Abstract
Objectives:
We sought to determine if use of a poly (ADP-ribose) polymerase (PARP) inhibitor is cost effective for maintenance treatment of platinum-sensitive recurrent ovarian cancer.
Methods:
A decision analysis model compared 4 maintenance strategies: 1) Observation 2) BRCA germline mutation testing and selective treatment of carriers (gBRCA only) 3) BRCA germline and tumor homologous recombination deficiency (HRD) testing andselective treatment of either BRCA carriers or those with tumor HRD (gBRCA and HRD only) 4) Treat all with niraparib to progression (treat all). Costs were estimated in 2016 US dollars. Incremental cost-effectiveness ratios (ICERs) were in dollars per progression-free quality adjusted life-year (PF-QALY). One-way sensitivity analyses tested multiple assumptions.
Results:
Maintenance PARP inhibitor was costlier and more effective than observation. Mean costs and PF-QALY were $827 and 3.4 months for observation, $46,157 and 5.7 for a BRCA-only strategy, $109,368 and 8.5 for a gBRCA and HRD only strategy, and $169,127 and 8.8 for a treat all strategy. gBRCA-only had an ICER of $243,092/PF-QALY compared to observation; other strategies did not approach cost effectiveness. Using the current FDA label for maintenance PARP inhibitor regardless of biomarker status, the third-party payer cost per month (28-day supply) would need to be reduced from approximately $14,700 to $3,600 to be considered cost effective compared to observation using a willingness to pay threshold of $100,000/PF-QALY.
Conclusion:
Maintenance PARP inhibitor therapy for platinum-sensitive recurrent ovarian cancer is not cost effective. Treatment of patients with BRCA mutation alone or with HRD + tumors are preferred strategies compared to a treat all strategy. Lowering the cost may make selective niraparib maintenance therapy cost effective compared to observation.
Precis:
Maintenance poly (ADP-ribose) polymerase inhibitor therapy is not cost effective in recurrent ovarian cancer patients regardless of biomarker status.
Introduction
In the United States, cancer is ranked second among the most expensive diseases to treat1. With an increasing proportion of cost incurred to patients as direct out of pocket co-payments or shared-payment plans, cancer patients are especially at risk of experiencing financial toxicity, resulting in a lower quality of life, further limiting access to the highest quality care, and increasingly leading to personal bankruptcy. Cost-effectiveness analyses to compare the relative value of treatment are urgently needed due to the recent proliferation of expensive and heavily marketed novel therapies. Advanced ovarian cancer is initially treated by a sequence of surgery and chemotherapy with a high likelihood of achieving disease remission, but with 80–90% rates of eventual relapse. Although broadly considered incurable at the time of recurrence, patients may enter a second or third remission with successful therapy. Medically prolonging the time in remission has been an area of active research. Poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) are a relatively recent FDA-approved class of drug approved as maintenance therapy in ovarian cancer. Although the three available PARP inhibitors (olaparib, niraparib, rucaparib) are selectively targeted to be clinically most effective in patients with a germline BRCA mutation or those having a tumor with homologous recombination deficiency (HRD), they were approved without restriction by biomarker2. The financial consequences of maintenance treatment are significant-patients may receive months to years of daily therapy. The cost-effectiveness literature to date has been limited to olaparib, and has not included HRD testing as a means of selecting patients where the relative value of PARP inhibitor may be higher3,4.
Widespread PARP inhibitor use as maintenance therapy in all patients with recurrent, platinum sensitive ovarian cancer has the potential to dramatically escalate US healthcare costs with unclear benefit. Given the known differences in reported effectiveness in biomarker-identified subgroups, this study aims to determine the cost effectiveness of maintenance PARP inhibitor using a decision analysis model of four different strategies to examine the implications of the broader FDA labeling compared to biomarker-driven use of PARP inhibitor.
