increased mortality risk in patients exposed to MER/VAN (Table 3).
Table 3.
Incidence of MAKE60 in the Patients With Available Follow-up Serum Creatinine Data in the Electronic Health Record at 60 Days
| MAKE60 Variable | Patients, No. (%) | P Value | |||
|---|---|---|---|---|---|
| PTZ/VAN (n = 604) |
CEF/VAN (n = 496) |
MER/VAN (n = 117) | Total (n = 1217) | ||
| SCr doubling at 60 ± 30 d | 16 (3) | 11 (2) | 7 (6) | 34 (3) | .081 |
| Death (within 90 d) | 34 (6) | 33 (7) | 13 (11) | 80 (7) | .091 |
| New RRT requirement | 7 (1) | 1 (0) | 1 (1) | 9 (1) | .15 |
| MAKE60 composite | 51 (8) | 43 (9) | 19 (16) | 113 (9) | .024 |
Abbreviations: CEF, cefepime; MAKE60, major acute kidney events at 60 days; MER, meropenem; PTZ, piperacillin-tazobactam; RRT, renal replacement therapy; SCr, serum creatinine; VAN, vancomycin.
DISCUSSION
Empiric selection of antibiotics in critically ill patients is a delicate balance between selecting the appropriate agents to treat the infection, limiting host toxicity, and optimizing antimicrobial stewardship. Current guidelines clearly prioritize broad-spectrum therapies for septic patients, which results in widespread use of intravenous antipseudomonal β-lactams and drugs to treat resistant gram-positive infections [26]. Notwithstanding slight differences in the spectrum of activity, choice of antipseudomonal β-lactam agent is often based on provider and institution preference.
Recently, nephrotoxicity has been raised as a possibility with PTZ/VAN therapy, which has led to concern over using this combination in the empiric setting. In contrast to previous literature, in this large cohort study of critically ill patients, we found that brief exposure to the combination of PTZ/VAN conferred no greater risk of moderate to severe AKI than similar such exposures to CEF/VAN or MER/VAN. In addition, PTZ/VAN did not increase the risk of persistent kidney dysfunction, need for renal replacement therapy, or death at 60 days.
Previous studies that identified a heightened risk for AKI with PTZ/VAN have predominately included hospitalized floor patients. In this lower-risk population, a 2–3-fold higher risk of AKI with PTZ/VAN has been observed [8, 16, 27]. In ICU patients with an already high baseline risk of AKI, findings have been more inconsistent. In a retrospective cohort study of 122 critically ill patients, PTZ/VAN was not associated with a higher incidence of AKI than CEF/VAN after adjustment for propensity to receive each treatment [7]. A meta-analysis of a small subgroup of critically ill patients found an increased risk of AKI associated with PTZ/VAN compared with VAN alone [16]. Recently, another meta-analysis found variability in relative AKI risk depending on the comparator group selected. When PTZ/VAN was compared with VAN alone, the risk of AKI was increased (odds ratio, 9.62; 95% confidence interval, 4.48–20.68). However, when PTZ/VAN was compared with CEF/VAN, carbapenem/VAN, or PTZ alone, no greater risk was observed [15].
Associations of PTZ/VAN and nephrotoxicity have occurred after 3–5 days of concurrent therapy, much longer than customarily used in the empiric setting [10, 12]. Whereas previous studies focused their analysis on ≥48 hours of combination antibiotic exposure, we focused on a more clinically relevant question for critically ill patients. By 48 hours, microbiological data are often available to guide deescalation, and treatment with extended-spectrum antibiotic combinations rarely continues for >72 hours [14]. This was supported in our study, where the median (IQR) duration of combination antibiotic therapy in patients from 2016 was 1.5 (0.9–2.6) days.
The outcomes evaluated also distinguish the current work from previous studies. Prior literature has historically included all stages of AKI, which assumes that all stages are equally deleterious. In the current analysis, we focused on moderate to severe AKI, which is most closely associated with heightened morbidity and mortality risks [2, 28]. We also defined AKI by both creatinine and urine output criteria, as recommended by the guidelines, a measure that has been inconsistently applied in previous investigations. In addition, critically ill patients have high baseline risk for AKI owing to their severity of illness and multiple comorbid conditions. Previous studies insufficiently adjusted for this risk, which may have affected findings. For example, the antimicrobial spectrums of PTZ and MER are slightly broader than that of CEF, which may result in preferential selection in patients with a greater severity of illness. We used multiple methods to determine the independent risk of the antibiotic combination group, including a validated AKI risk score as well as a robust multivariable model.
