Abstract
Background and Objective
Peficitinib pharmacokinetics and pharmacodynamics have been characterized mainly in Caucasian subjects. This study investigated the pharmacokinetics, pharmacodynamics, safety, and tolerability of peficitinib in healthy Japanese subjects compared with Caucasian subjects.
Methods
In this single-center, randomized, double-blind, placebo-controlled study, a cohort of healthy Japanese (n = 24) and Caucasian (n = 24) men received a single oral dose of peficitinib (20, 60, or 200 mg) or placebo. Another cohort of Japanese men (n = 24) received peficitinib (10, 30, or 100 mg) or placebo twice daily for 7 days. Pharmacokinetic and pharmacodynamic parameters were assessed, and adverse events (AEs) monitored throughout.
Results
Dose proportionality of maximum plasma drug concentration (Cmax) and area under the plasma concentration–time curve extrapolated to infinity (AUCinf) was demonstrated for both ethnicities. The geometric mean ratio for dose-normalized Cmax was 45.7–98.8% higher and AUCinf was 33.8–66.4% higher in Japanese versus Caucasian subjects. Mean peak inhibition of STAT5 phosphorylation was higher in Japanese than Caucasian subjects for a given peficitinib dose, but inhibition was comparable across ethnicities for a given plasma peficitinib concentration. In the multiple-dose study, plasma peficitinib concentrations were similar on day 1 and day 7. All AEs were mild, and none resulted in study discontinuation.
Conclusions
Peficitinib was well tolerated at doses up to 200 mg daily for 7 days in healthy Japanese subjects. Dose-proportional exposure was demonstrated across the single-dose range of 20–200 mg, with greater peficitinib exposure in Japanese compared with Caucasian subjects. The pharmacokinetic/pharmacodynamic relationships were considered comparable between these populations.
ClinicalTrials.gov identifier
Electronic supplementary material
The online version of this article (10.1007/s40261-020-00910-w) contains supplementary material, which is available to authorized users.
Key Points
| Seven days of peficitinib treatment at doses up to 200 mg/day did not reveal any significant safety concerns in healthy male Japanese subjects. |
| In Japanese and Caucasian subjects, the plasma peficitinib concentration increased proportionally with the dose administered. |
| After administration of peficitinib, the plasma drug concentration was slightly higher in Japanese than in Caucasian subjects during the first 24 h after administration, but drug activity (as measured by JAK inhibition) at a given plasma peficitinib concentration was comparable for both ethnicities. |
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by persistent joint inflammation, pain, and limited mobility [1–3]. Methotrexate, a synthetic disease-modifying antirheumatic drug (DMARD), is used as standard first-line treatment for RA [3, 4]. In patients who fail to respond adequately to methotrexate, combination with a biological DMARD, such as a tumor necrosis factor (TNF) inhibitor, may be initiated [3, 4]. However, about 20–40% of patients fail to respond sufficiently to treatment with a TNF inhibitor, and others may lose response over time or suffer adverse effects [5]. In recent years, targeted synthetic DMARDs with novel mechanisms of action, such as Janus kinase (JAK) inhibitors, have provided these patients with further treatment options [6, 7].
The JAK family (JAK1, JAK2, JAK3, and tyrosine kinase-2 [TYK2]) of non-receptor tyrosine kinases plays a crucial role in pro-inflammatory signaling pathways implicated in the pathogenesis of RA [7], and several JAK inhibitors with differential selectivity have been developed [7, 8]. Peficitinib is a pan-JAK inhibitor that inhibits JAK1, JAK2, JAK3, and TYK2 [8, 9]. Recent evidence suggests that peficitinib may suppress the proliferation and migration of RA synovial fibroblasts, which are responsible for the invasion and damage of healthy cartilage in RA [10].
The pharmacokinetics and pharmacodynamics of peficitinib have been characterized in two phase 1, randomized, placebo-controlled, escalating-dose studies conducted in the USA [11]. The studies, which evaluated single (3–300 mg) and multiple (30–200 mg twice daily) peficitinib oral dosing in healthy predominantly Caucasian subjects, demonstrated rapid absorption at all doses, and the attainment of steady-state plasma levels by day 3 of multiple twice-daily dosing. The plasma concentration of peficitinib increased proportionally with increasing single and multiple doses, and no significant differences in the pharmacokinetics and pharmacodynamics of peficitinib were observed between the sexes [11].
Following a successful clinical development program [12–14], peficitinib has recently received its first global approval in Japan as a once-daily treatment for patients with RA who have an inadequate response to conventional therapies [15, 16]. However, as the previous pharmacokinetic/pharmacodynamic studies were performed in mainly Caucasian subjects, and as these parameters can vary in different ethnicities [17], this study was performed to investigate the pharmacokinetics, pharmacodynamics, and safety and tolerability of peficitinib in Japanese subjects, as well as to compare the pharmacokinetic and pharmacodynamic profiles for Japanese and Caucasian subjects.
Methods
Study Design
This was a single-center, randomized, double-blind, placebo-controlled study to assess the pharmacokinetics, pharmacodynamics, and safety and tolerability of single and multiple oral doses of peficitinib in healthy male Japanese subjects (NCT01225224). To compare pharmacokinetics and pharmacodynamics in different races, healthy male Caucasian subjects were also included.
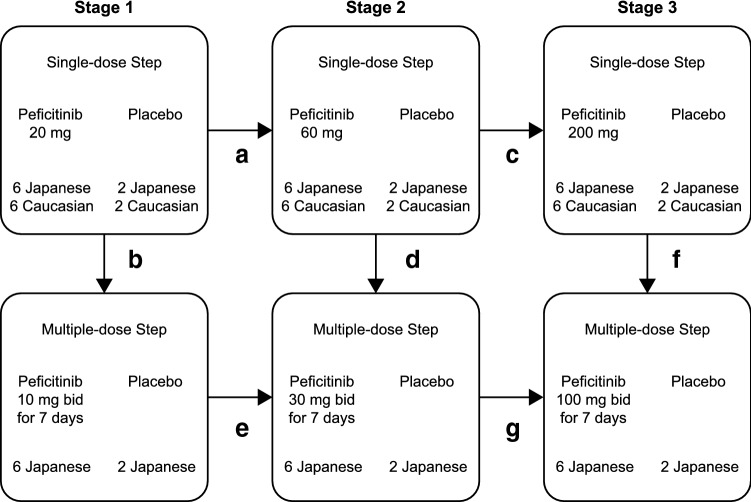
After screening (between day − 30 and day − 3), one cohort of Japanese and Caucasian subjects was randomized 3:1 to receive a single dose of peficitinib (20, 60, or 200 mg) or placebo (Figs. 1a, 2). Another cohort of Japanese subjects was randomized 3:1 to receive peficitinib (10, 30, or 100 mg) or placebo twice daily for 7 days (Figs. 1b, 2).