Methods
A decision analysis model was created to compare the cost effectiveness of four strategies for maintenance therapy in patients with platinum sensitive recurrent ovarian cancer using a societal perspective: 1) observation 2) BRCA germline mutation testing, followed by selective treatment of only those patients with germline BRCA mutations with maintenance PARP inhibitor (‘gBRCA only’); 3) both BRCA germline mutation testing and tumor HRD testing, followed by selective treatment of only those patients with either BRCA germline mutations or those with HRD positive tumors with maintenance PARP inhibitor (‘gBRCA and HRD only’); 4) treatment of all patients with maintenance PARP inhibitor (‘treat all’). The population of interest was those patients eligible for maintenance PARP inhibitor therapy according to FDA labeling, the estimated 5,570 cases of ovarian cancer patients with platinum sensitive recurrences in 2017 in the United States4,5. Strategies were compared using incremental cost-effectiveness ratios (ICERs), defined as the ratio of the difference in costs between strategies and the difference in quality-adjusted progression free-survival (PF-QALY) between strategies.
Models were generated and analysis was programmed using TreeAge Pro 2017 software (TreeAge Software, Williamstown, MA). The MD Anderson Cancer Center institutional review board reviewed and exempted this study from the approval process after determination that it is not human subjects research.
For our model’s base case, we primarily utilized the supporting data from the first FDA maintenance approval in PARP inhibitor, the NOVA study, a randomized, double blind, phase 3 trial of 553 patients with platinum sensitive recurrent ovarian cancer randomized to niraparib or placebo6. The median age ranged between 57–63, and most patients had stages III or IV disease at the time of diagnosis. Both germline BRCA mutation carriers and BRCA wild type subjects were included. As part of an exploratory analysis, the BRCA wild type subjects in this study had tumor testing for HRD to determine its use in predicting outcome. In this trial, the greatest progression-free survival (PFS) benefit of niraparib was seen in subjects with germline BRCA mutations (21 months). BRCA wild type subjects with tumor HRD also saw PFS benefit from niraparib (13 months). Less benefit was seen in subjects who were biomarker negative: BRCA wild type subjects without evidence of tumor HRD (7 months). Table 1 shows the base case estimates for median progression-free survival by subgroup according to biomarker. While our base case for our model and model structure primarily uses niraparib data from the NOVA study, similar PFS outcomes have been seen in olaparib as based on the SOLO-2 and Study 19 trials, and rucaparib based on the ARIEL3 trial7–9.
Table 1.
Estimates of clinical effectiveness by strategy
| Observation | Niraparib maintenance | |||
|---|---|---|---|---|
| Patient cohort | PFS (months) | Utility* | PFS (months) | Utility* |
| All patients | 4.22 | .83 | 11.64 | .84 |
| Germline BRCA1/2 mutant | 5.5 | .83 | 21 | .84 |
| Germline BRCA wild type | 3.9 | .83 | 9.3 | .84 |
| HRD testing, positive | 3.8 | .83 | 12.9 | .84 |
| HRD testing, negative | 3.8 | .83 | 6.9 | .84 |
Utility scores adapted from the European Quality of Life scale, 5-Dimensions (EQ-5D-5L) measurements from NOVA trial, Mirza et al. 2016
For the observation strategy, PFS was estimated from subjects with and without germline BRCA mutations who received placebo in the NOVA trial. Similarly, for the treat all strategy, PFS was estimated from subjects with and without germline BRCA mutations who received niraparib. Among the BRCA wild type cohort, the rate of HRD positivity was assigned as 54.7%, based on subjects without germline BRCA mutations who underwent HRD tumor analysis. PF-QALYs were calculated as the product of the progression-free survival for each strategy and its mean estimated utility as determined by the European Quality of Life scale, 5-Dimensions (EQ-5D)10. In this scale, an index value of 1 indicates full health. Mean utility was adapted from the supplemental material of the NOVA trial, and was similar between niraparib and placebo arms. In the absence of overall survival data, recent publications in oncology have used cost per progression-free life years gained or progression-free QALY as measures of cost effectiveness, utilizing a willingness-to-pay cost threshold of an incremental cost-effectiveness ratio greater than $50,000–100,000/PF-QALY3,4,11.
All patients in the model were assigned to have regular laboratory assessments with a CA-125 drawn every 3 months, regardless of receipt of maintenance therapy strategy. Patients on PARP inhibitor had a weekly complete blood count (CBC) for the first 4 weeks of treatment, followed by a CBC monthly. All patients on maintenance therapy had a monthly visit with their gynecologic oncology provider. If the patient was not on treatment (in the observation cohort), they had an office visit every 3 months. At the time of progression all patients received a CT scan and additional office visit. Patients were assumed to enter the model after receipt of platinum-based chemotherapy for their recurrence, and therefore clinical estimates and costs related to cytotoxic chemotherapy were not included.
