Abstract
It is over 4 years since the Prospective Comparison of angiotensin receptor/neprilysin inhibitor (ARNI) with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial was published in New England Journal of Medicine. The PARADIGM-HF trial was the one that contributed to the official approval to use ARNI simultaneously with cardiac resynchronisation therapy (CRT) or implantable cardioverter-defibrillator (ICD) in patients who receive optimal medical treatment and still presented NYHA II-IV class symptoms according to the 2016 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic heart failure. The aim of this article is to summarise current knowledge on the activity of ARNI in a selected group of patients with heart failure with reduced ejection fraction (HFrEF) based on a recent PARADIGM-HF subanalysis in the field of renal function in patients with and without chronic kidney disease, glycaemia control in patients with diabetes, ventricular arrhythmias and sudden cardiac death and health-related quality of life. This article includes also recently announced findings on the TRANSITION study which revealed that HFrEF therapy with ARNI might be safely initiated after an acute decompensated heart failure episode, including patients with heart failure de novo and ACEI/ARB naïve, both hospitalised or shortly after discharge, in contrary to the PARADIGM-HF trial, where patients had to be administered a stable dose of an ACEI/ARB equivalent to enalapril 10 mg a day for at least 4 weeks before the screening.
Keywords: Chronic heart failure, Angiotensin receptor/neprilysin inhibitor, Angiotensin-converting enzyme, PARADIGM-HF, Diabetes, Chronic kidney disease, Sudden cardiac death, Quality of life, TRANSITION
Introduction
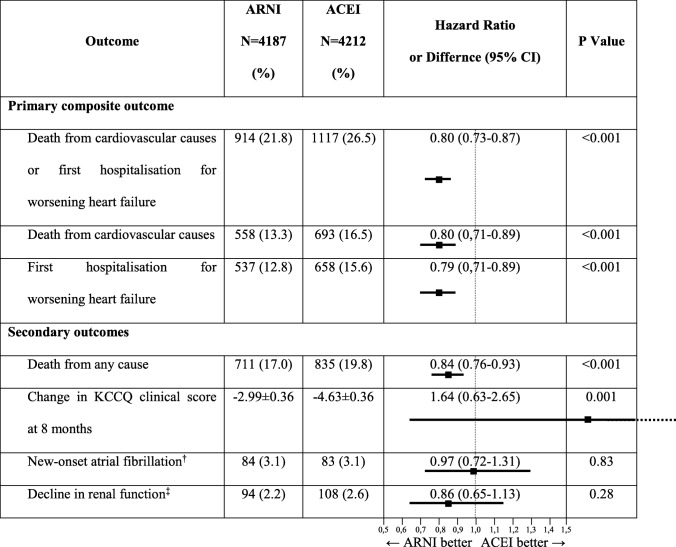
It is over 4 years since the Prospective Comparison of ARNI (angiotensin receptor/neprilysin inhibitor—editor’s note) with ACEI (angiotensin-converting enzyme inhibitor—editor’s note) to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial was published in New England Journal of Medicine. The purpose of the trial was to compare the efficacy of administering enalapril (ACEI) versus sacubitril/valsartan (ARNI) in a selected group of adult patients suffering from heart failure with reduced ejection fraction (HFrEF) of 35% or less and New York Heart Association (NYHA) class II-IV symptoms. The trial was terminated early after a median follow-up of 27 months because of a significantly reduced risk of the primary and secondary endpoint in the sacubitril/valsartan group compared with the enalapril group [1] (Table 1). The PARADIGM-HF trial was the one that contributed to the official approval to use ARNI simultaneously with cardiac resynchronisation therapy (CRT) or implantable cardioverter-defibrillator (ICD) in patients who receive optimal medical treatment and still presented NYHA II-IV class symptoms, according to the 2016 European Society of Cardiology Guidelines for the diagnosis and treatment of acute and chronic heart failure [2]. Glaggett et al. in their study confirmed that life expectancy of patients receiving ARNI might increase from 1 up to 2 years depending on their age compared with patients receiving ACEI [3]. This observation is the ground for strong recommendation to use a combined therapy with sacubitril and valsartan
Table 1.
Primary and secondary outcomes for the PARADIGM-HF trial, modified and adapted version [1]
ACEI angiotensin-converting enzyme inhibitor (enalapril), ARNI angiotensin receptor/neprilysin inhibitor (sacubitril/valsartan), KCCQ Kansas City Cardiomyopathy Questionnaire
†Atrial fibrillation not presented at randomisation
‡End-stage renal disease or a decrease of 50% or more in the estimated glomerular filtration rate (eGFR) from the value at randomisation or a decrease in the eGFR of more than 30 ml/min/1.73 m2 to less than 60 ml/min/1.73 m2
In the course of time, subsequent analyses of the PARADIGM-HF trial reveal the ARNI effect to be more and more beneficial for patients with heart failure. The aim of this article is to summarise current knowledge on the influence of ARNI in a selected group of patients with HFrEF, based on a recent PARADIGM-HF subanalysis.