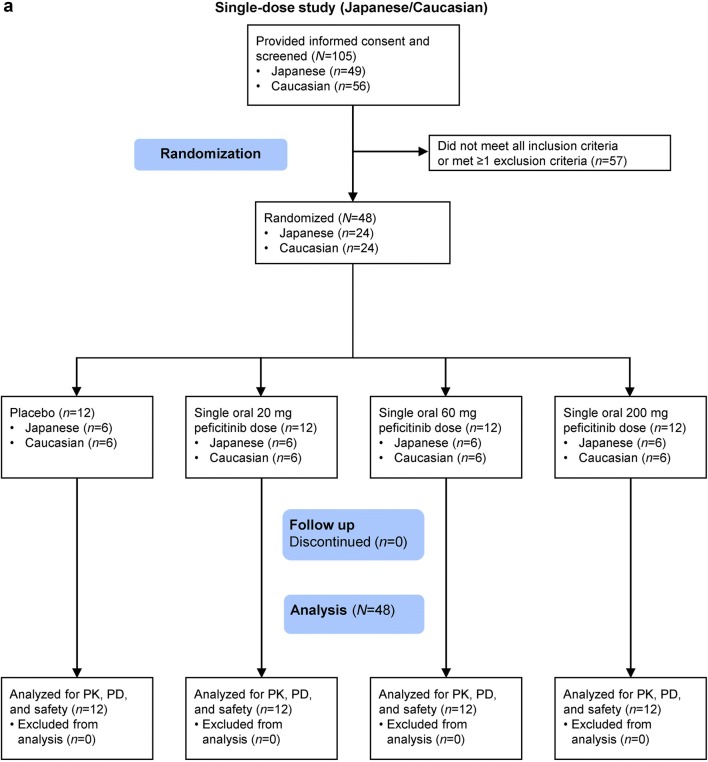
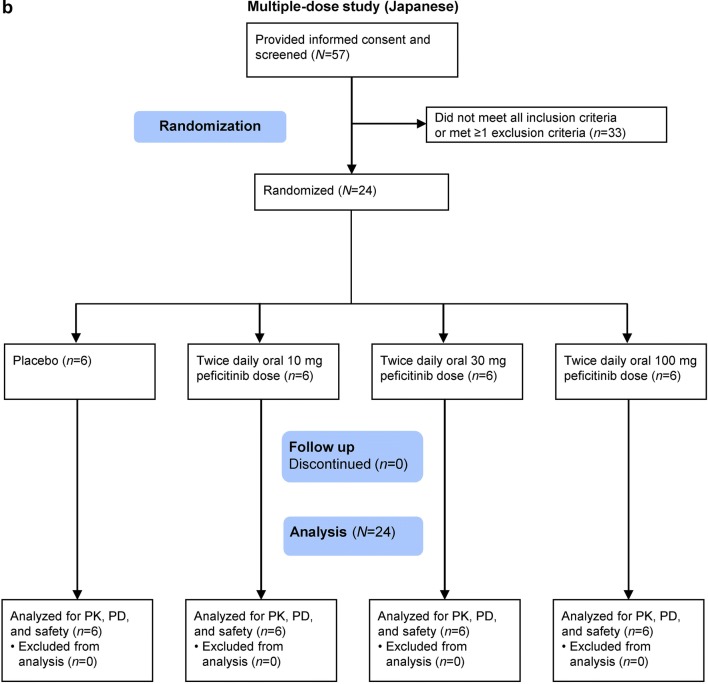
Fig. 1.
Participant flow for the a single-dose study and b multiple-dose study. PD pharmacodynamics, PK pharmacokinetics
Fig. 2.
The 3-stage peficitinib dose-escalation scheme. Dose escalation was conducted in steps A through to G. For example, after completion of the single-dose step in stage 1 dosing proceeded to the single-dose step in stage 2, and to the multiple-dose step in Stage 1. bid twice daily
Ethical Conduct
The study protocol and informed consent form were approved by the institutional review board of the CPC Clinic, Kagoshima, Japan. The study was conducted in compliance with the Declaration of Helsinki, Japanese Good Clinical Practice, and applicable laws and regulations. All subjects provided written informed consent before entering the study.
Study Participants
Inclusion and Exclusion Criteria
Healthy males aged 20–44 years with a body weight of ≥ 50 to < 80 kg (Japanese) or ≥ 50 to < 100 kg (Caucasian), body mass index of ≥ 17.6 to < 26.4 kg/m2 (Japanese) or ≥ 18.0 to < 30.0 kg/m2 (Caucasian), and no previous or concurrent clinically significant disease or abnormal medical or laboratory findings were eligible. Japanese and Caucasian subjects were required to have four grandparents of the relevant race. Japanese subjects had to have resided in Japan for at least 10 years while Caucasian subjects were required to have resided for less than 10 years.
Subjects were excluded if they had received any investigational drug in other clinical trials or post-marketing studies within 120 days before screening; received or were scheduled to receive any medications within 7 days prior to admission (day − 2); received peficitinib previously; consumed excessive alcohol (mean ≥ 45 g/day) regularly; or smoked excessively (mean ≥ 20 cigarettes/day). No concomitant therapies were allowed during the study except for topical preparations and treatments for adverse events (AEs).
Sample Size
The planned sample size was 72 subjects. A total of 48 subjects were to be enrolled in the single-dose study (eight Japanese [six receiving peficitinib, two receiving placebo] and eight Caucasian [six receiving peficitinib, two receiving placebo] subjects per dose level). A total of 24 subjects (Japanese only) were to be enrolled in the multiple-dose study (six receiving peficitinib and two receiving placebo per dose level). The sample size was based on practical considerations and on the sample sizes in the US single- and multiple-dose pharmacokinetic/pharmacodynamic studies [11].
Study Drug Administration
In the single-dose study, hospitalized subjects fasted overnight. Study medication was then administered with water on day 1, after which subjects fasted for at least a further 5 h. Subjects were discharged on day 3 and returned for post-study examinations on day 7.
In the multiple-dose study, the study drug was taken at 12-h intervals, approximately 30 min after breakfast and the evening meal on day 1–day 6. The last dose was administered after breakfast on day 7. Subjects were discharged on day 10 and returned for post-study examinations on day 13.
Pharmacokinetic Assessments
Blood samples for pharmacokinetic assessment of peficitinib were collected pre-dose, and at 0.5, 1, 1.5, 2, 3, 4, 6, 8,12, 24, 36, and 48 h after single-dose administration, and up to 72 h after the final dose of multiple-dose administration (day 1 pre-dose, and at 0.5, 1, 2, 3, 4, 6, 8, and 12 h post-dose; day 4 pre-dose; day 7 pre-dose, and at 0.5, 1, 2, 3, 4, 6, 8,12, 24, 36, 48, and 72 h post-dose). Urine samples were collected pre-dose, and at 0–6, 6–12, 12–24, and 24–48 h after single-dose administration, and at pre-dose and the same time points after study-drug administration on day 1 and day 7 of multiple-dose administration.
Plasma and urine samples were stored at − 70 °C and were sent to BML, Inc. Central Laboratory (Saitama, Japan) for analysis of peficitinib concentrations. Peficitinib concentrations in plasma and urine were measured using a validated liquid chromatography with tandem mass spectrometry method [18]. The lower limits of quantification for peficitinib in plasma and urine were 0.25 ng/mL and 2.5 ng/mL, respectively. All concentrations below the lower limit of quantitation were set to zero. Pharmacokinetic parameters were determined for each subject in the single-dose and multiple-dose (after the first and last doses) studies using non-compartmental analysis of concentrations of unchanged study drug in plasma. The maximum plasma drug concentration (Cmax), time to Cmax (tmax), area under the plasma concentration–time curve (AUC) from the time of dosing to 12 h post-dose (AUC12; multiple dose only), AUC from the time of dosing to the last measurable concentration (AUClast; single dose only), AUC from the time of dosing extrapolated to infinity (AUCinf; single dose only), terminal elimination half-life (t½), and apparent total clearance of the drug from plasma after oral administration were estimated using actual elapsed time from administration. The cumulative amount of unchanged drug excreted into the urine (Ae) was measured for each subject in the single- and multiple-dose (after the first and last doses) studies.