Adverse events were modeled to reflect relevant financial consequences of dose reductions and discontinuations based on niraparib use in the NOVA trial. For our base case, all grade ≥3 adverse events were modeled to occur within the first three weeks of treatment initiation. Using data from the NOVA trial, 74% of patients on maintenance niraparib would have grade ≥3 adverse events, of which the majority would require a dose reduction from the full dose of 300mg to 200mg. We modeled a minority of those patients, approximately 20%, would go on to discontinue treatment. For patients who experienced an adverse event requiring dose reduction, it was assumed that they received 3 weeks of full dose maintenance therapy (300mg per day), followed by a reduced dose of treatment (200mg per day) until the time of their estimated disease progression. For patients with an adverse event requiring dose reduction, weekly CBCs were taken for an additional 4 weeks before returning to the regular CBC monitoring schedule. Patients who experienced an adverse event requiring discontinuation received 3 weeks of full dose therapy followed by 1 week of dose reduction (200mg per day), followed by no further therapy. Patients continued therapy or observation until discontinuation due to adverse event or progression or death.
For our base case, the PFS estimate for patients who had a dose reduction to 200 mg was modeled to be equivalent to those patients who received the full dose of therapy. Given that adverse events were typically observed early in the treatment course, we assumed those patients on therapy who had a discontinuation had a PFS equal to their counterparts who had never taken PARP inhibitor (observation).
Selected costs of clinical care related to each strategy were included (Table 2). Given the short time horizon of less than 24 months, costs were not discounted. For laboratory testing (CA-125, CBC), diagnostic imaging (CT scan of the chest, abdomen, pelvis), office visits, and germline BRCA testing, costs were estimated using Healthcare Common Procedure Coding System (HCPCS) or Current Procedural and Terminology (CPT) codes from the clinical diagnostic lab fee schedule and physician fee schedule from the Centers for Medicare & Medicaid Services (CMS)12,13. In our base case, half of the patients were assumed to have received germline BRCA testing before entering into the model, and thus 50% of patients in selective strategies were assigned the costs of germline BRCA testing. Cost of HRD Testing was estimated using the out-of-pocket patient charge of $4,040 based on correspondence with Myriad Genetics regarding the used in the NOVA trial. For our base case, cost of niraparib was used, and taken from the wholesale acquisition cost of the medication14. A 28-day supply at 300 mg per day is $14,769, with a cost per 100 mg capsule of $175.59. No administration costs were included for niraparib beyond office visits with a gynecologic oncologist. Given that the highest proportion of adverse events in the NOVA trial was hematologic, costs of hematologic adverse events was applied, using the lower-end estimate of average per-episode cost adapted from a recent study on chemotherapy-related adverse events in breast cancer which incorporated both inpatient and outpatient costs15. While a minority of patients who experienced adverse events went on to discontinue niraparib in the NOVA trial, in order to provide a more conservative estimate of adverse event cost, a second cost of adverse events was not applied. All costs were estimated in 2017 U.S. dollars.
Table 2.
Cost estimates
| Item | Cost (2017 U.S. Dollars) | CPT/HCPCS Codes* |
|---|---|---|
| CA-125 | $38.58 | 86304 |
| CBC (with differential) | $14.41 | 85025 |
| CT abdomen/pelvis with constrast | $315 | 74177 |
| Office/outpatient visit | $108.74 | 99214 |
| Germline BRCA testing | $2,503 | 81162 |
| HRD testing | $4,040 | -- |
| 100 mg capsule of Niraparib | $175.59 | -- |
| Estimated cost of adverse hematologic event | $4,797 | -- |
Centers for Medicare and Medicaid Services. Physician fee schedule. Available at:https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
2017 clinical diagnostic Lab fee schedule. Available at: (https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/17CLAB.zip)
Multiple one-way sensitivity analyses were performed to test the clinical and cost assumptions utilized in the base case model. The rate of germline BRCA mutation carriers in the study population, estimated to be 20% in the base case16, was varied from 5% to 50%. The cost of adverse events and cost of HRD testing was varied down to $0. Finally, the cost of PARP inhibitor was tested to determine the cost at which treatment with PARP inhibitor would be cost effective compared to observation given a willingness-to-pay threshold of $100,000/PF-QALY.