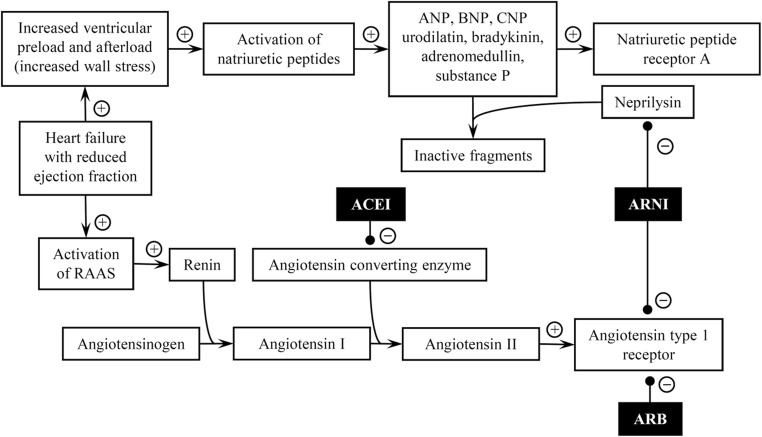
Randomised controlled trials have proved that the renin-angiotensin-aldosterone system (RAAS) plays an important role in the pathophysiology of heart failure, thus the morbidity and mortality of patients with HFrEF may be improved by blocking RAAS [4, 5]. In patients with HFrEF, upregulation of RAAS occurs, which in turn leads to excessive production of natriuretic peptides: B-type natriuretic peptide (BNP), atrial-derived A-type natriuretic peptide (ANP), endothelium-derived C-type natriuretic peptide (CNP) and kidney-derived urodilatin. In consequence, natriuretic peptides modulate the response to RAAS by promoting natriuresis and vasodilatation [6, 7]. It seems that the best strategy to improve outcomes in HFrEF would be inhibition of breakdown of the natriuretic peptides and blocking the RAAS at the same time [8, 9]. Neprilysin is a metalloendopeptidase and cleaves several different substrates such as ANP, BNP, CNP, endothelin, substance P, bradykinin and angiotensin I-II to inactive fragments, and as a consequence, it reduces the serum levels all of these peptides [9–11]. Inhibition of neprilysin with sacubitril results in an increase in serum levels of both natriuretic peptides and angiotensin II which stimulates the RAAS activity and counteracts the beneficial activity of natriuretic peptides [12]. Combination of sacubitril—neprilysin inhibitor—and valsartan—angiotensin receptor inhibitor—seems to be a better option than any other drug administered in heart failure management as it affects the pathophysiology of heart failure: it prevents degradation of natriuretic peptides and inhibits RAAS at the same time (Fig. 1) [9, 12]. Myhre et al. showed that during the treatment with ARNI, the serum BNP concentration increased up to 2–3 folds during the first 8–10 months compared to the initial BNP concentration while serum concentration of N-terminal prohormone of BNP (NT-proBNP) was relatively stable and its increase was not so dramatic as it was for BNP; an increase in the BNP concentration, accompanied by an increase in the NT-proBNP level, was associated with worse outcomes [13]. Nasrien et al. conducted a study on 23 subjects with HFrEF to assess the impact of sacubitril/valsartan on the level of natriuretic peptides, other than BNP in the serum with the use of different tests. It was revealed that the ANP concentration increased up to 2 folds by the first follow-up visit after on average 22 days of the treatment with ARNI, whereas the change in the CNP concentration remained inconsistent [14]. It is still unclear if the ARNI effect relies more on ANP or BNP activity, as BNP is a relatively poorer substrate for neprilysin than ANP or even CNP [15–17].
Fig. 1.
Pathways of activation renin-angiotensin-aldosterone and natriuretic peptide systems and points of interest for ACEI, ARB, and ARNI (“+” for activation and “–” for inhibition); adapted from: Jhund PS, McMurray JJV. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart. 2016 Sep 1;102(17):1342-7 [9]. ANP—A-type natriuretic peptide; ACEI—angiotensin-converting enzyme inhibitor; ARB—angiotensin receptor blocker; ARNI—angiotensin receptor/neprilysin inhibitor; BNP—B-type natriuretic peptide; CNP—C-type natriuretic peptide; RAAS—renin-angiotensin-aldosterone system
Effects of ARNI on renal function in patients with and without chronic kidney disease
Brenner et al. showed that RAAS inhibition reduces urinary albumin excretion and slows down the progression to end-stage renal disease, especially in patients without chronic kidney disease (CKD) or diabetes [18]. Treatment with RAAS inhibitors becomes limited in patients with renal impairment or diabetes because the risk of serum creatinine increase or hyperkalemia is greater than in patients with HFrEF and without the abovementioned co-morbidities [19].