Pharmacodynamic Assessments
Signal transducer and activator of transcription 5 (STAT5) phosphorylation activity (used as a biomarker for JAK enzyme activity [11, 19]) and lymphocyte subsets were assessed (see Supplementary Table S1). STAT5 phosphorylation was quantified using the difference in mean fluorescence intensity from flow cytometric analysis of CD3-positive lymphocytes stained with anti-phosphorylated STAT5 antibodies, with and without IL-2 stimulation. The lymphocyte subsets were also assessed by flow cytometry. Neither STAT5 phosphorylation nor lymphocyte subset had an upper or lower limit of quantitation. Percentage of STAT5 phosphorylation inhibition (i.e., JAK inhibition) was calculated as reported in a previous study [11].
Blood samples for assessment of the inhibition of STAT5 phosphorylation activity were collected pre-dose and at 1, 2, 4, 8, 24, and 48 h after single-dose administration, and up to 72 h after the final dose of multiple-dose administration (day 1 pre-dose and at 1, 2, 4, 8, and 12 h post-dose; day 4 pre-dose; day 7 pre-dose and at 1, 2, 4, 8, 12, 24, 48, and 72 h post-dose). Blood samples for lymphocyte subset analysis were collected pre-dose and at 24 h after single-dose administration, and up to 72 h after the final dose of multiple-dose administration (day 1 pre-dose; day 4 pre-dose; day 7 pre-dose, and 72 h post-dose).
Safety
AEs were monitored throughout the single- and multiple-dose studies. All AEs were coded by System Organ Class and preferred term using MedDRA version 12.1. For each treatment-emergent AE (TEAE), the investigator provided an assessment of the causal relationship with peficitinib and graded the severity as mild, moderate, or severe.
Statistical Analysis
Populations for Analysis
The safety analysis set consisted of all subjects who received at least one dose of study drug. The pharmacokinetic and pharmacodynamic analysis sets consisted of subjects who received the study drug and provided at least one pharmacokinetic or pharmacodynamic measurement, respectively.
Statistical Methodology
The dose proportionality of peficitinib exposure was assessed using the power model. The Cmax and AUCinf were plotted against dose, and the slope was estimated by SAS MIXED procedure. To explore the impact of potential factors of exposure variabilities on peficitinib Cmax and AUCinf, race (Caucasian = 0 and Japanese = 1), age, and body weight were included as fixed effects in the model below:
where α0 represents the intercept; α1, α2, and α3 represent the impact of each factor; and β represents the slope of the regression line. Dose proportionality was concluded if the 95% confidence interval (CI) for the slope contained 1. A covariate was considered to be statistically significant with α = 0.05 based on the Type III test for fixed effects.
The accumulation of peficitinib was investigated for the multiple-dose study using Cmax and AUC12. For each parameter, the least-squares mean difference between day 7 and day 1, and associated 90% CI, was back-transformed to the original scale to obtain the GMR and its 90% CI.
All data processing, summarization, and analyses were performed using SAS® Data Integration Studio (version 3.4; SAS Institute Inc., Cary, NC, USA), SAS® Drug Development (version 3.4), SAS® (version 8.2 and 9.4), and Phoenix® WinNonlin® (version 5.2.1; Certara USA, Inc., Princeton, NJ, USA).
Results
Participant Demographics and Baseline Characteristics
In the single-dose study, 24 Japanese subjects and 24 Caucasian subjects were randomized and received treatment with 20 mg, 60 mg, or 200 mg peficitinib, or placebo (Figs. 1a, 2). In the multiple-dose study, 24 Japanese subjects were randomized to receive 10 mg, 30 mg, or 100 mg peficitinib or placebo twice daily (Figs. 1b, 2). All randomized subjects completed the study and were included in the analysis sets.
The mean age range of Japanese subjects was 22.3–26.8 years in the single-dose study and 26.2–31.5 years in the multiple-dose study. The mean age of Caucasian subjects was 29.0–33.2 years and, as expected, they were heavier than the Japanese subjects (Table 1).
Table 1.
Demographic and baseline characteristics
| Parameter | Single dose | Multiple dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Japanese | Caucasian | Japanese | ||||||||||
| Placebo (n = 6)a | Peficitinib dose | Placebo (n = 6)a | Peficitinib dose | Placebo bid (n = 6)a | Peficitinib dose | |||||||
| 20 mg (n = 6) | 60 mg (n = 6) | 200 mg (n = 6) | 20 mg (n = 6) | 60 mg (n = 6) | 200 mg (n = 6) | 10 mg bid (n = 6) | 30 mg bid (n = 6) | 100 mg bid (n = 6) | ||||
| Age, years | 22.3 (2.1) | 26.8 (6.3) | 26.2 (5.9) | 22.8 (1.0) | 29.8 (4.8) | 29.0 (3.8) | 33.2 (4.7) | 31.0 (7.0) | 30.3 (5.7) | 26.2 (5.2) | 29.7 (8.2) | 31.5 (8.0) |
| Weight, kg | 64.5 (8.3) | 63.9 (6.1) | 64.4 (7.1) | 64.0 (6.4) | 76.0 (8.9) | 70.5 (10.7) | 78.7 (10.3) | 73.4 (5.9) | 67.9 (8.5) | 60.3 (7.0) | 64.0 (6.9) | 61.0 (5.2) |
| BMI, kg/m2 | 21.7 (1.3) | 20.3 (1.6) | 21.9 (1.7) | 21.3 (2.6) | 22.8 (1.1) | 22.3 (3.3) | 23.6 (1.8) | 23.0 (2.0) | 22.7 (1.7) | 21.2 (1.9) | 22.5 (2.3) | 21.2 (1.5) |
Data are expressed as mean (standard deviation)
bid twice daily, BMI body mass index
aFor both single- and multiple-dose steps, data for subjects receiving placebo were pooled over the respective steps
Pharmacokinetics
Single-Dose Study
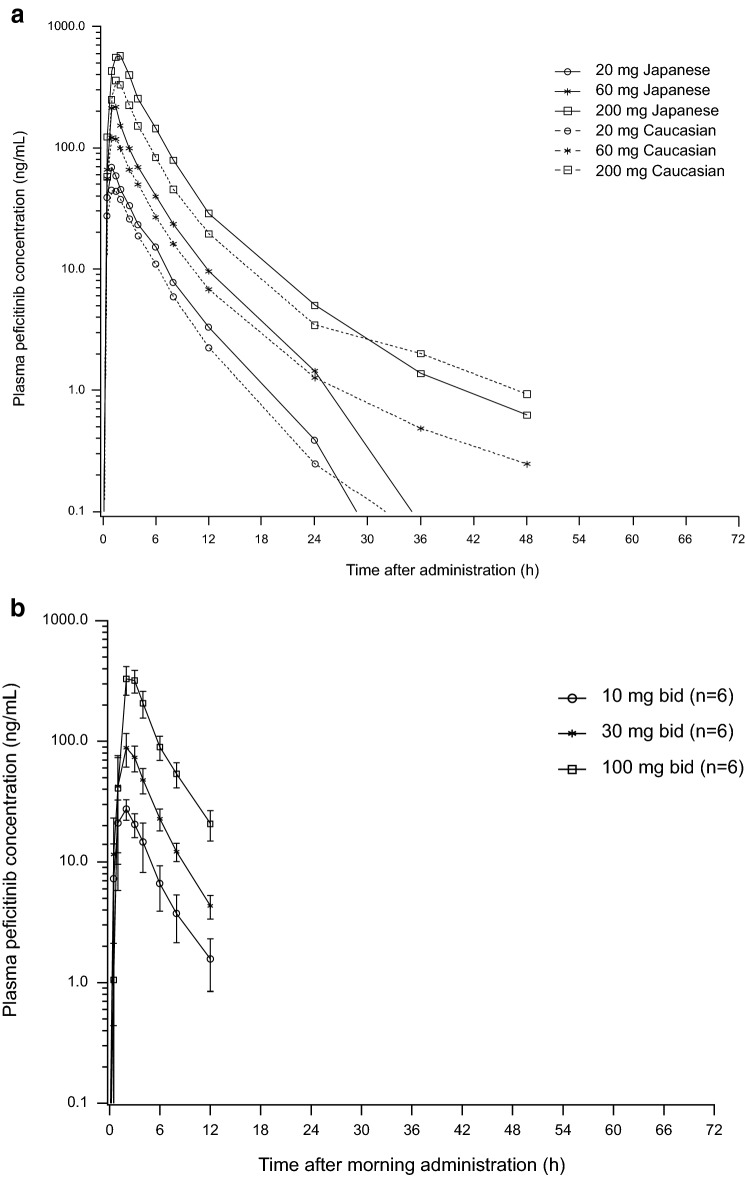
In the single-dose study, plasma concentrations of peficitinib were generally higher in Japanese than Caucasian subjects during the first 24 h after administration (Fig. 3a). Pharmacokinetic parameters are summarized in Table 2. As shown in this table, mean Cmax and AUC (AUCinf and AUClast) values were higher in Japanese subjects compared with Caucasian subjects, while median tmax values were similar in both populations (Table 2). Mean t½ ranged from 3.7 to 7.5 h in Japanese subjects and from 6.9 to 10.0 h in Caucasian subjects. The fraction of the peficitinib dose excreted unchanged in urine (Aelast%) ranged from 16.8% at 20 mg to 12.5% at 200 mg in Japanese subjects and from 14.2% at 20 mg to 9.9% at 200 mg in Caucasian subjects (Table 2).