The mechanism through which PTZ/VAN could increase the risk for AKI remains unknown. We observed a numerically higher incidence of stage 1 AKI in the PTZ/VAN group, which was driven by a higher frequency of creatinine-based AKI diagnosis rather than by oliguria or anuria and did not correspond to greater AKI severity stages or 60-day adverse renal outcomes. Based on these data, one could hypothesize that rather than direct tubular damage associated with PTZ/VAN, the change in serum creatinine observed could be a function of inhibition of the tubular secretion of creatinine by piperacillin [29]. Piperacillin has been previously shown to inhibit active secretion of multiple medications via active transporters in the proximal convoluted tubule [30–32]. Competition at this site could result in small increases in creatinine enough to signal AKI stage 1, without a corresponding increase in more severe AKI or 60-day renal dysfunction.
Interestingly, it was recently found that when cell cycle arrest marker kinetics were assessed (tissue inhibitor metalloproteinase 2 and insulin growth factor binding protein 7), biomarker levels were significantly elevated with the first dose of VAN or PTZ in those that went on to have stage 2 or 3 AKI. However, the investigators were unable to assess whether these elevations occurred before or after antibiotic administration owing to the lack of precise administration time data [33]. More mechanistic data are necessary to understand the impact of PTZ/VAN on the kidney.
This current study is not without limitations. Owing to the retrospective nature of this review, unmeasured factors that affected antibiotic choice or AKI risk could have influenced study findings, although we think this is unlikely. The study time frame was cut off in 2016 to avoid a possible bias from recent publications suggesting heightened risk with PTZ/VAN. Moreover, we fit several multivariable models to adjust for factors that would differ between treatment groups. In some cases, nephrotoxic medications assessed in our AKI risk prediction score were obtained from preadmission medication data. There is potential that incomplete documentation or recall bias may have affected the accuracy of the preadmission medication history and this component of our baseline AKI risk assessment. We also used a validated AKI sniffer based on objective criteria to determine the incidence of study end points. Our use of the AKIN criteria rather than the more recent Kidney Disease Improving Global Outcomes criteria in this tool may have impacted our detection of AKI [19]. However, these 2 sets of criteria similarly define stages 2 and 3 AKI, which limits the likelihood of any clinically meaningful difference in outcomes attributable to this distinction.
Death was the primary driver of the MAKE60 composite end point that suggested worse outcomes with MER/VAN. It is very unlikely that all deaths were due to adverse kidney outcomes, as supported by the similar incidences of persistent kidney dysfunction and new need for renal replacement therapy across groups. Instead this probably reflects more severe illness in the patients receiving MER/VAN. We were unable to incorporate antibiotic pharmacokinetics and pharmacodynamics into risk prediction models. Renally eliminated antibiotics are closely monitored at the study center by critical care clinical pharmacists as part of the multidisciplinary care team. No changes to dosing protocols occurred during the study time frame. Although we cannot exclude the impact of these factors on the study findings, we have no reason to believe that the treatment approach would have differed between groups.
In conclusion, data from this large cohort of critically ill patients suggests that short courses of PTZ/VAN were not associated with a greater risk of short-term or 60-day adverse renal outcomes compared with other similar empiric broad-spectrum combinations. Further research is needed to understand the mechanism underlying previously noted associations between PTZ/VAN and AKI.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We acknowledge Nicole M. Andrijasevic, study coordinator of the Anesthesia Clinical Research Unit, for her help with data extraction.
Disclaimer. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported in part by the Mayo Midwest Pharmacy Research Committee and the National Center for Advancing Translational Sciences (grant UL1 TR002377).
Potential conflicts of interest. S. N. serves on the advisory board of DoseMe. E. F. B. serves on the advisory board of Fast Biomedical. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Linder A, Fjell C, Levin A, Walley KR, Russell JA, Boyd JH. Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med 2014; 189:1075–81. [DOI] [PubMed] [Google Scholar]
- 2. Fuchs L, Lee J, Novack V, et al. Severity of acute kidney injury and two-year outcomes in critically ill patients. Chest 2013; 144:866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rimes-Stigare C, Frumento P, Bottai M, Mårtensson J, Martling CR, Bell M. Long-term mortality and risk factors for development of end-stage renal disease in critically ill patients with and without chronic kidney disease. Crit Care 2015; 19:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta RL, Pascual MT, Soroko S, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int 2004; 66:1613–21. [DOI] [PubMed] [Google Scholar]