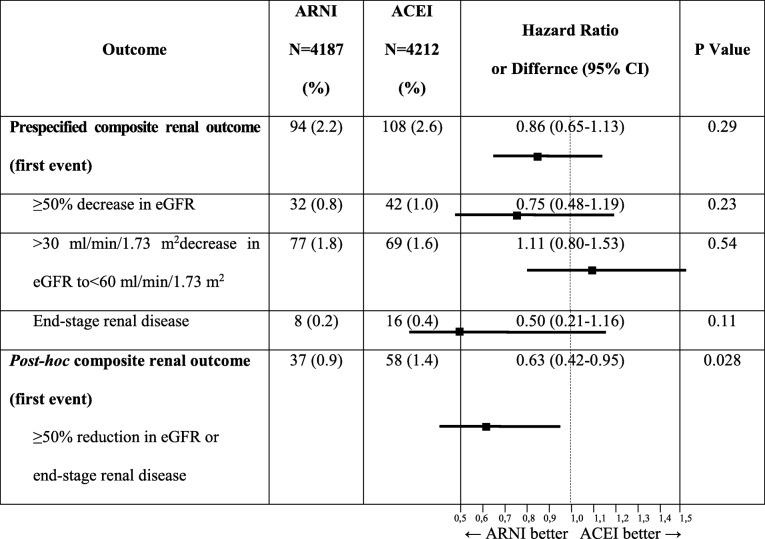
A subanalysis of the PARADIGM-HF trial, performed by Damman et al., concerning the effects of ARNI on renal function in comparison with ACEI, shows superiority of ARNI over ACEI both in patients with and without CKD [20]. At the screening, the eGFR in the trial was 70 ± 20 ml/min/1.73 m2 and 2745 patients of 8399 had CKD; the median urinary albumin/creatinine ratio (UACR) was 1.0 mg/mmol (UACR was available in 1872 patients). The rate of decline in eGFR was significantly lower in the sacubitril/valsartan than in the enalapril arm (− 1.61 ml/min/1.73 m2/year; [95% CI, − 1.77 to − 1.44] vs − 2.04 ml/min/1.73 m2/year [95% CI, − 2.21 to − 1.88], p < 0.001), despite a greater increase in UACR in the sacubitril/valsartan than in the enalapril arm (1.20 mg/mmol [95% CI, 1.04 to 1.36] vs 0.90 mg/mmol [95% CI, 0.77 to 1.03], p < 0.001). The results were similar in patients with and without CKD at screening (Table 2). Although the decrease in eGFR was similar in both groups and the incidence of the pre-specified renal outcome (a ≥ 50% decrease in eGFR or a > 30 ml/min/1.73 m2decrease in eGFR to < 60 ml/min/1.73 m2 or an end-stage renal disease) was similar between patients with or without CKD at screening, a post hoc analysis of a conventional renal composite outcome (an end-stage renal disease or a ≥ 50% decrease in the eGFR) revealed that end-stage renal disease occurred significantly less frequently in patients treated with sacubitril/valsartan, irrespective of presence of CKD at screening. What is more, the increase in UACR was associated with a higher risk of the pre-specified composite renal endpoint only in the enalapril group, but not in the sacubitril/valsartan group.
Table 2.
Renal endpoints for PARADIGM-HF trial; modified and adapted version [20]
ACEI angiotensin-converting enzyme inhibitor (enalapril), ARNI angiotensin receptor/neprilysin inhibitor (sacubitril/valsartan), eGFR estimated glomerular filtration rate
Packer et al. analysed again the PARADIGM-HF trial in the context of deterioration of renal function in patients with (3784 patients) and without concomitant diabetes (4615 patients). The deterioration of renal function was significantly lower in patients without diabetes than in diabetic patients (− 1.0 ml/min/1.73 m2/year [95% CI, − 1.2 to − 1.0] vs − 2.0 ml/min/1.73 m2/year [95% CI, − 2.1 to − 1.9], p < 0.0001) irrespective of the used treatment agent [21]. According to Packer et al., the rate of decline in eGFR was lower in the sacubitril/valsartan than in the enalapril arm but the statistically obtained values differ from those obtained by Damman et al. because of different determined criteria (− 1.3 ml/min/1.73 m2/year vs − 1.8 ml/min/1.73 m2/year, p < 0.0001). It is pointed out that the benefit of using ARNI was greater in diabetic than in non-diabetic patients (difference 0.6 ml/min/1.73 m2/year [95% CI, 0.4–0.8] vs 0.3 ml/min/1.73 m2/year [95% CI, 0.2–0.5], p = 0.038).