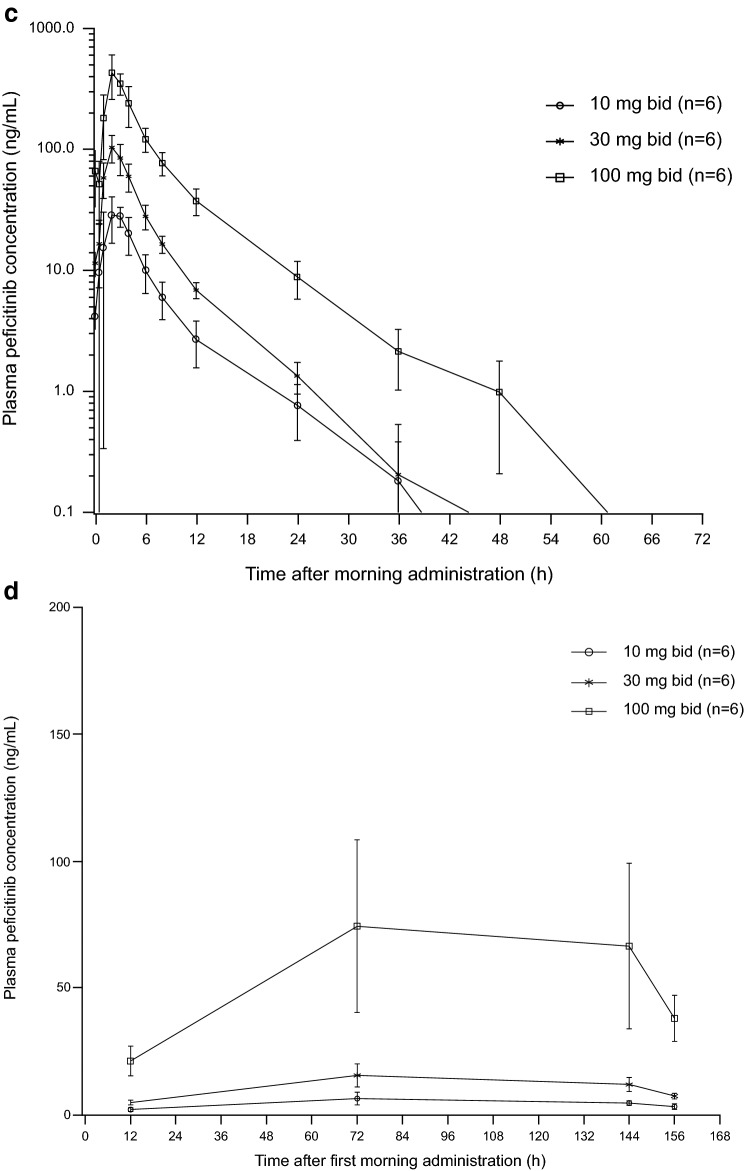
Fig. 3.
Mean plasma peficitinib concentration–time curves a after single doses in Japanese and Caucasian subjects, b on day 1 of multiple peficitinib dosing in Japanese subjects, c on day 7 of multiple peficitinib dosing in Japanese subjects, and d trough concentrations after first dose of multiple dosing. Each point in a represents the mean. Each point in b–d represents the mean and standard deviation
Table 2.
Plasma pharmacokinetic parameters of single-dose peficitinib in Japanese and Caucasian subjects
| Parameter | Japanese | Caucasian | ||||
|---|---|---|---|---|---|---|
| Peficitinib dose | ||||||
| 20 mg (n = 6) | 60 mg (n = 6) | 200 mg (n = 6) | 20 mg (n = 6) | 60 mg (n = 6) | 200 mg (n = 6) | |
| tmax (h) | ||||||
| Median | 1.0 | 1.3 | 2.0 | 1.0 | 1.0 | 1.8 |
| Range | 1.0–3.0 | 1.0–1.5 | 1.0–2.0 | 1.0–1.5 | 1.0–1.5 | 1.5–3.0 |
| Cmax (ng/mL) | ||||||
| Mean (SD) | 76.9 (24.2) | 241.1 (74.7) | 648.7 (55.5) | 48.9 (16.9) | 130.9 (19.4) | 381.2 (114.4) |
| %CV | 31.5 | 31.0 | 8.6 | 34.6 | 14.8 | 30.0 |
| AUCinf (ng∙h/mL) | ||||||
| Mean (SD) | 259.5 (42.9) | 782.8 (158.6) | 2524.9 (234.5) | 196.3 (54.6) | 528.6 (118.0) | 1520.3 (186.5) |
| %CV | 16.5 | 20.3 | 9.3 | 27.8 | 22.3 | 12.3 |
| AUClast (ng∙h/mL) | ||||||
| Mean (SD) | 256.2 (44.1) | 775.6 (155.9) | 2512.2 (232.3) | 191.1 (55.3) | 523.6 (117.4) | 1507.2 (192.4) |
| %CV | 17.2 | 20.1 | 9.2 | 28.9 | 22.4 | 12.8 |
| t½ (h) | ||||||
| Mean (SD) | 3.7 (0.7) | 4.0 (1.0) | 7.5 (4.9) | 7.3 (10.0) | 10.0 (5.0) | 6.9 (3.2) |
| %CV | 18.8 | 24.4 | 65.0 | 137.1 | 49.8 | 46.3 |
| CL/F (L/h) | ||||||
| Mean (SD) | 78.9 (13.5) | 79.8 (19.2) | 79.8 (8.2) | 108.5 (30.0) | 117.8 (23.6) | 133.1 (15.0) |
| %CV | 17.1 | 24.1 | 10.3 | 27.6 | 20.0 | 11.2 |
| Aelast (mg) | ||||||
| Mean (SD) | 3.4 (0.8) | 8.9 (1.7) | 25.1 (5.4) | 2.9 (0.7) | 6.4 (2.2) | 19.8 (2.4) |
| %CV | 24.6 | 19.0 | 21.6 | 25.3 | 33.9 | 11.8 |
| Aelast% (%dose) | ||||||
| Mean (SD) | 16.8 (4.1) | 14.8 (2.8) | 12.5 (2.7) | 14.2 (3.6) | 10.6 (3.6) | 9.9 (1.2) |
| %CV | 24.6 | 19.0 | 21.6 | 25.3 | 33.9 | 11.8 |
Aelast cumulative amount of unchanged drug excreted into the urine from the time of dosing to the last measurable concentration, Aelast% fraction of the drug dose excreted unchanged into the urine from the time of dosing to the last measurable concentration, AUCinf area under the plasma concentration–time curve from the time of dosing extrapolated to infinity, AUClast area under the plasma concentration–time curve from the time of dosing to the last measurable concentration, Cmax maximum plasma concentration, CL/F apparent total clearance of the drug from plasma after oral administration, CV coefficient of variation, SD standard deviation, t½, terminal elimination half-life, tmax time to maximum plasma concentration
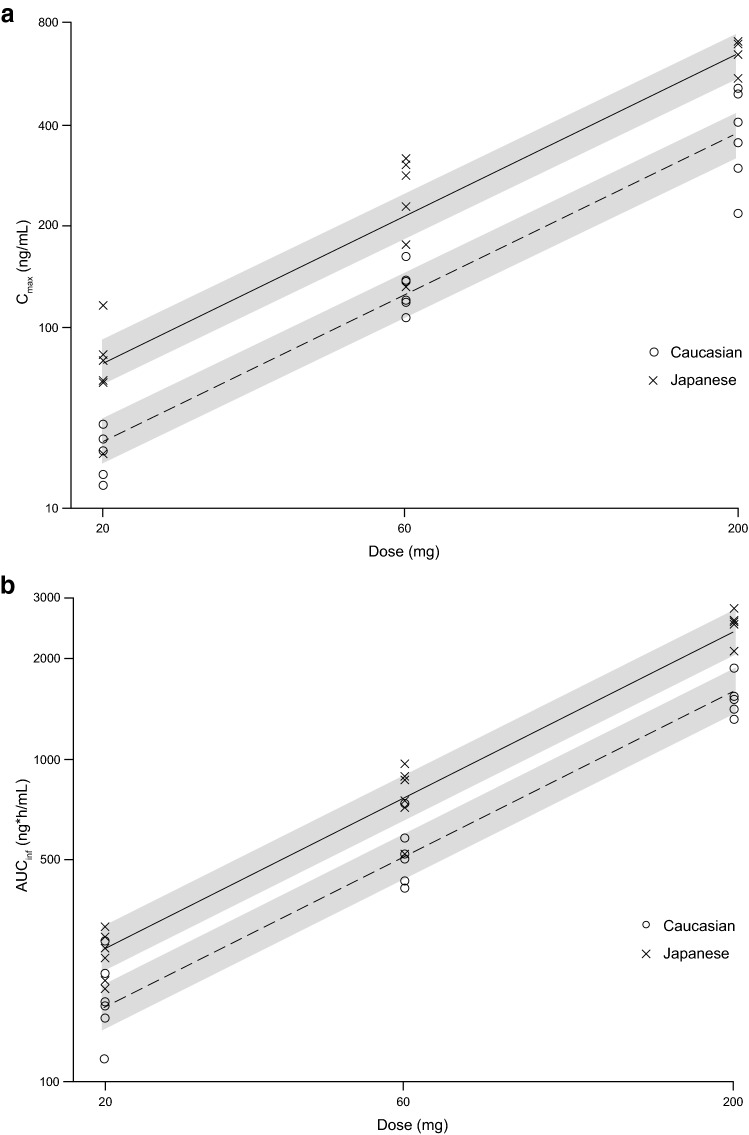