Two alternative scenarios were also modeled to test the key assumptions of the model. In the first scenario, all patients were assumed to have had germline BRCA mutation testing prior to entering the model. In the second scenario, patients who discontinued niraparib due to adverse effects were assumed to receive the same full PFS benefit as patients who continued therapy until progression, in order to test the effect of our base case assumption of equivalence in PFS between patients who discontinued niraparib and observation.
Results
Under our base case assumptions, all maintenance therapy strategies with PARP inhibitor for women with platinum sensitive recurrent ovarian cancer were both costlier and conferred greater PF-QALYs than observation (Table 3). Mean costs and PF-QALY were $827 and 3.4 months for observation, $46,157 and 5.7 for a BRCA-only strategy, $109,368 and 8.5 for a BRCA and HRD strategy, and $169,127 and 8.8 for a treat all strategy.
Table 3.
Base case costs and outcomes related to selected strategies for platinum-sensitive recurrent ovarian cancer
| Strategy | Cost/patient ($) | PF-QALY Benefit/patient (months) | PF-QALY Benefit/patient (years) | Incremental cost-effectiveness ratio ($/PF-QALY)* | Additional annual cost to US health system ($)** |
|---|---|---|---|---|---|
| Observation | $827 | 3.4 | 0.29 | -- | -- |
| gBRCA testing/selective treatment | $46,157 | 5.7 | 0.48 | $243,092/PF-QALY | $ 249 million |
| gBRCA testing + HRD testing/selective treatment | $109,368 | 8.5 | 0.71 | $269,883/PF-QALY | $ 597 million |
| Treat all | $169,127 | 8.8 | 0.74 | $2.2 million/PF-QALY | $ 926 million |
All ICERs refer to a comparison to the next less effective strategy in Table. The ICER threshold for considering a strategy cost effective was $100,000/PF-QALY.
Assuming 5,507 platinum sensitive recurrent ovarian cancer patients annually.
BRCA germline testing followed by selective PARP inhibitor treatment of only those patients with BRCA mutations had an ICER of approximately $240,000/PF-QALY compared to observation. Under a willingness-to-pay cost threshold of $100,000/PF-QALY, this strategy is not cost effective. The two other maintenance strategies, ‘treat all’ and ‘gBRCA and HRD only’ did not approach cost effectiveness. Compared to germline BRCA testing and selective treatment of patients with BRCA mutations, the addition of HRD screening and treatment of patients with HRD positive tumors had an ICER of $269,883/PF-QALY. Compared to selective treatment of BRCA mutation carriers and patients with HRD positive tumors, treatment of all platinum sensitive recurrent ovarian cancer patients with maintenance PARP inhibitor had an ICER exceeding $2 million/PF-YLS.
The estimated additional annual cost to the US healthcare system of maintenance PARP inhibitor is substantial. Under the least restrictive strategy, treatment of all platinum sensitive recurrent ovarian cancer patients with maintenance therapy would add an additional $926 million dollars to the healthcare system compared to observation. Notably, restriction to only germline BRCA patients under the base case 20% rate of germline BRCA mutation carriers in this population would save an additional $677 million compared to a treat all strategy.
In sensitivity analysis, the prevalence of germline BRCA mutations in the population was varied from 5% to 50%. Across this range, there was no change in cost-effectiveness rankings. In the extreme case of 50% of patients having BRCA mutations, the ICER of selective treatment of germline mutant patients still exceeded $200,000/PF-QALY compared to observation. As the likelihood of a germline BRCA mutation increases, all PARP inhibitor maintenance strategies become more cost effective compared to observation. Reduction in the cost of adverse events associated with niraparib treatment similarly did not impact cost-effectiveness rankings. In the extreme case where adverse events were assumed to add no additional cost to maintenance niraparib strategies, the ICER of selective treatment of germline mutant patients still exceeded $230,000/PF-QALY compared to observation. Reduction of the cost of HRD testing had no effect on cost-effectiveness rankings down to a cost of $0.