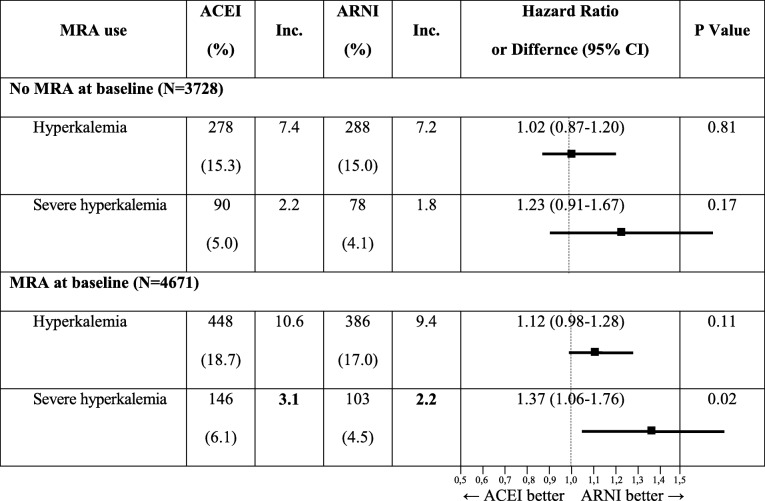
ARNI, apart from a decrease of lessening of the eGFR, also reduces the risk of hyperkalemia in patients who take mineralocorticoid receptor antagonists (MRAs) together with RAAS inhibitors. That was proved in a subanalysis conducted by Desai et al. [22]. The authors compared 4671 patients who were taking MRAs at baseline and who were randomly assigned to the group of sacubitril/valsartan or enalapril. Although the total number of incidences of hyperkalemia, identified for potassium level > 5.5 mEq/l, was similar in both treatment groups, incidences of severe hyperkalemia, identified for potassium level > 6.0 mEq/l, were more frequent in the enalapril than in the sacubitril/valsartan group (3.1/100 patient/year vs 2.2/100 patient/year; HR 1.37 [95% CI, 1.06–1.76], p = 0.02) (Table 3). An analysis of 791 patients with no MRAs at baseline who started taking MRAs during the PARADIGM-HF trial showed that severe hyperkalemia was more common in the group of enalapril than in the group of sacubitril/valsartan (3.3/100 patient/year vs 2.3/100 patient/year; HR 1.43 [95% CI, 1.13–1.81], p = 0.003). Although the risk of hyperkalemia between groups was not statistically significant, the risk of severe hyperkalemia was statistically significant, both for MRA non-recipients and MRA recipients [23]. Generally, patients treated with MRAs at baseline were younger, demonstrated more severe HF symptoms and higher potassium levels and used diuretics more frequently. It seems therefore that ARNI might attenuate the risk of hyperkalemia in patients with HF in the course of MRA therapy.
Table 3.
Prevalence of hyperkalemia and severe hyperkalemia in patients with and without MRAs at baseline assigned to the arms of PARADIGM-HF trial; modified and adapted version [22]
ACEI angiotensin-converting enzyme inhibitor (enalapril), ARNI angiotensin receptor/neprilysin inhibitor (sacubitril/valsartan), MRA mineralocorticoid receptor antagonist, Inc. incidence: no of cases/100 patients/year
The PARADIGM-HF study had several limitations, which makes it necessary to analyse the results particularly carefully. Firstly, patients were not randomised according to MRA use at baseline. Secondly, those who received MRAs were not monitored for dosing or compliance with MRAs during the study [22, 23]. Finally, the run-in period excluded patients who did not tolerate ARNI or ACEI or developed hyperkalemia during its administration. Due to residual differences between MRA-treated and MRA-naïve groups, some patients were excluded during the initial period of the trial. The true risk of hyperkalemia in both the groups randomised to the enalapril or sacubitril/valsartan arm may be underestimated and the hyperkalemia risk and the activity of ARNI, associated with the above risk, requires further investigation [22, 24].
Effects of ARNI on glycaemia control in patients with diabetes
Diabetes mellitus is a comorbidity frequently accompanying chronic heart failure and is an independent risk factor for heart failure progression [25, 26]. Findings of Kristensen et al. reveal that approximately 1 in 5 patients with HFrEF without a confirmed history of diabetes suffers from undiagnosed diabetes and 1 patient in 3 has actually pre-diabetes. The authors have divided all patients without history of diabetes into 3 groups, according to the concentration of glycated heamoglobin (HbA1c), measured at screening: normal < 6.0%, pre-diabetes 6.0–6.4% (2103 patients) and undiagnosed diabetes ≥ 6.5% (1106 patients); patients diagnosed previously with diabetes (2907 patients) were considered to have diabetes irrespective of HbA1c concentration [27]. An analysis showed that patients with a history of diabetes had a higher risk of the primary composite outcome of hospitalization due to heart failure or cardiovascular mortality, compared with those without a history of diabetes (HR 1.38 [95% CI, 1.25 to 1.52], p < 0.001); a higher risk was also observed in patients with undiagnosed diabetes and diagnosed diabetes, compared with those with normal HbA1c (HR 1.39 [95% CI, 1.17–1.64], p < 0.001 vs HR 1.64 [1.43–1.87], p < 0.001, respectively) and patients with pre-diabetes compared with those with normal HbA1c (HR 1.27 [95% CI, 1.10–1.47], p < 0.001).
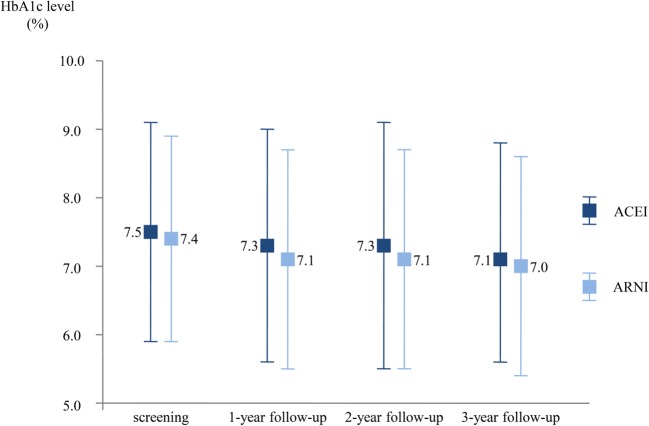
ARNI improves glycaemia control in patients with previously diagnosed diabetes and those with undiagnosed diabetes and HbA1c concentration ≥ 6.5% at screening that was shown by Seferovic et al. in their post hoc analysis [26]. Although no significant differences in HbA1c levels between randomised arms at screening were observed, HbA1c concentrations were lowered by 0.16% (± 1.4) in the enalapril arm while 0.26% (± 1.25) in the sacubitril/valsartan arm during the first year of follow-up (between-group reduction 0.13% [95% CI, 0.05–0.22], p = 0.0023) and remain lower over the 3-year follow-up (between-group reduction 0.14% [95% CI, 0.06–0.23], p = 0.0055) (Fig. 2). Additionally, new use of insulin or oral antihyperglycaemic drugs was lower in the group of sacubitril/valsartan than in the group of enalapril, which enhances the position of ARNI in optimal medical treatment of patients with HFrEF and concomitant diabetes.