The plots of Cmax and AUCinf versus dose are shown in Fig. 4. The slope of the regression lines for Japanese and Caucasian subjects appeared comparable for both the Cmax and AUCinf plots (Fig. 4). Among race, age, and body weight, only race was a significant covariate for the Cmax and AUCinf of peficitinib (Table 3). The estimated slopes and 95% CIs were 0.919 (0.820, 1.019) and 0.947 (0.878, 1.016) for Cmax and AUCinf, respectively, and the CIs for these parameters contained 1 (Table 3). Dose proportionality of Cmax and AUCinf after single doses of peficitinib was demonstrated across the dose range of 20–200 mg. The estimated coefficients of race showed that Cmax and AUCinf increased 1.60-fold (= exp(0.47)) and 1.40-fold (= exp(0.34)) in Japanese compared to Caucasian subjects.
Fig. 4.
Dose proportionality of peficitinib aCmax and b AUCinf for Japanese and Caucasian subjects. The solid and dashed lines are regression lines for Japanese and Caucasian, respectively. Grey bands are the 90% confidence intervals around the predicted values. X and y axis are shown with log scale. AUCinf area under the plasma concentration–time curve from time 0 to infinity, Cmax maximum plasma drug concentration
Table 3.
Dose proportionality of peficitinib and the effects of race, age, and body weight after single dosing
| Parameter | Estimate | 95% CI | p value |
|---|---|---|---|
| Cmax | |||
| Slope | 0.919 | 0.820, 1.019 | < 0.0001 |
| Intercept | 1.495 | 0.476, 2.513 | 0.005 |
| Effect on intercept | |||
| Race (Japanese) | 0.473 | 0.243, 0.704 | < 0.001 |
| Body weight | − 0.00515 | − 0.0182, 0.00790 | 0.43 |
| Age | − 0.00138 | − 0.0211, 0.0183 | 0.89 |
| AUCinf | |||
| Slope | 0.947 | 0.878, 1.016 | < 0.0001 |
| Intercept | 2.802 | 2.094, 3.509 | < 0.0001 |
| Effect on intercept | |||
| Race (Japanese) | 0.337 | 0.177, 0.497 | < 0.001 |
| Body weight | − 0.00479 | − 0.0139, 0.00428 | 0.29 |
| Age | − 0.00286 | − 0.0161, 0.0108 | 0.67 |
AUCinf area under the plasma concentration–time curve from the time of dosing extrapolated to infinity, CI confidence interval, Cmax maximum plasma concentration
Multiple-Dose Study
In the multiple-dose study, plasma concentration profiles of peficitinib were similar on day 1 and day 7 (Fig. 3b). Pharmacokinetic parameters are summarized in Table 4. Mean Cmax and AUC12 values increased as dose increased, and were higher on day 7 compared with day 1 (Table 4). Median tmax values ranged from 1.5 to 2.5 h, and mean t½ values on day 7 ranged from 6.0 to 7.4 h. The fraction of the peficitinib dose excreted unchanged in urine from the time of dosing on day 7–12 h post-dose (Ae12%) ranged from 26.4% at 30 mg to 37.7% at 100 mg (Table 4). Steady-state plasma concentrations were achieved by day 3, as assessed by visual prediction (Fig. 3c). The Cmax on day 7 was 13–28% higher than the Cmax after the first dose, while AUC12 on day 7 was 25–37% higher than AUC12 after the first dose (Table 5).
Table 4.