- 5. Uchino S, Kellum JA, Bellomo R, Doig GS, Tan I, Bouman C. Acute renal failure in critically ill patients. JAMA 2015; 294:813–818. [DOI] [PubMed] [Google Scholar]
- 6. Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 2015; 10:1510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hammond DA, Smith MN, Painter JT, Meena NK, Lusardi K. Comparative incidence of acute kidney injury in critically ill patients receiving vancomycin with concomitant piperacillin-tazobactam or cefepime: a retrospective cohort study. Pharmacotherapy 2016; 36:463–71. [DOI] [PubMed] [Google Scholar]
- 8. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy 2014; 34:670–6. [DOI] [PubMed] [Google Scholar]
- 9. Giuliano CA, Patel CR, Kale-Pradhan PB. Is the combination of piperacillin-tazobactam and vancomycin associated with development of acute kidney injury? a meta-analysis. Pharmacotherapy 2016; 36:1217–28. [DOI] [PubMed] [Google Scholar]
- 10. Rutter WC, Cox JN, Martin CA, Burgess DR, Burgess DS. Nephrotoxicity during vancomycin therapy in combination with piperacillin-tazobactam or cefepime. Antimicrob Agents Chemother 2017; 61:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rutter WC, Burgess DS, A# F. Incidence of acute kidney injury among patients treated with piperacillin-tazobactam or meropenem in combination with vancomycin. Antimicrob Agents Chemother 2018; 62:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navalkele B, Pogue JM, Karino S, et al. Risk of acute kidney injury in patients on concomitant vancomycin and piperacillin-tazobactam compared to those on vancomycin and cefepime. Clin Infect Dis 2017; 64:116–23. [DOI] [PubMed] [Google Scholar]
- 13. Barlam TF, Cosgrove SE, Abbo LM, et al. Executive summary: implementing an antibiotic stewardship program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis 2016; 62:1197–202. [DOI] [PubMed] [Google Scholar]
- 14. Baby N, Faust AC, Smith T, Sheperd LA, Knoll L, Goodmanb EL. Nasal methicillin-resistant Staphylococcus aureus(MRSA) PCR testing reduces the duration of MRSA-targeted therapy in patients with suspected MRSA pneumonia. Antimicrob Agents Chemother 2017; 61: e02432–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luther MK, Timbrook TT, Caffrey AR, Dosa D, Lodise TP, LaPlante KL. Vancomycin plus piperacillin-tazobactam and acute kidney injury in adults: a systematic review and meta-analysis. Crit Care Med 2018; 46:12–20. [DOI] [PubMed] [Google Scholar]
- 16. Hammond DA, Smith MN, Li C, Hayes SM, Lusardi K, Bookstaver PB. Systematic review and meta-analysis of acute kidney injury associated with concomitant vancomycin and piperacillin/tazobactam. Clin Infect Dis 2017; 64:666–74. [DOI] [PubMed] [Google Scholar]
- 17. Melton LJ. 3rd. The threat to medical-records research. N Engl J Med 1997; 337:1466–70. [DOI] [PubMed] [Google Scholar]
- 18. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kellum JA, Lameire N, Aspelin P, et al. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2:1–138. [Google Scholar]
- 20. Ahmed A, Vairavan S, Akhoundi A, et al. Development and validation of electronic surveillance tool for acute kidney injury: a retrospective analysis. J Crit Care 2015; 30:988–93. [DOI] [PubMed] [Google Scholar]
- 21. Levey AS, Bosch JP, Breyer Lewis J, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999; 130:461–70. [DOI] [PubMed] [Google Scholar]
- 22. Billings FT, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract 2014; 127:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malhotra R, Kashani KB, Macedo E, et al. A risk prediction score for acute kidney injury in the intensive care unit. Nephrol Dial Transplant 2017; 32:814–22. [DOI] [PubMed] [Google Scholar]
- 24. Singer M, Deutschman CS, Seymour C, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13:241–57. [DOI] [PubMed] [Google Scholar]
- 26. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45:486–552 [DOI] [PubMed] [Google Scholar]
- 27. Rutter WC, Burgess DR, Talbert JC, Burgess DS. Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: a retrospective cohort analysis. J Hosp Med 2017; 12:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sawhney S, Marks A, Fluck N, Levin A, Prescott G, Black C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017; 69:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang K, Kestenbaum B. Proximal tubular secretory clearance a neglected partner of kidney function. Clin J Am Soc Nephrol 2018; 13:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landersdorfer CB, Kirkpatrick CMJ, Kinzig M, Bulitta JB, Holzgrabe U, Sörgel F. Inhibition of flucloxacillin tubular renal secretion by piperacillin. Br J Clin Pharmacol 2008; 66:648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Najjar TA, Abou-Auda HS, Ghilzai NM. Influence of piperacillin on the pharmacokinetics of methotrexate and 7-hydroxymethotrexate. Cancer Chemother Pharmacol 1998; 42:423–8. [DOI] [PubMed] [Google Scholar]
- 32. Tjandramaga TB, Mullie A, Verbesselt R, De Schepper PJ, Verbist L. Piperacillin: human pharmacokinetics after intravenous and intramuscular administration. Antimicrob Agents Chemother 1978; 14:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ostermann M, McCullough PA, Forni LG, et al. Kinetics of urinary cell cycle arrest markers for acute kidney injury following exposure to potential renal insults. Crit Care Med 2018; 46:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.