Fig. 2.
Concentration of glycated haemoglobin at screening and over 3 years of follow-ups in patients with diagnosed diabetes and non-diabetic patients and HbA1c concentration ≥ 6.5% at screening randomised to enalapril or sacubitril/valsartan arm [21]. ACEI—angiotensin-converting enzyme inhibitor; ARNI—angiotensin receptor/neprilysin inhibitor; HbA1c—glycated haemoglobin
The pathophysiological mechanisms, underlying better glycaemia control when ARNI is administered, are not fully understood and need further investigation. Currently, it is usually attributed to higher concentration of active peptides which could not be degraded by inactive neprilysin like BNP, bradykinin and glucagon-like peptide-1 (GLP-1) (Fig. 3) [28–33]. Although the use of sacubitril/valsartan seems to be beneficial in patients with diabetes by potentiating antihyperglycaemic acting of endogenous GLP-1, the use of ARNI in concomitant treatment with GLP-1 long-acting analogues may be harmful in patients with both diabetes and heart failure. A deleterious effect of co-administering ARNI and GLP-1 analogues is explained by augmentation of GLP-1 influence on a heart rate and a concentration of cyclic adenosine monophosphate which limited ARNI efficacy [34].
Fig. 3.
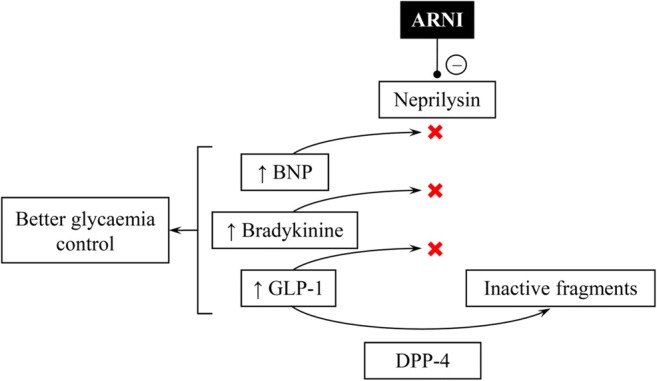
Mechanism of better glycaemia control when the use of sacubitril/valsartan underlies inter alia an increased concentration of peptides which cannot be degraded by actually blocked neprilysin (“–” for inhibition, “↑” for increase). ARNI—angiotensin receptor/neprilysin inhibitor; BNP—B-type natriuretic peptide; DPP-4—dipeptidyl peptidase-4; GLP-1—glucagon-like peptide-1
Effects of ARNI on all-cause mortality including sudden cardiac death
The impact of administration of sacubitril/valsartan on decrease in the number of cardiovascular deaths and all-cause mortality compared with enalapril in patients with chronic heart failure in the PARADIGM-HF trial, is incontestable. Desai et al. examined the data from the trial to better understand the mode of death [35]. According to the authors, most of the deaths were due to cardiovascular disorders (80.9%), and among them, the majority of cases were sudden cardiac deaths (44.8%) followed by worsening heart failure (26.5%) [35]. The risk of cardiovascular death was significantly reduced in the sacubitril/valsartan arm (HR 0.80 [95% CI, 0.72–0.89], p < 0.001) compared with enalapril and in deaths due to cardiovascular reasons; the reduction in mortality with sacubitril/valsartan was greater in comparison to enalapril and similar for both sudden cardiac deaths (HR 0.80 [95% CI 0.68–0.94], p = 0.008) and deaths due to increasing heart failure (HR 0.79 [95% CI 0.64–0.98], p = 0.034).
The precise mechanism of reduced mortality and sudden cardiac death in the group of sacubitril/valsartan remains unclear. However, Sarrias and Bayes-Genis suggest a direct involvement of neprilysin inhibition in the process [36]. Increase in natriuretic peptide concentration leads to molecular cascade, which in turn, reduces cardiac inflammation, myocyte death, hypertrophy and fibrosis; all these 4 factors are beneficial for patients with HFrEF as they reverse or reduce left ventricular remodeling. By inducing an antiarrhythmic effect with enkephalins, endorphins and bradykinin, they reduce the rate of ventricular tachyarrhythmias and ventricular premature beats which results in a decline of the rate of sudden cardiac death.