Pharmacokinetic parameters of peficitinib after multiple dosing for 7 days in Japanese subjects
| Parameter | Day 1 | Day 7 | ||||
|---|---|---|---|---|---|---|
| Peficitinib dose | ||||||
| 10 mg bid (n = 6) | 30 mg bid (n = 6) | 100 mg bid (n = 6) | 10 mg bid (n = 6) | 30 mg bid (n = 6) | 100 mg bid (n = 6) | |
| tmax (h) | ||||||
| Median | 1.5 | 2.0 | 2.0 | 2.5 | 2.0 | 2.0 |
| Range | 1.0–2.0 | 2.0–3.0 | 2.0–3.0 | 1.0–4.0 | 1.0–3.0 | 2.0–3.0 |
| Cmax (ng/mL) | ||||||
| Mean (SD) | 28.5 (6.1) | 93.5 (21.2) | 383.4 (26.0) | 36.4 (8.2) | 106.2 (25.8) | 482.8 (77.3) |
| %CV | 21.5 | 22.7 | 6.8 | 22.4 | 24.3 | 16.0 |
| AUC12 (ng∙h/mL) | ||||||
| Mean (SD) | 114.5 (29.1) | 353.9 (60.8) | 1335.7 (135.1) | 144.6 (24.1) | 443.6 (80.1) | 1832.8 (261.3) |
| %CV | 25.5 | 17.2 | 10.1 | 16.6 | 18.1 | 14.3 |
| t½ (h) | ||||||
| Mean (SD) | NC | NC | NC | 6.0 (1.2) | 6.1 (3.6) | 7.4 (3.5) |
| %CV | 20.7 | 59.5 | 47.1 | |||
| CL/F (L/h) | ||||||
| Mean (SD) | 87.7 (23.6) | 83.3 (14.6) | 71.2 (8.7) | 71.0 (13.6) | 69.6 (13.5) | 55.7 (9.3) |
| %CV | 26.9 | 17.6 | 12.2 | 19.2 | 19.4 | 16.6 |
| Ae12 (mg) | ||||||
| Mean (SD) | 1.5 (0.4) | 4.7 (1.2) | 19.2 (2.7) | 3.2 (0.3) | 7.9 (1.3) | 37.7 (9.8) |
| %CV | 29.5 | 25.4 | 14.1 | 10.6 | 16.0 | 26.0 |
| Ae12% (%dose) | ||||||
| Mean (SD) | 14.9 (4.4) | 15.6 (4.0) | 19.2 (2.7) | 32.0 (3.4) | 26.4 (4.2) | 37.7 (9.8) |
| %CV | 29.5 | 25.4 | 14.1 | 10.6 | 16.0 | 26.0 |
Ae12 cumulative amount of unchanged drug excreted into the urine from the time of dosing to 12 h post-dose, Ae12% fraction of the drug dose excreted unchanged into the urine from the time of dosing to 12 h post-dose, AUC12 area under the plasma concentration–time curve from the time of dosing to 12 h post-dose, bid twice daily, Cmax maximum plasma concentration, CL/F apparent total clearance of the drug from plasma after oral administration, CV coefficient of variation, NC not calculated, SD standard deviation, t½ terminal elimination half-life, tmax time to maximum plasma concentration
Table 5.
Comparison of peficitinib Cmax and AUC12 between days 1 and 7
| Parameter (day 7/day 1) | Dose | GMR | 90% CI |
|---|---|---|---|
| Cmax | 10 mg bid | 1.28 | 1.03, 1.58 |
| 30 mg bid | 1.13 | 0.90, 1.44 | |
| 100 mg bid | 1.25 | 1.10, 1.42 | |
| AUC12 | 10 mg bid | 1.28 | 1.02, 1.62 |
| 30 mg bid | 1.25 | 1.04, 1.51 | |
| 100 mg bid | 1.37 | 1.19, 1.57 |
AUC12 area under the plasma concentration–time curve from the time of dosing to 12 h post-dose, bid twice daily, CI confidence interval, Cmax maximum plasma concentration, GMR geometric mean ratio
Pharmacodynamics
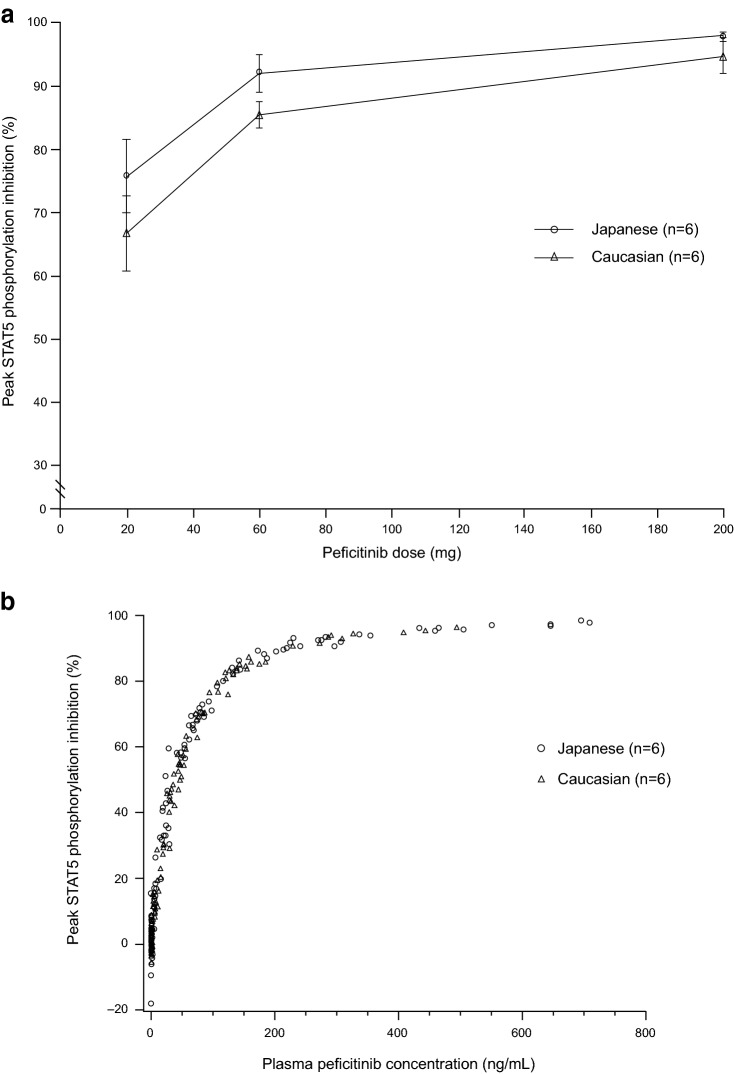
Mean peak inhibition of STAT5 phosphorylation, i.e. peak inhibition of JAKs, increased with increasing single doses of peficitinib and was generally higher in Japanese subjects than in Caucasian subjects (Fig. 5a). However, the extent of inhibition of STAT5 phosphorylation was similar for Japanese and Caucasian subjects when the plasma concentrations of peficitinib were comparable (Fig. 5b).
Fig. 5.
a Mean peak inhibition of STAT5 phosphorylation and b scatter plots for the percentage of STAT5 inhibition versus plasma peficitinib concentrations, after single doses of peficitinib in Japanese and Caucasian subjects. Each point in a represents the mean and standard deviation. STAT5 signal transducer and activator of transcription 5
The mean percentage change from baseline in peripheral lymphocyte subsets showed little variation (− 2.88% to 2.37%) by peficitinib dose after single doses of peficitinib. There was also no difference due to peficitinib dosing for the mean percentage change from baseline between Japanese and Caucasian subjects (Supplementary Table S2). After multiple doses of peficitinib in Japanese subjects, the mean percentage change in peripheral lymphocyte subsets again showed little variation (− 7.50% to 0.83%) by peficitinib dose or time of measurement (days 4 and 7 pre-dose and 72 h after last dosing). The largest change was a 7.50% decrease in the subset of CD3+, CD45RA+ and total naïve cells in patients administered with 100 mg peficitinib 72 h after twice-daily dosing on day 7. However, even in this subset, no dose dependency was apparent in the change from baseline (Supplementary Table S3).
Safety
In the single-dose study, four Japanese subjects each experienced a TEAE, one of which (increased blood potassium; 20-mg dose) was considered by the investigator to be possibly or probably related to peficitinib (Table 6). One Caucasian subject (60-mg dose) experienced diarrhea considered to be possibly or probably related to peficitinib. In the multiple-dose study, ten Japanese subjects experienced a total of 16 TEAEs, of which 14 TEAEs were considered possibly or probably related to peficitinib (Table 6). Of the 14 TEAEs, the most frequently reported was decreased neutrophil count at day 4 and day 7 (n = 3, receiving 100 mg twice daily). Neutrophil counts recovered in all three subjects by day 8.