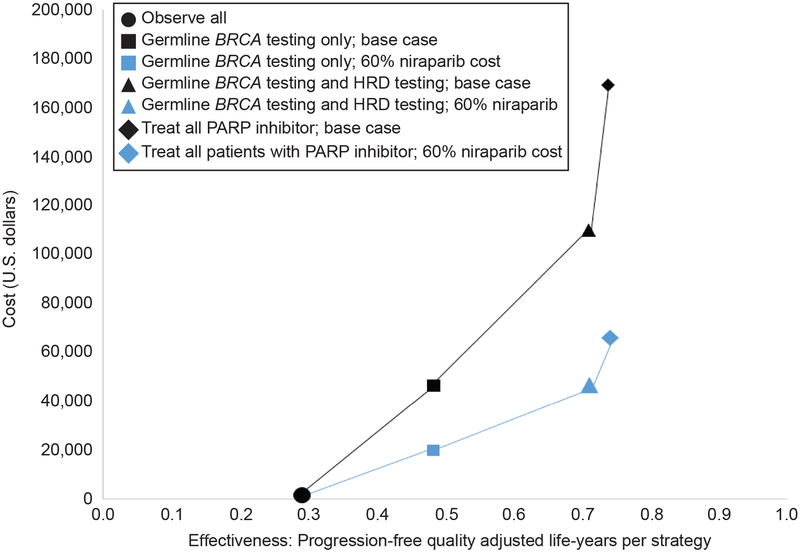
The cost of PARP inhibitor was varied over a wide range. Reductions in the 100mg capsule cost ($175) to the following values would render each strategy potentially cost effective compared to observation with a willingness to pay threshold of $100,000/PF-QALY: $66 (germline testing); $59 (germline and HRD testing); $43 (treat all). Using the current FDA label for maintenance PARP inhibitor for maintenance treatment regardless of biomarker status, the cost per month (28-day supply) would need to be reduced from approximately $14,700 to $3,600 to be considered cost effective compared to observation using a willingness to pay threshold of $100,000/PF-QALY. Figure 1 illustrates the relative cost effectiveness of each strategy using base case assumptions and at a 60% per-capsule reduction in cost of niarparib. Similar to HRD testing, reduction in the cost of germline testing had no effect on cost-effectiveness rankings. In the alternative scenario where the cost of germline testing was removed from the model, there was minimal effect on the overall model results. Similarly, cost-effectiveness rankings were unchanged in the alternative scenario where patients who prematurely discontinued niraparib due to adverse effects were assigned the full PFS benefit as patients who continued therapy until progression. In both cases, the ICER of selective treatment of germline BRCA mutant patients exceeded $200,000/PF-QALY compared with observation.
Figure 1.
Cost effectiveness of all strategies under base case assumptions and with 60% reduction in per-capsule cost of niraparib. HRD, homologous recombination deficiency; PARP, poly (ADP-ribose) polymerase.
Discussion
This study demonstrates that use of maintenance PARP inhibitor therapy in platinum sensitive recurrent ovarian cancer is not cost effective compared to observation. Selective maintenance treatment based on either germline BRCA mutation status or HRD tumor status has the most favorable cost-effectiveness ratio when compared to use in all patients.
To date, published studies of the cost effectiveness of maintenance PARP inhibitor therapy have examined maintenance olaparib. Results were not dissimilar from the current study; however, HRD status had not previously been included. Smith et al. used decision analysis models to compare maintenance olaparib to observation in germline mutant and wild-type BRCA1/2 patients with platinum sensitive recurrent ovarian cancer4. Maintenance olaparib was not found to be cost effective compared with observation in either population, however, the cost of germline BRCA testing or adverse effects was not included in the Smith et al. analysis. Previously, Secord et al. used decision analysis modeling to compare unselected maintenance olaparib in patients with platinum sensitive recurrent ovarian cancer and selective maintenance olaparib in patients with germline BRCA1/2 mutations with observation3. Similarly, use of maintenance olaparib was found not cost effective regardless of restriction by BRCA1/2 mutation status.