The beneficial effects of ARNI application, compared with ACEI on reduction of appropriate shocks, non-sustained ventricular tachycardia (nsVT) and premature ventricular contraction (PVC), were shown by de Diego et al. in a prospective, non-randomised trial which included 120 patients with HFrEF and implanted ICDs who were initially treated with ACEI or ARB within the first 9 months and then ACEI or ARB was subsequently changed to ARNI for the next 9 months of the follow-up [37]. ARNI, compared with ACEI or ARB, significantly decreased: nsVT episodes (5.4 ± 0.5 vs 15 ± 1.7, p < 0.002), sVT and appropriate ICD shocks (0.8% vs 6.7%, p < 0.02), PVCs per hour (33 ± 12 vs 78 ± 15, p < 0.0003) and increased biventricular pacing percentage (from 95% ± 6% to 98.8% ± 1.3%, p < 0.02). Such types of original studies provoke questions whether we still need ICDs if we have ARNI [38], but the answer is nowadays not obvious and the potential antiarrhythmic activity of ARNI needs further studies.
Effects of ARNI on health-related quality of life
The design of the PARADIGM-HF trial included not only objective and measureable data collection but also but also allowed patients to make a subjective analysis of their health-related quality of life (HRQL) when they used ARNI or ACEI, evaluated with the Kansas City Cardiomyopathy Questionnaire (KCCQ) completed, at randomisation and after 4, 8, 12, 24 and 36 months [39]. KCCQ includes both question about clinical status (i.e. extremities swelling, fatigue or shortness of breath) and social or physical activity (i.e. showering/bathing, hurrying/jogging or working/doing household chores).
This subanalysis performed by Lewis et al. showed that KCCQ clinical summary scores and KCCQ overall summary scores at 8 months were better in patients treated with sacubitril/valsartan compared with those treated with enalapril (+ 0.64 vs − 0.29, p = 0.008, for clinical summary scores and + 1.13 vs − 0.14, p < 0.001, for overall summary scores, respectively) [40].
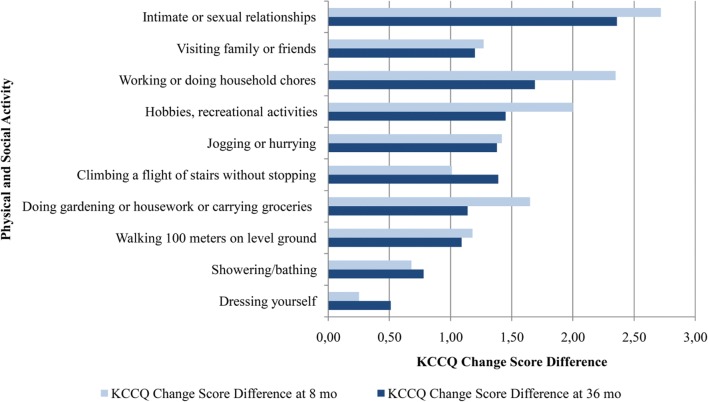
Similarly, Chandra et al. compared adjusted change scores in most physical and social activities at 8 months and during 36 months [41]. It is suggested that such types of activity like jogging and sexual relationships had the lowest mean scores whereas getting dressed and showering had the highest mean scores at baseline. The analysis performed at 8 months, reported significant adjusted change score in all social or physical activities except for getting dressed and showering (0.25 [95% CI, − 0.66–1.16], p = 0.59, and 0.68 [95% CI, − 0.24–1.59], p = 0.15, respectively), with the largest adjusted change score in household chores and sexual relationships (2.35 [95% CI, 1.19–3.50], p < 0.001, and 2.72 [95% CI, 0.97–4.46], p = 0.002, respectively) in the group of patients treated with sacubitril/valsartan (Fig. 4); the results were similar after 36 months. As a result, the improvement in both physical and social activity is claimed to be circa 9 years of aging (95% CI, 4–13 years, p < 0.001) in favour of sacubitril/valsartan arm.
Fig. 4.
Change score differences of KCCQ physical and social activities between enalapril and sacubitril/valsartan group at 8-month and at 36-month follow-up [37, 38]. KCCQ—Kansas City Cardiomyopathy Questionnaire; mo—months
When is the moment for ARNI initiation? TRANSITION study
TRANSITION (Comparison of Pre- and Post-discharge Initiation of LCZ696 Therapy in HFrEF Patients After an Acute Decompensation Event) is a randomised, simultaneous, open-label study which aimed at comparing pre- and post-discharge (1–14 days) initiation of sacubitril/valsartan in patients with HFrEF, LVEF ≤ 40% and NYHA class II–IV following hemodynamic stabilisation (defined as no need for intravenous diuretics in 24 h prior to screening and systolic blood pressure > 110 mmHg for at least 6 h prior to randomisation), after an episode of acute decompensated heart failure (ADHF), including patients with newly diagnosed HF and also ACEI/ARB naïve (no ACEI/ARB for ≥ 4 weeks before hospitalisation). In contrast, all patients of PARADIGM-HF were administered a stable dose of an ACEI/ARB equivalent to enalapril 10 mg/day for at least 4 weeks before the screening.