Table 6.
Summary of treatment-emergent adverse events after a single dose of peficitinib in Japanese and Caucasian subjects and during multiple peficitinib dosing in Japanese subjects for 7 days
| TEAE, n (%) | Japanese | Caucasian | ||||||
|---|---|---|---|---|---|---|---|---|
| Peficitinib dose | Peficitinib dose | |||||||
| Placeboa (n = 6) | 20 mg (n = 6) | 60 mg (n = 6) | 200 mg (n = 6) | Placeboa (n = 6) | 20 mg (n = 6) | 60 mg (n = 6) | 200 mg (n = 6) | |
| Single-dose steps | ||||||||
| Any TEAE | 0 | 1 (16.7) | 2 (33.3) | 1 (16.7) | 0 | 0 | 1 (16.7) | 0 |
| Blood potassium increased | 0 | 1 (16.7)b | 0 | 0 | 0 | 0 | 0 | 0 |
| White blood cell count increased | 0 | 0 | 2 (33.3) | 1 (16.7) | 0 | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 1 (16.7)b | 0 |
| TEAE, n (%) | Peficitinib dose | |||
|---|---|---|---|---|
| Placebo bida (n = 6) | 10 mg bid (n = 6) | 30 mg bid (n = 6) | 100 mg bid (n = 6) | |
| Multiple-dose steps | ||||
| Any TEAE | 1 (16.7) | 2 (33.3) | 3 (50.0) | 4 (66.7) |
| Diarrhea | 0 | 0 | 1 (16.7)b | 0 |
| Feeling abnormal | 0 | 1 (16.7)b | 0 | 0 |
| Malaise | 1 (16.7)c | 0 | 0 | 0 |
| Alanine aminotransferase increased | 0 | 1 (16.7)b | 0 | 1 (16.7)b |
| Aspartate aminotransferase increased | 0 | 1 (16.7)b | 0 | 0 |
| Blood creatinine phosphokinase increased | 0 | 1 (16.7)b | 0 | 0 |
| Neutrophil count decreased | 0 | 0 | 0 | 3 (50.0)b |
| White blood cell count increased | 0 | 1 (16.7)b | 2 (33.3)d | 0 |
| Headache | 0 | 1 (16.7)b | 0 | 0 |
| Rash | 0 | 0 | 1 (16.7)b | 0 |
| Hot flush | 0 | 1 (16.7)b | 0 | 0 |
bid twice daily, TEAE treatment-emergent adverse event
aIn both the single- and multiple-dose studies, data for subjects receiving placebo were pooled
bConsidered by the investigator to be possibly or probably related to peficitinib
cConsidered by the investigator to be possibly or probably related to treatment with placebo
dConsidered by the investigator to be possibly or probably related to peficitinib in one of the two subjects
All TEAEs were mild in severity. There were no serious TEAEs or TEAEs resulting in discontinuation from the studies.
Discussion
Ethnicity can lead to variability in the pharmacokinetics and pharmacodynamics of drugs, which may affect their efficacy and safety profiles [17]. Consequently, it is important to characterize the safety and pharmacokinetic/pharmacodynamic profiles of a drug in the target ethnic population. In the present single-dose study, some differences in peficitinib pharmacokinetic findings were observed between the Japanese and Caucasian populations.
After single-dose administration, peficitinib was absorbed rapidly, with a median tmax of 1–2 h in both Japanese and Caucasian subjects. These findings are consistent with previous observations in healthy, fasted, mainly Caucasian men [11], where median tmax for peficitinib ranged from 1 h at 3–30 mg to 1.8 h at 300 mg [11]. In agreement with the previous single-dose study in the USA [11], dose proportionality was demonstrated for Cmax and AUCinf across the range of single peficitinib doses from 20 to 200 mg in both Japanese and Caucasian subjects. Cmax was 70% higher and AUCinf was 49% higher in Japanese subjects compared with Caucasian subjects. The higher drug exposure in Japanese subjects is consistent with observations in a previous study, in which peficitinib Cmax and AUC were found to be higher in Asian subjects compared with non-Asian subjects [20]. Peficitinib is metabolized by the sulfotransferase (SULT) isoenzyme, SULT2A1, and the methyltransferase isoenzyme, nicotinamide N-methyltransferase [18]. To date no genetic polymorphisms of these isoenzymes have been reported, and the cause of higher peficitinib exposure in Asian patients remains unknown. The mean cumulative amounts of peficitinib excreted unchanged in urine after single doses ranged from 3.4 to 25.1 mg in Japanese subjects, and from 2.9 to 19.8 mg in Caucasian subjects. Our results are consistent with data from a previous pharmacokinetic/pharmacodynamic study conducted in a primarily Caucasian population in the USA, which reported a range of 3.9–22.1 mg after single oral peficitinib doses of 30–200 mg [11]. These findings indicate that the elimination pathway for peficitinib is comparable in Japanese and Caucasian subjects.
In the multiple-dose study in Japanese subjects, steady-state plasma concentrations were achieved by day 3 with twice-daily dosing, also consistent with the previous US multiple-dose pharmacokinetic/pharmacodynamic study [11]. A mild accumulation of peficitinib was observed after multiple dosing; Cmax and AUC12 on day 7 increased by 13–28% and 25–37%, respectively, compared with day 1. It is known that peficitinib exposure (Cmax and AUC) is increased by high fat and calorie meals [21], and while study drugs were administered under fasted conditions in the single-dose study, they were administered after meals in the multiple-dose study. Nevertheless, we found that the Cmax and AUCinf in the single-dose study were similar to the Cmax and AUC12 on day 1 in the multiple-dose study, suggesting that there was no food effect. A breakfast limited to 500 cal for subjects in the multiple-dose study might help to explain the absence of a food effect when comparing exposures for the single- and multiple-dose studies.
As the IL-2 signal is delivered from its receptor (IL-2R) via JAK1/JAK3, which then phosphorylates STAT5, STAT5 phosphorylation was used as a biomarker of JAK activity. Similar to the previous US pharmacokinetic/pharmacodynamic study in mainly Caucasian subjects [11], we observed a dose-dependent inhibition of STAT5 phosphorylation, which provided support for the mechanism of pharmacological action of peficitinib (JAK inhibition) in Japanese subjects. Mean peak inhibition of STAT5 phosphorylation was generally higher in Japanese subjects compared with Caucasian subjects following a single dose of peficitinib, consistent with the higher plasma concentrations observed in Japanese subjects. However, when plasma concentrations of peficitinib were comparable, a similar level of STAT5 phosphorylation inhibition was observed, regardless of ethnicity. Therefore, the pharmacokinetic/pharmacodynamic relationship seems similar in Japanese and Caucasian subjects.
For the mean percent change from baseline in lymphocyte subsets, a clinically meaningful change was not observed after single or multiple dosing of peficitinib (with no dose-dependent changes), which was similar to the results reported in a previous US study [11]. No differences were observed in the change from baseline regardless of ethnicity (Japanese or Caucasian) for all lymphocyte subsets, leading to the conclusion that peficitinib has no effect on the cell count of lymphocyte subsets in either Japanese or Caucasian subjects.