We acknowledge the limitations of this study that may stem from several assumptions made in our model. While the study used primarily the NOVA trial for base case estimates, our findings of the relative economic impact of use of a targeted therapy in a selected and unselected populations resulting from a broad FDA labeling are applicable across PARP inhibitors. While we did not seek to directly compared individual PARP inhibitor, the cost profile and outcomes for maintenance olaparib and rucaparib are comparable17. Additionally, use of the $100,000/PF-QALY ICER threshold may be criticized for lack of applicability in an updated healthcare landscape18. The significant burden of the cost of oncologic care may also suggest an exploration of a different willingness-to-pay threshold specific to cancer care.
Other limitations to our cost estimates should be pointed out. To estimate the cost of adverse hematologic effects, we used cost estimates adapted from the breast cancer literature, which may not accurately reflect cost of hematologic adverse events in ovarian cancer patients15. Interestingly, we found varying the costs of a hematologic adverse event related to niraparib in sensitivity analysis resulted had little impact on the overall results of the model. Finally, our use of the out-of-pocket charge to patients for estimate of the HRD testing cost may overestimate the true cost estimate of this test, as charges reflect a desired reimbursement rate. Varying the cost of HRD testing had minimal effect on the model results.
While PARP inhibitors have recently introduced an alternative to cytotoxic chemotherapy or biologics in both the treatment and maintenance settings for ovarian cancer, the high cost of this therapy is significant. With spending on cancer drugs exceeding $100 billion annually, awareness and considered scrutiny of the value of new therapies is warranted19. The Society of Gynecologic Oncology (SGO) has released a position statement on high drug prices and spending and have proposed a number of potential solutions to address this issue20. In addition to suggesting increased transparency in drug pricing and improving access to generics, the authors highlight the inability of Medicare to negotiate drug pricing, which has resulted in industry setting cancer drug pricing at whatever point the market will bear21. The subsequent downstream effect is that private insurers largely follow Medicare’s lead.
The SGO position statement also recommends the increased study of value-based pricing at the federal level. In the current U.S. healthcare system, there is currently no formal relationship that exists between the efficacy of cancer drugs and drug pricing22. Solely benchmarking pricing on absolute or relative efficacy of cancer therapy, however, is only one piece of a complex problem. The meaning of value in cancer treatment may differ between patient, physician, and payer, and the magnitude of effect of a therapy on progression-free or overall survival may be weighted differently than the novelty, convenience, impact on quality of life, or adverse effects of a therapy23. In a recent statement by the American Society of Clinical Oncology on the affordability of cancer drugs, the unfortunate conclusion that there is no simple solution to escalating drug prices is unsurprising. While sobering, this reinforces both the opportunity and need for ongoing research in this area with the inclusion of a diversity of knowledge and experience of physicians, patients, policymakers, payers to help inform potential change.
Supplementary Material
Acknowledgments
Supported by T32 training grant for training of academic gynecologic oncologists (T32CA101642), the MD Anderson Cancer Center Support Grant (P30CA016672)
Footnotes
Presented at the Society for Gynecologic Oncology on March 24–27, 2018 in New Orleans, LA.
Financial Disclosure
Dr Alvarez Secord discloses that she has received clinical trial grant funding from AbbVie, Amgen, Astellas Pharma Inc., Astex Pharmaceuticals Inc., AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Endocyte, Exelixis, Incyte, Merck, PharmaMar, Prima Biomed, Roche/Genentech, TapImmune and TESARO. She has also received honoraria for Advisory Boards f Alexion, Aravive, Astex, AstraZeneca, Boehringer Ingelheim, Clovis, Janssen/Johnson & Johnson, Merck, Mersano, Myriad, Oncoquest, Precision Therapeutics, Roche/Genentech, and TESARO within the past 36 months. Laura J. Havrilesky discloses that she has received clinical trial grant funding from AstraZeneca and Tesaro. The other authors did not report any potential conflicts of interest.