The primary endpoint was the proportion of patients achieving 200 mg sacubitril/valsartan twice a day at 10 weeks’ post-randomisation, and it was reached by 45.0% of 493 patients in the pre-discharge arm and 50.4% of 490 patients in the post-discharge arm (relative risk ratio [RRR] 0.893; [95% CI, 0.783–1.019], p = 0.092) [42, 43].
As the incidence of adverse events of sacubitril/valsartan and their discontinuations was similar in pre- and post-discharge groups, it is concluded that HFrEF therapy with ARNI might be safely initiated after an ADHF episode, including patients with new-onset and ACEI/ARB naïve, both in-hospital or shortly after discharge.
Conclusions
The PARADIGM-HF was a well-designed clinical trial that confirmed benefits due to initiation of sacubitril/valsartan in patients diagnosed with HFrEF, LVEF ≤ 35% and NYHA II–IV symptoms despite optimal medical treatment accompanied by cardiac resynchronisation therapy (CRT) or implantable cardioverter-defibrillator (ICD). Although, we do not understand all mechanisms of ARNI activity and the way it decreases HF symptoms, some of the subanalyses provide us invaluable experience on how ARNI reduce the decline in eGFR and risk of hyperkalemia in patients with and without chronic kidney disease, improve glycaemia control in diabetic patients, prevent ventricular arrhythmias or sudden cardiac death and ameliorate health-related quality of life. The new TRANSITION study results, where sacubitril/valsartan is initiated earlier than previously, suggest that ARNI will be used more frequently soon. It brings a hope for patients, affected by HF and who have not responded really positively to a conventional therapy.
Acknowledgements
None declared.
Compliance with ethical standards
Conflict of interest
Małgorzata Lelonek participated in clinical trials with molecule LCZ696. Marcin Książczyk declares that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 3.Claggett B, Packer M, McMurray JJ, Swedberg K, Rouleau J, Zile MR, Jhund P, Lefkowitz M, Shi V, Solomon SD, PARADIGM-HF Investigators Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373(23):2289–2290. doi: 10.1056/NEJMc1509753. [DOI] [PubMed] [Google Scholar]
- 4.Investigators SOLVD, Yusuf S, Pitt B, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 5.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 6.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Schulz-Knappe P, Forssmann K, Herbst F, Hock D, Pipkorn R, Forssmann WG. Isolation and structural analysis of “urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin Wochenschr. 1988;66:752–759. doi: 10.1007/BF01726570. [DOI] [PubMed] [Google Scholar]
- 8.Rademaker MT, Charles CJ, Espiner EA, et al. Combined inhibition of angiotensin II and endothelin suppresses the brain natriuretic peptide response to developing heart failure. ClinSci. 2004;106:569–576. doi: 10.1042/CS20030366. [DOI] [PubMed] [Google Scholar]
- 9.Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart. 2016;102(17):1342–1347. doi: 10.1136/heartjnl-2014-306775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 11.Dalzell JR, Seed A, Berry C, et al. Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: studies with thiorphan and omapatrilat. Cardiovasc Ther. 2014;32:13–18. doi: 10.1111/1755-5922.12053. [DOI] [PubMed] [Google Scholar]
- 12.Bayés-Genís A. Neprilysin in heart failure: from oblivion to center stage. JACC Heart Fail. 2015;3(8):637–640. doi: 10.1016/j.jchf.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Myhre PL, Vaduganathan M, Claggett B, Packer M, Desai AS, Rouleau JL, Zile MR, Swedberg K, Lefkowitz M, Shi V, McMurray J, Solomon SD. B-Type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019;73(11):1264–1272. doi: 10.1016/j.jacc.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim NE, McCarthy CP, Shrestha S, Gaggin HK, Mukai R, Szymonifka J, Apple FS, Burnett JC Jr, Iyer S, Januzzi JL Jr Effect of neprilysin inhibition on various natriuretic peptide assays. J Am Coll Cardiol. 2019;73(11):1273–1284. doi: 10.1016/j.jacc.2018.12.063. [DOI] [PubMed] [Google Scholar]
- 15.Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278(11):1808–1817. doi: 10.1111/j.1742-4658.2011.08082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pankow K, Schwiebs A, Becker M, Siems WE, Krause G, Walther T. Structural substrate conditions required for neutral endopeptidase-mediated natriuretic peptide degradation. J Mol Biol. 2009;393(2):496–503. doi: 10.1016/j.jmb.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe Y, Nakajima K, Shimamori Y, Fujimoto Y. Comparison of the hydrolysis of the three types of natriuretic peptides by human kidney neutral endopeptidase 24.11. Biochem Mol Med. 1997;61(1):47–51. doi: 10.1006/bmme.1997.2584. [DOI] [PubMed] [Google Scholar]
- 18.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 19.Clark H, Krum H, Hopper I. Worsening renal function during 16ennin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur J Heart Fail. 2014;16:41–48. doi: 10.1002/ejhf.13. [DOI] [PubMed] [Google Scholar]