Peficitinib was generally well tolerated. One Japanese subject and one Caucasian subject each had a TEAE that was potentially drug related (increased blood potassium and diarrhea, respectively) after a single dose. In the multiple-dose study, the most common TEAE that was potentially drug related was decreased neutrophil count, which was reported for three subjects receiving peficitinib 100 mg twice daily. This is consistent with the decline in neutrophil count observed in the highest dose groups (100 mg and 200 mg twice daily) of the previous US pharmacokinetic/pharmacodynamic study where subjects were treated with peficitinib for 14 days [11]. Post-treatment follow-up in our study demonstrated that this reduction in neutrophil count was rapidly reversible. In the previous US pharmacokinetic/pharmacodynamic study, a similar decrease in neutrophil count recovered within 3 days of administration of the last peficitinib dose [11]. Overall, the safety findings in this study were similar to those reported in the US single- and multiple-dose pharmacokinetic/pharmacodynamic studies in mostly Caucasian subjects [11].
Limitations of our study include the short duration of the study and the inclusion of healthy subjects instead of patients with RA. The study population was also young and not representative of the age demographic of the target population in which peficitinib is indicated. Furthermore, the study population was entirely male, although sex has been previously found to have no significant effect on peficitinib pharmacokinetic parameters [11]. As peficitinib is intended for long-term use in patients with RA, longer-term pharmacokinetic, pharmacodynamic, and safety assessments are required in male and female patients taking concomitant medications. Short- and longer-term safety were investigated in the phase 2b and phase 3 studies performed with male and female RA patients, even though some concomitant medication use was not allowed [12–14].
The major strength of our study is that it provided a direct comparison of the effects of ethnicity. Comparison or pooling of pharmacokinetic/pharmacodynamic data across studies involving different ethnicities and use of different dosage forms or regimens can confound data analysis and make interpretation of the findings difficult [22]. The Asian population has previously been under-represented in peficitinib pharmacokinetic studies. Our results indicate that following a single dose of peficitinib, at comparable plasma peficitinib concentrations, the level of JAK inhibition was similar in Japanese and Caucasian subjects.
Conclusion
Peficitinib was well tolerated when administered at doses up to 200 mg daily for 7 days in healthy, non-elderly, male Japanese subjects. All AEs were mild in severity and none resulted in study discontinuation. Linear increases in Cmax and AUC for peficitinib were observed within the investigated single-dose range, and peficitinib plasma concentrations were similar on day 1 and day 7 in the multiple-dose study. After single-dose administration, pharmacokinetic measures of exposure were approximately 70–80% higher in Japanese subjects than in Caucasian subjects. However, as a similar level of JAK inhibition was observed in Japanese and Caucasian subjects with comparable plasma concentrations of peficitinib, the pharmacokinetic/pharmacodynamic relationship seems similar regardless of ethnicity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Hiroyuki Fukase, M.D., who conducted this study as a principal investigator.
Author contributions
Study concept: TN. Study design and conduct: TN, TH. Data acquisition: TH, KO. Analysis and interpretation: MS, YK, JT, TN, MS. Writing and reviewing of manuscript: All authors.
Data availability
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at http://www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Compliance with Ethical Standards
Funding
This study was sponsored by Astellas Pharma Inc. Medical writing support was provided by Sarah Whitfield, Ph.D., for Cello Health MedErgy (Europe) and funded by Astellas Pharma Inc.
Conflict of interest
M. Shibata, T. Hatta, M. Saito, J. Toyoshima, Y. Kaneko, and T. Nishimura are employees of Astellas Pharma Inc. K. Oda is an employee of Astellas Research Institute of America LLC.
Ethics approval
The study was reviewed and approved by the Institutional Review Board. The study was conducted in accordance with Good Clinical Practices, International Conference on Harmonisation guidelines, applicable regulations, and guidelines governing clinical study conduct and the ethical principles that have their origin in the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Cheung TT, McInnes IB. Future therapeutic targets in rheumatoid arthritis? Semin Immunopathol. 2017;39:487–500. doi: 10.1007/s00281-017-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji J, Zhang L, Zhang Q, et al. Functional disability associated with disease and quality-of-life parameters in Chinese patients with rheumatoid arthritis. Health Qual Life Outcomes. 2017;15:89. doi: 10.1186/s12955-017-0659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36:685–695. doi: 10.1007/s00296-015-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320:1360–1372. doi: 10.1001/jama.2018.13103. [DOI] [PubMed] [Google Scholar]
- 5.Rubbert-Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther. 2009;11:S1. doi: 10.1186/ar2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen JS, Aletaha D, Bijlsma JWJ, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jegatheeswaran J, Turk M, Pope JE. Comparison of Janus kinase inhibitors in the treatment of rheumatoid arthritis: a systemic literature review. Immunotherapy. 2019;11:737–754. doi: 10.2217/imt-2018-0178. [DOI] [PubMed] [Google Scholar]
- 8.Hamaguchi H, Amano Y, Moritomo A, et al. Discovery and structural characterization of peficitinib (ASP015K) as a novel and potent JAK inhibitor. Bioorg Med Chem. 2018;26:4971–4983. doi: 10.1016/j.bmc.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Yamazaki S, Yamagami K, et al. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci. 2017;133:25–33. doi: 10.1016/j.jphs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Diller M, Hasseli R, Hülser ML, et al. Targeting activated synovial fibroblasts in rheumatoid arthritis by peficitinib. Front Immunol. 2019;10:541. doi: 10.3389/fimmu.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao YJ, Sawamoto T, Valluri U, et al. Pharmacokinetics, pharmacodynamics, and safety of ASP015K (peficitinib), a new Janus kinase inhibitor, in healthy subjects. Clin Pharmacol Drug Dev. 2016;5:435–449. doi: 10.1002/cpdd.273. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75:1057–1064. doi: 10.1136/annrheumdis-2015-208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Takeuchi T, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3) Ann Rheum Dis. 2019;78:1320–1332. doi: 10.1136/annrheumdis-2019-215163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi T, Tanaka Y, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. 2019;78:1305–1319. doi: 10.1136/annrheumdis-2019-215164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Astellas Pharma Inc. Oral JAK inhibitor Smyraf® tablets approved in Japan for the treatment of rheumatoid arthritis (including prevention of structural joint damage) in patients who have an inadequate response to conventional therapies [Press release]. 2019. https://www.astellas.com/en/news/14651. Accessed 12 June 2019.
- 16.Markham A, Keam SJ. Peficitinib: first global approval. Drugs. 2019;79:887–891. doi: 10.1007/s40265-019-01131-y. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda SU, Zhang L, Huang S-M. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–423. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
- 18.Oda K, Cao YJ, Sawamoto T, et al. Human mass balance, metabolite profile and identification of metabolic enzymes of [14C]ASP015K, a novel oral janus kinase inhibitor. Xenobiotica. 2015;45:887–902. doi: 10.3109/00498254.2015.1026864. [DOI] [PubMed] [Google Scholar]
- 19.Leonard WJ, Mitra S, Lin J-X. Immunology: JAK3 inhibition—is it sufficient? Nat Chem Biol. 2016;12:308–310. doi: 10.1038/nchembio.2066. [DOI] [PubMed] [Google Scholar]
- 20.Zhu T, Parker B, Wojtkowski T, et al. Drug interactions between peficitinib, an orally administered, once-daily Janus kinase inhibitor, and rosuvastatin in healthy subjects. Clin Pharmacokinet. 2017;56:747–757. doi: 10.1007/s40262-016-0474-4. [DOI] [PubMed] [Google Scholar]
- 21.Pharmaceuticals and Medical Devices Agency (PMDA) Japan. Smyraf tablets® 50 mg and 100 mg: package insert v3. 2019. https://image.packageinsert.jp/pdf.php?mode=1&yjcode=3999046F1023. Accessed 19 Feb 2020.
- 22.Chen ML. Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2006;45:957–964. doi: 10.2165/00003088-200645100-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Researchers may request access to anonymized participant-level data, trial-level data, and protocols from Astellas-sponsored clinical trials at http://www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.