References
- 1.Soni A Top 10 most costly conditions among Men and Women, 2008: estimates for the U.S. civilian noninstituionalized adult population, age 18 and older Statistical Brief # 331, July 2011, Agency for Healthcare Research and Quality, Rockville, MD: Available at: http://www.meps.ahrq.gov/mepsweb/data_files/publications/st331/stat331.shtml [PubMed] [Google Scholar]
- 2.Berchuck A, Secord AA, Moss HA, Havrilesky LJ. Maintenance poly (ADP-ribose) polymerase inhibitor therapy for ovarian cancer: Precision oncology or one size fits all? J Clin Oncol 2017;35(36):3999–4002. [DOI] [PubMed] [Google Scholar]
- 3.Secord AA, Barnett JC, Ledermann JA, Peterson BL, Myers ER, Havrilesky LJ. Cost-Effectiveness of BRCA1 and BRCA2 mutation testing to target parp inhibitor use in platinum-sensitive recurrent ovarian cancer. Int J Gynecol Cancer 2013;23(5):846–52. [DOI] [PubMed] [Google Scholar]
- 4.Smith HJ, Walters Haygood CL, Arend RC, Leath CA, Straughn JM. PARP inhibitor maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer: A cost-effectiveness analysis. Gynecol Oncol 2015;139(1):59–62. [DOI] [PubMed] [Google Scholar]
- 5.Howlader N Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, et al. SEER Cancer Statistics Review 1975–2014 National Cancer Institute SEER Cancer Statistics Review 1975–2014 National Cancer Institute. 2017;(April):2012–4. [Google Scholar]
- 6.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N Engl J Med 2016;375(22):2154–64. [DOI] [PubMed] [Google Scholar]
- 7.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 2014;15(8):852–61. [DOI] [PubMed] [Google Scholar]
- 8.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18(9):1274–84. [DOI] [PubMed] [Google Scholar]
- 9.Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390(10106):1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group TE. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy (New York) 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 11.Oh A, Tran DM, Mcdowell LC, Keyvani D, Barcelon JA, Merino O et al. Cost-effectiveness of nivolumab-ipilimumab combination therapy compared with monotherapy for first-line treatment of metastatic melanoma in the United States. J Manag Care Spec Pharm. 2017;23(6):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. Physician fee schedule. Available at: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx.
- 13.2017 clinical diagnostic Lab fee schedule. Available at: https://www.cms.gov/apps/ama/license.asp?file=/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/17CLAB.zip
- 14.TESARO Announces Availability of Zejula™ ( Niraparib ) for Patients With Recurrent Ovarian Cancer in the U.S. Available at: https://globenewswire.com/news-release/2017/04/19/962337/0/en/TESARO-Announces-Availability-of-Zejula-Niraparib-for-Patients-With-Recurrent-Ovarian-Cancer-in-the-U-S.html. Last retrieved October 2nd, 2018.
- 15.Rashid N, Koh HA, Baca HC, Lin KJ, Malecha SE, Masaquel A. Economic burden related to chemotherapy-related adverse events in patients with metastatic breast cancer in an integrated health care system. Breast Cancer Targets Ther 2016;8:173–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsop K, Fereday S, Meldrum C, deFazio A, Emmanuel C, George J et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian ovarian cancer study group. J Clin Oncol 2012;30(21):2654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute for clinical and economic review Poly ADP-ribose polymerase (PARP) Inhibitors for Ovarian Cancer: Effectiveness & Value Draft Evidence Report. Available at: https://icer-review.org/wp-content/uploads/2017/02/MWCEPAC_OVARIAN_FINAL_EVIDENCE_REPORT_10112017.pdf. Last retrieved October 2nd, 2018.
- 18.Neumann PJ, Cohen JT, Weinstein MC. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. N Engl J Med 2014;371(9):796–7. [DOI] [PubMed] [Google Scholar]
- 19.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011;103(2):117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Society of Gynecologic Oncology Policy Statement: Addressing the High Cost of Drugs for Oncology Patients: A National Priority Available at: (https://www.sgo.org/public-policy/addressing-the-high-cost-of-drugs-for-oncology-patients/.)
- 21.Kesselheim AS, Avorn J, Sarpatwari A. The high cost of prescription drugs in the United States origins and prospects for reform. JAMA - J Am Med Assoc 2016;316(8):858–71. [DOI] [PubMed] [Google Scholar]
- 22.Mailankody S, Prasad V. Five years of cancer drug approvals: Innovation, efficacy, and costs. JAMA Oncol 2015;1(4):539–40. [DOI] [PubMed] [Google Scholar]
- 23.Cohn DE, Ko E, Meyer LA, Wright JD, Temkin SM, Foote J et al. The “value” of value in gynecologic oncology practice in the United States: Society of Gynecologic Oncology evidence-based review and recommendations. Gynecol Oncol 2017;145(1):185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.