- 20.Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, Zile MR, Packer M, Desai AS, Solomon SD, McMurray J. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–498. doi: 10.1016/j.jchf.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Packer M, Claggett B, Lefkowitz MP, McMurray J, Rouleau JL, Solomon SD, Zile MR. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the ennin-angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2018;6(7):547–554. doi: 10.1016/S2213-8587(18)30100-1. [DOI] [PubMed] [Google Scholar]
- 22.Desai AS, Vardeny O, Claggett B, et al. Reduced risk of hyperkalemia during treatment of heart failure with mineralocorticoid receptor antagonists by use of sacubitril/valsartan compared with enalapril. A secondary analysis of the PARADIGM-HF Trial. JAMA Cardiol. 2017;2(1):79–85. doi: 10.1001/jamacardio.2016.4733. [DOI] [PubMed] [Google Scholar]
- 23.Masoudi FA, MD, MSPH, FACC, FAHA reviewing Desai AS et al. JAMA Cardiol 2016 Nov 14 Ezekowitz JA. JAMA Cardiol 2016 Nov 14 Does sacubitril/valsartan reduce hyperkalemia in HFrEF patients taking MRAs? https://www.jwatch.org/na42871/2016/12/20/does-sacubitril-valsartan-reduce-hyperkalemia-hfref. Accessed 28 Aug 2019
- 24.Ezekowitz JA (2016) Hyperkalemia, sacubitril/valsartan, and mineralocorticoid antagonists in patients with heart failure. JAMA Cardiol. 10.1001/jamacardio.2016.4731 [DOI] [PubMed]
- 25.Tomova GS, Nimbal V, Horwich TB. Relation between hemoglobin (1c) and outcomes in heart failure patients with and without diabetes mellitus. Am J Cardiol. 2012;109:1767–1773. doi: 10.1016/j.amjcard.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5(5):333–340. doi: 10.1016/S2213-8587(17)30087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristensen SL, Preiss D, Jhund PS et al (2016) Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial. Circ Heart Fail 9(1). 10.1161/cirheartfailure.115.002560 [DOI] [PMC free article] [PubMed]
- 28.Chrysohoou C, Tousoulis D. The role of sacubitril/valsartan therapy on renal function and glucose metabolism in chronic heart failure patients. Integr Obesity Diabetes. 2017;3(6):1–6. doi: 10.15761/IOD.1000196. [DOI] [Google Scholar]
- 29.Heinisch BB, Vila G, Resl M, Riedl M, Dieplinger B, Mueller T, Luger A, Pacini G, Clodi M B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men. Diabetologia. 55(5):1400–1405. 10.1007/s00125-011-2392-1 [DOI] [PubMed]
- 30.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122(12):4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori MA, Sales VM, Motta FL, et al. Kinin B1 receptor in adipocytes regulates glucose tolerance and predisposition to obesity. PLoS One. 2012;7(9):e44782. doi: 10.1371/journal.pone.0044782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plamboeck A, Holst JJ, Carr RD, et al. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48(9):1882–1890. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- 33.Willard JR, Barrow BM, Zraika S, et al. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia. 2017;60(4):701–708. doi: 10.1007/s00125-016-4172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Packer M. Augmentation of glucagon-like peptide-1 receptor signalling by neprilysin inhibition: potential implications for patients with heart failure. Eur J Heart Fail. 2018;20(6):973–977. doi: 10.1002/ejhf.1185. [DOI] [PubMed] [Google Scholar]
- 35.Desai AS, McMurray J, Packer M, Swedberg K, Rouleau JL, Chen F, Gong J, Rizkala AR, Brahimi A, Claggett B, Finn PV, Hartley LH, Liu J, Lefkowitz M, Shi V, Zile MR, Solomon SD. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36(30):1990–1997. doi: 10.1093/eurheartj/ehv186. [DOI] [PubMed] [Google Scholar]
- 36.Sarrias A, Bayes-Genis A. Is sacubitril/valsartan (also) an antiarrhythmic drug? Circulation. 2018;138(6):551–553. doi: 10.1161/circulationaha.118.034755. [DOI] [PubMed] [Google Scholar]
- 37.de Diego C, González-Torres L, Núñez JM, Centurión Inda R, Martin-Langerwerf DA, Sangio AD, Chochowski P, Casasnovas P, Blazquéz JC, Almendral J. Effects of angiotensin-neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm. 2018;15(3):395–402. doi: 10.1016/j.hrthm.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich JR. Do we still need ICDs if we have ARNi? Heart Rhythm. 2018;15(3):403–404. doi: 10.1016/j.hrthm.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi: 10.1016/S0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 40.Lewis EF, Claggett BL, McMurray JJV et al (2017) Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail 10(8). 10.1161/circheartfailure.116.003430 [DOI] [PubMed]
- 41.Chandra A, Lewis EF, Claggett BL, Desai AS, Packer M, Zile MR, Swedberg K, Rouleau JL, Shi VC, Lefkowitz MP, Katova T, McMurray J, Solomon SD. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. 2018;3(6):498–505. doi: 10.1001/jamacardio.2018.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in hospitalized patients with HFrEF after hemodynamic stabilization: Baseline characteristics of the TRANSITION study compared with TITRATION and PARADIGM-HF. Poster P6531 presented at ESC congress, Munich, Germany, 25-29 August 2018
- 43.Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in hospitalized patients with heart failure with reduced ejection fraction after hemodynamic stabilization: Primary results of the TRANSITION study. Poster P886 presented at ESC Congress, Munich, Germany, 25-29, 2018 August, 2018